Abstract
The goal was to determine the age-related changes in accommodative movements of the lens and ciliary body in rhesus monkeys. Varying levels of accommodation were stimulated via the Edinger-Westphal (E-W) nucleus in 26 rhesus monkeys, aged 6-27 years, and the refractive changes were measured by coincidence refractometry. Centripetal ciliary process (CP) and lens movements were measured by computerized image analysis of goniovideographic images. Ultrasound biomicroscopy (UBM) at 50 MHz was used to visualize and measure accommodative forward movements of the ciliary body in relation to age, accommodative amplitude, and centripetal CP and lens movements. At ∼3 diopters of accommodation, the amount of centripetal lens movement required did not significantly change with age (p=0.10; n=18 monkeys); however, the amount of centripetal CP movement required significantly increased with age (p=0.01; n=18 monkeys), while the amount of forward ciliary body movement significantly decreased with age (p=0.007; n=11 monkeys). In the middle-aged animals (12-16.5 years), a greater amount of centripetal CP movement was required to induce a given level of lens movement and thereby a given level of accommodation (p=0.01), compared to the young animals (6-10 yrs). Collectively, the data suggests that, with age, the accommodative system may be attempting to compensate for the loss of forward ciliary body movement by increasing the amount of centripetal CP movement. This, in turn, would allow enough zonular relaxation to achieve the magnitude of centripetal lens movement necessary for a given amplitude of accommodation.
Keywords: accommodation, presbyopia, ciliary muscle, ciliary body, lens, monkey, rhesus, ultrasound biomicroscopy
Introduction
Accommodation in the human eye occurs with the forward and centripetal movement of the ciliary muscle during its contraction, releasing tension on the zonula that are attached to the lens and allowing the lens to thicken and increase in curvature. Presbyopia is the loss of the eye's ability to accommodate as it ages and has been attributed to increased hardening of the lens with age (Fisher, 1971, 1977; Pau and Krantz, 1991; Glasser and Campbell, 1998, 1999), or to the inability of the ciliary muscle to undergo configurational changes with age (Tamm et al., 1991, 1992a).
Existing evidence supports the theory that the lens plays a role in presbyopia (Fisher, 1969, 1971, 1977; Bito and Miranda, 1989; Koretz et al., 1989; Pau and Krantz, 1991; Glasser and Campbell, 1998, 1999; Heys et al., 2004; Croft et al., 2006a). Indeed, age-related loss of deformability in the older excised human lens (i.e., above ∼40 years of age) can account entirely for presbyopia (Glasser and Campbell, 1998, 1999). However, lens hardening may occur as a result of reduced accommodative effect on the lens due to reduced ciliary muscle configurational change during accommodation. Decreased centripetal lens movement could be consequent to decreased ciliary body forward movement, given that there is a significant correlation between them (Croft et al., 2006a).
The ciliary muscle does not lose the ability to contract with age, but it does lose the ability to move forward and centripetally with age, perhaps due to an increasingly inelastic posterior attachment (Tamm et al., 1992a, 1992b; Croft et al., 2006a). The loss of muscle movement with age is sufficient to explain losses in centripetal lens movement and in accommodative amplitude (Croft et al., 2006a) and may be involved in the pathophysiology of presbyopia.
The rhesus monkey provides an excellent model with which to study human accommodation and presbyopia. Although there are some differences between the species, the accommodative mechanism in the rhesus is virtually identical to that in humans, and both species develop presbyopia on the same relative timescale.
In rhesus monkeys, we studied accommodation and the magnitude of the movements made by the components of the accommodative apparatus, to determine if any early differential age-related changes occurred between components that could provide clues to the presbyopia puzzle.
Materials and Methods
Details of all experimental preparations, equipment, iridectomy, goniovideography and ultrasound biomicroscopic (UBM) imaging, electrode implantation, central stimulation, measurement of accommodation, image calibration, etc., have been thoroughly described previously (Kaufman and Lütjen-Drecoll, 1975; Crawford et al., 1989; Vilupuru and Glasser, 2002; Croft et al., 2006a, 2006b). Brief descriptions and illustrations are provided below.
Monkeys
Twenty-six rhesus monkeys (Macaca mulatta), of either sex, aged 6 to 27 years and weighing 5.5 to 15.1 kg, were used for this study. Prior to the start of the study, all animals included had normal ocular biomicroscopic slit-lamp examinations, with no signs of ocular pathology (other than age-related lenticular opacification).
Details of all animal handling procedures and anesthesia, surgical and experimental preparations, iridectomy, etc., have been described previously (Kaufman et al., 1975; Crawford et al., 1989; Vilupuru et al., 2002; Croft et al., 2006a, 2006b). All procedures conformed to the ARVO Statement for the Use of Animals in Research and were in accordance with institutionally approved animal protocols.
Measurement Procedures
Edinger-Westphal (E-W) Stimulation and Accommodation
Accommodation was stimulated via the E-W nucleus (Crawford et al., 1989; Croft et al., 2006a). A Hartinger coincidence refractometer (aus Jena, Jena, Germany) was used to measure resting refractive error and accommodation in response to stimulation of the E-W nucleus.
Definitions–Accommodative Stimulus
Maximal Stimulus: the level of E-W stimulus current necessary to induce maximum accommodative change. Supramaximal Stimulus: any level of E-W stimulus current above the maximal stimulus. Submaximal Stimulus: any level of E-W stimulus current below the maximal stimulus.
Goniovideography
Various stimulus levels were given to induce accommodation from zero diopters up to and beyond that necessary to induce maximum accommodation. Centripetal lens movement and centripetal ciliary process (CP) movement (reflects centripetal ciliary body movement) were measured by computerized image analysis of goniovideographic images (Fig. 1) (Croft et al., 2006a). The amount of accommodation was tabulated along with the corresponding amount of CP and lens movement at each stimulus level (Croft et al., 2006a).
Figure 1.
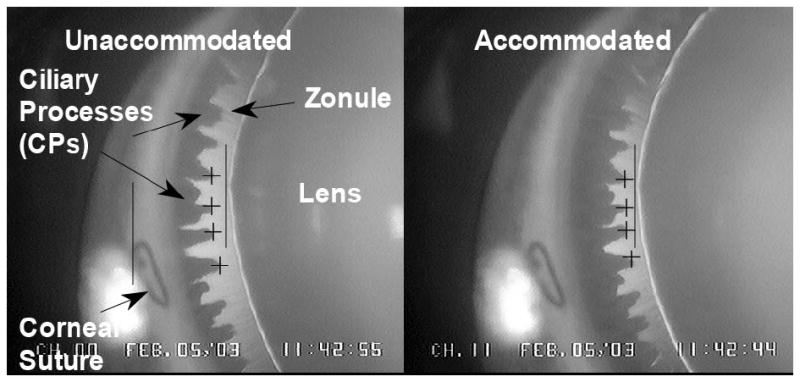
Goniovideography images of normal lens and ciliary process (CP) configuration in the accommodated and unaccommodated states. To obtain quantitative measurements, a 9-0 nylon suture placed at the corneoscleral limbus served as a reference point (left solid vertical line) from which to measure distances to the lens equator (right solid vertical line) and the CPs (cross-hairs) for each image during a 2.2-sec stimulus period. Reprinted with permission from: Croft et al. Accommodative Ciliary Body and Lens Function in Rhesus Monkeys I. Normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci 2006;47:1076-1086.
Ultrasound Biomicroscopy (UBM)
Ultrasound biomicroscopy (UBM) at 50 MHz was used to visualize accommodative movements of the ciliary body (Fig. 2). Using these images, the angle between the anterior aspect of the ciliary body and the inner aspect of the cornea (CB-Cornea angle) was measured in the unaccommodated (resting) eye and during supramaximal stimulation to induce accommodation (Croft et al., 2006a). The extent that the CB-Cornea angle narrowed in the accommodated versus the unaccommodated state (defined as the accommodative CB-Cornea angle change) was used as a surrogate indicator of forward ciliary body movement (Fig. 2) (Croft et al., 2006a) and is referred to as such hereafter. Forward ciliary body movement was examined in relation to age, accommodative amplitude, and centripetal lens and centripetal CP movement.
Figure 2.
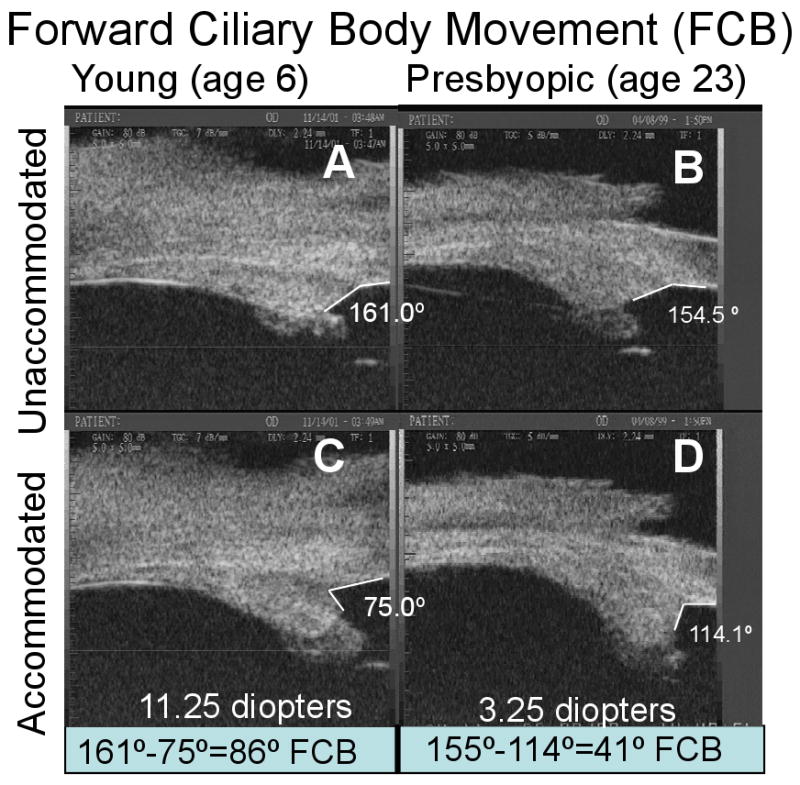
Ultrasound biomicroscopy images of two normal monkey eyes, aged 6 years (A, C) and 23 years (B, D), in the unaccommodated and accommodated states. The change in angle between the anterior aspect of the ciliary body and the inner aspect of the cornea during supramaximal central stimulation was used as a surrogate indicator of forward ciliary body movement (FCB) (Croft et al., 2006a). Panels B and D adapted with permission from: Croft et al. Accommodative Ciliary Body and Lens Function in Rhesus Monkeys I. Normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci 2006;47:1076-1086.
Statistical Analysis
Simple linear regression (i.e., centripetal lens or forward ciliary body movement versus age, and centripetal lens versus centripetal CP movement) and multiple regression analysis (i.e., accommodation versus lens and centripetal CP movement) were undertaken.
Further, general linear regression models were used to assess the relationships of accommodation versus centripetal CP movement, accommodation versus lens movement, and accommodation versus both centripetal CP movement and lens movement in all monkeys. The correlation structure of the observations within each monkey (i.e., instances in which there were two eyes from the same monkey, and instances in which there were varying stimulus levels within a single eye) was modeled by generalized estimating equations (GEEs). Models with linear and quadratic terms were applied to all variables of interest to determine the best fit, and such models have no associated correlation coefficient. In addition, log transformation of accommodation was undertaken in order to stabilize the residuals so that the proposed models were not unduly affected by variance fluctuations. There was no specific pattern in the residual plots and therefore no concern as to the validity of the model.
For some analyses, the data were grouped according to monkey age: young (6-10 years); middl-aged (12-16.5 years); and older (above 20 years). Based on a life span of 35 years and 75 years for monkeys and humans, respectively, equivalent age divisions in the human would be: young (12-21 years); middle-aged (26-35 years); and older (above 43 years). Middle-age in the human is generally considered to be older than 35; however, for the sake of comparison in this manuscript the above age groupings were used and referred to as such hereafter. According to Duane's curve (Duane, 1922), the average accommodative amplitude (over each age range specified) in the young human eye is ∼13.5 diopters, which declines to ∼9.0 diopters by middle age (33% loss in accommodation), and further declines to ∼2.0 diopters in the older group (85% loss in accommodative amplitude). Duane's curve also indicates that ∼2/3 of human accommodative amplitude is lost by the single age point of 35 years. The ∼2.0 diopters that remain in the older human eye (i.e., above age 50) could be attributed to depth of focus, optical aberrations, etc.
Results
Amplitudes of accommodation, forward ciliary body movement, centripetal CP movement, and centripetal lens movement for the young, middle-aged, and older monkey eyes in response to supramaximal stimulation (∼25% above maximal stimulation) are summarized in Table 1. In the middle-aged eyes compared to the young eyes, all four variables declined significantly: forward ciliary body movement declined most dramatically (54.8%); followed by the decline in accommodative amplitude (46.7%); centripetal lens movement (30.4%); and centripetal CP movement (19.4%). While the 55% loss in forward ciliary body movement occurred by middle age, a 55% loss in centripetal lens movement did not occur until older age.
Table 1. Average Maximum Amplitudes of Accommodation, Forward Ciliary Body, Ciliary Process (CP) and Lens Movement in Young, Middle-Aged and Older Eyes.
A) Data are mean ± s.e.m. accommodative amplitude (diopters; D) at supramaximal (∼25% above that necessary to induce maximum accommodation) stimulus levels in 28 eyes of 23 rhesus monkeys. B) Data are mean ± s.e.m. forward ciliary body movement (FCB; in units of degrees as previously defined (Fig. 2) (Croft et al., 2006a)); centripetal ciliary process movement (CP); and lens movement amplitude (mm) at standard supramaximal stimulus settings. Age ranges: young eyes (6 to 9.5 years); middle-aged eyes (12 to 15 years); and older eyes (17 to 27 years). A p≤0.05 represents a significant difference between the young age group versus the other age groups by two sample t-test. C) Percent decrease is calculated as [(middle-aged/young)-1]*100 or ((older/young)-1)*100 for each variable. For instances in which there were two eyes from one monkey, the data were averaged to provide one data point. A subset of the CP and centripetal lens equator movement data (16 eyes of 12 monkeys) was adapted with permission from: Croft et al. Accommodative Ciliary Body and Lens Function in Rhesus Monkeys. Invest Ophthalmol Vis Sci 2006;47:1076-1086.
| A. | B. | C. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Centripetal Movement (mm) | % Decline | |||||||||
| Centripetal | ||||||||||
| Accommodation (D) | FCB (°) | n | CP | Lens | Accommodation (D) | FCB | CP | Lens | ||
| Young | Mean | 15.2 | 61.8 | 8 | 0.44 | 0.31 | ---- | ---- | ---- | ---- |
| (6-9.5 yrs) | s.e.m. | 1.0 | 5.7 | 0.02 | 0.01 | |||||
| Middle-Aged | Mean | 8.1 | 27.9 | 7 | 0.35 | 0.22 | 46.7 | 54.8 | 19.4 | 30.4 |
| (12-15 yrs) | s.e.m. | 0.5 | 4.4 | 0.03 | 0.03 | |||||
| Middle-Aged vs Young | p= | 0.001 | 0.001 | 0.012 | 0.022 | |||||
| Older | Mean | 2.4 | 23.9 | 8 | 0.32 | 0.14 | 84.0 | 61.3 | 26.7 | 54.3 |
| (17-26 yrs) | s.e.m. | 0.6 | 3.0 | 0.05 | 0.03 | |||||
| Older vs Young | p= | 0.001 | 0.001 | 0.08 | 0.001 | |||||
| Middle-Aged vs Older | p= | 0.001 | 0.258 | 0.584 | 0.166 | 37.3 | 6.5 | 7.3 | 23.9 | |
Centripetal lens movement per diopter of accommodation (mm/D) was the same in the young versus the middle-aged monkeys (Fig. 3).
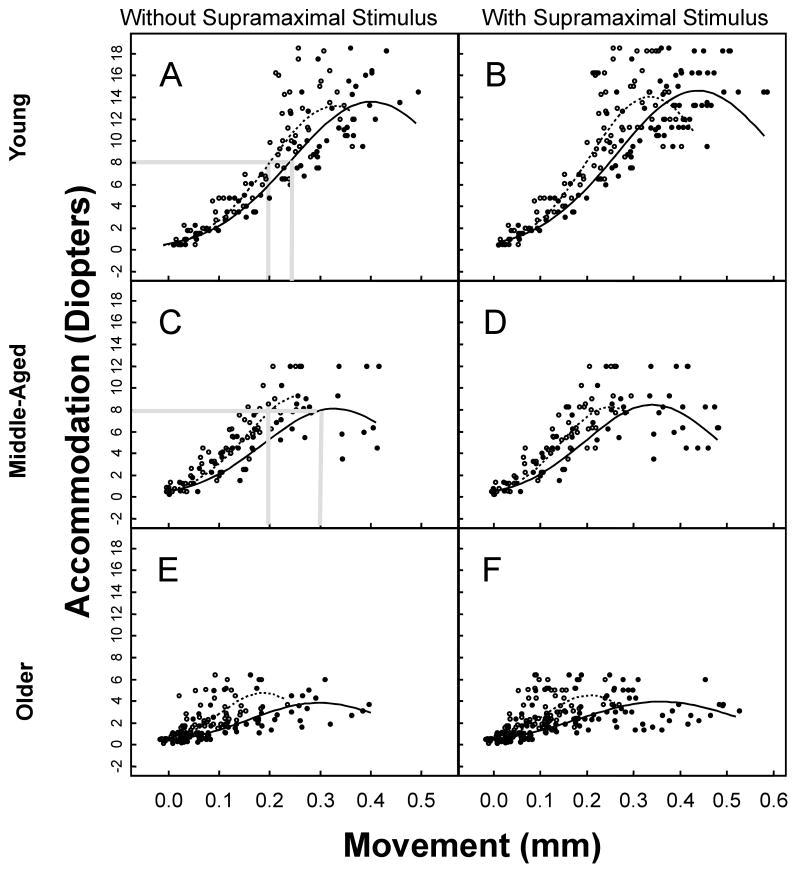
Figure 3.
Accommodation versus centripetal ciliary process (CP) or centripetal lens movement in young (ages 6-10 years; n=8 eyes; 5 monkeys); middle-aged (ages 12-16.5 years; n=5 eyes; 5 monkeys); and older (ages 20 years and above; n=15 eyes; 10 monkeys) monkey eyes. A, C, E: Maximal and Submaximal Stimulus Levels. Various levels of accommodation, from zero diopters up to maximum accommodation, were induced and plotted versus the corresponding centripetal CP and centripetal lens movement for each monkey eye. Centripetal CP and centripetal lens movements began to plateau at 0.3 mm of movement. The fitted regression curve of the amplitude of the centripetal CP (solid line) or centripetal lens (dashed line) movement versus accommodation is shown for each panel. The p-values are obtained from the Likelihood Ratio Test. B, D, F: Supramaximal, Maximal and Submaximal Stimulus Levels. Includes data collected during various supramaximal stimulation levels for each monkey eye plus data contained in panels A, C, and E. The results were similar when data from the supramaximal stimulus levels were included in the analysis.
UBM Imaging
By qualitative examination of dynamic UBM images of a 16-year-old rhesus monkey eye during accommodation, one can observe dampened forward ciliary body movement (compared to the young eye in Fig. 2) with substantial muscle apex thickening and centripetal CP movement still present (Video Clip #1).
Goniovideography
Accommodative Amplitude versus Centripetal Ciliary Process (CP) or Centripetal Lens Movement Measured Gonioscopically
Submaximal and Maximal Stimulus Levels (Figs. 3 A, C, E)
In regard to CP movement versus accommodation, a statistically significant difference existed between the curves of all three age groups (p<0.01). In regard to the lens movement versus accommodation, there was weak evidence of a statistically significant difference between the curves of the middle-aged versus older eyes (p=0.064), and between the older versus the young eyes (p=0.071), but not between the middle-aged versus the young eyes (p=0.49). It is important to note that the amount of accommodation per mm of centripetal lens movement (D/mm) was the same in the young and middle-aged monkeys (Fig. 3). The amount of accommodation per mm of centripetal CP movement (D/mm) was less in the middle-aged compared to the young monkeys (Fig. 3). This means that a greater amount of centripetal CP movement was required to induce a given level of lens movement for a given level of accommodation in the middle-aged eyes compared to the young eyes (Figs. 3 A, C; Fig. 4).
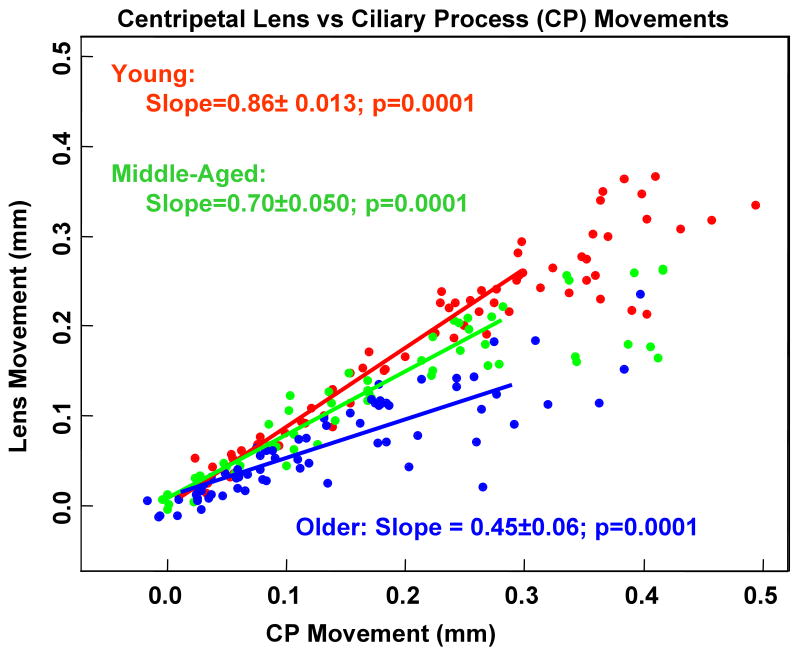
Figure 4.
Lens versus corresponding centripetal ciliary process (CP) movement. There was a significant increase in units of centripetal CP movement per unit of centripetal lens movement between the young and middle-aged eyes (p<0.01) and between the middle-aged and older eyes (p<0.01). A linear model was the best fit for the data. This model excluded data points at the high end of the response curve (above 0.3 mm CP movement) where the data begins to plateau.
Submaximal, Maximal and Supramaximal Stimulus Levels (Figs. 3 B, D, F)
The results were similar with the data from the supramaximal stimulus levels included in the analysis. Further, with supramaximal stimulation included (Figs. 3 B, D, F), greater centripetal CP movement was induced but with no further centripetal lens movement or accommodation. In the middle-aged eyes, there was an upper limit (∼0.28 mm) to centripetal lens movement (Fig. 3 D), thus limiting accommodative amplitude. The loss in centripetal lens movement amplitude in the middle-aged compared to the young eyes could have been due to loss in forward ciliary body movement (see section below reporting maximum forward ciliary body movement amplitudes).
Results were similar if the centripetal CP movement data above 0.3 mm were removed from the analysis (Supplemental Figure 1). In this case, the best fit for both the centripetal CP and centripetal lens data remained quadratic.
Thus, the best-fit equation describing the relationship between accommodation and CP or lens movement was log(accommodation) = b0 + b1*CP + b2*CP2 or log accommodation) = b0+b1*lens+b2*lens2, respectively. The coefficients of these more complex regression equations cannot be interpreted. To compare the results between age groups, one must plot the curves of the regressions equations (Fig. 3).
Multiple and Stepwise Regression Analysis
In all three age groups, the multiple regression model (Table 2 A) explained accommodative amplitude better than the model used in Figs. 3 A, C, and E above. This was not surprising, since both centripetal CP and centripetal lens movements are needed for accommodation. With all of the parameters included, the best predictor of accommodative amplitude over the full accommodative range for all age groups was centripetal CP movement (Table 2).
Table 2. Multiple Regression Analysis of Accommodation vs Centripetal Ciliary Process (CP) and Lens Movement.
Summary of the significance of the fitted regressions in which accommodation is modeled as a function of centripetal lens and ciliary process (CP) movement. The p-value is associated with the test of the null hypothesis that the multiple regression coefficient of a particular parameter (i.e., CP or lens) is zero. If p<0.05 for a particular measurement (CP or lens), then the particular parameter better predicted accommodation. A) The analysis includes data during maximal and various submaximal stimulation levels to induce accommodation for each monkey eye. B) Includes data during various supramaximal stimulation levels, as well as maximal and various submaximal stimulation levels, to induce accommodation for each eye.
| A. Maximal and Submaximal Stimulus Levels | ||||
|---|---|---|---|---|
| Accommodation vs. Lens and CP Movement | ||||
| Variable | Estimate | Std. Error | p-value | |
| Young | CP | 13.5 | 4.32 | 0.0018 |
| CP2 | -15.0 | 5.35 | 0.0051 | |
| Lens | 3.9 | 5.22 | 0.4495 | |
| Lens2 | -9.8 | 7.75 | 0.2044 | |
| Middle-Aged | CP | 11.5 | 3.23 | 0.0004 |
| CP2 | -19.9 | 5.44 | 0.0003 | |
| Lens | 6.2 | 4.98 | 0.2101 | |
| Lens2 | -4.1 | 13.60 | 0.7604 | |
| Older | CP | 10.6 | 3.48 | 0.0022 |
| CP2 | -19.1 | 5.47 | 0.0005 | |
| Lens | 9.9 | 6.83 | 0.1487 | |
| Lens2 | -22.2 | 21.90 | 0.3104 | |
| B. Supramaximal, Maximal and Submaximal Stimulation Levels | ||||
| Variable | Estimate | Std. Error | p-value | |
| Young | CP | 10.5 | 2.22 | <0.0001 |
| CP2 | -10.7 | 2.52 | <0.0001 | |
| Lens | 6.5 | 2.84 | 0.0229 | |
| Lens2 | -12.7 | 4.03 | 0.0017 | |
| Middle-Aged | CP | 9.0 | 2.34 | 0.0001 |
| CP2 | -14.3 | 3.74 | 0.0001 | |
| Lens | 11.29 | 4.79 | 0.0184 | |
| Lens2 | -20.0 | 12.87 | 0.1199 | |
| Older | CP | 7.4 | 2.60 | 0.0042 |
| CP2 | -11.1 | 3.42 | 0.0012 | |
| Lens | 9.2 | 6.27 | 0.1413 | |
| Lens2 | -19.4 | 17.11 | 0.2579 | |
A stepwise regression was undertaken to choose the best model with which to predict accommodation (Table 3). The results showed that both CP and lens movements were important in predicting accommodation.
Table 3. Stepwise Multiple Regression Analysis of Accommodation vs Centripetal Ciliary Process (CP) and Lens Movement.
Analogous to Table 2, this table reports the results for the best models from the stepwise regression analysis.
| A. Maximal and Submaximal Stimulus Levels | ||||
|---|---|---|---|---|
| Accommodation vs. Lens and CP Movement | ||||
| Variable | Estimate | Std. Error | p-value | |
| Young | CP | 3.4 | 0.88 | 0.0001 |
| CP2 | ||||
| Lens | 15.6 | 1.51 | <.0001 | |
| Lens2 | -30.1 | 2.17 | <.0001 | |
| Middle-Aged | CP | 12.5 | 2.81 | <.0001 |
| CP2 | -21.5 | 4.20 | <.0001 | |
| Lens | 4.7 | 1.22 | 0.0001 | |
| Lens2 | ||||
| Older | CP | 3.0 | 1.40 | 0.0335 |
| CP2 | ||||
| Lens | 19.8 | 5.09 | <.0001 | |
| Lens2 | -64.7 | 15.97 | <.0001 | |
| B. Supramaximal, Maximal and Submaximal Stimulation Levels | ||||
| Variable | Estimate | Std. Error | p-value | |
| Young | CP | 10.5 | 2.22 | <0.0001 |
| CP2 | -10.7 | 2.52 | <0.0001 | |
| Lens | 6.5 | 2.84 | 0.0229 | |
| Lens2 | -12.7 | 4.03 | 0.0017 | |
| Middle-Aged | CP | 13.1 | 3.63 | 0.0003 |
| CP2 | -20.6 | 5.74 | 0.0003 | |
| Lens | 3.4 | 1.57 | 0.0300 | |
| Lens2 | ||||
| Older | CP | 9.8 | 1.33 | <.0001 |
| CP2 | -14.8 | 1.99 | <.0001 | |
| Lens | 3.7 | 2.00 | 0.0621 | |
| Lens2 | ||||
Forward Ciliary Body Movement, Centripetal Lens or Centripetal Ciliary Process (CP) Movement versus Age during ∼3 Diopters of Accommodation
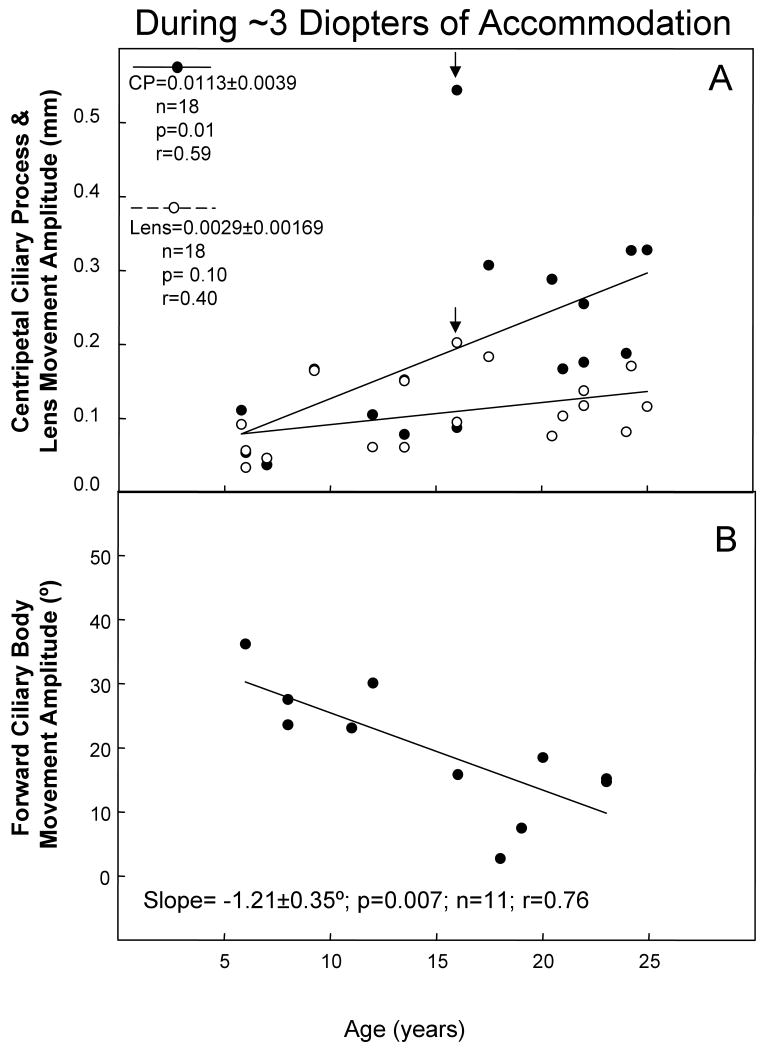
We examined the age-related change in forward ciliary body movement, or centripetal lens or centripetal CP movement amplitude, during ∼3 diopters of accommodation (Fig. 5). The amount of centripetal lens movement required to induce ∼3 diopters of accommodation did not change significantly with age (p=0.10). However, the amount of centripetal CP movement significantly increased (p=0.01), while the amount of forward ciliary body movement significantly decreased with age (p=0.007).
Figure 5.
Amplitude of centripetal ciliary process (CP), centripetal lens, and forward ciliary body (FCB) movements during ∼3 diopters of accommodation. A) Data are gonioscopically measured centripetal CP and lens movement amplitudes plotted versus age in 25 eyes of 18 rhesus monkeys. The CP and lens regression analysis showed one outlier at age 16 (arrows). The CP movement value was almost twice the next nearest value at this age. Results of a regression analysis without this monkey included are: (CP slope=0.0112 ± 0.0022, n=17, p= 0.001, r=0.79; Lens slope=0.0029 ± 0.00150, n=17, p=0.07, r=0.45). B) Ultrasound biomicroscopically measured FCB movement (Croft et al., 2006a), plotted versus age in 17 eyes of 11 rhesus monkeys. The lines represent least squares linear regression of the CP, FCB, or lens response amplitude versus age. Numbers represent slopes ± s.e.m.; P, probability that the slope=0.0; r=correlation coefficient. For both panels A and B, for instances in which there were two eyes from one monkey, the data were averaged to provide one data point.
Forward Ciliary Body, Centripetal Lens, and Centripetal Ciliary Process (CP) Movement at Maximum Amplitudes (during supramaximal stimulus; 28 eyes, 23 monkeys)
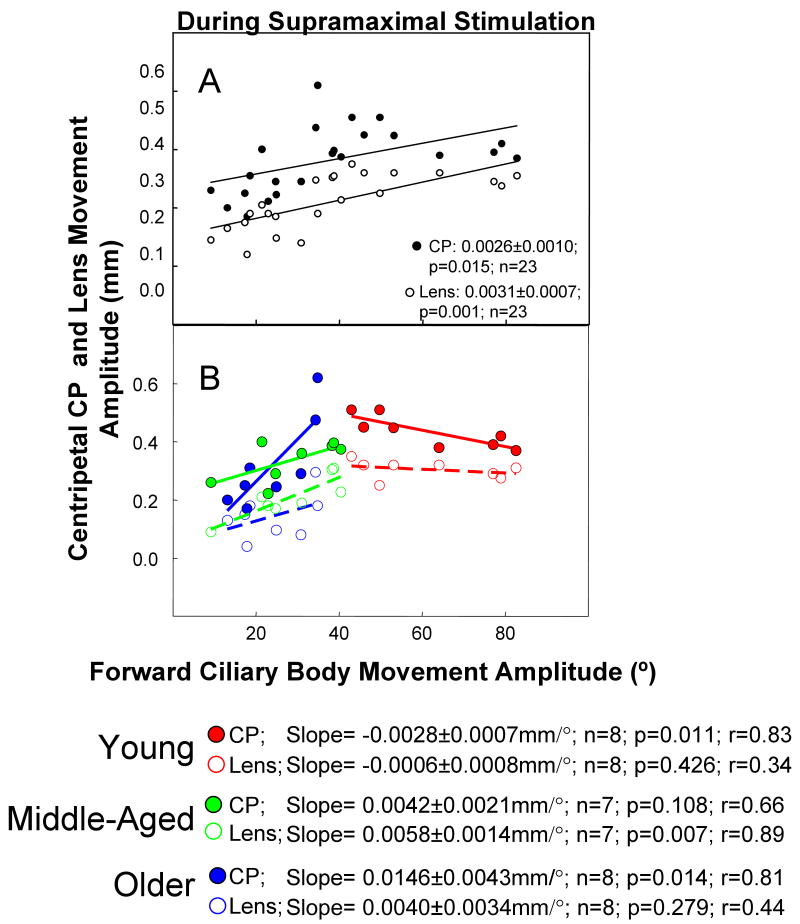
Maximum accommodative forward ciliary body movement was linearly correlated with maximum amplitude of both centripetal CP and centripetal lens movement (Fig. 6 A; p<0.02); however, these relationships clearly changed depending on the age group. Centripetal CP and centripetal lens movement versus accommodative forward ciliary body movement were plotted for the three age groups (Fig. 6 B). In the young eyes, as forward ciliary body movement amplitude increased, there was a slight but statistically significant decline in centripetal CP but not centripetal lens movement (Fig. 6 B). In the middle-aged eyes, the average amount of forward ciliary body movement dropped dramatically (see above), and there was a significant increase in CP movement per degree of forward ciliary body movement and an increase in centripetal lens movement per degree of forward ciliary body movement (Fig. 6 B). In the older eyes, the increase in centripetal CP movement per degree of forward ciliary body movement was even more dramatic, while the increase in lens movement per unit of forward ciliary body movement was less pronounced but similar to that found for the middle-aged eyes.
Figure 6.
Ciliary body and lens movements during supramaximal stimulation.
A) Temporal accommodative forward ciliary body (FCB) movement versus centripetal ciliary process (CP) and lens movement during a supramaximal stimulus current ∼25% above the maximal stimulus in 28 eyes of 23 rhesus monkeys ranging in age from 6 to 27 years. A subset of the centripetal lens equator movement data (16 eyes of 12 monkeys) was adapted with permission from: Croft et al. Accommodative Ciliary Body and Lens Function in Rhesus Monkeys. Invest Ophthalmol Vis Sci 2006;47:1076-1086. B) Panel B is the same data as Panel A; however, the data were separated and analyzed according to age. For instances in which there were two eyes from one monkey the data were averaged to provide one data point. Age ranges: young eyes (6 to 9.5 years, n=9 eyes, 8 monkeys); middle-aged eyes (12 to 15 years, n=8 eyes, 7 monkeys); and older eyes (17 to 27 years, n=11 eyes, 8 monkeys). The lines represent least squares linear regression of the amplitude of the CP (solid line) or lens (dashed line) centripetal movement versus FCB. Numbers represent slopes ± s.e.m.; P, probability that the slope=0.0; r=correlation coefficient.
In the middle-aged eyes, there was a weak positive relationship between centripetal CP and forward ciliary body movement (0.0042 mm/°; p=0.108) and a significant relationship between the lens centripetal movement and forward ciliary body movement (0.0058 mm/°; p=0.007; Fig. 6 B). The positive relationship between centripetal CP and forward ciliary body movement was significant and most pronounced in the older eyes, in which the unit of centripetal CP movement per unit of forward ciliary body movement (0.0146 mm/°; p=0.014) was the highest of all three age groups (Fig. 6 B). In the older monkey eyes, lens movement was not significantly correlated with forward ciliary body movement (p=0.279) but was significantly related to CP movement (p=0.0001, Fig. 4).
Discussion
We have documented the age-related functional changes of various components of the accommodative apparatus of the rhesus monkey. These results demonstrate that study of the entire age range is required to determine which component of the accommodative apparatus changes first with age.
Collectively, the data of the current study show that the loss in forward ciliary body movement with age occurs sooner than the loss in centripetal lens equator movement. The loss in forward muscle movement is partially compensated for by the level of increased centripetal muscle movement required to achieve zonular relaxation and lens rounding. Overall, the amplitude of the centripetal ciliary body movement is somewhat reduced with age. It is unlikely that the lenses of the middle-aged monkeys changed substantially in internal refractive properties or hardness compared to those of the young animals since the centripetal lens movement per diopter of accommodation was the same in both the young and middle-aged animals.
The higher amplitudes of centripetal lens movement were not achieved in the middle-aged eyes, possibly due to the 55% loss in forward ciliary body movement. Even during supramaximal stimulation, despite the compensating centripetal CP movement, no further lens movement was induced in the middle-aged eyes. There are hundreds of zonular fibers extending from the valleys of the ciliary processes to the anterior and posterior lens surfaces, in addition to those that extend between the ciliary processes and the lens equator (Glasser and Campbell, 1998; Rohen, 1979). Some of these zonular attachments may be more dependent on forward ciliary body movement than centripetal CP movement to achieve relaxation and allow lens rounding.
Without the supramaximal stimulation data included, multiple regression analysis showed that most of the variability in accommodative amplitude was explained by centripetal CP movement in all three age groups, while the centripetal lens movement was also important to explain some part of accommodative amplitude (based on the stepwise regression analysis; Table 3). This was not surprising, since it is known that both parameters are needed for accommodation. Centripetal CP movement may reflect both the centripetal lens equatorial movement and the thickening of the lens, which may be why centripetal CP movement was so important in the stepwise regression models to predict accommodation.
Goniovideographically measured centripetal CP movements predominantly represent centripetal ciliary body movements, but these measurements do not really distinguish centripetal from forward ciliary body movement. Thus, centripetal CP movements that we report here may actually be a hybrid or composite, in contrast to movements measured by UBM that can isolate measurement of forward ciliary body movement. Nonetheless, the techniques of measuring forward ciliary body movement by UBM and centripetal CP movement by goniovideography clearly provide separate and distinct information about ciliary body function and its change with age (Croft et al., 2006a).
Presbyopia is by definition the loss in accommodative amplitude, due to the loss in the ability of the lens to change shape. Given the CP versus lens relationships in Fig. 4, one might argue that the CP movement has not dampened with age but the lens movement has. However, the same amount of lens movement per diopter of accommodation exists in both the young and middle-aged eyes (Fig. 3).
Given the difference between young and middle-aged eyes in CP and forward ciliary body movement, one might consider the possibility of a Type I error (the possibility of a false assumption of a significant difference between the groups, with no biological basis). The probability of such an error is low (typically p=0.05 or a 5% chance) and, based on an overall examination of the data, we considered the existence of a Type I error an unlikely possibility.
The active movement of the ciliary body is possible only by ciliary muscle contraction. Due to the posterior restriction of muscle movement in the aging eye, the longitudinal portion of the muscle may undergo more of an isometric contraction than the circular portion—thus the marked loss in forward movement. However, the circular portion of the muscle during contraction applies its force centripetally, a direction that is perpendicular to the restriction and that is not in direct opposition to the restriction. In support of this idea, Tamm et al. (1992) reported that the area encompassing the circular portion of the ciliary muscle increases with age in excised human eyes, while the area of the longitudinal portion of the ciliary muscle decreases with age. This aging change may be adaptive to compensate for the muscle's posterior restriction or to further support the accommodative effort as the lens thickens with age. The aging change mentioned above would likely not be to compensate for the decreased deformability of the lens (at least in the middle-aged monkey eyes), since, by extrapolation from human data, changes in lens deformability do not occur until after age 19 in monkeys (∼age 40 in humans; see second to last paragraph of the discussion, below).
By examination of dynamic UBM images of a 16-year-old rhesus monkey eye during supramaximal stimulation to induce accommodation, one can understand how there could be an age-related loss in forward ciliary body movement while substantial centripetal CP movement remained, based on ocular geometry (Video Clip #1). Age-related stiffening of the posterior attachments (i.e., choroid, posterior muscle tendons (Tamm et al., 1992), and/or posterior vitreous zonule, which extend in a straight line from the ora serrata to the zonular plexus in the valleys of the ciliary processes (Lütjen-Drecoll et al., Unpublished results)) could dampen forward ciliary body movement, with substantial centripetal CP movement remaining.
With the lens substance removed (leaving an empty capsular bag), the centripetal muscle movement was enhanced but forward muscle movement was unchanged compared to the normal iridectomized eye. With the lens substance and capsule removed (thus severing the anterior zonular attachments between the ciliary muscle and the lens capsule), the loss of forward muscle movement was far more pronounced (50%) than the loss in centripetal muscle movement (10-15%) (Croft et al., 2008) (Wasilewski et al., 2008). This suggests that there may be agonistic tractional forces, supplied by the attachment of the anterior zonula/lens complex to the ciliary muscle during accommodation, that enhance muscle movement and are far more important to forward movement than to centripetal movement of the muscle. These forces provide anterior traction to the muscle and counterbalance those forces that pull the muscle back into the resting state (i.e., posterior elastic tendons, choroid). Alternatively, the anterior attachments of the muscle to the anterior zonula/lens complex may simply be an anchor by which the muscle pulls itself forward and thereby contribute passively to forward muscle movement. Whether the attachment of the anterior zonula/lens complex to the ciliary muscle provides traction or plays a “passive anchor” role in ciliary muscle contraction, it facilitates forward muscle movement until zonular relaxation is achieved during the accommodative response.
Formation of chemical bonds between lens fibers might also cause dampened lens equator movement, but it was beyond the scope of this study to determine bonding between the lens fiber cells in the young and middle-aged rhesus eyes. In excised human eyes, age-related changes in lens compliance (Weeber et al., 2005) and lens resistance to deformation (Glasser and Campbell, 1999) were minimal prior to age 40 (age in monkey years ∼19). The lens resistance to deformation increased dramatically after the age of 40 in excised human lenses (Glasser and Campbell, 1999). Age-related lens thickening by itself could be considered a significant change and could play a role in the pathophysiology of presbyopia. However, Alió et al. (2005) reported that, while human lens thickness begins increasing before the age of 40, the density of the human lens nucleus only begins increasing after age 40, and does so linearly with age. Alió also reported an increase in intraocular light scattering and aberrations after age 40, which decreases the optical image quality (Alió et al., 2005). This suggests that significant cumulative lens changes are not apparent until after the age of 40 in humans, by which time, as mentioned previously, more than 2/3 of the accommodative ability has been lost (Duane, 1922).
This study demonstrates that ciliary body function begins to change with age before lens function changes. Our data show that the lens accommodative response (i.e., centripetal lens movement required to induce a given level of accommodation) was not significantly changed in the middle-aged monkeys (12-16.5 years) compared to the young monkeys; thus, it is unlikely that the resting lenses of the middle-aged monkeys changed in refractive power compared to the young animals. The age-related loss in ciliary body function (i.e., loss of forward ciliary body movement) that we measured could be due to decreasing elasticity of the choroid, the posterior ciliary muscle tendons, or the posterior vitreous zonule. Chemical or physical lysis or other treatment of these inelastic attachments may sustain the ability of the ciliary body to move forward during accommodative effort and thus prevent or delay secondary age-related lenticular changes and perhaps facilitate the mobility/deformability of accommodating IOLs.
Supplementary Material
These data are a subset of Fig. 3 and include the data only up to 0.30 mm of CP movement. The data are plotted along with the regression curves. The results were similar but not exactly identical to the analysis results in Fig. 3.
Acknowledgments
NEI (EY10213); Ocular Physiology Research & Education Foundation; Research to Prevent Blindness (Unrestricted Grant and Physician-Scientist Award); Core Grant for Vision Research grant # P30 EY016665; and Retina Research Foundation (Walter H. Helmerich Chair). This publication was made possible in part by Grant Number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
We also acknowledge James Reed, for technical expertise with image analysis systems.
Support: This work was funded by NEI grants EY10213 & R21EY018370 to PLK, by an unrestricted gift from the Ocular Physiology Research & Education Foundation, and by the Walter H. Helmerich Chair of the Retina Research Foundation. We also acknowledge the Wisconsin National Primate Research Center, University of Wisconsin-Madison base grant # 5P51 RR 000167 and the Core Grant for Vision Research grant # P30 EY016665.
Footnotes
Portions of these results were presented at the Association for Research in Vision and Ophthalmology annual meeting, Fort Lauderdale, Florida, May 1, 2005 and May 3, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alió JL, Schimchak P, Negri HP, Montés-Micó R. Crystalline lens optical dysfunction through aging. Ophthalmology. 2005;112:2022–2029. doi: 10.1016/j.ophtha.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Bito LZ, Miranda OC. Accommodation and presbyopia. In: Reinecke RD, editor. Ophthalmology annual. New York: Raven Press; 1989. pp. 103–128. [Google Scholar]
- Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB, Nadkarni NV, Kaufman PL. Accommodative ciliary body and lens function in rhesus monkeys: I. Normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006a;47:076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Nadkarni NV, Kaufman PL. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006b;47:1087–1095. doi: 10.1167/iovs.04-1524. [DOI] [PubMed] [Google Scholar]
- Croft MA, McDonald JP, James RJ, Heatley GA, Lin TL, Lütjen-Drecoll E, Kaufman PL. Surgical intervention and accommodative responses, I: Centripetal ciliary body, capsule, and lens movements in rhesus monkeys of various ages. Invest Ophthalmol Vis Sci. 2008;49:5484–5494. doi: 10.1167/iovs.08-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane A. Studies in monocular and binocular accommodation with their clinical applications. Am J Ophthalmol. 1922;5:867–877. [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. Elastic constants of the human lens capsule. J Physiol (Lond) 1969;201:1–19. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. The elastic constants of the human lens. J Physiol (Lond) 1971;212:147–180. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF. The force of contraction of the human ciliary muscle during accommodation. J Physiol (Lond) 1977;270:51–74. doi: 10.1113/jphysiol.1977.sp011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. [PubMed] [Google Scholar]
- Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol. 1975;14:766–771. [PubMed] [Google Scholar]
- Koretz JF, Kaufman PL, Neider NW, Goeckner PA. Accommodation and presbyopia in the human eye. II. Aging of the anterior segment. Vision Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Ludwig K, Wegscheider E, Hoops JP, Kampik A. In vivo imaging of the human zonular apparatus with high-resolution ultrasound biomicroscopy. Graefes Arch Clin Exp Ophthalmol. 1999;237:361–371. doi: 10.1007/s004170050245. [DOI] [PubMed] [Google Scholar]
- Pau H, Krantz J. The increasing sclerosis of the human lens with age and its relevance to accommodation and presbyopia. Graefes Arch Clin Exp Ophthalmol. 1991;229:294–296. doi: 10.1007/BF00167888. [DOI] [PubMed] [Google Scholar]
- Rohen JW. Scanning electron microscopic studies of the zonular apparatus in human and monkey eyes. Invest Ophthalmol Vis Sci. 1979;18:133–144. [PubMed] [Google Scholar]
- Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol. 1992a;110:871–876. doi: 10.1001/archopht.1992.01080180143043. [DOI] [PubMed] [Google Scholar]
- Tamm E, Lütjen-Drecoll E, Jungkunz W, Rohen JW. Posterior attachment of ciliary muscle in young, accommodating old, presbyopic monkeys. Invest Ophthalmol Vis Sci. 1991;32:1678–1692. [PubMed] [Google Scholar]
- Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mech Ageing Dev. 1992b;62:209–221. doi: 10.1016/0047-6374(92)90057-k. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Wasilewski R, McDonald JP, Heatley G, Lütjen-Drecoll E, Kaufman PL, Croft MA. Surgical intervention and accommodative responses, II. Forward ciliary body accommodative movement is facilitated by zonular attachments to the lens capsule. Invest Ophthalmol Vis Sci. 2008;49:5495–5502. doi: 10.1167/iovs.08-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RGL. Dynamic mechanical properties of human lenses. Exp Eye Res. 2005;80:425–434. doi: 10.1016/j.exer.2004.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These data are a subset of Fig. 3 and include the data only up to 0.30 mm of CP movement. The data are plotted along with the regression curves. The results were similar but not exactly identical to the analysis results in Fig. 3.