Abstract
BACKGROUND
Steatosis decreases survival of liver grafts after transplantation due to poorly understood mechanisms. We examined the effect of steatosis on the survival of liver grafts in a rat liver transplantation model and the viability of cultured rat hepatocytes after hypoxia and reoxygenation.
MATERIALS AND METHODS
Rats were fed a choline and methionine-deficient diet (CMDD) to induce hepatic steatosis and the livers were transplanted into recipient rats after 6 h of cold storage. Cultured hepatocytes were made steatotic by incubation for 3 days in fatty acid-supplemented medium. Hypoxia and reoxygenation were induced by placing the cultures in a 90% N2/10% CO2 atmosphere for 4 h, followed by return to normoxic conditions for 6 h. Hepatocyte viability was assessed by lactate dehydrogenase release and mitochondrial potential staining.
RESULTS
Transplanted steatotic livers exhibited 0% viability compared to 90% for lean liver controls. When donor CMDD rats were returned to a normal diet, hepatic fat content decreased while viability of the grafts after transplantation increased. Cultured steatotic hepatocytes generated more mitochondrial superoxide, exhibited a lowered mitochondrial membrane potential, and released significantly more lactate dehydrogenase after hypoxia and reoxygenation than lean hepatocyte controls. When steatotic hepatocytes were defatted by incubating in fatty acid-free medium, they became less sensitive to hypoxia and reoxygenation as the remaining intracellular triglyceride content decreased.
CONCLUSIONS
Hepatic steatosis reversibly decreases viability of hepatocytes after hypoxia and reoxygenation in vitro. The decreased viability of steatotic livers after transplantation may be due to a direct effect of hypoxia and reoxygenation on hepatocytes, and can be reversed by defatting.
Keywords: steatosis, liver transplantation, choline and methionine-deficient diet, intracellular triglyceride, defatting
INTRODUCTION
Hepatic steatosis is usually an asymptomatic condition but is a significant risk factor in liver transplantation; in fact, greater than 30% steatosis constitutes one of the single greatest risk factors, similar to the risk associated with donors after cardiac death for liver transplantation (1). Transplanted steatotic livers exhibit increased rates of primary nonfunction and initial poor graft function (2–4). The current consensus is that donors with greater than 60% fat content should not be used, while mild to moderately steatotic grafts can perform as well as nonsteatotic grafts as long as there are no other risk factors with the donor or recipient. In living donors with steatotic livers, combination therapies of diet, exercise and drugs for 2 to 8 weeks have been successfully used to reduce macrovesicular steatosis prior to transplantation, indicating that measures to reduce steatosis in steatotic donors can be used to reduce the risk of transplantation (5).
Based on steatotic animal models, fatty livers tend to have enlarged hepatocytes due to cytoplasmic lipid droplets with a concomitant reduction in sinusoidal space, as well as reduced total hepatic blood flow and microvascular perfusion (6, 7). Furthermore, experimental animal data suggest that steatotic livers are much more sensitive to ischemia-reperfusion than normal “lean” livers. For example, hepatic warm ischemia in obese Zucker rats had a much more pronounced effect on animal survival and led to more hepatocyte necrosis, microvascular disruption, and oxidative damage than in lean animals (8). Rats fed a choline- and methionine-deficient diet (CMDD) exhibited more functional impairment after ischemia-reperfusion in situ, and increased oxidative stress compared to animals fed a normal diet (8, 9). Studies using the same animal model on the effect of cold storage of liver followed by rewarming and perfusion also show more extensive damage in fatty livers than in lean livers, and a reduced “safe” preservation time before transplantation with steatotic livers (9, 10).
The mechanism whereby steatosis increases the sensitivity of the liver to ischemia-reperfusion injury is poorly understood. More specifically, the primary event responsible for the exacerbated response has not been identified, and it is unclear whether the enhanced inflammatory response is the result of or a causative factor in this response. In this study, we describe a cell culture model to investigate the effect of hypoxia-reoxygenation on hepatocyte viability. We show that steatotic hepatocytes are more sensitive to warm hypoxia-reoxygenation than normal “lean” hepatocytes. Furthermore, we show that steatotic hepatocytes generate more mitochondrial superoxide and exhibit a lowered mitochondrial membrane potential. Thus, it is plausible that steatotic hepatocytes are primary responders to warm ischemia-reperfusion injury in vivo.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco’s modified Eagle medium (DMEM) with 4.5 g/L glucose, penicillin-streptomycin stock solution, epidermal growth factor, and fetal bovine serum, were purchased from Invitrogen Life Technologies (Carlsbad, CA); glucagon from Lilly (Indianapolis, IN); hydrocortisone from Upjohn (Kalamazoo, MI); insulin from Squibb (Princeton, NJ). JC-1 (full name 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolyl-carbocyanine iodide, cat. #T3168), MitoSOX Red dye (cat. #M36008), menadione (cat. #M5625), and all other chemicals, unless otherwise noted, were purchased from Sigma-Aldrich Chemicals (St. Louis, MO).
Standard hepatocyte culture medium consisted of DMEM supplemented with 7 ng/mL glucagon, 7.5 μg/mL hydrocortisone, 0.5 U/mL insulin, 20 ng/mL epidermal growth factor, 200 U/mL penicillin, 200 μg/mL streptomycin, and 10% v/v fetal bovine serum. Type I collagen (1.11 mg/mL in 1 mM HCl) was prepared by extracting acid-soluble collagen from rat tail tendons as described elsewhere (11).
Induction of Hepatic Steatosis in Donor Rats
All procedures with animals were approved by the Subcommittee on Research Animal Care, Massachusetts General Hospital and in accordance with National Research Council guidelines. Male Lewis rats (Charles River, Wilmington, Massachusetts) weighing 280 to 320 g were housed in a 12 h day - light cycle and allowed free access to food and water. To induce fatty liver, the rats were fed a choline and methionine-deficient diet (Test Diet, Richmond, Indiana) for 40 to 44 days (12, 13).
Liver Transplantation
Isogenic orthotopic liver transplantation was performed as described by Kamada and Calne, and later by Mokuno et al. (14, 15). Briefly, the bile duct was cannulated with a short intraluminal polyethylene stent. Veins emptying into the portal vein and the hepatic artery were ligated and divided, and the portal vein divided at the level of the inferior mesenteric vein. The infrahepatic and suprahepatic vena cava, including part of the diaphragm, were transected. The liver was flushed with 10 mL cold saline containing 50 U heparin and then 5 ml hetastarch-free University of Wisconsin solution (Viaspan ™, Barr Laboratories, Pomona, NY) and stored for 6 h at 0°C. After cold storage, orthotopic liver transplantation was performed without hepatic artery reconstruction. The donor liver was flushed with 6 ml cold Ringer’s solution, the suprahepatic vena cava anastomosed with a 7-0 nylon running suture, and the portal vein anastomosed using the cuff technique. Blood was allowed to flow into the donor liver, and the infrahepatic vena cava anastomosed using the cuff technique. After revascularization of the graft, the rat was given 8 ml/kg Ringer’s solution and 2 mL/kg 7% w/v NaHCO3 intravenously, and intramuscular injections of 80 mg/kg penicillin and 100 mg/kg streptomycin. The bile duct was connected and wrapped around the omentum. Anhepatic time ranged from 14 to 16 min. The animals were returned to standard housing facilities and monitored for up to one week.
Hepatocyte Isolation, Seeding and Culture
Female Lewis rats (Charles River Laboratories, Wilmington, MA) weighing 180 to 200 g (2 to 3 months old), were used as a hepatocyte source. Hepatocytes were isolated by the two-step collagenase perfusion technique originally described by Seglen (16) and slightly modified by Dunn (11). Typically, 200 to 300 million hepatocytes were isolated from a single isolation with viabilities ranging between 85% and 95%, as determined by trypan blue exclusion. Nonparenchymal cells, as judged by their size (less than 10 μm in diameter) and morphology (nonpolygonal or stellate), were <1%.
Twelve-well tissue culture plates were coated with a thin layer of type I collagen gel. Briefly, 200 μl of an ice-cold mixture of 1 part of 10X concentrated DMEM and 9 parts of acid solution of type I collagen was evenly distributed over the bottom of each well. The collagen solution was allowed to gel at 37°C for 60 min before cell seeding.
One-half million of primary hepatocytes in suspension in 0.5 ml of hepatocyte culture medium was added into each well of the plate. Cultures were incubated in 90% air/10% CO2 at 37°C. Twenty-four hours later, the medium was aspirated along with unattached cells, and a second layer of collagen was put on top of the culture. After allowing to gel for 60 min, 0.5 ml fresh culture medium was added to the cultures, and changed daily thereafter.
Induction of Steatosis and Defatting of Hepatocytes
Seven days after seeding of hepatocytes, the medium was switched to fatty acid-supplemented medium. This “fatty medium” consisted of standard hepatocyte culture medium supplemented with 20 mg/ml albumin, 2 mM linoleic acid, and 4 mM sodium oleate. Hepatocytes were cultured for 2 days in this medium, with daily medium changes. Subsequent defatting was induced by switching the cultures back to standard hepatocyte culture medium for 3 days, with daily medium changes.
Exposure of Hepatocytes to Hypoxia-Reoxygenation
A hypoxic chamber consisting of a hermetically closed plexiglass box (12 x 12 x 10 in) with drilled ports for gassing and an oxygen probe was used. The tissue culture plates were placed in the chamber, the chamber closed tightly, and purged continuously with humidified 90% N2 / 10% CO2 for up to 4 hours. After the prescribed hypoxia time, the plates were retrieved from the chamber, the culture medium collected for further analysis, and the cells fed with fresh culture medium pre-equilibrated at normoxic conditions. The plates were then placed back in a regular incubator, and the medium sampled 3 and 6 hours later.
In some cultures, Kupffer cells were isolated with a modified double step Percoll protocol (17, 18). The day before the ischemia-reperfusion experiment, 5x105 Kupffer cells were seeded on top of the double-gel hepatocyte cultures. Less than 24 h after seeding, Kupffer cells were found to migrate through the collagen matrix and settle down close to the hepatocytes.
Triglyceride Content and Lactate Dehydrogenase Release Assays
Triglyceride content in liver was determined by sonicating liver tissue in 20 volumes of 0.25 M sucrose, 50 mM Tris-HCl, 1 mM EDTA for 1 min at 4°C. Triglyceride concentration in the homogenate was measured using a commercial kit (Sigma). Similarly lipid content of hepatocyte cultures was determined in the homogenate obtained by sonication of hepatocytes that were recovered from the collagen gel sandwich using collagenase digestion.
Cell injury was assessed by measuring lactate dehydrogenase (LDH) released by hepatocytes in the culture medium using a commercial kit (Promega, Madison, WI). Results were normalized to the maximal LDH release measured in similar cultures after induction of complete cell lysis.
Measurement of Mitochondrial Membrane Potential and Superoxide Generation
Hepatocytes were seeded in 6-well plates pre-coated with 500 μl collagen gel per well at a density of 106 cells per well and 1 ml medium per well. One day after seeding, the cultures were overlaid with a 500 μl collagen gel per well, and fed 1 ml standard hepatocyte culture medium. The plates were maintained in 90% air/10% CO2 at 37°C, which daily medium changes. After one week of culture, the cells were switched to the fatty acid-supplemented medium for 3 days.
Mitochondrial membrane potential was measured using JC-1, a cationic dye which accumulates to form aggregates in negatively charged mitochondria, causing its fluorescence emission maximum to shift from 527 nm to 590 nm (19). A stock solution of JC-1 containing 5 mg JC-1 in 12 mL dimethylsulfoxide was prepared and stored at room temperature shielded from ambient light. To stain the cells, the medium was replaced with 1 ml fresh standard hepatocyte culture medium containing 52 μL JC-1 stock solution per mL of medium, and the plates were incubated at 37°C for 2 hours. To wash off excess JC-1, the JC-1-containing medium was aspirated and replaced with fresh medium containing no JC-1. Cells were incubated for another 30 min at 37°C/10% CO2. Fluorescence readings were taken every half-hour thereafter until stable (usually within 2–3 h after wash) in an Fmax fluorescence plate reader (Molecular Devices) using an excitation of 485 nm and an emission of 510 nm for the monomeric form of JC-1 and an excitation of 485 nm and an emission of 590 nm for the aggregate form.
For mitochondrial superoxide generation measurement, hepatocytes were cultured and made steatotic as described above. A stock solution of MitoSOX Red dye containing 200 μg MitoSOX Red dye/80 μl dimethylsulfoxide was prepared immediately before use. The culture medium was replaced with 1 ml fresh standard hepatocyte culture medium supplemented with 8 μl/mL of MitoSOX Red solution and incubated for 30 min. Positive controls consisted of lean hepatocytes incubated with the MitoSOX Red dye-supplemented medium as well as 1 μl/mL of a solution of 50 μM menadione in dimethylsulfoxide. Fluorescence intensity was measured in the plate reader using excitation and emission wavelengths of 510 and 580 nm, respectively.
RESULTS
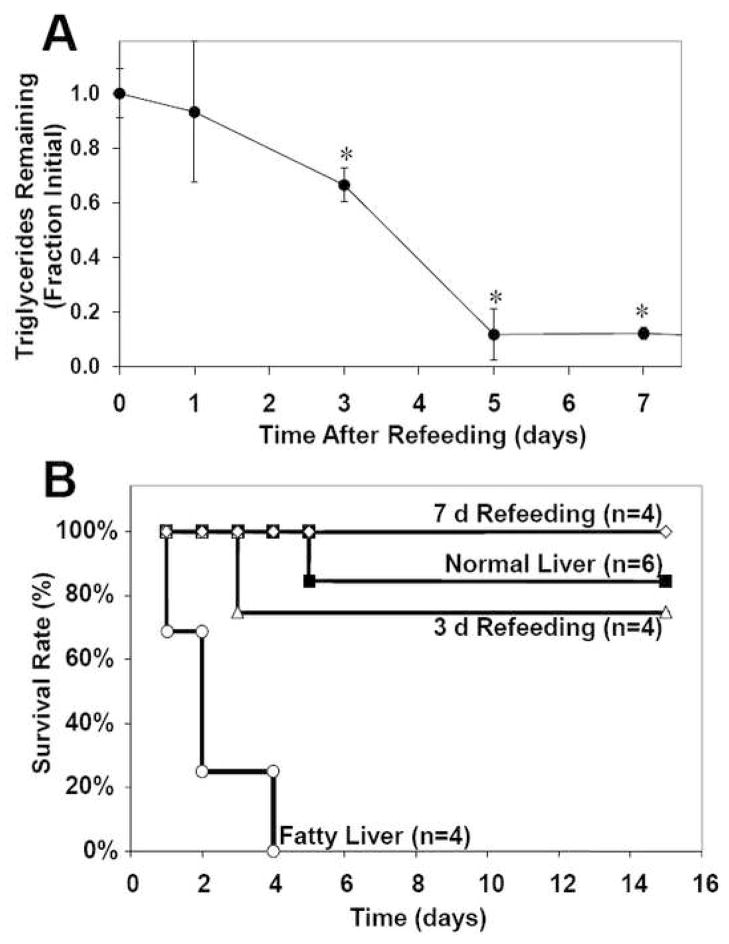
First, we investigated the effect of steatosis on liver and recipient survival in fatty liver rat transplantation model. Fatty liver was induced by CMDD feeding for about 6 wk, during which time the liver triglyceride content increased 20 fold. Macrovesicular steatosis was observed in more than 60% of the hepatocytes from these rats, a histological feature that is very similar to severe-grade human steatohepatitis (data not shown). To establish a correlation between extent of steatosis and survival, CMDD fed rats were returned to a regular diet up to 7 d before harvesting donor livers. Triglyceride content decreased in all livers (Figure 1A). The livers were stored in a hetastarch-free University of Wisconsin (UW) preservation solution for 6 h at 4°C, and then transplanted into recipient rats. This protocol mimics a typical clinical situation where the liver is cold stored in UW solution for several hours while it is being transported to the recipient site. Fatty livers from CMDD-fed rats showed no survival after 4 days (Figure 1B). In contrast, recipients of defatted livers showed survival rates that were comparable to normal livers, i.e. greater than 75%.
Figure 1.
Correlation between liver triglyceride content and survival rate after transplantation in a fatty liver rat model. Fatty liver was induced by feeding a choline and methionine-deficient diet for 6 weeks, after which the rats were returned to a normal diet. (A) Effect of refeeding time on liver triglyceride content. Initial content was 220±20 mg/g liver. *: significantly different (p<0.05) compared to initial triglyceride content by Student’s t-test. (B) Effect of refeeding time on survival curves of rats. Fatty liver curve is significantly different (p<0.05) from the other groups.
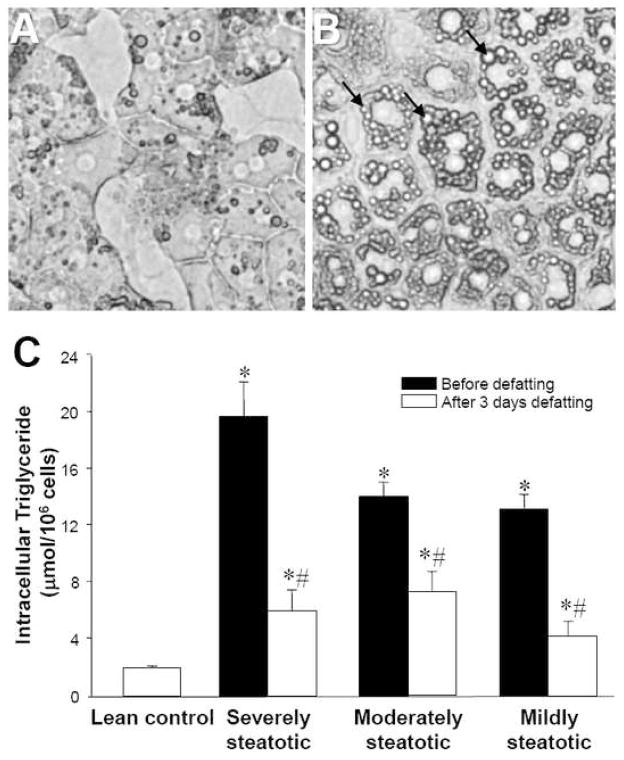
To elucidate the underlying mechanism of increased sensitivity of fatty liver to ischemia-reperfusion injury, we used a stable rat hepatocyte culture system fed fatty acid-supplemented culture medium. After 2 days of fatty acid exposure, hepatocytes exhibited an accumulation of cytoplasmic lipid droplets (Figure 2A, B). These droplets stained positively with oil red-O, a liposoluble dye (data not shown). Hepatocytes harvested after 2 days of fatty acid exposure accumulated amounts of triglycerides in proportion to the levels of fatty acids used during induction of steatosis (Figure 2C). Furthermore, steatosis was largely reversed by switching the steatotic hepatocytes back to normal culture medium for 3 days.
Figure 2.
Effect of fatty medium on lipid content in cultured hepatocytes. (A, B) Bright-field microscopy of primary rat hepatocytes cultured in standard hepatocyte culture medium, and after 2 days of culture in “fatty medium,” respectively. Arrows point to lipid droplets. (C) Hepatocytes were cultured for two days in media containing different proportions of fatty medium and subsequently defatted in regular medium for 3 days. Data shown are triglyceride content of hepatocytes as a function of proportion of fatty medium and defatting, expressed as averages ± SD of triplicate samples. *: significantly different (p<0.05) compared to lean control; #: significantly different compared to initial triglyceride content in same group by Student’s t-test. Each experiment was repeated at least twice with a different cell batch with similar findings.
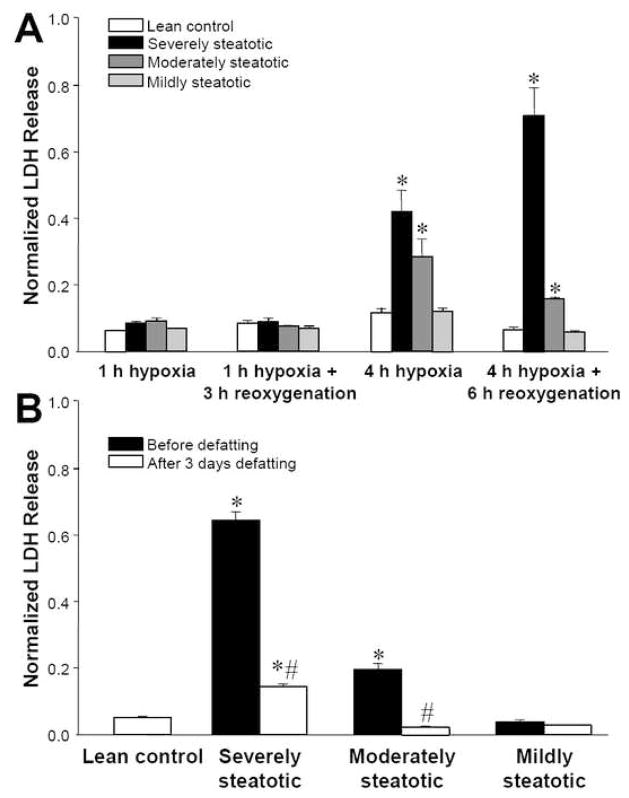
Cultured hepatocytes made fatty to varying degrees by exposure to various levels of fatty acids for 2 days were placed in a hypoxic chamber for 1 or 4 h, and then returned to normoxic conditions. Figure 3A shows that while 1 h of hypoxia did not cause much release of cytoplasmic lactate dehydrogenase (LDH) in any of the cultures tested, the 4 h hypoxic protocol led to >4 fold higher release of LDH compared to lean hepatocytes. Furthermore, the extent of LDH release was proportional to the levels of fatty acids used during induction of steatosis. These results suggest that fatty hepatocytes are intrinsically more sensitive to hypoxia-reoxygenation than lean hepatocytes.
Figure 3.
Effect of steatosis on LDH released in the culture medium by hepatocytes after hypoxia and reoxygenation. Prior to the experiment, hepatocytes were made steatotic by culture for 2 days in fatty medium (severely steatotic), a 1:1 mixture of fatty and regular medium (moderately steatotic), a 1:7 mixture of fatty and regular medium (mildly steatotic). Lean controls were placed in fresh regular medium. Hypoxia was induced by incubation in a 90% N2/10% CO2 atmosphere. For reoxygenation, the medium was replaced with fresh medium and the cultures incubated in a standard 90% air/10% CO2 atmosphere. (A) LDH release as a function of severity of steatosis and protocol of hypoxia and reoxygenation. *: significantly different (p<0.05) compared to lean control by Student’s t-test. (B) Comparison of LDH release after exposure to 4 h hypoxia followed by 6 h reoxygenation for fatty hepatocytes before and after defatting in regular medium for 3 days. *: significantly different (p<0.05) compared to lean control; #: significantly different compared to before defatting in same group by Student’s t-test. Data shown are expressed as averages ± SD of triplicate samples from one experiment. Each experiment was repeated at least twice with a different cell batch with similar findings.
The data shown in Figure 1 suggested that the sensitivity to ischemia-reperfusion in vivo can be reversed by defatting. We observed a similar trend when fatty hepatocytes were returned to regular hepatocyte culture medium. As the fat content of hepatocytes decreased, the response to hypoxia-reoxygenation tended towards the lean controls (Figure 3B).
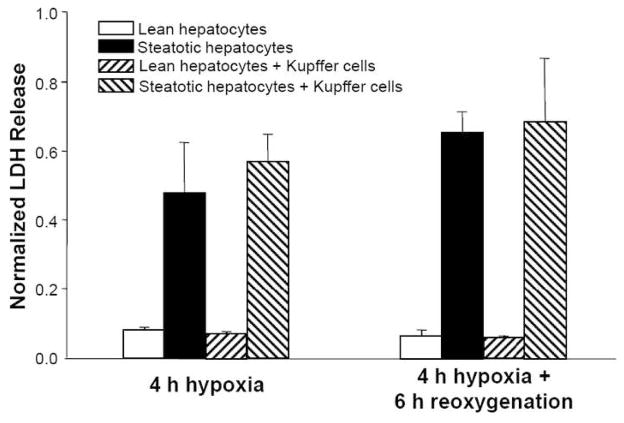
Prior studies have suggested that Kupffer cells can release factors that affect hepatocyte function upon exposure to inflammatory signals, such as endotoxin (20). We therefore investigated the response of hepatocytes co-cultured with Kupffer cells to hypoxia-reoxygenation. Using the 4 h hypoxia and 6 h reoxygenation protocol, no difference in LDH release was observed in the presence of Kupffer cells (Figure 4). Furthermore, we attempted to measure tumor necrosis factor-α (TNF-α) released in the medium, but levels were undetectable by ELISA (data not shown), suggesting no activation of Kupffer cells following hypoxia-reoxygenation in this cell culture model.
Figure 4.
Effect of hepatocyte co-culture with Kupffer cells on LDH release after 4 h hypoxia followed by 6 h reoxygenation. Data shown are averages ± SD of triplicate samples from one experiment. Each experiment was repeated at least twice with a different cell batch with similar findings.
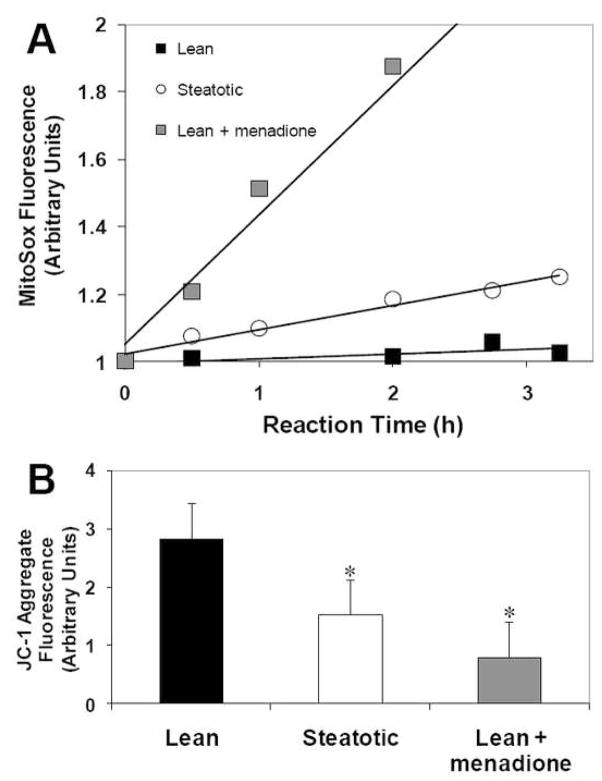
Since excessive lipid storage has been associated with increased free radical production, we hypothesized that fatty hepatocytes may produce more free radicals than lean hepatocytes. Figure 5A shows that mitochondrial generation of superoxide, as detected by the redox-sensitive dye MitoSox Red, was 20% greater than in lean hepatocytes, although 60% less than the positive control treated with menadione. We also found that mitochondrial membrane potential (MMP) of fatty hepatocytes was significantly reduced based on lower accumulation of the MMP-sensitive dye JC-1 (Figure 5B). Taken together, these data suggest that mitochondrial function is perturbed in fatty hepatocytes.
Figure 5.
Effect of steatosis on mitochondrial membrane potential and mitochondrial superoxide generation. Hepatocytes were made steatotic by culture for 3 days in fatty medium, while lean controls remained in regular medium. (A) Effect of steatosis on superoxide generation as detected by the Mitosox dye. Menadione was used as a positive control. (B) JC-1 aggregate fluorescence indicating mitochondrial membrane potential. Data shown are averages ± SD of triplicate samples from one experiment. *: significantly different (p<0.05) compared to lean control by Student’s t-test. Each experiment was repeated at least twice with a different cell batch with similar findings.
DISCUSSION
The results shown herein indicate that cultured primary rat hepatocytes made steatotic by preincubation with high levels of fatty acids are more sensitive to hypoxia-reoxygenation injury compared to similar control “lean” cultures. Furthermore, the sensitizing effect of steatosis was proportional to fat content and reversible both in vitro and in vivo, suggesting that the intracellular lipid content is a key parameter that determines hepatocyte sensitivity to hypoxia-reoxygenation.
The mechanism whereby steatosis increases the sensitivity of the liver to ischemia-reperfusion injury in vivo is poorly understood. Prior studies have shown that fatty livers exhibit serious microcirculatory disturbances after ischemia-reperfusion (21–23). Furthermore, there is evidence of a greater inflammatory response in fatty livers after transplantation (15), and treatments that reduce the inflammatory response, such as heat shock (24) and endotoxin antibodies (25), improve survival of fatty livers. None of these studies can identify the primary or triggering event leading to hepatic failure, as the inflammatory and circulatory changes may or may not be secondary to other earlier damage mechanisms. Our findings with cultured fatty hepatocytes in the absence of the various confounding factors found at the tissue and systemic levels suggest that steatotic hepatocytes are intrinsically more sensitive to hypoxia-reoxygenation. Therefore, it is plausible that a primary mechanism of fatty liver damage during ischemia-reperfusion involves direct injury to the hepatocytes.
Liver nonparenchymal cells, and in particular Kupffer cells, have been implicated in ischemia-reperfusion injury of the liver (26). Interestingly, coculturing Kupffer cells with the hepatocytes had no effect on the response of the hepatocytes to hypoxia-reoxygenation (Figure 4). This result with steatotic hepatocytes is consistent with prior published data with cultured lean hepatocytes (27). It is also noteworthy that Kupffer cells did not get activated (based on the lack of TNF-α secretion) in spite of the hepatocellular damage. It is therefore likely that Kupffer cell activation in vivo depends on the presence of exogenous factors, such as gut-derived endotoxin (25), which were not present in our culture system. Literature data also show that complement activation plays a key role in the postischemic activation of Kupffer cells in vivo (28). It is plausible that the absence of an effect of Kupffer cells is due to the lack of activatable complement in our culture conditions, since the culture medium is expected to contain low or negligible levels of complement (the medium contains 10% v/v heat-inactived serum). Such exogenous factors could be added to the co-culture system to investigate whether or not they can mediate an interaction between the steatotic hepatocytes and Kupffer cells during hypoxia-reoxygenation.
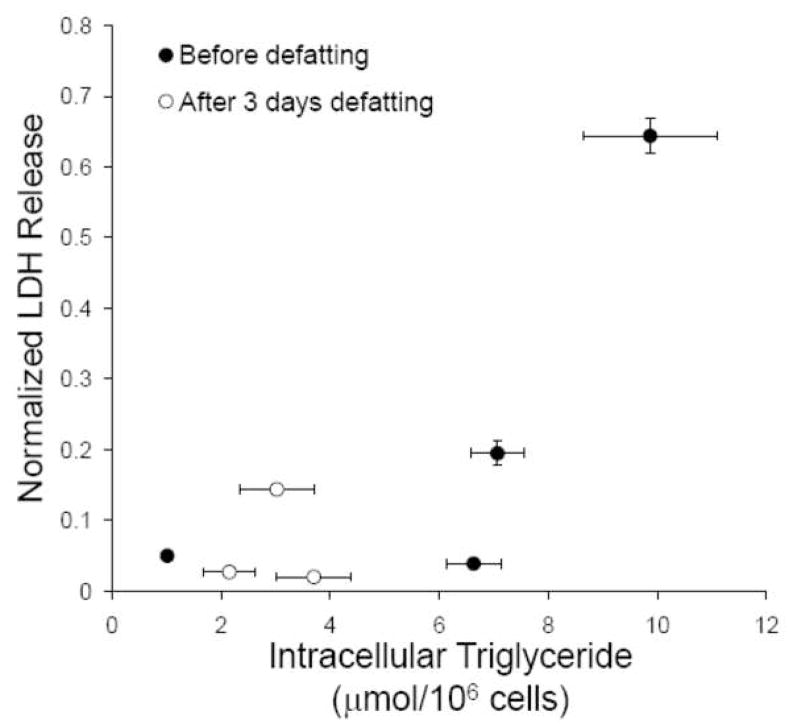
It is noteworthy that survival after transplantation increased from 0 to 75% after 3 days of refeeding, at which point fat content of the liver had been reduced by only ~30% (Figure 1). This suggests that there may be a threshold of fat content above which failure occurs. A similar observation can be made when analyzing the cell culture data. Plotting a correlation between intracellular triglyceride content (from Figure 2C) and LDH release upon hypoxia-reoxygenation (from Figure 3B) suggests a similar threshold phenomenon (Figure 6), where a sudden increase in LDH release occurs when triglyceride content reaches about 8 μmol/106 cells.
Figure 6.
Correlation between triglyceride content and LDH release after 4 h hypoxia followed by 6 h reoxygenation. Data from Figures 2C and 3B were combined.
We found a lowered mitochondrial membrane potential along with increased mitochondrial superoxide production in cultured steatotic hepatocytes, which is consistent with a prior study reporting that mitochondria isolated from steatotic rat livers exhibit altered functions and increased superoxide generation (29). A recent report indicated that a large load of exogenous free fatty acids can induce mitochondrial membrane depolarization and “lipoapoptosis” in cultured hepatocytes (30). Although we did not observe significant cell death prior to hypoxia-reoxygenation, it is possible that the decreased basal mitochondrial membrane potential increases the probability of depolarization when the cells are subjected to an additional stress. Excess storage of intracellular lipids in hepatocytes has been reported to increase sensitivity to select apoptosis-causing signals (31). The mechanism whereby steatosis reduced the mitochondrial membrane potential was not investigated in this study; however, in vivo studies of steatotic livers have reported increased expression of mitochondrial uncoupling protein-2 (UCP-2), which decouples the respiratory chain from the ATP synthase (32). Furthermore, treatments that decrease lipid content, such as epigallocatechin gallate administration, also decrease UCP-2 levels and ischemia-reperfusion-induced hepatic damage (33).
In summary, we have described a cell culture model to investigate the effect of hypoxia-reoxygenation on hepatocyte viability. We show that steatotic hepatocytes are more sensitive to warm hypoxia-reoxygenation than normal “lean” hepatocytes. Furthermore, we show that steatotic hepatocytes generate more mitochondrial superoxide and exhibit a lowered mitochondrial membrane potential. Thus, it is plausible that steatotic hepatocytes are primary responders to warm ischemia-reperfusion injury in vivo. Consequently, interventions that reduce intracellular lipid content in hepatocytes may be a potential avenue to precondition steatotic livers before surgical interventions that involve stopping blood flow for significant periods of time, such as in transplantation procedures. Such interventions could be implemented in the donor prior to liver explantation (34), or in a normothermic perfusion system (35). The cell culture system described herein may be useful to screen various metabolic treatments or preconditioning strategies to increase the resistance of steatotic hepatocytes to hypoxia-reoxygenation injury prior to testing in more complex animal models.
Acknowledgments
This work was partially supported by the National Institutes of Health grants R01DK059766, R01DK043371, P41EB002503, and the Shriners Hospitals for Children.
List of abbreviations
- CMDD
choline- and methionine-deficient diet
- DMEM
Dulbecco’s modified Eagle’s medium
- EDTA
ethylenediamine tetraacetic acid
- LDH
lactate dehydrogenase
- ELISA
enzyme-linked immunosorbent assay
- JC-1
5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolyl-carbocyanine iodide
- MMP
mitochondrial membrane potential
- TNF-α
tumor necrosis factor-α
- UCP-2
uncoupling protein-2
- UW
University of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, Kafetzis I, Castaing D, Bismuth H. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538–1540. [PubMed] [Google Scholar]
- 3.Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, Tzakis A, Starzl TE. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47:903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chui AK, Shi LW, Rao AR, Verran DJ, Painter D, Koorey D, McCaughan GW, Sheil AG. Donor fatty (steatotic) liver allografts in orthotopic liver transplantation: a revisit. Transplant Proc. 2000;32:2101–2102. doi: 10.1016/s0041-1345(00)01587-6. [DOI] [PubMed] [Google Scholar]
- 5.Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, Yoshimitsu K, Enjoji M, Kotoh K, Taketomi A, Uchiyama H, Shimada M, Nawata H, Maehara Y. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608–612. doi: 10.1097/01.tp.0000166009.77444.f3. [DOI] [PubMed] [Google Scholar]
- 6.Takeda Y, Arii S, Kaido T, Niwano M, Moriga T, Mori A, Hanaki K, Gorrin-Rivas MJ, Ishii T, Sato M, Imamura M. Morphologic alteration of hepatocytes and sinusoidal endothelial cells in rat fatty liver during cold preservation and the protective effect of hepatocyte growth factor. Transplantation. 1999;67:820–828. doi: 10.1097/00007890-199903270-00007. [DOI] [PubMed] [Google Scholar]
- 7.Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780–784. doi: 10.1097/00007890-199909270-00009. [DOI] [PubMed] [Google Scholar]
- 8.Koneru B, Reddy MC, Torre ANd, Patel D, Ippolito T, Ferrante RJ. Studies of hepatic warm ischemia in the obese Zucker rat. Transplantation. 1995;59:942–946. doi: 10.1097/00007890-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Nakano H, Nagasaki H, Barama A, Boudjema K, Jaeck D, Kumada K, Tatsuno M, Baek Y, Kitamura N, Suzuki T, Yamaguchi M. The effects of N-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the rat steatotic liver produced by a choline-methionine-deficient diet. Hepatology. 1997;26:670–678. doi: 10.1053/jhep.1997.v26.pm0009303498. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Tokunaga Y, Fujita T, Tanaka K, Yamaoka Y, Ozawa K. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282–287. doi: 10.1097/00007890-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Dunn JCY, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 12.Koneru B, Dikdan G. Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation. 2002;73:325–330. doi: 10.1097/00007890-200202150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Teramoto K, Bowers JL, Khettry U, Palombo JD, Clouse ME. A rat fatty liver transplant model. Transplantation. 1993;55:737–741. doi: 10.1097/00007890-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 15.Mokuno Y, Berthiaume F, Tompkins RG, Balis UJ, Yarmush ML. Technique for expanding the donor liver pool: heat shock preconditioning in a rat fatty liver model. Liver Transpl. 2004;10:264–272. doi: 10.1002/lt.20014. [DOI] [PubMed] [Google Scholar]
- 16.Seglen PO. Preparation of isolated rat liver cells. Meth Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Borderie D, Sogni P, Soubrane O, Houssin D, Calmus Y. NO-mediated vasodilation in the rat liver. Role of hepatocytes and liver endothelial cells. J Hepatol. 1997;26:1348–1355. doi: 10.1016/s0168-8278(97)80471-0. [DOI] [PubMed] [Google Scholar]
- 18.Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–265. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- 19.Berthiaume F, MacDonald AD, Kang YH, Yarmush ML. Control analysis of mitochondrial metabolism in intact hepatocytes: effect of interleukin-1β and interleukin-6. Metabol Eng. 2003;5:108–123. doi: 10.1016/s1096-7176(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 20.Kurose I, Miura S, Higuchi H, Watanabe N, Kamegaya Y, Takaishi M, Tomita K, Fukumura D, Kato S, Ishii H. Increased nitric oxide synthase activity as a cause of mitochondrial dysfunction in rat hepatocytes: roles for tumor necrosis factor alpha. Hepatology. 1996;24:1185–1192. doi: 10.1002/hep.510240534. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, McCuskey RS, Jaeschke H. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1385–1395. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ijaz S, Yang W, Winslet MC, Seifalian AM. The role of nitric oxide in the modulation of hepatic microcirculation and tissue oxygenation in an experimental model of hepatic steatosis. Microvasc Res. 2005;70:129–136. doi: 10.1016/j.mvr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation. 2001;72:1625–1631. doi: 10.1097/00007890-200111270-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mokuno Y, Berthiaume F, Tanimura Y, Yarmush ML. Heat shock preconditioning inhibits CD4+ T lymphocyte activation in transplanted fatty rat livers. J Surg Res. 2006;135:92–99. doi: 10.1016/j.jss.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Fiorini RN, Shafizadeh SF, Polito C, Rodwell DW, Cheng G, Evans Z, Wan C, Belden S, Haines JK, Birsner J, Lewin D, Wasiluk KR, Dunn DL, Schmidt MG, Chavin KD. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–1573. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 26.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 27.Samarasinghe DA, Farrell GC. The central role of sinusoidal cells in hepatic hypoxia-reperfusion injury in the rat. Hepatology. 1996 doi: 10.1002/hep.510240541. [DOI] [PubMed] [Google Scholar]
- 28.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. The American journal of physiology. 1993;264:G801–809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 29.Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, Morselli-Labate AM, Trevisani F, Palasciano G, Altomare E, Bernardi M. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res. 2005;124:160–168. doi: 10.1016/j.jss.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 31.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchino S, Yamaguchi Y, Furuhashi T, Wang FS, Zhang JL, Okabe K, Kihara S, Yamada S, Mori K, Ogawa M. Steatotic liver allografts up-regulate UCP-2 expression and suffer necrosis in rats. J Surg Res. 2004;120:73–82. doi: 10.1016/j.jss.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Fiorini RN, Donovan JL, Rodwell D, Evans Z, Cheng G, May HD, Milliken CE, Markowitz JS, Campbell C, Haines JK, Schmidt MG, Chavin KD. Short-term administration of (−)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005;11:298–308. doi: 10.1002/lt.20348. [DOI] [PubMed] [Google Scholar]
- 34.Chavin KD, Fiorini RN, Shafizadeh S, Cheng G, Wan C, Evans Z, Rodwell D, Polito C, Haines JK, Baillie GM, Schmidt MG. Fatty acid synthase blockade protects steatotic livers from warm ischemia reperfusion injury and transplantation. Am J Transplant. 2004;4:1440–1447. doi: 10.1111/j.1600-6143.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- 35.Tolboom H, Pouw R, Uygun K, Tanimura Y, Izamis ML, Berthiaume F, Yarmush ML. A model for normothermic preservation of the rat liver. Tissue engineering. 2007;13:2143–2151. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]