Summary
The analysis of age-specific mortality can yield insights into how anti-aging interventions operate that cannot be matched by simple assessment of longevity. Mortality, as opposed to longevity, can be used to assess the effects of an anti-aging intervention on a daily basis, rather than only after most animals have died. Various gerontogene mutations in Caenorhabditis elegans have been shown to increase longevity as much as ten-fold and to decrease mortality at some ages even more.. Environmental alterations, such as reduced food intake (dietary restriction) and lower temperature also result in reduced mortality soon after the intervention. Here we ask how soon anti-aging interventions, applied during adult life, affect age-specific mortality in nematodes. Using Maximum Likelihood Analysis, we estimated the Gompertz parameters after shifts of temperature, and of food concentration and maintenance conditions. In separate experiments, we altered expression of age-1 and daf-16, using RNAi. Using about 44,000 nematodes in total, to examine daily mortality, we find that for both types of environmental shift, mortality responded immediately in the first assessment, while RNAi-induced changes resulted in a slower response, perhaps due to delayed mechanics of RNAi action. However, under all conditions there is a permanent “memory” of past states, such that the initial mortality component [a] of the Gompertz equation [μ(x)= aebx] bears a permanent “imprint” of that earlier state. However, “b” (the rate of mortality increase with age) is always specified by the current conditions.
Keywords: Aging, Mortality, Life Tables, Gerontogenes, Life Span, C. elegans, Genetics, Caloric Restriction, Demography, Longevity, Temperature Shift, RNAi
Introduction
“Lifespan extension” has been the hallmark of successful anti-aging studies in a diversity of organisms ranging across invertebrate and vertebrate animal models. In our model organism, the nematode Caenorhabditis elegans, this same surrogate marker for successful intervention has dominated the field for 25 years because it is central to most definitions of aging and relatively straight forward to measure (Johnson & Wood, 1982; Finch, 1990). The analysis of mortality is superior to simply measuring longevity because it can reveal the dynamics of the interventions in question; on the down-side of mortality measurements is the fact that much larger populations need to be employed (Vaupel et al., 1998).
Life-extension has been achieved by numerous methods (Tissenbaum & Johnson, 2008): including genetic approaches (Johnson & Wood, 1982; Kenyon, 2005; Tissenbaum & Johnson, 2008), interrupting development (Klass & Hirsh, 1976; Johnson et al., 1984), dietary restriction (DR; Klass, 1977; Johnson et al., 1990; Houthoofd et al., 2007; Greer and Brunet, 2009), hormesis (Lithgow et al., 1995; Cypser et al., 2006), and drugs (Melov et al., 2000; Wood et al., 2004; Evason et al., 2005). Two important questions for treatments associated with aging are whether interventions that prolong life will work at advanced ages and what effect these interventions have on late-life mortality, the latter of which can only be answered by analyzing the dynamics of age-specific mortality. (Finch, 1990; Vaupel et al., 1998; Carey, 2003).
Here we examine daily mortality in large populations (about 44,000 nematodes assessed) of C. elegans, focusing on shifts of conditions specifying mortality during the adult phase. Although requiring vastly larger sample sizes, we use daily mortality rate (the fraction of the population dieing on a given day of life) because mortality-in contrast to longevity, as is typically studied in C. elegans-can be assessed at all ages and provides an immediate feedback of the effect of the longevity intervention. We apply three interventions, all of which cause a shift from high-mortality to low-mortality and vice versa: DR, temperature shifts, and RNAi (Timmons et al., 2001) to down regulate gene expression,. Notably, environmental shifts affect mortality almost immediately, but there is a permanent “memory” of prior life conditions, showing that late-life interventions into aging can reduce the rate of future aging but do not cause “rejuvenation”. Genetic shifts also show a memory effect but the effects of the intervention take longer to observe, perhaps because of the delay necessary for the effects of RNAi to be established.
Results
Mortality Condition
We altered mortality by varying two environmental parameters: temperature (25°C vs 16°C), or food concentration (109/ml of E. coli, strain RW2, in liquid medium vs. confluent RW2 on NGM agar plates). We also affected mortality by targeting gene expression using RNAi; we shifted the wild-type animals to daf-2 RNAi to reduce mortality, and we shifted age-1 mutants to daf-16 RNAi to increase mortality. In all cases, we used populations of thousands of genetically identical nematodes (Supplementary Fig. S1) that had been carefully maintained in a uniform environment and were switched from one condition to another at a variety of adult ages.
Temperature Switch
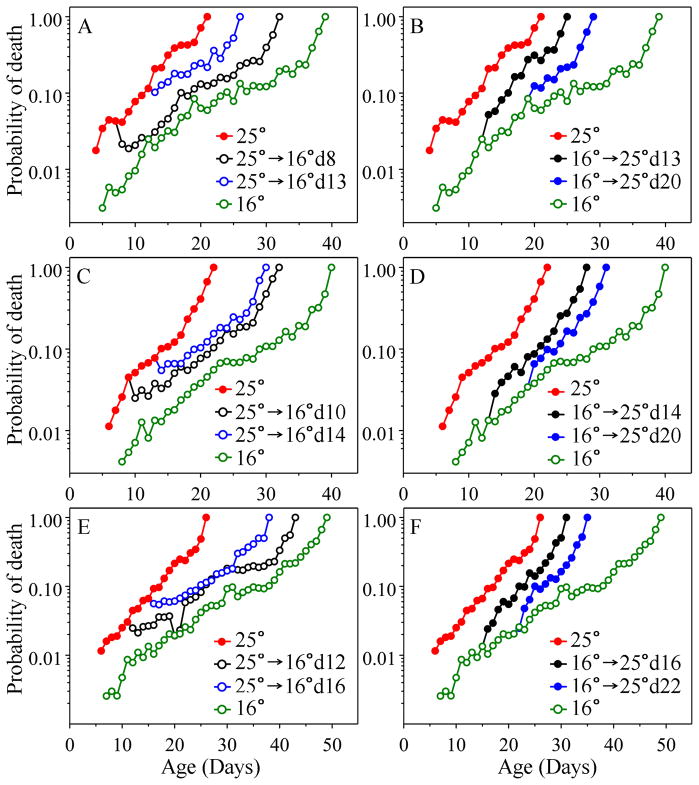
First we examined temperature with the expectation that when we switched the population from a high temperature (and high mortality) to a low temperature (low mortality) that mortality would change rapidly to that specified by the new temperature condition. Before performing such switches, we assessed mortality and analyzed it using the Gompertz equation: (μ(x) = aebx). We already knew that temperature dramatically affects life span in C. elegans (Klass, 1976; Friedman and Johnson, 1982), decreasing it more than 2-fold when comparing 16°C and 25°C. In three replicate control experiments switching between 16°C and 25°C, and vice versa (Fig. 1 and SI Table 1), we found that the rate of increase in mortality (b) was consistently about two-fold higher at the higher temperature, varying between 0.23 and 0.29 at 25°C, compared with 0.12 to 0.15 at 16°C. In all cases, this difference in slope was astronomically significant. In contrast, the other Gompertz parameter (a, initial mortality; SI Table 1), was affected little if at all, showing a non-significant higher value at 25°C.
Fig. 1.
Age-specific probability of death in response to temperature shift (The sample size and day of shift are detailed in the same order in SI Figure 1(A)). The red and green symbols represent controls maintained at 25°C and at 16°C, respectively. The rates of increase in mortality of 25°C controls are significantly higher than that of 16 °C controls in three replicates with p values of 3.0E-88, 2.1E-88 and 5.3E-93, respectively (SI Table 1). The black and the blue lines with open circles (A, C, E) represent the first and the second down shift from 25°C to 16 °C, respectively, whereas the black and blue lines with filled circles (B, D, F) represent the first and the second up shift from 16°C to 25°C, respectively. (A) Down shifts on day 8 and day 13 in the first experiment. For both the day-8 down-shift population and the day-13 down-shift population, the probability of death becomes significantly lower than that of the 25°C control as soon as the first day post-shift (p = 0.0094 and p = 4.1E-05, respectively, SI Table 2). Their mortality trajectories are significantly lower than that of 25°C (p = 2.1E-45 and p = 3.9E-19, respectively, SI Table 1), but are not significantly different from 16°C control (p = 0.1454 and p = 0.8875). (B) Up shifts on day 13 and 20 in the first experiment. After shifting to 25°C from 16°C, both up-shift populations show significantly higher daily probability of death as soon as the first day post-shift, with p values of 1.9E-05 and 0.0003, respectively and also significantly higher mortality trajectories (p = 3.5E-20 and p = 9.9E-09, respectively) in comparison to 16°C. However, their rates of increase in mortality are not significantly different from that of the 16°C control (p = 0.0385 and p = 0.1110, respectively). (C) Down shifts on day 10 and day 14 in the second experiment. Results are similar to A. (D) Up shifts on day 14 and day 20 in the second experiment. Results are similar to B. (E) Down shifts on day 12 and day 16 in the third experiment. Results are similar to A. (F) Up shifts on day 16 and 22 in the third experiment. Results are similar to B.
When we switched populations to the alternate temperature at a variety of different ages, we found that the rate of increase in mortality of the switched population (Gompertz parameter b) was not significantly different from the control population maintained continuously at the new temperature throughout adult life but very different from b of the unshifted control (Fig. 1, SI Table 1). In every experiment, the rate of increase in mortality was determined only by the concurrent temperature; i.e., the slope was always the same as that of the control at the same temperature (SI Table 1). In contrast, initial mortality (a) was dependent upon the age at which the switch was made; there was a monotonic increase in initial mortality that varied with increasing age at which the population was switched (SI Table 1). This initial mortality after the switch was consistently different from that of populations raised from young adulthood at that temperature. These results show that prior conditions matter in setting initial mortality but that present conditions determine the trajectory of current and future mortality, in response to temperature.
These significant impacts on mortality occurred within one day following the shift (SI Table 2); significant differences in mortality were seen on the first day and every subsequent day following the shift (with only one exception). When we examined populations at a finer time scale, we found that mortality again seemed to change within one-day following the temperature shift, but not within the first 12 hours. This suggests that temperature effects may not be immediate, although problems of power may also play a role in these findings (SI Figure 2A, B, SI Table 3). These results show that rate of aging (b) differs at the two temperatures; in fact, the rate of aging is determined only by the concurrent temperature condition. More strikingly, however, these results also show that there is a “memory” of the earlier state such that a population raised at higher temperature can never reach as low a level of age-specific mortality as that of a control population maintained continually at the lower temperature, and vice versa with high temperatures.
Switch in Food Concentration
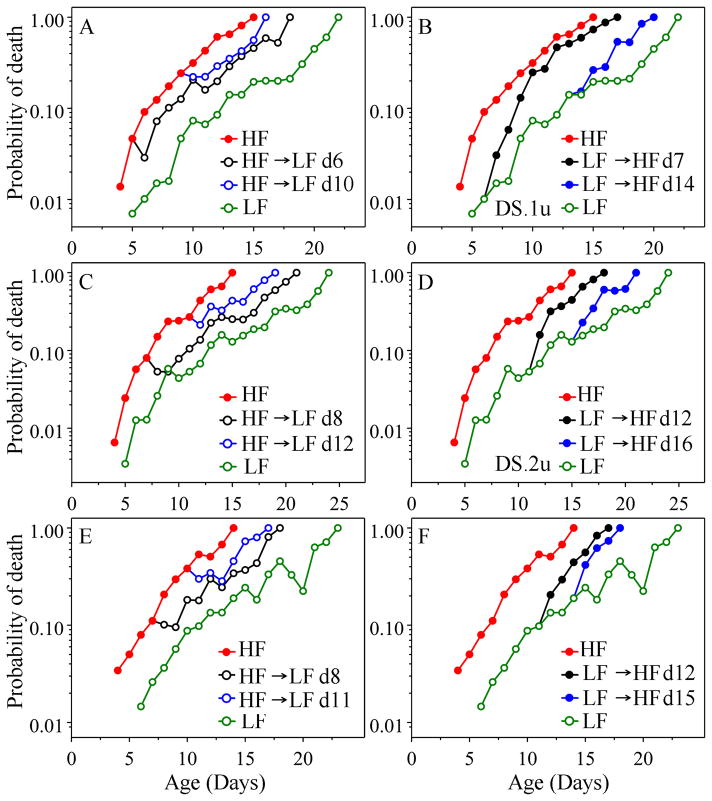
There are several reasons to examine the effects of altered food consumption on longevity and mortality. DR is the most-studied intervention known to extend longevity and decrease mortality in vertebrates, notably rodents (Weindruch & Walford, 1988). DR also extends life span in nematodes (Klass, 1977; Johnson et al., 1990; Houthoofd et al., 2007) and does this by decreasing “b”, the rate of mortality increase (Fig. 2, SI Table 4). As with temperature, every comparison between the rate of mortality increase under high-food and low-food conditions (which involved changed bacterial concentration as well as maintenance of the aging worms on both solid and liquid) showed highly significant differences (p = 7.5 × 10−61). There was no effect of food condition on initial mortality (a), which varied between 0.0025 and 0.0029 for the high-food condition and between 0.0021 and 0.0028 for the-low food condition.
Fig. 2.
Age-specific probability of death in response to dietary shift. Three replicates were represented by panels A–B, C–D and E–F, respectively. The sample sizes and days of shift are detailed in the same order in SI Figure 1(B). The red line with filled circles and the green line with open circles are the high food (HF, confluent RW2 on NGM plates) control without shift and the low food (LF, 1 × 109/ml RW2 in liquid medium) control without shift, respectively. The rates of increase in mortality of HF controls are significantly higher than that of LF controls in all three replicates, with p values of 7.5E-61, 4.8E-86 and 4.7E-67, respectively (SI Table 4). The black line with open circles and the blue line with open circles represent the first and the second down shift, respectively, from HF to LF. The black line with filled circles and the blue line with filled circles represent the first and the second up shift from LF to HF, respectively. (A) Down shifts on day 6 and 10 in the first experiment. In comparison to HF control, the probability of death of day 6 down-shift population decreases significantly with p values of 8.5E-07, 0.0006 and 7.1E-05 on the first, second and third days post-shift, respectively (SI Table 5). Its rate of increase in mortality is significantly lower than that of HF control as well (p = 4.4E-32, SI Table 4). However, its rate of increase in mortality is not significantly different from that of the LF control (p = 0.2774). For the day-10 down-shift population, the probability of death decreases significantly as soon as the first day post shift (p = 0.0029), and the rate of increase in mortality is significantly lower than that of HF control (p = 1.2E-07) but not significantly different from that of LF control (p = 0.8875). (B) Up shifts from LF to HF on day 7 and 14 in the first experiment. For both the day-7 up-shift population and the day-14 up-shift population, the rates of increase in mortality are significantly higher than that of the LF control (p = 2.7 E-15 and p = 1.7E-06, respectively) but not significantly different from the HF control (p = 0.0719 and p = 0.2301, respectively), and daily probability of death increase significantly with p values on the second day post-shift of 2.6 E-08 and 0.0086, respectively. (C) Down shifts on day 8 and 12 in the second experiment. Results are similar to A. (D) Up shifts on day 12 and 16 in the second experiment. Results are similar to B. (E) Down shifts on day 8 and 11 in the third experiment. Results are similar to A. (F) Up shifts on day 12 and 15 in the third experiment. Results are similar to B.
When we examined the populations shifted from high food to low food or vice versa we found a very clear effect on “b” and an effect on “a” that was monotonic with age. As with temperature, the rate of increase in mortality was always determined by concurrent food conditions, mimicking that of the control consistently maintained under the same food condition. Every switch from low food to high food showed an age-specific mortality (b) after the switch that did not differ from the high-food control, and every switch to low food displayed subsequently reduced rates of mortality increase that did not differ from the low-food control. Again every slope (b) differed with high significance from that of the opposite control (SI Table 4). This mortality switch again took place within the first day after the switch, the first time period in which population mortality data were collected typically (SI Table 5). In 11 of 12 switches, at ages varying from the sixth day to the sixteenth day of adult life and in both directions (high to low and the opposite), we saw significant differences in mortality, when compared with the non-switched control. As time passed, the effects of the new food condition only became more significant (SI Table 5). Age-specific mortality after the switch never moved to that of the control, always showing a “memory” of the previous food concentration.
Not an Effect of Bacterial Toxicity
One of the effects of gerontogene mutations in C. elegans is to make the worm more resistant to death by a variety of toxic bacteria (Aballay et al., 2000; Garsin et al., 2003). Since our food restriction regimen involved growth on two different concentrations of bacteria (109/ml vs confluency), we wanted to rule out infection by bacteria as a possible reason for differential death, especially considering that many have interpreted the lower bacterial concentration to represent dietary restriction. We did this by growing worms under conditions where further bacterial growth was limited by kanamycin, thus preventing infection of the worms by their bacterial diet. Under these conditions both initial mortality (a) and the rate of increase in mortality (b) were similar to what was seen in food-switch studies where kanamycin was not used (SI Figure 2C, D; SI Table 6, 7). Again the rate of mortality increase was determined only by the concurrent food condition and the change occurred rapidly following the food switch.
Switch of gene-expression by RNAi
We have seen that switching environmental conditions, either temperature or food, results in rapid changes in mortality to that specified by the new environment. However, in both cases there is a “memory” of the initial environmental condition. There is considerable interest in knowing whether similar results are seen when a change in gene-expression occurs as might happen when a human takes a drug designed to complex with a protein encoded by a gene known to limit life span. Many such drugs have now been tested in the nematode (Melov et al., 2000; Wood et al., 2004; Evason et al., 2005). Such a switch of gene-expression can be engineered in the worm by using RNAi and indeed some studies of such switching by RNAi have been previously performed (Dillin et al., 2002a; Dillin et al., 2002b). However those studies used populations of worms too small for mortality analyses, making it necessary for us to perform our own switch-of-gene-expression experiments to study the effects of such switches on mortality and its trajectory.
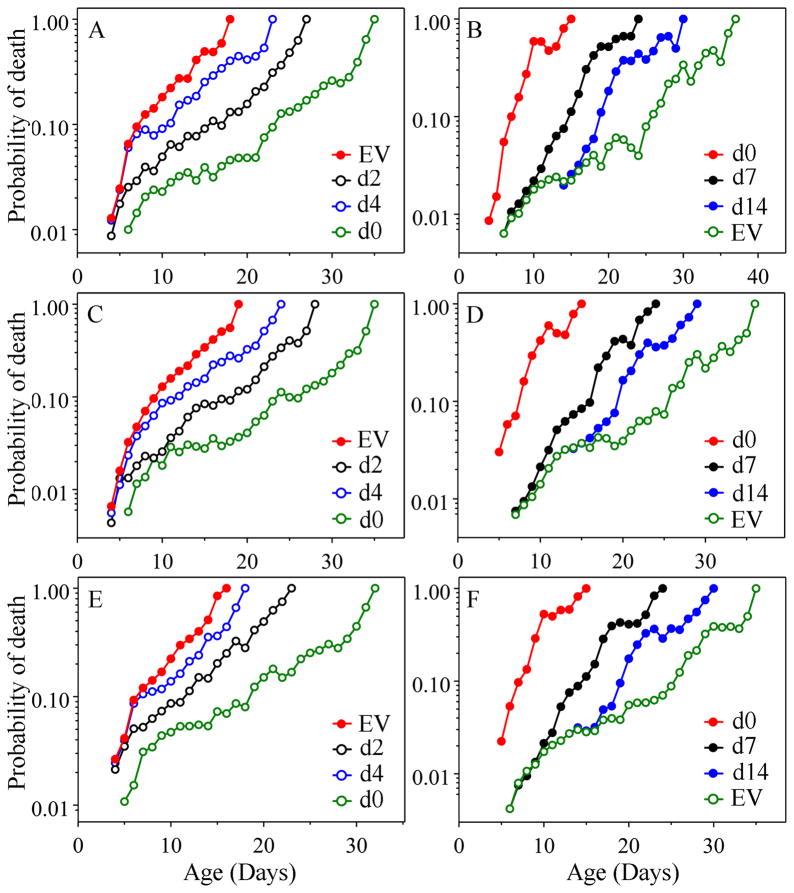
We switched gene-expression in two ways. First, we performed a switch to low mortality and longer life by switching wild-type worms from a diet of bacteria containing an empty RNAi feeding vector (EV) to one containing a feeding vector expressing daf-2. In the converse switch to a high mortality condition, we started with an age-1 strain and reversed its long-life phenotype to a short-lived one by feeding it an RNAi vector down-regulating daf-16 expression. As before, when we compared worms that were fed EV with those fed daf-2 or daf- 16 RNAi from day one of adult life, we found little effect on initial mortality (a), which varied less than 10% in most experiments. In contrast, the rate of increase in mortality (b) was altered dramatically (Fig. 3, SI Table 8), always varying coordinately with the vector being fed, in a highly significant manner and typically varying about three-fold. Also similar to earlier results, initial mortality following a switch to daf-2 RNAi (i.e., high- to low mortality) was dependent only on the age at which the switch was made, consistent with a memory of former conditions affecting mortality of the worms. However, unlike environmental switches, genetic switches at older ages did not achieve statistically significant changes in subsequent mortality (b) on the first day after the switch; instead, one day after switching, mortality of switched populations was statistically indistinguishable from the control that had not been switched. Switching to daf-2 RNAi at later ages did reduce mortality (b) highly significantly in all cases, but mortality never achieved rates as low as that of the population switched at day 0 (the first day of adulthood). Similarly, the switch from age-1 to daf-16 (i.e., low-to high-mortality) by RNAi consistently resulted in increasing initial mortality (a) and altered rates of aging (b), whenever the switch occurred at later adult ages, suggesting continual expression of the insulin/IGF-1 signaling pathway and of DAF-16 action throughout life (Dillin et al., 2002a; b). In contrast to the situation for environmental shifts, it typically took several days (>10 % of the worm’s lifespan) after the shift in gene-expression for the changes in mortality to become significant (Fig. 3, SI Table 9). This delay may be due to reduced ability to inhibit RNA-expression by RNAi at later ages and/or to stability of the DAF-2 and DAF-16 protein even after the shift in expression, or to a delay in the action of the protein on mortality. Regardless, in all of our experiments, whether using environmental or RNAi manipulations of mortality, we found consistent impacts on the rate of aging and consistent lack of rejuvenation.
Fig. 3.
Age-specific probability of death in response to RNAi. The sample size and day of RNAi treatment are detailed in SI Figure 1(C and D) in the same order. For both the down-shift and the up-shift, empty vector (EV) is pL4440. In the three replicates of daf-2 RNAi shift (A, C, E), red line with filled circles represents worms on empty vector only, whereas green, black, and blue lines with open circles represent shifts from empty vector to daf-2 RNAi on day 0, day 2 and day 4, respectively. In the three replicates of the daf-16 RNAi shift (B, D, F), green line with open circles represents TJ1062 worms on empty vector without shift; while red, black and blue lines with filled circles represent shifts from empty vector to daf-16 RNAi on day 0, day 7 and day 14, respectively. (A) The first experiment of daf-2 RNAi down shift. The first days on which daily probability of death becomes significantly lower than that of EV control for shift populations on day 0, day 2 and 4 are the 5th day post-shift, the 5th day post-shift and 6th day post-shift, respectively (SI Table 9). The rates of increase in mortality of all three shifted populations are significantly lower than that of the EV control (p = 2.9E-93, p = 1.4E-63 and p = 6.9E-51, respectively, SI Table 8). However, neither the day-2 shift nor the day-4 shift display the same rate of increase in mortality as does the group shifted on day 0; instead their rates are significantly higher than that of the group shifted on day 0 (p = 0.0092 and p = 2.5E-07, respectively). (B) the first experiment of daf-16 RNAi up shift. The first days on which daily probability of death becomes significantly higher than that of the EV control for populations shifted on day 0, day 7 and 14 are the 5th day post-shift, the 6th day post-shift and 6th day post-shift, respectively (SI Table 9). The rates of increase in mortality of all three shift populations are significantly higher than that of the EV control (p = 1.7E-94, p = 4.1E-40 and p = 1.9E-26, respectively, SI Table 8). In comparison with the population shifted on day 0, both populations shifted on day 7 and day 14 show significantly lower rates of increase in mortality (p = 7.2E-13 and p = 3.6 E-23, respectively). (C) Second replicate of daf-2 RNAi down shift. Results are similar to A. (D) Second replicate of daf-16 RNAi up shift. Results are similar to B. (E) Third replicate of daf-2 RNAi down shift. Results are similar to A. (F) Third replicate of daf-16 RNAi up shift. Results are similar to B.
Discussion
Age is by far the most significant predictor of mortality and morbidity in human populations, as well as in numerous animal models (Finch, 1990; Carey, 2003). Thus, slowing the rate of aging could significantly affect overall levels of morbidity and significantly delay expenditures on elder health care (Olshansky et al., 2001). Both environmental and genetic alterations can lead to the extension of adult life span, which can be interpreted as slowed aging. Temperature, food restriction, and genetic alterations have been demonstrated to lead to significant life extensions in yeast, nematodes, flies, and mice. Indeed genetic mutations in the age-1 gene in the nematode worm, C. elegans (where the most robust responses to these interventions have been seen [Kenyon, 2005; Tissenbaum & Johnson, 2008]) can lead to as much as a ten-fold longer life (Ayyadevara et al., 2008). When coupling single-gene mutations with food restriction, as much as an eight-fold extension of life has been seen (Houthoofd et al., 2004).
Advocates of intervention to slow the aging process, of necessity, suggest that interventions be started later in life, but there is relatively little data suggesting that late-life switches can actually result in the slowing of aging and the delay of late-onset pathology. A few switches of food availability in rodents late in life have shown that life extension can still be seen when mice are switched to DR conditions at later ages (Weindruch & Walford, 1982; Goodrick et al., 1983; Means et al., 1993; Berrigan et al., 2003; Dhahbi et al., 2004) and the same is true in rats, Drosophila, and in Mediterranean fruit flies, where the story may be more complex (Carey et al., 1998; Tatar et al., 2001; Mair et al., 2003; Skorupa et al., 2008). In a recent study, B6C3F1 male mice were shifted to a 40% DR diet at 19 months of age (Dhahbi et al., 2004), and when compared with 10% restricted controls, the DR shift resulted in a statistically significant change in monthly mortality only after 9 months of DR (our recalculation using the author’s data). This long period to see an effect probably resulted from the low power due to the small sample size (n = 60), which is very typical for mouse studies. Reports of aging reversal or “rejuvenation” appear less frequently in the literature (Carey et al., 1998; Tatar et al., 2001; Mair et al., 2003; Dhahbi et al., 2004; Giannakou et al., 2007), and our results show no such reversal, which would be manifested as a reduction in daily mortality. Work on med-flies shows that flies fed protein late in life are restored to a lower mortality rate and have renewed fertility (Carey et al., 1998). Recently the Interventions Testing Program has identified compounds resulting in life extension during the adult phase of mice, and notably rapamycin can increase life expectancy at 600 days (when feeding begins) by as much as 28 to 48% (Strong et al., 2008; Harrison et al., 2009). These studies did not examine mortality, only longevity.
A series of elegant experiments in Drosophila (Mair et al., 2003; Giannakou et al., 2007) examined mortality in flies that were switched from high mortality conditions to low mortality conditions and vice versa, using both a temperature and a food switch, as well as a change in gene expression to establish such differences. The data showed that mortality after a temperature shift was determined by both current conditions and previous conditions. Surprisingly, mortality after a dietary shift (Mair et al., 2003) and mortality after a shift of gene expression (Giannakou et al., 2007) were determined solely by concurrent conditions. In other words there was no memory of earlier conditions. In analyzing human mortality data after the German reunification, Vaupel et al. (2003) found also that mortality of several cohorts of aged East and West Germans were no longer different ten years after reunification. Further analysis of other human populations undergoing similar changes in lifestyle and mortality are therefore warranted.
In comparing mortality alteration in populations of C. elegans subjected to a switch in environmental or genetic condition at various times during the adult phase with those observed in Drosophila we find similarities and differences. We have replicated the temperature-shift results observed in Drosophila; however, our results for both food and gene-expression shifts are very different from what has been observed in the fly. In both Drosophila and C. elegans, a shift in temperature, food quantity or gene-expression results in an immediate impact of the new condition on mortality rate. However, the remarkable reduction in mortality to that of the control seen after food shift and shift of gene expression in Drosophila were not replicated in C. elegans. Unlike the fly, “short-term risk of death” (Mair et al., 2003) is not the only thing affected by DR and by altered gene expression. In C. elegans past history also plays a role in determining mortality. Mortality in the worm appears to be cumulative and a function of integrating all the worm’s past mortality history.
In the case of genetic switches we employed an RNAi feeding methodology, which has been used by many labs to identify genes showing altered adult longevity (Lee et al., 2003; Hamilton et al., 2005; Hansen et al., 2005; 2007; Curran & Ruvkun, 2007, Chen et al., 2007; Samuelson et al., 2007. Our methodology was similar in design to that used by Dillin et al (2002a, 2002b) to study switching in several other longevity mutants. (The studies of Dillin and colleagues used small populations of worms, making it necessary for us to perform our own switch-of-gene-expression experiments to study the effects of such switches on mortality and its trajectory.) Here the results were the same as with the environmental shifts in that the rate of increase in mortality (b) rapidly changed to a new trajectory, determined by both current as well as past gene expression. In contrast to the environmental interventions, genetic alteration never achieved a rate of mortality increase that was identical to that of the unshifted population of the same gene-expression pattern. When temperature is shifted the physiologic processes of a cold-blooded organism are almost immediately determined by the new environment and thus mortality under altered temperature may be determined primarily by such processes in both worms and flies. Also in C. elegans a new food regimen is almost immediately recognized and determines a new mortality trajectory, i.e. rate of aging. In contrast, the inhibition of gene function by RNAi involved a molecular response to the RNAi and it appears to take several days for this response to be put into effect (SI Table 9). Both shifts to higher mortalities (shift to daf-16 RNAi) and to lower mortalities (shift to daf-2 RNAi) resulted in significant changes on day 5. In contrast, both shifts of temperature and of food condition produced alterations in mortality by the end of the first day after the shift. There was no difference with chronologic age in the time it took to see a significant effect on mortality after RNAi shifts. This is consistent with a lack of age effects either on the rate of food consumption or on the rate with which RNAi takes place.
Mair et al. (2003) discuss two types of factors that may influence adult age-specific mortality: those that are inherent to the system and which modulate accumulation of irreversible damage and those that are either external to the system or reflect an inherent instability in the system and which modulate short-term vulnerability to death (risk). In this formulation, aging in C. elegans can be seen to be determined by both “aging-related damage”, which we have termed a memory component, as well as by risk that is determined by current environment and genes. (By culturing worms in the presence of kanamycin to prevent bacterial growth, we have ruled out acute food-source toxicity as a factor influencing mortality in our food-switch experiments.) A similar conclusion was reached by Lenaerts and colleagues (2007), studying mortality trajectories in worms maintained on agar plates comprised of axenic growth medium supplemented with different concentrations of radiation-killed bacteria. In that study, the rate of increase in mortality (0.08) of worms shifted on day 0 from the fully-fed condition to the DR condition was the same as that (0.08) of worms maintained under the DR condition from eggs; and the rate of increase of mortality (0.15) of worms shifted on day 0 from DR to fully fed condition was very similar to that (0.18) of worms maintained under fully fed condition from eggs. Although there was only one replicate, the studies of Lenaerts and co-workers support our conclusion that the rate of increase in mortality is determined by concurrent food conditions.
We fail to identify any rationale in methodology that could underlie the striking differences between fruit flies and nematodes after food switch in terms of their memory of past food conditions. Fertility was modified in both populations so that neither was effectively reproducing. In our studies this was not done by sterilizing the animals but rather by inhibiting development of progeny by using 5-fluorodeoxy- Uridine (FUdR), which has become a standard method in nematode aging research and has been repeatedly shown by us to be without detectable effect on longevity. In the case of a switch of gene-expression, where we also observed overt differences between C. elegans and Drosophila melanogaster in terms of their mortality response after switching, we employed temperature-sensitive sterile mutants, negating the confound of FUdR altogether. Such different responses between worms and flies may result from strain, species or even phyla differences. An examination of mortality data in mice after dietary shift fails to identify or disprove a memory effect, due to lack of power (Weindruch & Walford, 1982; Goodrick et al., 1983; Means et al., 1993; Berrigan et al., 2002; Dhahbi et al., 2004). Therefore, for now, the intriguing phenomenon of memory, as reported here, and the question of whether it is a general phenomenon across taxa remains an open question.
Experimental procedures
Nematode maintenance and conditions for lifespan assessments
Standard nematode culturing techniques were employed (Sulston & Hodgkin, 1988). The following strains were utilized: N2 (wild type), TJ1060 [spe-9(hc88) I; fer-15(b26) II, and TJ1062 [spe-9(hc88) I; fer-15(b26) age-1(hx546) II]. Techniques for RNAi treatment of synchronized populations were described in Rea et al. (2007). Lifespan was assessed at 16 °C and 25 °C in liquid culture with 109 bacteria per ml (Johnson & Wood, 1982) for temperature-shift, or at 20 °C on NGM plates for dietary-shift and RNAi-shift, using synchronous populations of about 50 worms per plate as previously described (Johnson & Wood, 1982), unless otherwise described.
Temperature shift conditions
Synchronized N2 hermaphrodites grew up at 20 °C on NGM/OP50 plates supplemented with 2% peptone. When they were young adults, we transferred worms to 25 °C and 16 °C, respectively, in standard liquid food with the concentration of OP50 at 1 × 109/ml. To sterilize worms, we treated them with 25 μM FUdR when they were young adults in liquid plates. Worms were shifted on specified days as shown in SI Figure 1(A).
For large-scale cultivation, a synchronized N2 population was grown at 20 °C on NGM/OP50 plates supplemented with 2% peptone. They were transferred as young adults to standard liquid food in 20 10-cm glass plates in which 25 μM FUdR was added. Ten plates were put at 25 °C and 10 at 16 °C. We washed and transferred them to fresh liquid plates with FUdR every day. When they were 6 days old, those worms at 25 °C were washed and transferred into 6-cm liquid food plates with 80 worms each. We scored and transferred those worms at 25 °C every 12 hours, and shifted almost half of them to 16 °C on day 9. Three days after shift, we threw away both unshifted worms at 25 °C and worms shifted to 16 °C. When those worms kept at 16 °C were 17 days old, we transferred them into 6-cm liquid food plates with 80 worms each. We scored them every 12 hours and shifted almost half of them to 25 °C on day 20. Three days after shift, the experiment ended.
Dietary shift conditions
The high-mortality food condition was 25 °C on NGM plates, using RW2 (wild type E. coli, grown to confluency) plates supplemented with 2% peptone and the low-mortality condition was 25 °C in plates containing liquid S-basal and RW2 at 1 × 109/ml. Synchronized TJ1060 populations grew up on NGM plates spotted with RW2 at 25 °C. When they were young adults, we transferred them into high-food and low-food conditions. Worms were shifted on specified days, as shown in SI Figure 1(B).
Kanamycin treatment
RW2 was grown to saturation overnight in LB. We then added 200 μg/mL kanamycin (filter sterilized), and incubated overnight. An aliquot of cells was streaked out onto a plain LB plate to insure that bacteria were dead and no colonies grew up. NGM Plates with 2% peptone had kanamycin (a final concentration of 200 μg/mL) added by pouring it onto the plate.
RNAi feeding conditions
For gene-expression shifts, establishment of lower mortality entailed use of TJ1060 fed RNAi against daf-2 (SI Figure 1C), while shifts to higher mortality entailed use of TJ1062 fed RNAi against daf-16 (SI Figure 1D). RNAi feeding constructs have been described previously (Rea et al., 2007). RNAi feeding methodology was based on the procedure of Kamanth and Ahringer (2003) with the following modifications: Overnight HT115(DE3) cultures containing empty vector (pL4440), daf-2 or daf-16 were first adjusted to an OD590 value of 0.9 then 500 μL was spread onto 10 cm RNAi plates (NGM agar + 1 mM IPTG, 100μg/ml ampicillin and 5μg/mL tetracycline). Lawns were grown overnight at 23 °C then stored at 4 °C until use. Large populations of TJ1060 and TJ1062 were maintained at 16 °C on NGM/OP50 plates. Synchronous populations were generated by a 4-hour limited egg lay onto NGM/OP50 lawns. Eggs were collected by washing (S-Basal), counted, then a stock population immediately transferred onto vector-only RNAi plates at 25 °C. The first day of adulthood was deemed Day 0. Stock animals were transferred each day to fresh vector-only plates by washing (S-Basal). On the desired days, an unbiased selection of stock animals was manually transferred to daf-2 (TJ1060) or daf-16 (TJ1062) RNAi plates and then life span recorded. All subsequent manipulations of these selected populations were done using platinum picks.
Statistical assessments of lifespan
Probability of death is estimated and plotted to reflect mortality trajectory with age. It is estimated by the formula
where qx is the probability of death at age (day) x; dx is the number of worms that died in the age interval (x, x+1) and nx is the number of worms at risk at the beginning of age x.
Comparison of probability of death between two populations was made by calculating the normalized statistics
where and are probability of death at age x and number of worms at risk in two populations.
The force of mortality is fitted with the following Gompertz model to reflect the initial mortality and the rate of increase in mortality with age
where μ(x) represents the force of mortality at age (day) x, a is the initial mortality, and b is the rate of increase in mortality with age (the slope of the mortality curve). Gompertz parameters were estimated by using the Maximum likelihood method. We used Gauss to do MLE fitting. The Log Likelihood function can be expressed in the form
Where qx is described by the equation:
Likelihood ratio test is employed for the comparison of the rates of increase in mortality between two populations.
Supplementary Material
Acknowledgments
Technical assistance was provided by Ben Gurney (B.Sc.), Alison Kell (B.Sc.), Adya Mishra, KC Sweta, Lauren Temmer and Mike Gleason. Supported by the National Institute on Aging to TEJ and JWV and the Polis Foundation to TEJ and SLR.
Footnotes
Author Contributions
DW performed the environmental shifts, made the figures, analyzed all the data, wrote much of the paper and especially the Methods, and submitted the manuscript. SR developed and performed the RNAi-shift experiments and supervised much of the training. JC helped to collect data, organize the manuscript, and conduct training. TEJ conceived and supervised the experiments, obtained funding, and wrote the manuscript. All helped to design and interpret experiments.
References
- Aballay A, Yorgey P, Ausubel F. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 2000;10:1539–1542. doi: 10.1016/s0960-9822(00)00830-7. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Muller HG, Wang JL, Vaupel JW. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- Carey JR. The Biology and Demography of Life Span. Princeton: Princeton University Press; 2003. [Google Scholar]
- Chen D, Kally ZP, Palter JE, Kapahi P. Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell. 2007;6:525–533. doi: 10.1111/j.1474-9726.2007.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genetics. 2007;4:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Kim H-J, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci USA. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for Insulin/IGF-1 signaling in C. elegans. Science. 2002a;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AJ, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002b;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–262. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- Finch CE. Senescence, Longevity, and the Genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Jacobson J, Vinti G, Leevers SJ, Partridge L. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007;6:429–38. doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38:36–45. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic screen for longevity genes in C. elegans. Genes Develop. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Hsu A-L, Dillin A, Kenyon C. new genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genetics. 2005;1:e17. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Extending lifespan in C. elegans. Science. 2004;305:1238–1239. doi: 10.1126/science.305.5688.1238c. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Gems D, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. Interdiscip Top Gerontol. 2007;35:98–114. doi: 10.1159/000096558. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1982;79:6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Aging can be genetically dissected into component processes using long-lived lines of Caenorhabditis elegans. Proc Natl Acad Sci USA. 1987;84:3777–3781. doi: 10.1073/pnas.84.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Mitchell DH, Kline S, Kemal R, Foy J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech Ageing Dev. 1984;28:23–40. doi: 10.1016/0047-6374(84)90150-7. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Friedman DB, Foltz N, Fitzpatrick PA, Shoemaker JE. Genetic variants and mutations of Caenorhabditis elegans provide tools for dissecting the aging processes. In: Harrison DE, editor. Telford; Caldwell, NJ: 1990. pp. 101–126. [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in C. elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Klass M, Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976;260:523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Lenaerts I, Van Eygen S, Vanfleteren F. Adult-limited dietary restriction slows gompertzian aging in Caenorhabditis elegans. Ann N Y Acad Sci. 2007;1100:442–8. doi: 10.1196/annals.1395.049. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Means LW, Higgins JL, Fernandez TJ. Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav. 1993;54:503–508. doi: 10.1016/0031-9384(93)90243-9. [DOI] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy D, Doctorow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Olshansky JS, Carnes BA, Butler RN. If humans were built to last. Sci Am. 2001;284:50–55. doi: 10.1038/scientificamerican0301-50. [DOI] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development and life extension in Caenorhabditis elegans. PLOS Biology. 2007;5:2312–2329. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson AV, Carr CE, Ruvkun G. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Develop. 2007;21:2976–2994. doi: 10.1101/gad.1588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, Hodgkin J. In: The Nematode Caenorhabditis elegans. Wood WB, editor. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1988. pp. 587–606. [Google Scholar]
- Tatar F, Chien SA, Priest NK. Negligible senescence during reproductive dormancy in Drosophila melanogaster. Am Nat. 2001;158:248–258. doi: 10.1086/321320. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Johnson TE. In: Molecular Biology of Aging. Guarente L, Partridge L, Wallace D, editors. Cold Spring Harbor, N.Y: 2008. pp. 153–183. [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Khazaeli A, Liedo P, Longo VD, et al. Biodemographic Trajectories of Longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K. It’s never too late. Science. 2003;301:1679–1681. doi: 10.1126/science.1090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction of mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C. Thomas; Springfield, IL: 1988. [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.