Abstract
Background
Viral load (VL) is a critical marker for monitoring HIV disease progression and response to antiretroviral therapy. In resource-constrained settings, there is a need for a simple and inexpensive assay to monitor infected adults and children.
Methods
We compared versions 2 and 3 of the ExaVir™ Load assay, Cavidi AB (HIV RT) with the Roche, COBAS® Amplicor® HIV-1 Monitor assay (HIV RNA) for quantifying HIV VL.
Results
The HIV RT version 2 assay showed good sensitivity with detection in 94% of samples with HIV RNA >1000 copies/ml. Adult samples were tested using HIV RT version 2 (n=35) and version 3 (n=23) assays with plasma volumes of 1ml (recommended), 0.5ml and 0.25ml in comparison with HIV RNA. The HIV RT and HIV RNA assay results were comparable when tested using different volumes. Comparison of results from pediatric samples (n=27), tested using 1ml and a smaller volume by HIV RT version 2 were not significantly different.
Conclusion
The HIV RT assay was comparable to the HIV RNA assay with sensitivity approaching that of RT-PCR. Smaller volumes than the recommended 1ml can be used, improving utility of this assay for pediatric monitoring.
Keywords: Monitoring HIV infection, HIV reverse transcriptase, viral load, resource constrained countries, pediatric testing
Introduction
Together with CD4+T cell counts, viral load (VL) assays are critical markers for monitoring HIV disease progression and response to antiretroviral therapy (ART). VL is commonly assessed by measuring plasma HIV RNA levels however the use of nucleic acid based VL assays is limited in many resource-constrained settings as they are expensive, rely on complex equipment and highly trained laboratory staff 1–3 and are susceptible to viral DNA contamination. Alternative, affordable assays for determining VL have been assessed including the ExaVir™ Load assay (Cavidi AB)4 which measures virion-associated reverse-transcriptase (RT) activity rather than virion-associated RNA, and several in-house and real time PCR assays which quantify HIV RNA 2,5–9. The in-house real time PCR assays, although usually less expensive than commercially available assays, require sophisticated equipment, skilled staff, monitoring of reagent quality and remain prone to contamination 2,3,10. The ExaVir™ Load assay is relatively inexpensive, requires simple, robust equipment and is easier to perform than the RNA-based assays 1,3,11,12. In this study, we compared the version 2 and recently released version 3 ExaVir™ Load assay (HIV RT) to the COBAS® Amplicor® HIV-1 Monitor assay version 1.5 (HIV RNA). As the recommended plasma volume of 1ml plasma can be difficult to obtain from pediatric patients, we also assessed whether the assay could be used with smaller plasma volumes (0.5 and 0.25ml).
Materials and Methods
Sample Population
Two hundred and one plasma samples obtained from HIV seropositive adults attending the Infectious Diseases Unit at the Alfred Hospital (Melbourne, Australia) were tested retrospectively for RT activity. Twenty seven plasma samples obtained from pediatric patients (<18 months) as part of a study into PMTCT care at Kenyatta National Hospital (ethics obtained from the Institutional Review Board, University of Washington and the Ethical Review Board, University of Nairobi) were tested retrospectively for RT activity. Six of these samples had sufficient volume to test at the recommended 1ml sample volume as well as a smaller sample.
VL assays
HIV RT activity in patient plasma samples was determined using the ExaVir™ Load version 2 assay (HIV RT version 2; Cavidi AB, Sweden) according to manufacturer’s instructions 4. Selected samples were also tested using the ExaVir™ Load version 3 assay (HIV RT version 3) during beta-testing also at the Burnet Institute. Results from samples tested using a plasma volume of <1ml were automatically adjusted for the appropriate dilution factor by the supplied computer software (version 2: ExaVir™ Load Analyzer version 1.62/COLO 2–6 and version 1.62/COLO 2–9 (dilutions analysis only); version 3: ExaVir Load version 3 colo3.1 2007-04-17). HIV RNA testing was performed using the COBAS® Amplicor® HIV-1 Monitor assay version 1.5 ultra-sensitive preparation (RT-PCR; Roche Diagnostics, USA)13 according to the manufacturer’s instructions.
Statistical Analysis
Both variables (HIV RT activity (copies/ml equivalents) and HIV RNA (copies/ml)) were transformed using a log10 transformation and their correlation determined using Pearson’s correlation coefficient (r). Samples above and below the limit of detection for both the HIV RNA assay and the HIV RT assay were excluded from this statistical analysis as they were outside the linear range of the assay; samples below the limit of detection were included in all other analysis. ANOVA was used to assess the difference between the adult dilutions tested on the HIV RT version 2 assay, Friedman’s test was used for analysis of adult dilutions tested on the HIV RT version 3 assay (due to small sample size) whilst the Wilcoxon signed-rank test was used for pediatric dilutions tested using HIV RT version 2 assay. A clustered linear regression was performed when assessing the differences in the pediatric samples regardless of plasma volume.
RESULTS
Sample Volume Analysis
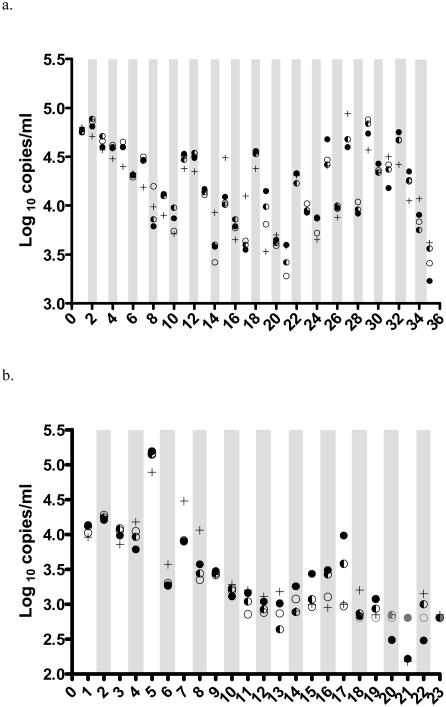
To determine the validity of using smaller plasma volumes for VL testing, volumes of 0.5ml and 0.25ml were compared with the recommended 1ml sample volume using the HIV RT version 2 assay (n=35). When a plasma volume of <1ml was tested in the HIV RT assay, volume was adjusted to 1ml with HIV-1 seronegative human plasma. Results corrected for sample volume by the HIV RT were not different to the results using the recommended 1ml testing volume or to HIV RNA (p>0.3). The median of the greatest difference between the 1ml sample and the diluted sample using the HIV RT assay for all samples was 0.03 log10 copies/ml equivalents (IQR: −0.08 to 0.08 log10; range: −0.41 to 0.35 log10); the median of the largest difference between volumes tested using the HIV RT assay and the matched HIV RNA results was −0.14 log10 copies/ml (IQR: −0.22 to 0.07 log10; range: −0.62 to 0.55 log10; Fig. 1a).
Figure 1.
HIV viral load results using different plasma volumes from 35 adult HIV+ patients tested using the HIV RT assay version 2 (a) and version 3 (b). HIV RNA values are shown using +. HIV RT values using the recommended 1 ml of plasma are shown using ●, 0.5ml plasma results using ◐ and 0.25 ml plasma results using ○. Samples shown in grey are below the detection limit of the HIV RT assay and have been assigned the assay detection limit.
Testing of neat and diluted adult plasma sample volumes (n=23) using the HIV RT version 3 assay showed similar results to the version 2 assay. Diluted samples were below the detection limit of the HIV RT version 3 assay in at least one of the volumes tested for six patient samples (HIV RNA median: 700 copies/ml; range: 150 to 1,600 copies/ml). The median of the greatest difference between the 1ml sample and the dilutions tested using the HIV RT version 3 assay for all samples was 0.05 log10 copies/ml equivalents (IQR: −0.09 to 0.25 log10; range: −0.59 to 1.01 log10); the median of the greatest difference between the HIV RNA result and the matched volumes tested using the HIV RT version 3 assay was 0.06 log10 copies/ml (IQR: −0.27 to 0.38 log10; range: −0.98 to 0.71 log10; Fig. 1b). Results within the detectable range from samples tested at smaller sample volumes using the HIV RT version 3 assay were not significantly different (p > 0.3) to the results using the recommended testing volume.
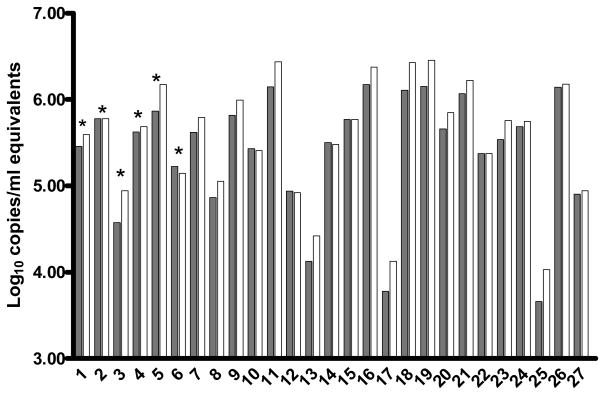
Samples from pediatric patients (n=6) were tested using the HIV RT version 2 assay and results using the recommended 1ml were compared to those obtained using 0.25ml (n=2) and 0.5ml (n=4). Results using the smaller volumes were not significantly different to those obtained from 1ml of plasma (p=0.17). A further 21 samples from pediatric patients were tested using two volumes but where insufficient plasma was available to test using the recommended 1ml. The median of the greatest difference between the largest and smallest samples tested using the HIV RT assay for all samples was 0.17 log10 copies/ml (IQR: −0.29 to −0.02 log10; range: −0.37 to 0.08 log10; n=27, Fig. 2).
Figure 2.
HIV viral load results using different plasma volumes from 27 pediatric HIV+ individuals tested using the HIV RT assay version 2. HIV RT results using the larger volume of plasma (range: 1 to 0.3ml) are shown in dark grey and the smaller volume (range: 0.15 to 0.5ml, adjusted for dilution factor) are shown in light grey, * Results from 1ml plasma tested (n=6) as the recommended sample volume.
Sensitivity of HIV RT assay and correlation with HIV RNA
Samples (n=144) from 128 patients were tested using both the HIV RNA and HIV RT version 2 assays with 116 samples giving results in the detectable range using both assays. A strong positive association was observed between detectable samples using the HIV RT and the HIV RNA assays (r=0.91; p=<0.0001). The sensitivity of the HIV RT version 2 assay approached that of the HIV RNA assay with detection by HIV RT of 94% of all samples with HIV RNA >1000 copies/ml (n=93; Table 1).
Table 1.
Sensitivity of the HIV RT assay ExaVir™ Load kit version 2 compared to HIV RNA assay (COBAS® Amplicor® HIV-1 Monitor assay version 1.5)
| HIV RNA (copies/ml) | Number of samples tested | Number (%) of samples detectable by HIV RT version 2 |
|---|---|---|
| 50–400 | 20 | 11 (55) |
| 401–1,000 | 31 | 22 (71) |
| 1,001–10,000 | 47 | 42 (89) |
| 10,001–50,000 | 30 | 29 (97) |
| >50,000 | 16 | 16 (100) |
Discussion
This is the first study evaluating the Cavidi HIV RT version 2 and 3 assays using small plasma volumes (0.25–0.5ml) which are often needed for testing in pediatric populations. Our data, using both adult and pediatric samples, suggest that the performance of the HIV RT assay is largely unaffected by plasma volumes ≥0.25ml, as the minimal bias of 0.05 log10 is not clinically significant. For plasma samples with low VL of less than 800 copies/ml (detection limit of HIV RT version 3 of 200 copies/ml equivalents then adjusted for dilution factor) the sensitivity of the HIV RT assay using volumes <1ml may be compromised. The lowest HIV RNA viral load that we have tested providing a detectable HIV RT result with plasma volumes of 1ml, 0.5ml and 0.25ml is 800 HIV RNA copies/ml. Further testing at low RNA/RT values is required due to the small sample size.
In this study, results from version 2 of the HIV RT assay correlated well with those of the HIV RNA assay (r=0.91), which is consistent to previous studies using both version 2 4,12,14–16 and version 1 12,14,17,18 of the HIV RT assay. The HIV RT version 2 assay showed good sensitivity with 94% of samples with HIV RNA >1,000 copies/ml detectable by HIV RT, similar to our earlier data and those of others using spiked plasma 12 or clinical samples 4,14–16. The HIV RT assay has been greatly improved from version 1, where the sensitivity was reported to be in the range of 7,000 to 10,000 HIV RNA copies/ml 12,14. The level of detection appears comparable to or better than the standard nucleic acid based assays. Preliminary data from our laboratory (not published) with the HIV RT version 3 assay indicate that this assay is more sensitive than the version 2 assay with a reported detection limit of 200 copies/ml equivalents.
In our laboratory the cost of the HIV RT assay including labour and consumables is approximately one-fifth of the price of the Roche COBAS® Amplicor® HIV-1 Monitor test, providing a significant cost-saving to the laboratory. The HIV RT assay should be considered as an effective low-cost alternative for monitoring HIV levels of children and adults particularly in, but not limited to, resource-constrained settings.
Acknowledgments
We would like to acknowledge Gary Corrigan for his assistance, Cavidi AB for the donation of reagents, Dr. Irene Iwani for assistance in obtaining the pediatric specimens, Maxwell Majiwa and Joseph Ochieng for technical assistance at University of Nairobi, Nairobi, Kenya, the Clinical Research Department and the Infectious Diseases Department, The Alfred Hospital, Melbourne, Australia for assistance in obtaining the adult specimens and Jenny Lewis for statistical analysis
Footnotes
Data previously presented in part at XVI International AIDS Conference, Toronto, Canada, 13–18 August 2006 and Australasian Society for HIV Medicine, 18h Annual Conference, Melbourne, Australia, 11–14 October 2006.
References
- 1.Crowe S, Turnbull S, Oelrichs R, et al. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin Infect Dis. 2003;37(Suppl 1):S25–35. doi: 10.1086/375369. [DOI] [PubMed] [Google Scholar]
- 2.Rouet F, Rouzioux C. The measurement of HIV-1 viral load in resource-limited settings: how and where? Clin Lab. 2007;53(3–4):135–148. [PubMed] [Google Scholar]
- 3.Fiscus SA, Cheng B, Crowe SM, et al. HIV-1 Viral Load Assays for Resource-Limited Settings. PLoS Medicine. 2006;3(10):e417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malmsten A, Shao XW, Sjodahl S, et al. Improved HIV-1 viral load determination based on reverse transcriptase activity recovered from human plasma. J Med Virol. 2005;76(3):291–296. doi: 10.1002/jmv.20360. [DOI] [PubMed] [Google Scholar]
- 5.Kamat A, Ravi V, Desai A, et al. Quantitation of HIV-1 RNA levels in plasma and CSF of asymptomatic HIV-1 infected patients from South India using a TaqMan real time PCR assay. J Clin Virol. 2007;39(1):9–15. doi: 10.1016/j.jcv.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Muller J, Eis-Hubinger AM, Daumer M, et al. A novel internally controlled real-time reverse transcription-PCR assay for HIV-1 RNA targeting the pol integrase genomic region. J Virol Methods. 2007;142(1–2):127–135. doi: 10.1016/j.jviromet.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rekhviashvili N, Stevens W, Marinda E, et al. Clinical performance of an in-house real-time RT-PCR assay using a fluorogenic LUXtrade mark primer for quantitation of human immunodeficiency virus type-1 (HIV-1) J Virol Methods. 2007;146(1–2):14–2. doi: 10.1016/j.jviromet.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Schvachsa N, Turk G, Burgard M, et al. Examination of real-time PCR for HIV-1 RNA and DNA quantitation in patients infected with HIV-1 BF intersubtype recombinant variants. J Virol Methods. 2007;140(1–2):222–227. doi: 10.1016/j.jviromet.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43(6):2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greengrass V, Plate MM, Steele PM, et al. HIV monitoring in laboratories in resource limited areas. J Chinese Clin Med. 2007;2(8):469–479. [Google Scholar]
- 12.Jennings C, Fiscus SA, Crowe SM, et al. Comparison of two human immunodeficiency virus (HIV) RNA surrogate assays to the standard HIV RNA assay. J Clin Microbiol. 2005;43(12):5950–5956. doi: 10.1128/JCM.43.12.5950-5956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiDomenico N, Link H, Knobel R, et al. COBAS AMPLICOR: fully automated RNA and DNA amplification and detection system for routine diagnostic PCR. Clin Chem. 1996;42(12):1915–1923. [PubMed] [Google Scholar]
- 14.Greengrass VL, Turnbull SP, Hocking J, et al. Evaluation of a Low Cost Reverse Transcriptase Assay for Plasma HIV-1 Viral Load Monitoring. Curr HIV Res. 2005;3(2):183–190. doi: 10.2174/1570162053506955. [DOI] [PubMed] [Google Scholar]
- 15.Sivapalasingam S, Essajee S, Nyambi PN, et al. Human immunodeficiency virus (HIV) reverse transcriptase activity correlates with HIV RNA load: implications for resource-limited settings. J Clin Microbiol. 2005;43(8):3793–3796. doi: 10.1128/JCM.43.8.3793-3796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steegen K, Luchters S, De Cabooter N, et al. Evaluation of two commercially available alternatives for HIV-1 viral load testing in resource-limited settings. J Virol Methods. 2007;146(1–2):178–87. doi: 10.1016/j.jviromet.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Seyoum E, Wolday D, Girma M, et al. Reverse transcriptase activity for quantitation of HIV-1 subtype C in plasma: relation to RNA copy number and CD4 T-cell count. J Med Virol. 2006;78(2):161–168. doi: 10.1002/jmv.20523. [DOI] [PubMed] [Google Scholar]
- 18.Stevens G, Rekhviashvili N, Scott LE, et al. Evaluation of two commercially available, inexpensive alternative assays used for assessing viral load in a cohort of human immunodeficiency virus type 1 subtype C-infected patients from South Africa. J Clin Microbiol. 2005;43(2):857–861. doi: 10.1128/JCM.43.2.857-861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]