Abstract
Background
Anatomical evidence of brain damage from electroconvulsive therapy (ECT) is lacking, but there are no modern stereological studies in primates documenting its safety. Magnetic seizure therapy (MST) is under development as a less invasive form of convulsive therapy, and there is only one prior report on its anatomical effects. We discerned no histological lesions in the brains of higher mammals subjected to electroconvulsive shock (ECS) or MST, under conditions that model closely those used in humans. We sought to extend these findings by determining whether these interventions affected the number of neurons or glia in the frontal cortex or hippocampus.
Methods
Twenty-four animals received 6 weeks of ECS, MST, or anesthesia alone, 4 days per week. After perfusion fixation, numbers of neurons and glia in frontal cortex and hippocampus were determined by unbiased stereological methods.
Results
We found no effect of either intervention on volumes or total number or numerical density of neurons or glia in hippocampus, frontal cortex, or subregions of these structures.
Conclusions
Induction of seizures in a rigorous model of human ECT and MST therapy does not cause a change in the number of neurons or glia in potentially vulnerable regions of brain. This study, while limited to young, healthy, adult subjects, provides further evidence that ECT and MST, when appropriately applied, do not cause structural damage to the brain.
Keywords: Stereology, Frontal cortex, Hippocampus, Antidepressant, Transcranial magnetic stimulation
Introduction
Electroconvulsive therapy (ECT) is arguably the most effective antidepressant treatment available. Its use is limited by concern about memory impairment, stigma, and fears of brain damage, although structural damage has not been verified. Aside from the current study [from which qualitative neuropathological data were reported previously (Dwork et al., 2004)], animal models to determine whether cells are lost as a result of ECT have been limited to rodent models that do not include modern safeguards (general anesthesia, muscle paralysis, and respiratory support). Induction of seizures by magnetic stimulation (magnetic seizure therapy, MST), which produces a more localized current than electrical stimulation, is under development as a less invasive form of ECT intended to improve its risk/benefit ratio by sparing memory. Studies to date demonstrate the feasibility of MST (Lisanby et al., 2001) and its enhanced focality (Lisanby et al., 2003) and reduced amnesia (Moscrip et al., 2006; Spellman et al., 2008) relative to ECT in an animal model. Open studies of MST in humans provide preliminary evidence that seizures are produced safely (Lisanby et al., 2001). Initial reports suggest that MST improves mood in refractory depression (Kayser et al., 2008; Kosel et al., 2003; White et al., 2006) and demonstrates a side effect profile superior to that of ECT (Lisanby et al., 2003). However, only one report, our qualitative study, has addressed the anatomical effects of MST (Dwork et al., 2004). We now report a stereological study of frontal cortex and hippocampus, with an expanded sample, in animal models that closely replicate ECT and MST as applied clinically.
Materials and Methods
Twenty-four adolescent Macaca mulatta were divided into eight cohorts of three, matched for age, weight and sex. There were 4 male cohorts, aged 959 to1045, 990 to1092, 1011 to 1046, and 1142 to1308 days, and 4 female cohorts, aged 809 to 863, 1098 to1134, 1101 to 1112, and 1142 to 1308 days at sacrifice. Each cohort was group-housed. Within each cohort, subjects were randomly assigned to ECS, MST, or sham interventions. All staff not involved in the delivery of the interventions were masked to group assignment. This study was approved by the Institutional Animal Care and Use Committee of New York State Psychiatric Institute. Qualitative histological observations from the first four cohorts were reported previously (Dwork et al., 2004).
The study was designed to allow observation of either acute or delayed pathology. Interventions were performed for 6 weeks. Treatments were given 4 days per week (Monday, Tuesday, Thursday, and Friday). On Wednesday, animals in all 3 treatment groups (ECS, MST, and sham) received the sham intervention. A 5-week recovery period was interposed before the last intervention week, to permit maturation of possible neuropathological effects. Animals were sacrificed 3 days after the last intervention, so as not to miss acutely injured (eosinophilic) neurons or transient reactions, such as inflammatory infiltrates, microglial nodules, or astrocytic hypertrophy.
Subjects were sedated with ketamine (5 mg/kg IM) and xylazine (0.35 mg/kg IM). Like human ECT, interventions were administered under general anesthesia with methohexital (0.5 mg/kg IV), muscle relaxation with succinlycholine (3.5 mg/kg IV), and continuous ventilatory support (100% O2 positive pressure). Bilateral ECS and MST were administered at 2.5 times the individual subject’s seizure threshold, approximating high dosage bilateral ECT in patients.
The ECS electrodes were placed in the bilateral frontotemporal position, and the MST coil was centered on the vertex, as described in Moscrip et al. (2006). ECS was delivered with the Spectrum 5000Q (MECTA Corp.). MST used a custom repetitive stimulator capable of 50 Hz, 100% maximal stimulator output, in 8-second trains, with a round coil (Magstim Company Limited). Sham interventions were identical, but without brain stimulation. Physiological monitoring followed guidelines for human ECT.
Before removal from the skull, brains were fixed by transcardiac perfusion with 50 mM Na2S followed by 4% formaldehyde in phosphate buffer. Right frontal lobes were dissected into dorsal prefrontal, ventral prefrontal, and posterior frontal regions as described previously (Christensen et al., 2007). Frontal blocks were embedded in agar and cut in the coronal plane into 14 to 20 two-mm-thick slabs with a random start point within the slab thickness. A 100-micron-thick section was cut, with a vibratome, from the top of every second slab and stained with cresyl violet. For the first 4 cohorts, the same procedure was followed for the hippocampus, except that a section was taken from each slab (typically 9). For the last 4 cohorts, the left hippocampus was sampled. A block comprising the entire coronal extent of the left hemisphere, extending from the rostral pole of the temporal lobe to the caudal end of the hippocampus, was cryoprotected in 30% sucrose and exhaustively sectioned on a freezing sliding microtome. From a random start point, approximately ten 80-micron sections were collected at 1.6 mm intervals, mounted on slides, and stained with cresyl violet.
Volume measurements and cell counts were performed with the Cavalieri estimator and optical disector. Anatomical boundaries, stereological parameters, criteria for identifying neurons and glia, hardware, software, and formulae are specified and illustrated in a previous publication employing the brains of sham-treated animals to estimate total numbers of cortical neurons and glia (Christensen et al., 2007). Briefly, the sampling step size was 2 mm × 2 mm in the frontal cortex, 0.5 mm × 0.5 mm in CA1, and 0.35 mm x 0.35 mm in CA2–3. Counting frame height was 15 microns, representing a height sampling fraction of 2.3 to 2.9; a 5 mm upper guard zone was used. Counting frame areas were approximately 1550 square microns in the frontal cortex, 1300 in CA1, and 1350 in CA2–3. Objects counted varied from 139 to 400, representing 0.6 to 2.2 objects per discector. CAST software (Olympus Denmark) was employed for counting. The criteria for CA1 and CA2–3 were as illustrated in studies of humans (West and Gundersen, 1990) and macaca mulatta (Keuker et al., 2003). Volumes and cell counts in CA1 and CA2–3 were limited to the pyramidal cell layer. Neurons were distinguished by the presence of a prominent nucleolus and cytoplasmic Nissl substance. No effort was made to subclassify glial cells (Christensen et al., 2007).
Results
The coefficient of error (Gundersen and Jensen, 1987; Gundersen et al., 1999) for each individual measurement was < 0.10. Summarized values of the coefficients of error and coefficients of variation appear in the Table. There were no significant effects of treatment on the coefficient of error of any measurement.
Table.
CE (coefficient of error) is computed separately for each measure in each animal and represents the theoretical standard deviation/mean for that estimate, based on the distribution of counted objects. Values presented are mean and standard deviation (SD) of each CE, based on individual values of CE for each animal. Coefficient of variation (CV) is the observed standard deviation/mean for all 8 or 24 estimates (i.e., one per subject) of each measure. Probability (P) values are from one-way analysis of variance of CE by treatment.
| Coeficient of Error | Coefficient of Variation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neurons | Glia | Volume | Neurons | Glia | Volume | |||||
| region | treatment | Mean | Standard Deviation | Mean | Standard Deviation | Mean | Standard Deviation | |||
| CA1 | ECT | 0.083 | 0.015 | 0.073 | 0.015 | 0.038 | 0.011 | 0.11 | 0.20 | 0.16 |
| MST | 0.078 | 0.018 | 0.068 | 0.016 | 0.036 | 0.011 | 0.17 | 0.18 | 0.17 | |
| Sham | 0.079 | 0.017 | 0.071 | 0.017 | 0.037 | 0.008 | 0.13 | 0.17 | 0.16 | |
| Total | 0.080 | 0.016 | 0.071 | 0.016 | 0.037 | 0.010 | 0.13 | 0.18 | 0.16 | |
| P | 0.81 | 0.84 | 0.91 | |||||||
| CA2–3 | ECT | 0.092 | 0.028 | 0.082 | 0.028 | 0.051 | 0.014 | 0.18 | 0.26 | 0.22 |
| MST | 0.091 | 0.016 | 0.080 | 0.013 | 0.060 | 0.011 | 0.13 | 0.11 | 0.20 | |
| Sham | 0.089 | 0.013 | 0.079 | 0.013 | 0.056 | 0.012 | 0.27 | 0.26 | 0.37 | |
| Total | 0.091 | 0.019 | 0.080 | 0.018 | 0.055 | 0.012 | 0.21 | 0.24 | 0.28 | |
| P | 0.96 | 0.95 | 0.37 | |||||||
| DPFC | ECT | 0.082 | 0.011 | 0.086 | 0.033 | 0.045 | 0.005 | 0.25 | 0.41 | 0.24 |
| MST | 0.081 | 0.006 | 0.099 | 0.012 | 0.044 | 0.005 | 0.15 | 0.22 | 0.18 | |
| Sham | 0.089 | 0.009 | 0.093 | 0.005 | 0.049 | 0.004 | 0.23 | 0.17 | 0.21 | |
| Total | 0.084 | 0.009 | 0.093 | 0.020 | 0.046 | 0.005 | 0.24 | 0.28 | 0.23 | |
| P | 0.18 | 0.51 | 0.26 | |||||||
| PPFC | ECT | 0.065 | 0.006 | 0.065 | 0.006 | 0.027 | 0.003 | 0.17 | 0.17 | 0.21 |
| MST | 0.062 | 0.009 | 0.062 | 0.008 | 0.026 | 0.004 | 0.16 | 0.17 | 0.17 | |
| Sham | 0.057 | 0.007 | 0.060 | 0.008 | 0.027 | 0.003 | 0.18 | 0.24 | 0.22 | |
| Total | 0.061 | 0.008 | 0.062 | 0.007 | 0.026 | 0.003 | 0.17 | 0.19 | 0.20 | |
| P | 0.14 | 0.33 | 0.79 | |||||||
| VPFC | ECT | 0.064 | 0.009 | 0.074 | 0.013 | 0.033 | 0.003 | 0.13 | 0.31 | 0.10 |
| MST | 0.059 | 0.017 | 0.069 | 0.016 | 0.033 | 0.002 | 0.26 | 0.30 | 0.08 | |
| Sham | 0.063 | 0.005 | 0.078 | 0.011 | 0.032 | 0.005 | 0.15 | 0.24 | 0.29 | |
| Total | 0.062 | 0.011 | 0.074 | 0.013 | 0.033 | 0.003 | 0.18 | 0.29 | 0.18 | |
| P | 0.68 | 0.40 | 0.79 | |||||||
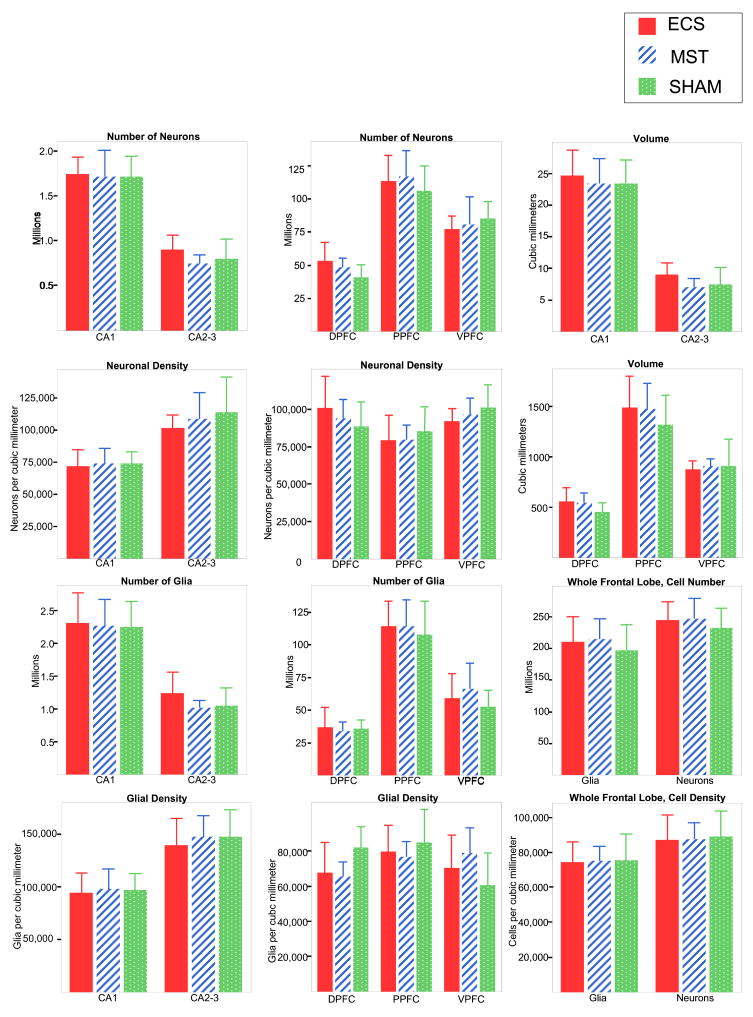
Cell counts, volumes, and densities are shown in Figure 1. One-way ANOVA revealed no significant effects of treatment on volume, neuronal density, glial density, total number of neurons, or total number of glia in any of the frontal and hippocampal subregions, nor in the frontal lobe as a whole. Among these 30 comparisons, even without correction for multiple tests, only one, for posterior frontal glial density, approached statistical significance (p = 0.06), whereas the random probability of at least one significant (α = 0.05) result among 30 comparisons is 0.79. In CA1, arguably the region most vulnerable to loss of neurons (see below), even the nominal differences in mean number of neurons are negligible (ECS 1.3% greater than sham, MST 0.1% less than sham).
Figure 1.
Means and standard deviations of neuronal numbers and densities, glial numbers and densities, and volumes of hippocampal and frontal subregions and entire frontal cortex. Abbreviations: CA1, CA2–3, subfields of hippocampus. DPFC, PPFC, VPFC, dorsal, posterior, and ventral portions of prefrontal cortex.
Several measurements were significantly correlated with the weight of the hemisphere: (dorsal prefrontal volume (r = 0.50), total frontal volume (r = 0.47), CA2–3 volume;(r= 0.46), total dorsal prefrontal glia (r = 0.65), total ventral prefrontal glia (r = 0.61) and total frontal glia (r=.66)). Covarying for hemispheric weight, there were still no significant effects of treatment on regional volumes or total numbers of glia or neurons (all p values > 0.1).
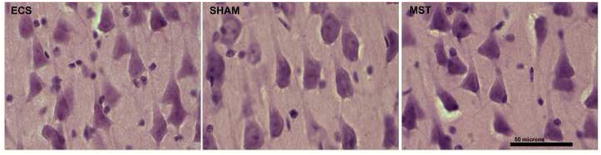
Routine histological examination of the left cerebral hemisphere, as previously reported for the first 4 cohorts (Dwork et al., 2004), revealed no neuropathological lesions. In particular, except for a rare eosinophilic neuron noted previously (Dwork et al., 2004) in one sham-treated animal, the neurons of CA1 had an entirely unremarkable appearance regardless of treatment (Figure 2). Although we had found greater hippocampal immunoreactivity for glial fibrillary acidic protein in animals that received active treatments (Dwork et al., 2004), qualitative assessment of hematoxylin and eosin stains by an experienced neuropathologist (AJD) yielded no evidence of astrocytic hypertrophy or proliferation.
Figure 2.
Typical, healthy appearance of hippocampal CA1 pyramidal cells in a cohort of 3 animals treated with ECS, anesthesia alone (sham), or MST, as indicated. Hematoxylin and eosin on 40 micron frozen sections. Scale bar =50 microns.
Discussion
Published studies show no evidence of destructive lesions from clinical ECT as currently applied, with muscle paralysis and oxygenation, nor in animal models mimicking these conditions (Devanand et al., 1994; Dwork et al., 2004; Perera et al., 2007; Scalia et al., 2007). While there have been no quantitative autopsy studies of ECT in humans, there are several reports of individuals who had received large numbers of treatments and showed no.qualitative abnormalities on neuropathological examinationi (Heyck, 1955; Lippman et al., 1985; Scalia et al., 2007). On the other hand, up to 90% of cases of temporal lobe epilepsy are accompanied by hippocampal sclerosis (Thom et al., 2002). A stereological study in rats found decreases of 17–32% in neuronal density throughout CA1 and CA3 after 30 kindled seizures, and subregions of CA1 and CA3 showed 17–18% decrements after only three kindled seizures (Cavazos et al., 1994)., In humans, the pyramidal neurons of CA1 (Sommer’s sector) are among the most vulnerable to generalized excitotoxic, hypoxic, ischemic and hypoglycemic insult (Auer et al., 2008). Thus, it would be reasonable to postulate that ECS or MST, while not producing hippocampal sclerosis, might effect a more subtle loss of neurons in CA1 or CA3, and that the absence of such loss would be strong evidence for the absence of excitotoxicity. Since both ECS and MST induce electrical current and ictal activity in the frontal lobes (Lisanby et al., 2003), we chose also to explore the possibility of focal cellular loss within this region. Furthermore, ECT impairs memory in depressed patients, as do ECS and MST in animal models (although the effects of MST are significantly less than those seen with ECS) (Moscrip et al., 2004; 2006; Spellman et al., 2008)., suggesting that hippocampus and prefrontal cortex might suffer pathological damage. However, rigorous measurements of hippocampal and frontal subregions revealed no loss of volume or cells.
The current study is the first stereological assessment of ECS or MST in higher mammals, and the first in any animal model of ECT or MST with muscle paralysis and supported oxygenation. Quantitative studies of unmodified ECS in rats found no effects on neuronal number in neocortex (Colon and Notermans, 1975), hippocampus (Dam et al., 1980; Gombos et al., 1999; Laursen et al., 1991), cerebellum (Dam et al., 1984), or thalamus (Laursen et al., 1991; Vaidya et al., 1999). In the only stereological study to include hippocampal granular cell neurons, these were greater in number, and the granular cell layer and hilus were greater in volume, in rats receiving daily unmodified ECS for 10 days, compared with rats receiving sham treatments; CA1 and CA2–3 were unaffected (Chen et al., 2009). Vaidya et al. (1999) found no evidence of injury to hippocampal hilar neurons with Nadler’s (Nadler and Evenson, 1983) silver method. Dalby et al. (1996) found no effect of a large number of seizures on numbers of somatostatinergic neurons in the hippocampal hilus. Okada et al (Okada et al., 2002), using reverse transriptase polymerase chain reaction (RT-PCR), found transient (at least 12 hours but less than 24) elevations of cortical mRNA levels of interleukin (IL)-1b, IL-6, and cyclooxygenase (COX)-2, but nonconvulsive transcranial magnetic stimulation (TMS) did not affect gene expression. Using rigorous stereological procedures in rats, Helsten et al. (2005) found increased numbers of endothelial cells and increased total capillary length in the dentate gyrus following single or multiple ECS, with or without preoxygenation that effectively precluded hypoxia. This result is consistent with our finding, 4 weeks after intravenous bromodeoxyuridine (BrdU) injection, of greater numbers of BrdU-labeled endothelial cells in bonnet macaque hippocampus when the injections were preceded by 4 weeks of ECS (with anesthesia, paralysis, and ventilatory support) than when they were preceded by a sham intervention. (Perera et al., 2007). These newly-generated endothelial cells may facilitate neurogenesis (Palmer et al., 2000).
In contrast to studies meant to model ECT, neuronal loss does occur in models of epilepsy. For example, Cardoso et al. (2008) used stereological methods to examine the entorhinal cortex and the hippocampal dentate gyrus and hilus in 3-month-old rats treated at age 2 months with daily ECS for 5 days and a sixth ECS 2 hours after the fifth. This protocol, specifically designed to induce the last seizure while glutamate reuptake capacity was impaired (i.e., to facilitate seizure-induced excitotoxicity), resulted in lower numbers of neurons but normal volume in the hippocampal hilus, and in lower numbers of neurons and smaller volume in layer 3 of the entorhinal cortex.
Our results for MST were also negative. To our knowledge, no other study has looked for histological damage or loss of cells following cases of seizures induced by MST. Studies of repetitive transcranial magnetic stimulation (rTMS), with or without unintended seizures, are mostly negative. Magnetic resonance imaging studies of rTMS have revealed no abnormalities of structure or diffusion (Li et al., 2003; Nahas et al., 2004). The only histological study in humans qualitatively examined temporal lobectomy specimens from two epileptic subjects who underwent rTMS with approximately 2,000 stimulations over the preceding 2–4 weeks (Gates et al., 1992). In one, rTMS induced an unintended partial motor seizure. Neither temporal lobectomy specimen showed pathological changes, except for a vascular malformation that obviously preceded rTMS. In lower mammals, most studies are negative, but two report subtle, qualitative abnormalities. There is a report of cortical microvacuolar changes, visible by light microscopy, following rTMS in unanesthetized rats (Matsumiya et al., 1992). Another study (Zyss et al., 2001) found no abnormalities by light microscopy of the brains of rats treated with ECS or rTMS, but reported “edematous changes” visible by electron microscopy that were milder after rTMS than after ECS. Sgro et al. (1991) found no qualitative abnormalities after rTMS of anesthetized rats. Post et al. (1999), in a qualitative study, found neither pathological changes nor increased immunoreactivity for glial fibrillary acidic protein (GFAP) in anesthetized or unanesthetized rats after rTMS, and they specifically ruled out microvacuolation. Ravnborg et al. (1990) found no change in the permeability of cerebral blood vessels after rTMS in anesthetized or unanesthetized rats. Qualitative studies by Nishikiori (1996) and Counter (1993) found no abnormalities of the brain after extensive rTMS in unanesthetized rabbits. Using quantitative in situ hybridization, Fujiki and Steward (1997) found an increase in mRNA for GFAP after rTMS in unanesthetized mice, but they did not examine glial morphology or GFAP immunoreactivity. In summary, there is little evidence that transcranial magnetic stimulation produces structural changes, whether or not seizures are induced.
A potential limitation of our study is the use of ketamine to sedate the animals for insertion of an intravenous line prior to treatment. While, in theory, ketamine could protect against excitotoxic damage, this is unlikely to have occurred with the low dose (5 mg/kg) that we employed. In a rat model of cerebral ischemia, a dose of 60 mg/kg of S(+) ketamine (2–3 times more potent than the racemic mixture that we used) was required to reduce neuronal loss in cerebral cortex, and even 90 mg/kg did not reduce neuronal loss in any sector of the hippocampus (Proescholdt et al., 2001). Furthermore, the seizure thresholds in our animals were similar to those in a subsequent study by our group, in which the animals were trained to accept the placement of the intravenous line, so that ketamine was not necessary (Moscrip et al., 2006; Spellman et al., 2008).
Since glia were not subclassified, we cannot rule out a loss of one type (e.g., oligodendrocytes or NG2 glia), accompanied by an increase in another type, (e.g. astrocytes or microglia). However in grey matter, an astrocytic or microglial reaction is likely to be related to loss of neurons, which was not observed; furthermore, on qualitative examination of hematoxylin and eosin-stained sections (reported previously for half of the animals (Dwork et al., 2004) and subsequently confirmed for the remainider), we did not see microglial nodules or hypertrophic (reactive) astrocytes, which would be characteristic of reactions to dying cells. Counting specifically of activated microglia may provide a more sensitive indicator of cellular reaction to damage, and in the future may confirm or challenge these negative results. However, this cannot be done on Giemsa-stained sections, and instead will require immunohistochemical stains whose consistency and sensitivity must be rigorously established.
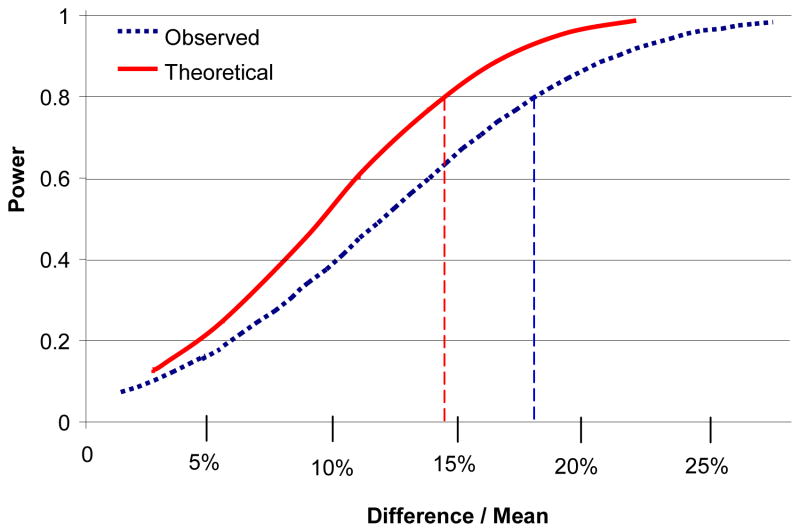
An inevitable limitation of this study is its statistical power, even though our sample is large compared with other terminal studies of nonhuman primates. With 8 subjects per group, the one-way ANOVA has a power of 0.8 to detect an effect size f (= SD of the 3 group means/SD of all subjects) of 0.7. Since the standard deviations of our measures are in the range of 11%–29% of the means, f = 0.7 when the highest and lowest means differ by 14% to 42%, so the power to detect smaller differences is limited. Nonetheless, if one considers the a priori hypothesis that the number of neurons in CA1 will be lower in ECS-treated than in sham-treated animals, a 1-tailed t-test would have a power of 0.8 to detect a decrement of 12%, 0.95 to detect a decrement of 16%, and 0.99 to detect a decrement of 19%, which is comparable to the decrement seen after 3 kindled seizures in the rat (Cavazos et al., 1994). This calculation is based on the observed coefficient of variation for each of the two groups (0.11 and 0.13), which encompasses both the imprecision of the measurement for each animal and the true variability among animals. However, the observed values for the two conditions were nearly identical: the mean number of neurons in CA1 was 1% greater (95% confidence interval 12% lower to 15% greater) in the ECS-treated group than in the sham-treated group (1-tailed p=0.43), and the mean neuronal density was 3% lower (1-tailed p=0.34). We note (Table) that the ratio of mean coefficient of error (CE, predicted ratio of standard deviation of estimate to value of estimate) to coefficient of variation (CV, observed ratio of standard deviation to mean for all subjects) is greatest, among all of the measures reported, for neuronal number in CA1. However, comparing. the relative contributions of imprecision of the estimate and between-subject biological variability (BV), from CV2 = BV2 + CE2 ((Mouton, 2002), p. 23), it is apparent that little statistical power would be gained even were the imprecision of the estimate eliminated entirely (Figure 3). This conclusion applies even more strongly to the other measures, where the contribution of the CE to the total variance is smaller.
Figure 3.
Statistical power for detection of indicated differences in total neuronal number in CA1 by 1-tailed t-test, α = 0.05, with 8 subjects per group. Broken blue curve indicates power based on observed standard deviation. Solid red curve indicates power based on theoretical standard deviation if imprecison of estimates could be eliminated entirely (i.e., CE = 0). Intersections of vertical lines with x-axis indicate differences detectable with power of 0.8. Power calculations from G*Power Version 3.0.1 (Faul et al., 2007).
The animals in these experiments were healthy young adults. The clinical use of ECT and MST in the treatment of depression are not so restricted. We do not know whether depression itself is associated with susceptibility to neural injury by therapeutically-induced seizures. Furthermore, ECT is often used in elderly subjects, and there are no absolute medical or neurological contraindications (American Psychiatric Association Committee on Electroconvulsive Therapy, 2001; Royal College of Psychiatrists Special Committe on ECT, 2005). Thus, until similar experiments are carried out in the context of animal models of aging or of neurological or medical diseases, and confirmed by autopsy studies of patients with such conditions, any inferences about the clinical safety of these methods must be made with these limitations in mind.
Using a model similar to the current study, we have shown increased hippocampal neurogenesis in ECS-treated Macaca radiata (Perera et al., 2007). We have likewise identified increased cell proliferation in the subgranular layer of the hippocampal dentate gyrus of the subjects treated with ECS, but not MST, in the current study (unpublished data). While it would be of interest to employ stereological methods to determine the effects of adult neurogenesis, such a study would focus on granular cells of the dentate gyrus. In contrast, the current study was focused on safety and therefore examined the hippocampal pyramidal cell layer and frontal cortex, likely areas for neuronal damage, but in which adult neurogenesis, if present, is much more limited (Cameron and Dayer, 2008).
The current study suggests that, like ECS, neither high doses of rTMS nor rTMS-invoked seizures result in structural damage to brain tissue. The dosage of MST tested here was 2.5 times the seizure threshold, selected to match the ECT dosage. The safety of higher MST dosages is under investigation (Kirov et al., 2008; Spellman et al., 2008).
Acknowledgments
NIMH: MH60884, MH60877, MH64168, MH62185, MH40210, Stanley Medical Research Institute, National Alliance for Research on Schizophrenia and Depression, and equipment upgrades from Magstim Company.
Abbreviations
- BV
biological variability
- CA1
cornu ammonis 1 (field of hippocampus)
- CA3
cornu ammonis 3 (field of hippocampus)
- CE
coefficient of error
- CV
coefficient of variation
- ECS
electroconvulsive shock
- ECT
electroconvulsive therapy
- GFAP
glial fibrillary acidic protein
- mRNA
messenger ribonucleic acid
- MST
magnetic seizure therapy
- rTMS
repetitive transcranial magnetic stimulation
- SD
standard deviation
- TMS
transcranial magnetic stimulation
Footnotes
Financial Disclosures
Dr. Lisanby has received research grants from Neuronetics, Brainsway, Cyberonics, NIH, AFAR, NARSAD, Stanley Medical Research Foundation, DARPA, Advanced Neuromodulation Systems/St. Jude, and NYSTAR, and equipment support from Magstim Company. She Chaired a Data Safety and Monitoring Board for a study sponsored by NorthStar. Columbia University has filed patent applications for novel TMS technology developed in Dr. Lisanby’s laboratory, not related to the topic presented here. None of the other authors has any real or apparent conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association Committee on Electroconvulsive Therapy. The practice of electroconvulsive therapy. 2. American Psychiatric Publishing; 2001. [Google Scholar]
- Auer RN, Dunn JF, Sutherland GR. Hypoxia and related conditions. In: Love S, Louis D, Ellison D, editors. Greenfield’s Neuroopathology. Edward Arnold; London: 2008. pp. 63–119. [Google Scholar]
- Cameron HA, Dayer AG. New interneurons in the adult neocortex: small, sparse, but significant? Biol Psychiatry. 2008;63:650–655. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso A, Assuncao M, Andrade JP, Pereira PA, Madeira MD, Paula-Barbosa MM, Lukoyanov NV. Loss of synapses in the entorhinal-dentate gyrus pathway following repeated induction of electroshock seizures in the rat. J Neurosci Res. 2008;86:71–83. doi: 10.1002/jnr.21474. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP, Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19:329–338. doi: 10.1016/j.euroneuro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 2007;290:330–340. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- Colon EJ, Notermans SL. A long-term study of the effects of electro-convulsions on the structure of the cerebral cortex. Acta Neuropathol. 1975;32:21–25. doi: 10.1007/BF00686064. [DOI] [PubMed] [Google Scholar]
- Counter SA. Neurobiological effects of extensive transcranial electromagnetic stimulation in an animal model. Electroencephalography and clinical neurophysiology. 1993;89:341–348. doi: 10.1016/0168-5597(93)90074-y. [DOI] [PubMed] [Google Scholar]
- Dalby NO, Tonder N, Wolby DP, West M, Finsen B, Bolwig TG. No loss of hippocampal hilar somatostatinergic neurons after repeated electroconvulsive shock: a combined stereological and in situ hybridization study. Biol Psychiatry. 1996;40:54–60. doi: 10.1016/0006-3223(95)00355-x. [DOI] [PubMed] [Google Scholar]
- Dam AM, Hertz M, Bolwig TG. The number of hippocampal neurons in rats after electrically-induced generalized seizures. Brain Res. 1980;193:268–272. doi: 10.1016/0006-8993(80)90965-8. [DOI] [PubMed] [Google Scholar]
- Dam M, Bolwig T, Hertz M, Bajorec J, Lomax P, Dam AM. Does seizure activity produce Purkinje cell loss? Epilepsia. 1984;25:747–751. doi: 10.1111/j.1528-1157.1984.tb03486.x. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Dwork AJ, Hutchinson ER, Bolwig TG, Sackeim HA. Does ECT alter brain structure? Am J Psychiatry. 1994;151:957–970. doi: 10.1176/ajp.151.7.957. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Arango V, Underwood M, Ilievski B, Rosoklija G, Sackeim HA, Lisanby SH. Absence of histological lesions in primate models of ECT and magnetic seizure therapy. Am J Psychiatry. 2004;161:576–578. doi: 10.1176/appi.ajp.161.3.576. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Steward O. High frequency transcranial magnetic stimulation mimics the effects of ECS in upregulating astroglial gene expression in the murine CNS. Brain Res Mol Brain Res. 1997;44:301–308. doi: 10.1016/s0169-328x(96)00232-x. [DOI] [PubMed] [Google Scholar]
- Gates JR, Dhuna A, Pascual-Leone A. Lack of pathologic changes in human temporal lobes after transcranial magnetic stimulation. Epilepsia. 1992;33:504–508. doi: 10.1111/j.1528-1157.1992.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Gombos Z, Spiller A, Cottrell GA, Racine RJ, McIntyre Burnham W. Mossy fiber sprouting induced by repeated electroconvulsive shock seizures. Brain Res. 1999;844:28–33. doi: 10.1016/s0006-8993(99)01924-1. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hellsten J, West MJ, Arvidsson A, Ekstrand J, Jansson L, Wennstrom M, Tingstrom A. Electroconvulsive Seizures Induce Angiogenesis in Adult Rat Hippocampus. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Heyck H. Histological findings in a brain without ganglion cell damage after 355 electric convulsive shock treatments. Monatsschrift fur Psychiatrie und Neurologie. 1955;129:128–137. [PubMed] [Google Scholar]
- Kayser S, Bewernick B, Axmacher N, Schlaepfer TE. Magnetic Seizure Therapy of Treatment-Resistant Depression in a Patient With Bipolar Disorder. J ECT. 2008 doi: 10.1097/YCT.0b013e31817dc45a. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Kirov G, Ebmeier KP, Scott AI, Atkins M, Khalid N, Carrick L, Stanfield A, O’Carroll RE, Husain MM, Lisanby SH. Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. Br J Psychiatry. 2008;193:152–155. doi: 10.1192/bjp.bp.107.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- Laursen H, Gjerris A, Bolwig TG, Barry DI. Cerebral Edema and Vascular Permeability to Serum Proteins Following Electroconvulsive Shock in Rats. Convuls Ther. 1991;7:237–244. [PubMed] [Google Scholar]
- Li X, Nahas Z, Lomarev M, Denslow S, Shastri A, Bohning DE, George MS. Prefrontal cortex transcranial magnetic stimulation does not change local diffusion: a magnetic resonance imaging study in patients with depression. Cogn BehavNeurol. 2003;16:128–135. doi: 10.1097/00146965-200306000-00006. [DOI] [PubMed] [Google Scholar]
- Lippman S, Manshadi M, Wehry M, Byrd R, Past W, Keller W, Schuster J, Elam S, Meyer D, O’Daniel R. 1,250 electroconvulsive treatments without evidence of brain injury. Br J Psychiatry. 1985;147:203–204. doi: 10.1192/bjp.147.2.203. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Luber B, Finck AD, Schroeder C, Sackeim HA. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates.[erratum appears in Arch Gen Psychiatry 2001 May;58(5):515] Arch Gen Psychiatry. 2001;58:199–200. doi: 10.1001/archpsyc.58.2.199. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Moscrip T, Morales O, Luber B, Schroeder C, Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Supplements to Clinical neurophysiology. 2003;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- Matsumiya Y, Yamamoto T, Yarita M, Miyauchi S, Kling JW. Physical and physiological specification of magnetic pulse stimuli that produce cortical damage in rats. J Clin Neurophysiol. 1992;9:278–287. doi: 10.1097/00004691-199204010-00008. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. A primate model of anterograde and retrograde amnesia produced by convulsive treatment. J ECT. 2004;20:26–36. doi: 10.1097/00124509-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) Int J Neuropsychopharmacol. 2006;9:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- Mouton P. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. JHU Press; 2002. [Google Scholar]
- Nadler JV, Evenson DJ. Use of excitatory amino acids to make axon-sparing lesions of hypothalamus. Methods Enzymol. 1983;103:393–400. doi: 10.1016/s0076-6879(83)03027-x. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Li X, Kozel FA, Mirzki D, Memon M, Miller K, Yamanaka K, Anderson B, Chae JH, Bohning DE, Mintzer J, George MS. Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. DepressAnxiety. 2004;19:249–256. doi: 10.1002/da.20015. [DOI] [PubMed] [Google Scholar]
- Nishikiori O. Studies on transcranial magnetic stimulation - Part 2. Pathological findings of rabbit brain after long-term stimulation based on characteristics of intracerebral induced voltage. Journal of Japanese Ophthalmological Society. 1996;100:489–495. [PubMed] [Google Scholar]
- Okada K, Matsunaga K, Yuhi T, Kuroda E, Yamashita U, Tsuji S. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002;957:37–41. doi: 10.1016/s0006-8993(02)03582-5. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A, Muller MB, Engelmann M, Keck ME. Repetitive transcranial magnetic stimulation in rats: Evidence for a neuroprotective effect in vitro and in vivo. Eur J Neurosci. 1999;11:3247–3254. doi: 10.1046/j.1460-9568.1999.00747.x. [DOI] [PubMed] [Google Scholar]
- Proescholdt M, Heimann A, Kempski O. Neuroprotection of S(+) ketamine isomer in global forebrain ischemia. Brain Res. 2001;904:245–251. doi: 10.1016/s0006-8993(01)02465-9. [DOI] [PubMed] [Google Scholar]
- Ravnborg M, Knudsen GM, Blinkenberg M. No effect of pulsed magnetic stimulation on the blood-brain barrier in rats. Neuroscience. 1990;38:277–280. doi: 10.1016/0306-4522(90)90392-h. [DOI] [PubMed] [Google Scholar]
- Royal College of Psychiatrists Special Committe on ECT. The ECT handbook: the third report of the Royal College of Psychiatrists’ Special Committee on ECT. Royal College of Psychiatrists; 2005. [Google Scholar]
- Scalia J, Lisanby SH, Dwork AJ, Johnson JE, Bernhardt ER, Arango V, McCall WV. Neuropathologic examination after 91 ECT treatments in a 92-year-old woman with late-onset depression. J ECT. 2007;23:96–98. doi: 10.1097/YCT.0b013e31804bb99d. [DOI] [PubMed] [Google Scholar]
- Sgro JA, Ghatak NR, Stanton PC, Emerson RG, Blair R. Repetitive high magnetic field stimulation: the effect upon rat brain. Electroencephalography & Clinical Neurophysiology - Supplement. 1991;43:180–185. [PubMed] [Google Scholar]
- Spellman T, McClintock SM, Terrace H, Luber B, Husain MM, Lisanby SH. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163–1170. doi: 10.1016/j.biopsych.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Sisodiya SM, Beckett A, Martinian L, Lin WR, Harkness W, Mitchell TN, Craig J, Duncan J, Scaravilli F. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002;61:510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- White PF, Amos Q, Zhang Y, Stool L, Husain MM, Thornton L, Downing M, McClintock S, Lisanby SH. Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesth Analg. 2006;103:76–80. doi: 10.1213/01.ane.0000221182.71648.a3. [DOI] [PubMed] [Google Scholar]
- Zyss T, Adamek D, Zieba A, Vetulani J, Mamczarz J, Mika J, Roman A. Transcranial magnetic stimulation versus electroconvulsive shocks - Neuroanatomical investigations in rats. Archives of Psychiatry and Psychotherapy. 2001;3:13–29. [PubMed] [Google Scholar]