Abstract
Aims
Inducible cyclooxygenase (COX-2) has been implicated in the process of inflammation and carcinogenesis. Chamomile has long been used in traditional medicine for the treatment of inflammatory diseases. In this study we aimed to investigate whether chamomile interferes with the COX-2 pathway.
Main Methods
We used lipopolysaccharide (LPS)-activated RAW 264.7 macrophages as an in vitro model for our studies.
Key Findings
Chamomile treatment inhibited the release of LPS-induced prostaglandin E(2) in RAW 264.7 macrophages. This effect was found to be due to inhibition of COX-2 enzyme activity by chamomile. In addition, chamomile caused reduction in LPS-induced COX-2 mRNA and protein expression, without affecting COX-1 expression. The non-steroidal anti-inflammatory drug, sulindac and a specific COX-2 inhibitor, NS398, were shown to act similarly in LPS-activated RAW 264.7 cells. Our data suggest that chamomile works by a mechanism of action similar to that attributed to non-steroidal anti-inflammatory drugs.
Significance
These findings add a novel aspect to the biological profile of chamomile which might be important for understanding the usefulness of aqueous chamomile extract in the form of tea in preventing inflammation and cancer.
Keywords: inflammation, cyclooxygenase-2, chamomile, chemoprevention, macrophages, non-steroidal anti-inflammatory agents, carcinogenesis
INTRODUCTION
There is increasing evidence that longstanding inflammation plays a critical role in the initiation and development of various human illnesses, including cancer (Federico et al., 2007; MacLennan et al., 2006; Khansari et al., 2009). Inflammation and disease are linked through the production of inflammatory mediators by macrophages and neutrophils (Federico et al., 2007; O’Shea and Murray, 2008). Inflammation results in induced expression and enzyme activity of cyclooxygenase-2 (COX-2), which produces inflammatory mediators such as prostaglandin E2 (PGE2) (Hussain et al., 2003). COX-2, unlike COX-1 which is constitutively expressed in most mammalian tissue, is not detectable in normal tissues, but is rapidly induced by growth factors, tumor promoters, oncogenes and carcinogens (Hussain et al., 2003; Simmons et al., 2004). Aberrant or increased expression of COX-2 has been implicated in the pathogenesis of various inflammatory disorders including lupus, multiple sclerosis, arthritis, Alzheimer’s disease and cancer (Kapoor et al., 2005; Wang et al., 2007). It was observed in early clinical studies that levels of prostaglandins and COX-2 were higher in tumor tissue than in normal tissue, suggesting a role of COX-2 in tumorigenesis (Taketo, 1998; Buskens et al., 2002). Experimental studies demonstrate that deletion of COX-2 suppresses the development of intestinal polyps in Apc delta716 knockout mice, whereas overexpression of COX-2 is sufficient to induce mammary gland tumors in multiparous (nonvirgin) females, suggesting a pivotal role of COX-2 in tumorigenesis (Oshima et al., 1996; Liu et al., 2001). In light of the above findings, COX-2 has become the focal point for the development of anti-inflammatory and anticancer drugs. Non-steroidal anti-inflammatory drugs (NSAIDs), nonselective non-aspirin NSAIDs and COX-2 selective inhibitors are being widely used for various inflammatory disorders and cancer prevention (Thun and Blackard, 2009). Selective inhibitors of COX-2, however, are associated with a small but definite risk of myocardial infarction and stroke. In view of the gastric side-effects of conventional NSAIDs and the recent withdrawal of selective COX-2 inhibitors from the market due to their adverse cardiovascular side-effects, there is considerable impetus to develop alternative anti-inflammatory agents with reduced gastric and cardiovascular side-effects (Ortiz, 2004; Coruzzi et al., 2007). Plant-derived natural agents may potentially be useful in this regard.
Chamomile has been used for centuries as a medicinal plant for its anti-inflammatory and analgesic properties (McKay and Blumberg, 2006; Srivastava and Gupta, 2009). It is consumed in the form of tea at a frequency of more than a million cups per day (Speisky et al., 2006). Chamomile has been approved by the German Commission E for oral consumption in the management of various inflammatory diseases of the gastrointestinal tract, and for topical application in the treatment of various skin disorders and inflammatory disorders of certain mucosal surfaces, such as the oral cavity and ano-genital areas (Ross, 2008). Recent studies have demonstrated its antioxidant, hypocholesteroemic, anti-parasitic, anti-aging, and anticancer properties, supporting its longstanding traditional use for treating various human ailments (Babenko and Shakhova, 2006; Lee and Shibamoto, 2002; Srivastava and Gupta, 2009). Several constituents of chamomile, including apigenin 7-O-glucoside, luteolin, terpene compounds, chamazulene, and (−)-alpha-bisabolol, patuletin, quercetin, myricetin, and rutin have been studied with respect to their anti-inflammatory activities. Of these, chamazulene, alpha-bisabolol, and apigenin have been shown to possess the highest anti-inflammatory activity against pro-inflammatory agents (McKay and Blumberg, 2006). The anti-inflammatory effects of azulenes may be related to an influence on the pituitary and adrenal glands, through increased cortisone release and reduced histamine production (Rekka et al., 1996). In cell culture studies, both bisabolol and bisabolol oxide have been shown to inhibit 5-lipoxygenase activity (Braga et al., 2009). Apigenin 7-O-glucoside application has been shown to inhibit skin inflammation caused by application of xanthine-oxidase and cumene hydroperoxide in rats (Fuchs and Milbradt, 1993). In light of accumulated investigative evidence, we speculated that chamomile may contain constituents that interfere with the actions of COX-2. To investigate this hypothesis, we used LPS-activated murine RAW 264.7 macrophages as a cell model, since they express high levels of COX-2 and are the most relevant model for our studies.
MATERIALS AND METHODS
Materials
Dry chamomile flower of Egyptian origin was purchased from Bec’s Tea Nirvana, Cleveland, Ohio. Cell culture medium, DMEM, fetal bovine serum, penicillin streptomycin cocktail and phosphate buffer saline were purchased from Cellgro Mediatech, Inc. (Herndon, VA). Lipopolysaccharide (LPS, E coli), acetylsalicylic acid, sulindac, arachidonic acid and apigenin 7-O-glucoside (>95% pure) were purchased from Sigma (St. Louis, MO). NS-398 was purchased from Calbiochem. All reagents used in the experiments were of analytical reagent grade or HPLC grade where applicable.
Preparation of extracts
Dry chamomile flowers were weighed and crushed to powder with a marble pestle and mortar and a 5% w/v suspension was prepared in a flask by adding hot boiled water. The flask was then placed on a shaker (200 rpm) for 4 h and the temperature was maintained at 37°C. After shaking, the flask was brought to room temperature the suspension was filtered through a series of Whatman filters and finally passed through 0.22 micron filter (Millipore, Billerica, MA). The filtered aqueous extract was freeze dried and stored at −20°C until use. For cell culture studies, the dried material from aqueous extract was weighed and dissolved in culture medium to achieve desired concentration.
Cell culture
Murine RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified essential medium in appropriate culture conditions. Cell stimulation was performed with 1 μg/mL of LPS.
PGE2 release
To determine PGE2 accumulation from endogenous arachidonic acid, cells were seeded in 96-well plates (5 × 104/200 μL/well), cultured for two days and, after supernatants were replaced by fresh medium, incubated with or without LPS in the absence or presence of the test agents for 24 h. PGE2 was measured in cell culture supernatants and cell lysate of RAW 264.7 macrophages by using PGE2 enzyme immunometric EIA kit (Cayman Chemical, Ann Arbor, MI). Experiments were performed at least three times in triplicate.
COX-2 enzyme activity
RAW 264.7 cells (1 × 105 cells in a 96 well plate) were pretreated with acetylsalicylic acid (250 μM) for 30 min to irreversibly inactivate COX-1. Thereafter, cells were washed with PBS and fed with fresh medium. Induction of COX-2 was achieved by adding LPS for 24 h. Then, medium was aspirated and cells washed with PBS again and supplied with fresh medium (fetal bovine serum-free). Test compounds were pre-incubated for 30 min before exogenous arachidonic acid was added. After 15 min, supernatants were removed and analyzed by PGE2 enzyme immunometric EIA assay. Experiments were performed at least three times in triplicate.
Cell viability assay
Cell respiration, an indicator of cell viability, was determined by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan. After the supernatants were removed for PGE2 determination, cells were incubated at 37° with MTT (0.5 mg/mL) for 45 min. The medium was aspirated and cells were solubilized in dimethyl sulfoxide (250 μL) for at least 2 h in the dark. The extent of reduction of MTT was quantified by optical density measurement at 550 nm.
Western blot analysis
Macrophages, grown in 6-well plates to confluence, were incubated with or without LPS in the absence or presence of the test agents. Cells were washed with ice-cold PBS and stored at −70° until further analysis. Frozen plates were put on ice and cells were lysed in 1% Triton X-100, 0.15 M NaCl, and 10 mM Tris-HCl pH 7.4 for 30 min. Lysates were homogenized through a 22 G needle and centrifuged at 10,000 g for 10 min at 4°. The supernatants were collected and protein was measured by the method according to Bradford, 1976. Cell lysates, containing equal amounts of protein, were boiled in SDS sample buffer for 5 min before running on a 10% SDS–polyacrylamide gel. Proteins were transferred to polyvinylidene fluoride membranes (Invitrogen, Carlsbad, CA). Membranes were blocked with 5% fat-free dry milk in TBS-T pH 8.0 (Tris-buffered saline [50 mM Tris, pH 8.0, 150 mM NaCl] with 0.1% Tween 20) and then incubated with a mouse immunoglobulin G1 against COX-1 (SC-7950) and COX-2 (SC-7951) or monoclonal anti-β-actin (SC-47778) antibody obtained from SantaCruz, SantaCruz, CA, diluted to 1:250 and incubated overnight at 4°. After washing 3 times with TBS-T, COX-1 and COX-2 was visualized by an anti-mouse IgG:horseradish peroxidase conjugate and the enhanced chemiluminescence system (ECL™, Amersham Pharmacia Biotech). Signal intensities were evaluated by densitometric analysis (Kodak Digital Science™ Image Station 2000R Life Science Products).
Reverse transcriptase (RT)-PCR analysis
RAW 264.7 cells (5 × 106 cells-10 cm dish) were incubated for 24 h with or without various concentrations of chamomile and LPS (1 μg/ml). After washing with PBS twice, total RNA was isolated from the cell pellet using RNA isolation kit (Invitrogen, CA). The total amount of RNA was determined by absorbance at 260 nm. One microgram (μg) of RNA was reverse transcribed into cDNA using avian myeloblastosis virus (AMV) reverse transcriptase and oligo(dT)15 primer (Promega Co., Madison, WI, USA). The PCR samples contained 50 μl of the reaction mixture, comprised of 50 mM KCl, 5 mM MgCl2, 0.16 mM dNTP, 5.0 units of Taq DNA polymerase (Qiagen, Valencia, CA, USA), and 20 pmol of sense and antisense primers in 10 mM Tris-HCl (pH 8.3). The primer for COX-1 were 5′-ACTGGCTCTGGGAATTTGTG-3′ (sense) and 5′-AGAGCCGCAGGTGATACTGT-3′ (antisense), COX-2 were 5′-GGAGAGACTATCAAGATAGT-3′ (sense) and 5′-ATGGTCAGTAGACTTTTACA-3′ (antisense) and those for GAPDH 5′-AGGCCGGTGCTGAGTATGTC-3′ (sense) and 5′-TGCCTGCTTCACCACCTTCT-3′ (antisense). The PCR amplification was performed under the following conditions: 38 cycles of denaturation at 94 °C for 15 sec, annealing at 55 °C for 1 min and extension at 72 °C for 1 min, using a thermal cycler (Px2, Thermo Electron Corporation). The amplified PCR products were run on a 2% agarose gel and visualized by SYBR Gold staining.
Statistical analysis
PGE2 determination and cell viability were performed in triplicate. All experiments were repeated at least twice. Results are expressed as mean values ± SEM. Statistical comparisons were made by ANOVA followed by a Dunnett’s multiple comparison test. P values <0.05 were considered significant.
RESULTS
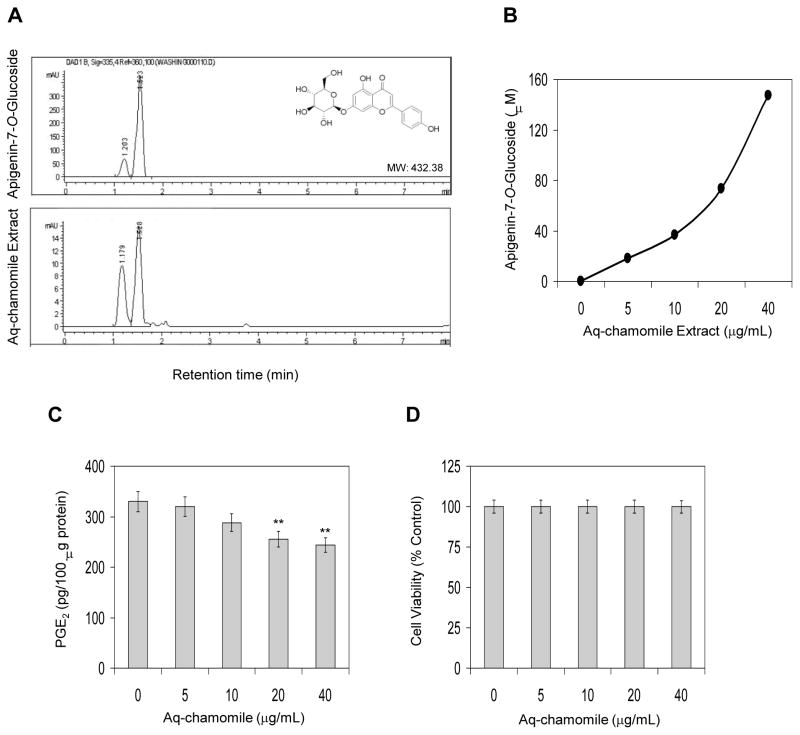
HPLC analysis of aqueous chamomile extract demonstrated two major peaks with retention times of 1.179 minutes (27.7%) and 1.520 minutes (63.3%) and five other minor peaks which together constitute 9% of the total flavonoids (Figure 1A). The two major peaks in the aqueous chamomile extract correspond to apigenin 7-O-glucoside (63.3%) and apigenin 7-O-neohespridoside (27.7%). The presence of these constituents in the aqueous chamomile extract was also confirmed by LC-MS analysis (data not shown).
Figure 1.
(A) HPLC chromatogram of apigenin 7-O-glucoside and aqueous chamomile extract demonstrating apigenin 7-O-glucoside as major constituent, (B) standardization of chamomile extract with apigenin 7-O-glucoside concentration, (C) effect of chamomile on PGE2 accumulation in cell culture supernatants of RAW 264.7 macrophages. Bars represent mean ± SEM of at least three independent experiments, each performed in triplicate. **p <0.01 (ANOVA), and (D) effect of chamomile on cell viability as determined by MTT assay. Details are described in ‘Materials and Methods’ section.
In the process of standardization of aqueous chamomile extract for ongoing studies, we defined the doses in equivalent molar concentration corresponding to apigenin 7-O-glucoside, as it is the major constituent of aqueous chamomile extract. For this, different concentrations of apigenin 7-O-glucoside were prepared in methanol and subjected to HPLC (Figure 1A). The peak area (retention time 1.5 to 1.7 min) were calculated and plotted to obtain a standard curve, which corresponded to the concentration of apigenin 7-O-glucoside in the aqueous extract on the basis of peak area (Figure 1B).
Next we determined the effect of aqueous chamomile extract on inhibition of endogenous prostaglandin E2 (PGE2) levels in RAW 264.7 macrophages. As shown in figure 1C, treatment of macrophages with chamomile caused a decrease in endogenous PGE2 levels in RAW 264.7 macrophages which was more pronounced at 20 and 40 μg/mL doses of chamomile. Chamomile exposure did not affect cell viability at the test concentration up to 40 g/mL doses as determined by MTT reduction assay (>95% cell viability; Figure 1D).
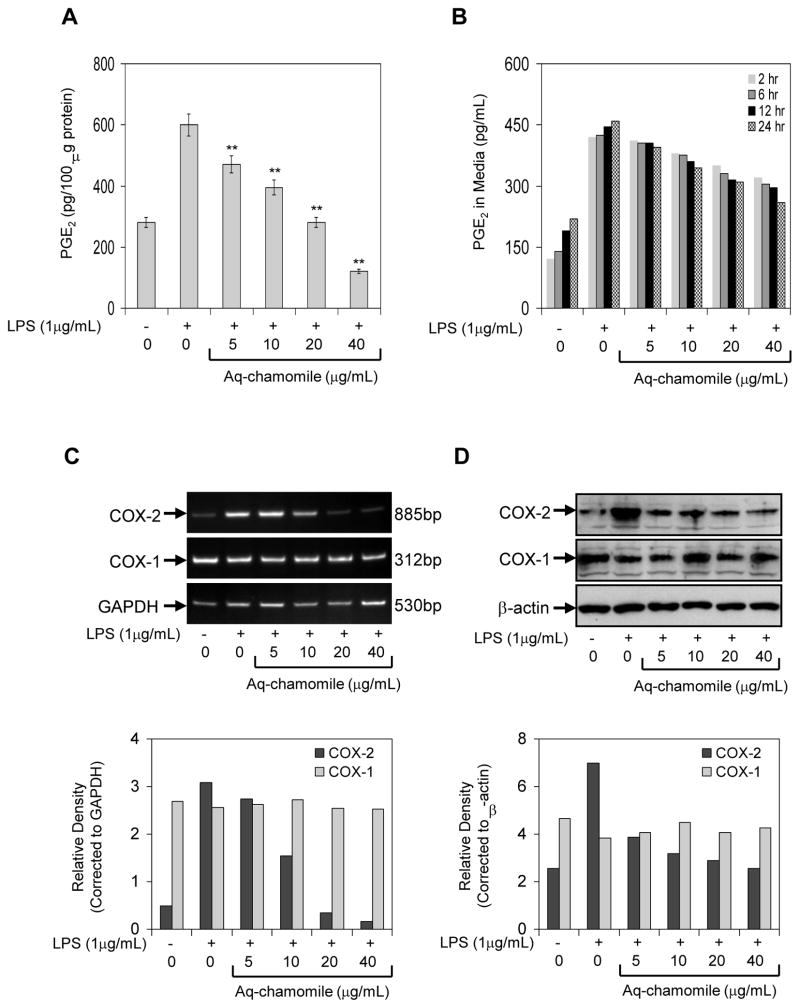
In the next series of experiments, we used LPS challenge as treatment of RAW 264.7 macrophages, which causes induction of COX-2, and converts LPS-induced endogenous arachidonic acid to PGE2 (Simmons et al., 2004). RAW macrophages treated with LPS (1 g/mL) in the presence of chamomile (5–40 g/ml) for 24 h exhibited a dose-dependent decrease in endogenous PGE2 production (Figure 2A). As shown in figure 2B, exposure of cells to chamomile caused a time-dependent decrease in PGE2 release in the cell culture medium. The IC50 value was calculated to be 24.0 μg/mL for apigenin 7-O-glucoside corresponding to 15.0 μM concentration of aglycone, apigenin.
Figure 2.
Effect of chamomile on PGE2, COX-1 and COX-2 expression in RAW 264.7 macrophages. (A) effect of chamomile on PGE2 accumulation in cell supernatant activated with 1 μg/mL LPS in the absence and presence of chamomile (5–40 μg/mL) for 24 h. (B) time-dependent accumulation of PGE2 in cell culture medium after chamomile treatment. Bars represent mean ± SEM of at least three independent experiments, each performed in triplicate. **p <0.01 (ANOVA) (C) Western blots for COX-1 and COX-2 protein expression, and (D) mRNA expression of COX-1 and COX-2 in RAW 264.7 macrophages stimulated with LPS and LPS and chamomile. Graph represents mRNA and protein levels of COX-1 and COX-2 corrected to GAPDH and β-actin, represents loading controls. Details are described in ‘Materials and Methods’ section.
In an attempt to find the underlying mechanism leading to reduced PGE2 production and release after chamomile exposure, we first examined the influence of chamomile on LPS-induced COX-2 mRNA levels. As shown in figure 2C, COX-2 mRNA levels were significantly elevated after LPS challenge to RAW 264.7 macrophages. A marked decrease in COX-2 mRNA expression was noted after treatment of cells with at 20 and 40 μg/mL doses of chamomile. Surprisingly, treatment of RAW 267.4 cells with test doses of chamomile did not cause any significant changes in the COX-1 mRNA levels. Since increased COX-2 mRNA steady state levels may lead to increased COX-2 protein, we examined the influence of chamomile on LPS-induced COX-2 protein expression. As shown in figure 2D, treatment of cells at 5–40 μg/mL doses of chamomile caused a significant decrease in COX-2 protein expression, whereas COX-1 protein expression remained unchanged at these doses of chamomile.
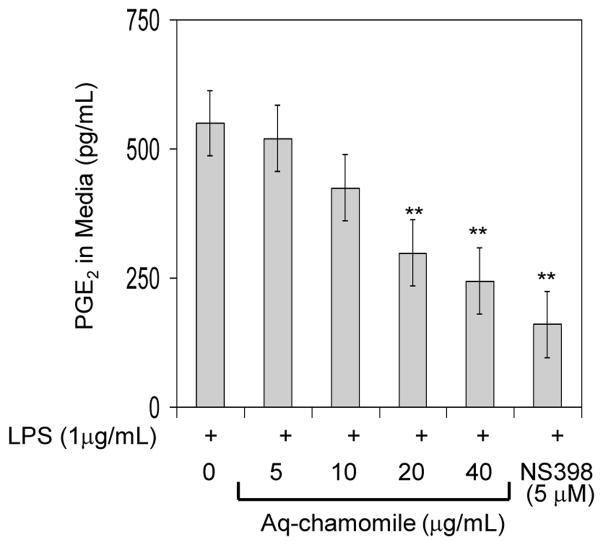
Next we determined the efficacy of chamomile in inhibition of COX-2 activity and compared its effects with those of NS398, a selective COX-2 inhibitor. To determine this, we used RAW 264.7 macrophages in which COX-2 was induced by LPS and exogenous arachidonic acid was added as substrate. Since exogenous arachidonic acid can be utilized by either COX-1 or COX-2 to produce PGE2, COX-1 was irreversibly blocked by acetylsalicylic acid before COX-2 was induced with LPS. As shown in figure 3, chamomile was able to inhibit the conversion of exogenous arachidonic acid to PGE2 in a dose-dependent manner which corresponded to inhibition of COX-2 activity. The IC50 value determined (28.0 μg/mL for apigenin 7-O-glucoside corresponding to 17.6 μM apigenin) was very similar to that calculated for chamomile-dependent inhibition of PGE2 production in LPS-activated RAW 264.7 macrophages.
Figure 3.
Effect of chamomile on COX-2 enzyme activity in LPS activated RAW 264.7 macrophages. RAW 264.7 macrophages were treated with 5–40 μg/mL doses of chamomile, in which COX-1 was irreversibly inactivated by acetylsalicylic acid and activated with 1 μg/mL LPS to induce COX-2. The cells were supplemented with fresh culture medium treated with or without chamomile or NS-398. The reaction was started by adding arachadonic acid and PGE2 levels were measured. Bars represent mean ± SEM of at least three independent experiments, each performed in triplicate. **p <0.01 (ANOVA)
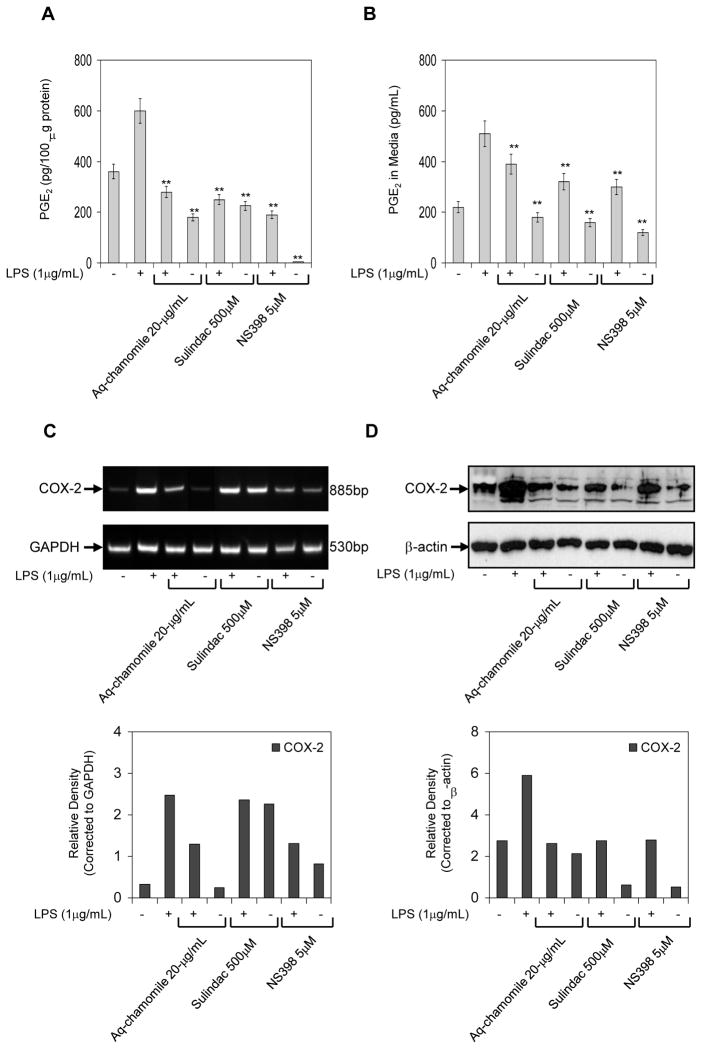
Inhibition of prostaglandin synthesis by direct interference with the cyclooxygenase enzymes is a common mechanism of non-steroidal anti-inflammatory drugs (NSAIDs). Since chamomile appeared to have actions similar to those of NSAIDs, we hypothesized that sulindac, an NSAID that inhibits the enzyme activity of COX-1 and COX-2, and NS398, a specific COX-2 inhibitor, might affect PGE2 production and release in LPS-activated RAW 264.7 macrophages in a similar way as chamomile. As shown in figure 4A & B, sulindac and NS398 significantly inhibited endogenous PGE2 production and release in RAW cells with and without LPS treatment, similar to that observed after chamomile treatment. The decrease in PGE2 levels after chamomile treatment corresponded with the decrease in COX-2 mRNA and protein expression; however, the expression of only COX-2 protein was decreased after treatment with sulindac and NS398 treatments. Treatment of RAW 264.7 macrophages with sulindac and NS398 did not affect the mRNA COX-2 levels, demonstrating that NSAIDs action in murine macrophages is not directed towards the transcription of COX-2.
Figure 4.
Comparative effects of chamomile, sulindac and NS398 on PGE2, COX-2 mRNA and protein levels in RAW 264.7 macrophages. (A) effect of chamomile (20 μg/mL), sulindac (500 μM) and NS398 (5 μM) on PGE2 accumulation in cell supernatant activated with 1 μg/mL LPS in the absence and presence of these agents for 24 h. (B) PGE2 release in cell culture medium after similar treatments. Bars represent mean ± SEM of at least three independent experiments, each performed in triplicate. **p <0.01 (ANOVA) (C) mRNA expression of COX-2, and (D) Western blot for COX-2 protein expression in RAW 264.7 macrophages stimulated with LPS and LPS and chamomile (20 μg/mL), sulindac (500 μM) and NS398 (5 μM). Graph represents mRNA and protein levels of COX-2 corrected to GAPDH and β-actin, represents loading controls. Details are described in ‘Materials and Methods’ section.
DISCUSSION
The present study demonstrates that aqueous chamomile extract has the ability to inhibit release of PGE2 from LPS activated RAW 264.7 macrophages (IC50 value: 24.0 μg/mL for apigenin 7-O-glucoside or 15.0 μM apigenin). The inhibitory activity of chamomile was due to a dose-dependent inhibition of COX-2 enzyme activity (IC50 value: 28.0 μg/mL for apigenin 7-O-glucoside or 17.6 μM apigenin). In addition, chamomile reduced COX-2 mRNA and protein expression, but did not affect the activity or expression of COX-1, the constitutive form of cyclooxygenase. Chamomile inhibits prostaglandin synthesis by a mechanism similar to that induced by NSAIDs. Sulindac, an NSAID, and NS398, a specific COX-2 inhibitor, caused a significant decrease in PGE2 levels and also inhibited COX-2 activity and protein expression, but not COX-2 mRNA expression in RAW 264.7 macrophages, challenged with LPS.
Many phenolic compounds of plant origin, especially flavonoids, possess anti-inflammatory, anti-carcinogenic and free radical scavenging properties and the number of molecules isolated and characterize continues to increase (Rahman et al, 2006; Stevenson and Hurst, 2007). Previous studies have demonstrated that individual constituents of chamomile such as chalmuzene, luteolin and apigenin are efficacious in inhibiting COX-2, iNOS and leukotrine expression in cell culture (McKay and Blumberg, 2006). A freeze-dried extract of chamomile has been shown to suppress the inflammatory effects and leukocyte infiltration in hind paw edema test induced by simultaneous administration of carrageenan and prostaglandin E1 in Wistar albino rats (Shipochliev et al., 1981). Ethyl acetate and ethanol extracts of chamomile showed strong inhibition towards 48/80-induced scratching in mice (Kobayashi et al, 2003). Furthermore, lyophilized ethanol extract of chamomile inhibited carrageenan-induced paw edema in Wistar rats (Al-Hindawi et al., 1989). More recently, we have demonstrated that aqueous and methanolic chamomile extracts possess anticancer effects; specifically, reduced proliferation and enhanced induction of apoptosis in various human cancer cells (Srivastava and Gupta, 2007). In the current study, we have shown that COX-2 is preferentially inhibited by chamomile. We also observed that the activities and mechanisms of action of chamomile resemble those of NSAIDs, which have been demonstrated to possess chemopreventive properties by their common ability to inhibit prostaglandin synthesis.
Numerous studies have demonstrated that the expression of murine macrophages, COX-2 is largely regulated by transcriptional activation (Kang et al., 2006; Mestre et al., 2001). Lipopolysaccharide and other pro-inflammatory cytokines activate NF-κB which is a mammalian transcription factor that regulates several genes important in immunity and inflammation. NF-κB binding sites have been identified on the murine COX-2 promoter which plays a role in LPS-mediated induction of COX-2 in macrophages. In addition, binding of CCAAT-enhancer-binding proteins (C/EBPs), c-AMP response element binding proteins (CREBs) and c-Jun to COX-2 promoter enhances its transcriptional activation. Further studies are needed to determine the effect of chamomile on NF-κB, C/EBP, CREB and c-Jun proteins.
In summary, our findings provide insight into the mechanism(s) through which chamomile, as an aqueous infusion in the form of tea, and possibly other related flavonoids, may prove beneficial in the prevention and management of various inflammatory and neoplastic disorders.
CONCLUSIONS
The mechanism of action of chamomile on the inhibition of PGE2 production was due to the suppression of the COX-2 gene expression and direct inhibition of COX-2 enzyme activity. This may be important in the prevention of inflammation and may contribute to the anti-inflammatory, anti-neoplastic and immunoregulatory effects of chamomile.
Acknowledgments
This work was supported by research grants from United States Public Health Services RO1 AT002709 and RO1 CA108512. The authors are thankful to Dr Sanjeev Shukla for technical assistance and densitometric analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hindawi MK, Al-Deen IH, Nabi MH, Ismail MA. Anti-inflammatory activity of some Iraqi plants using intact rats. Journal of Ethnopharmacology. 1989;26(2):163–168. doi: 10.1016/0378-8741(89)90063-9. [DOI] [PubMed] [Google Scholar]
- Babenko NA, Shakhova EG. Effects of Chamomilla recutita flavonoids on age-related liver sphingolipid turnover in rats. Experimental Gerontology. 2006;41(1):32–39. doi: 10.1016/j.exger.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braga PC, Dal Sasso M, Fonti E, Culici M. Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology. 2009;83(2):110–115. doi: 10.1159/000186049. [DOI] [PubMed] [Google Scholar]
- Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, Offerhaus GJ, Van Lanschot JJ, Ristimäki A. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122(7):1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Venturi N, Spaggiari S. Gastrointestinal safety of novel nonsteroidal antiinflammatory drugs: selective COX-2 inhibitors and beyond. Acta Bio Medica. 2007;78(2):96–110. [PubMed] [Google Scholar]
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. International Journal of Cancer. 2007;121(11):2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Milbradt R. Skin anti-inflammatory activity of apigenin-7-glucoside in rats. Arzneimittelforschung. 1993;43(3):370–372. [PubMed] [Google Scholar]
- Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Letters. 2003;191(2):125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Wingerd BA, Arakawa T, Smith WL. Cyclooxygenase-2 gene transcription in a macrophage model of inflammation. Journal of Immunology. 2006;177(11):8111–22. doi: 10.4049/jimmunol.177.11.8111. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Shaw O, Appleton I. Possible anti-inflammatory role of COX-2-derived prostaglandins: implications for inflammation research. Current Opinion Investigative Drugs. 2005;6(5):461–466. [PubMed] [Google Scholar]
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents on Inflammation and Allergy Drug Discovery. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Nakano Y, Inayama K, Sakai A, Kamiya T. Dietary intake of the flower extracts of German chamomile (Matricaria recutita L.) inhibited compound 48/80-induced itch-scratch responses in mice. Phytomedicine. 2003;10(8):657–664. doi: 10.1078/0944-7113-00283. [DOI] [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. Journal of Agricultural Food Chemistry. 2002;50(17):4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. Journal of Biological Chemistry. 2001;276(21):18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. Journal of Urology. 2006;176(3):1012–1016. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytotherapy Research. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- Mestre JR, Rivadeneira DE, Mackrell PJ, Duff M, Stapleton PP, Mack-Strong V, Maddali S, Smyth GP, Tanabe T, Daly JM. Overlapping CRE and E-box promoter elements can independently regulate COX-2 gene transcription in macrophages. FEBS Letters. 2001;496(2–3):147–51. doi: 10.1016/s0014-5793(01)02422-x. [DOI] [PubMed] [Google Scholar]
- Ortiz E. Market withdrawal of Vioxx: is it time to rethink the use of COX-2 inhibitors? Journal of Managed Care Pharmacy. 2004;10(6):551–554. doi: 10.18553/jmcp.2004.10.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28(4):477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical Pharmacology. 2006;72(11):1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rekka EA, Kourounakis AP, Kourounakis PN. Investigation of the effect of chamazulene on lipid peroxidation and free radical processes. Research Communication in Molecular Pathology & Pharmacology. 1996;92(3):361–364. [PubMed] [Google Scholar]
- Ross SM. Chamomile: a spoonful of medicine. Holistic Nursing Practice. 2008;22(1):56–57. doi: 10.1097/01.HNP.0000306329.41708.c6. [DOI] [PubMed] [Google Scholar]
- Shipochliev T, Dimitrov A, Aleksandrova E. Anti-inflammatory action of a group of plant extracts. Preventive Veterinary Medicine. 1981;18(6):87–94. [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacology Reviews. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Speisky H, Rocco C, Carrasco C, Lissi EA, López-Alarcón C. Antioxidant screening of medicinal herbal teas. Phytotherapy Research. 2006;20(6):462–467. doi: 10.1002/ptr.1878. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. Journal of Agricultural Food Chemistry. 2007;55(23):9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- Srivastava JK, Gupta S. Health promoting benefits of chamomile in the elderly population. In: Watson Ronald R., editor. Complementary and Alternative Therapies in the Aging Population. Elsevier Inc., Academic Press; 2009. [Google Scholar]
- Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sciences. 2007;64(22):2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II) Journal of National Cancer Institute. 1998;90(21):1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Blackard B. Pharmacologic effects of NSAIDs and implications for the risks and benefits of long-term prophylactic use of aspirin to prevent cancer. Recent Results in Cancer Research. 2009;181:215–221. doi: 10.1007/978-3-540-69297-3_20. [DOI] [PubMed] [Google Scholar]
- Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Reviews. 2007;26(3–4):525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]