Abstract
The precise contribution(s) of skin dendritic cells (DCs) to immune responses in the skin has not been well delineated. We developed an intradermal (i.d.) injection model in which CD8+ T (OT-I) cells that express ovalbumin (OVA)-peptide-specific T-cell receptors (Vα2/Vβ5) are delivered directly to the dermis of transgenic (Tg) mice expressing OVA in the epidermis. Following i.d. injection, these mice reliably develop skin GvHD by day 7. To determine the relative contribution of LCs to the ensuing graft-versus-host (GvHD) - like reaction, we generated K14-OVA × Langerin-Diphtheria-Toxin-Receptor (Langerin-DTR) Tg mice to allow conditional ablation of LCs in the epidermis. To delineate the role of dermal dendritic cells (dDCs) in the reaction we also generated K14-OVA Tg chimeras using beta-2-microglobulin-deficient (b2m) congenic donor bone marrow cells. Dermal DCs in these mice cannot present OVA to OT-I cells while the LC are antigen presentation competent. Unexpectedly, OT-I cell injection into diphtheria toxin (DT)-treated b2m→K14−OVA × Langerin-DTR Tg mice resulted in skin GvHD. Thus, in vivo, both LC and dDC appear to be dispensable for induction of keratinocyte-directed, CD8-mediated effector immune responses. Furthermore and surprisingly, OVA-expressing epidermal cells depleted of LCs that could not initiate allogeneic epidermal lymphocyte reactions, activated naïve OT-I cells in vitro. These results indicate that keratinocytes may function as accessory cells-competent to prime naïve skin-reactive T cells.
Keywords: Langerhans cells, Dendritic cells, GvHD, Langerin KO mice
Introduction
Dendritic cells (DCs) are the primary antigen-presenting cells (APCs) that mediate T cell immunity and tolerance (Banchereau and Steinman, 1998; Hawiger et al., 2001; Probst et al., 2003). In skin, it is thought that epidermal Langerhans cells (LCs) and dermal dendritic cells (dDCs) serve this role by taking up antigen (Ag) in the periphery and acting as sentinels. In the immature state, DCs express low levels of surface costimulatory molecules and induce tolerance upon interaction with naïve T cells (Hawiger et al., 2001; Probst et al., 2003; Steinman and Nussenzweig, 2002). Following activation by pathogenic stimuli skin DCs upregulate MHC II, CD40, CD80, and CD86 molecules and migrate to the skin-draining lymph nodes (LNs) to prime naïve T cells (Hemmi et al., 2001; Carbone et al., 2004; Stoitzner et al., 1999). It has been suggested that LC continuously migrate to the cutaneous lymph node (CLN) to maintain tolerance to self-Ag, however, a series of recent studies suggested that LCs may have an immunostimulatory function even in the steady-state (Azukizawa et al., 2003; Shibaki et al., 2004; Mayerova et al., 2004). Thus, the precise role of LCs in the induction of tolerance and immunity remains unclear. The role of dDCs has remained even more elusive.
Recent studies suggest several possibilities regarding the relative contributions of skin DCs (LCs and dDCs) to cross-presentation of skin-derived Ag, T cell priming, viral immunity, and contact hypersensitivity (CHS). With regard to CHS, elimination of LCs repeatedly resulted in three outcomes: no effect, attenuation, and enhancement of CHS responses (Kissenpfennig et al., 2005; Bennett et al., 2005; Kaplan et al., 2005). Despite varying results, each of these studies indicated that dDCs are sufficient to elicit a significant CHS response. However, the relative contributions of LCs and dDCs to the presentation of skin-associated protein antigen to CD8+ T cells remain unclear.
We previously described a systemic graft-versus-host-disease (GvHD) model in which mice that expressed a membrane-bound form of ovalbumin (mOVA) regulated by a keratin 14 (K14) promoter developed weight loss and skin and esophageal lesions following intravenous (i.v.) transfer of OT-I cells (Shibaki et al., 2004). To better understand the mechanisms underlying Ag-presentation within the skin, we developed an intradermal (i.d.) injection model in which OT-I cells are delivered directly into the dermis of different lines of K14-OVA Tg mice and elicit localized GvHD reactions. To examine the role of LCs in this reaction, we generated K14-OVA × Langerin-Diphtheria-Toxin-Receptor (Langerin-DTR) double Tg mice in order to allow conditional ablation of LCs after administration of diphtheria toxin (DT) (Bennett et al., 2005). To determine the contribution of dDCs (and potential circulating DC precursors), we chimerized K14-OVA Tg mice with congenic β2-microglobulin-deficient (b2m) bone marrow cells to impair the presentation of OVA Ag via MHC class I molecules. Surprisingly, we determined that elimination of epidermal LCs and/or the impairment of dDCs did not abrogate localized GvHD reactions in vivo. We also demonstrate that the elimination of LCs from the epidermis could abrogate mixed epidermal-lymphocyte reactions (MELRs) but did not attenuate OT-I T cell priming. These results indicate that keratinocytes can present self-Ag that is expressed in epidermis to naïve CD8 T cells.
Results
OVA mRNA expression level varies between the different lines of K14-OVA Tg mice
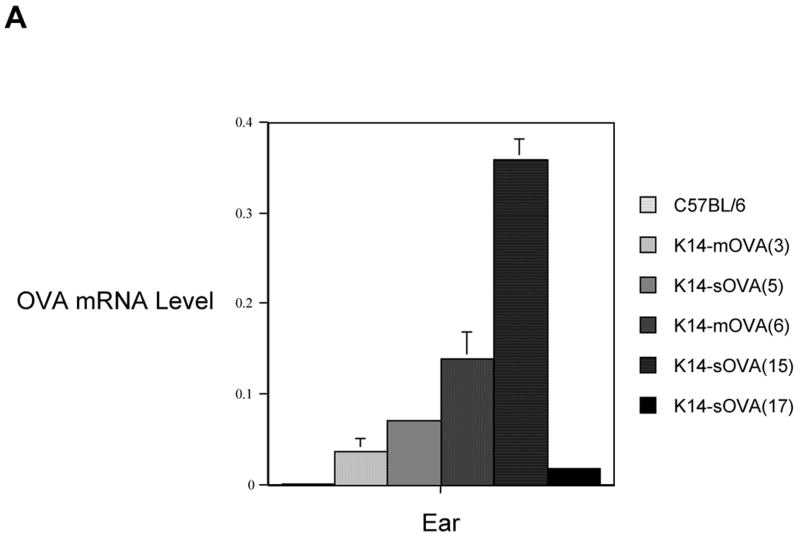
Five Tg mouse lines that express either soluble (K14-sOVA(5), K14-sOVA(15), and K14-sOVA(17) Tg) or membrane-bound (K14-mOVA(3) and K14-mOVA(6) Tg) forms of OVA in epidermis were established. OVA mRNA expression levels were quantified by real-time RT-PCR in ears (Fig 1) and trunk skin (data not shown). K14-mOVA(6) Tg mice develop systemic GvHD following i.v. transfer of OT-I cells manifested by histological signs and 30% weight loss by day 7 (Shibaki et al., 2004). K14-sOVA(15) Tg mice that express the highest level of OVA mRNA (Fig 1) developed more severe disease and died by day 4-5 after adoptive transfer of OT-I cells (data not shown). In contrast to the K14-mOVA(6) and K14-sOVA(15) Tg mice, other strains (K14-mOVA(3), K14-sOVA(5), and K14-sOVA(17) Tg) that expressed lower levels of OVA mRNA did not develop systemic GvHD following i.v. transfer of OT-I cells (data not shown). Thus, development of systemic GvHD reactions after i.v. transfer of OT-I cells correlates well with the level of OVA mRNA expression.
Figure 1. K14-OVA Tg mice express different levels of OVA mRNA.
OVA mRNA expression was quantified by real-time RT-PCR using total RNA extracted from the ears of all strains of K14-OVA Tg mice (-mOVA(3), -sOVA(5), -mOVA(6), -sOVA(15), and −sOVA(17)) and control C57BL/6 mice. All measurements were performed in triplicates and normalized to endogenous levels of β-actin. Standard error bars are included. The notations m and s indicate whether the epidermal OVA is targeted to the keratinocyte membrane (m) or is in soluble (s) form.
All lines of K14-OVA Tg mice develop localized GvHD following i.d. injection of OT-I cells
To additionally investigate the mechanism of GvHD in skin, 2 × 106 OT-I cells were injected i.d. into ears of all lines of K14-OVA Tg mice. Following i.d. injection, skin GvHD developed in all mice manifested on day 7 with widespread vacuolar changes, apoptosis of keratinocytes, and lymphocytic infiltration of the dermis and epidermis (Fig 2B-F). In contrast, i.d. injection of the same number of OT-I cells into the ears of control C57BL/6 (syngeneic) (Fig 2A) and BALB/c (allogeneic) (data not shown) mice did not cause skin GvHD. Injection of purified control CD8+ C57BL/6 T cells into the ears of K14-mOVA(6) Tg and C57BL/6 mice also did not cause local reactions (data not shown). Thus, localized GvHD reactions were specific for OT-I cells encountering OVA antigen within skin of all K14-OVA Tg mouse lines, independent of the level of OVA mRNA expression, or susceptibility of lines to develop systemic GvHD after i.v. transfer of OT-I cells.
Figure 2. GvHD-like skin lesions developed following i.d. injection of OT-I cells.
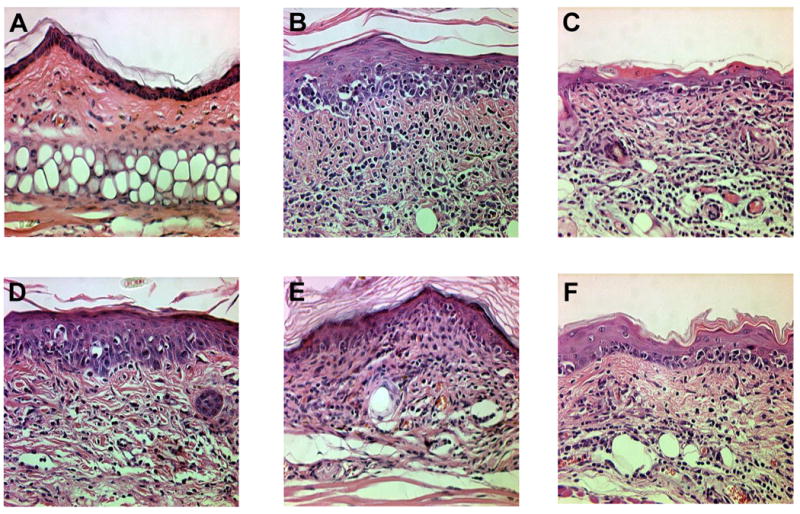
H&E histology (400×) of the ears from (a) control C57BL/6, (b) K14-mOVA(3), (c) K14-sOVA(5), (d) K14-mOVA(6), (e) K14-sOVA(15), and (f) K14-sOVA(17) Tg mice after i.d. injection of 2 × 106 OT-I cells. Skin biopsies from all K14-OVA Tg mouse strains exhibited basal vacuolar changes, apoptotic keratinocytes, and lymphocytic infiltration characteristic of GvHD on day 7 after treatment.
I.d. injection of OT-I cells causes activation of LCs within the skin on day 2 and GvHD-like changes by day 3
Histologically, GvHD reactions in skin following i.d. injection were much more severe than previously observed in the systemic GvHD model (Shibaki et al., 2004). To understand the mechanism of this local reaction, epidermal sheets were stained with antibodies against MHC II molecules on day 2 following i.d. injection of 2 × 106 OT-I cells in 20 μl of PBS. Clusters of enlarged LCs with elongated dendrites were evident, suggesting maturation in the epidermis (Fig 3B). Maturation of LCs was also evident after injection of 20 μl of PBS alone, suggesting that the i.d. injection itself was sufficient to elicit maturation of LCs (Fig 3C).
Figure 3. I.d. injection of OT-I cells caused activation of LCs and GvHD-like histological changes.
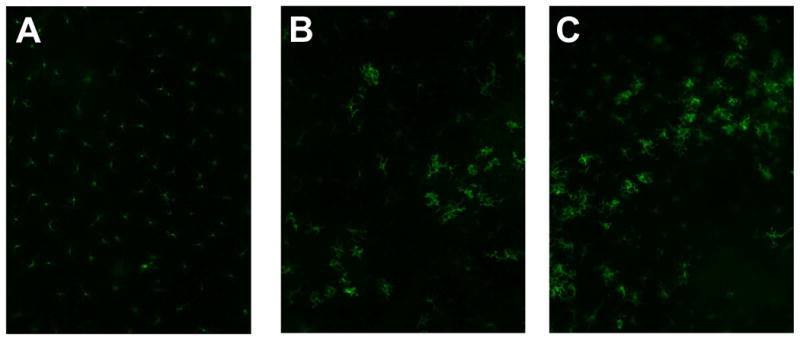
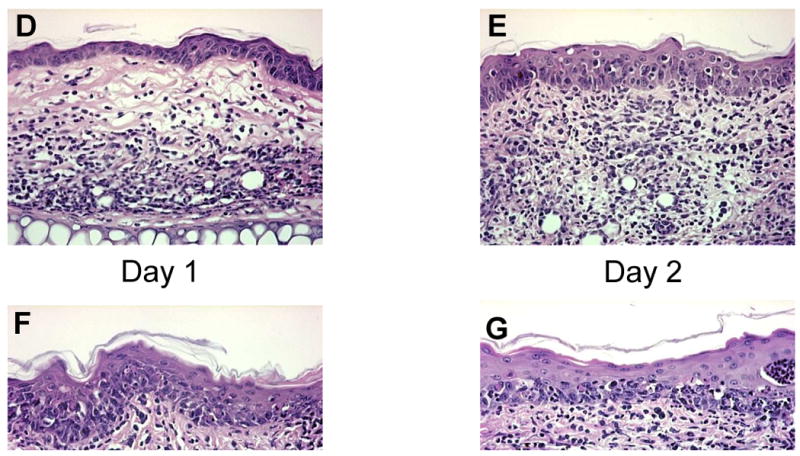
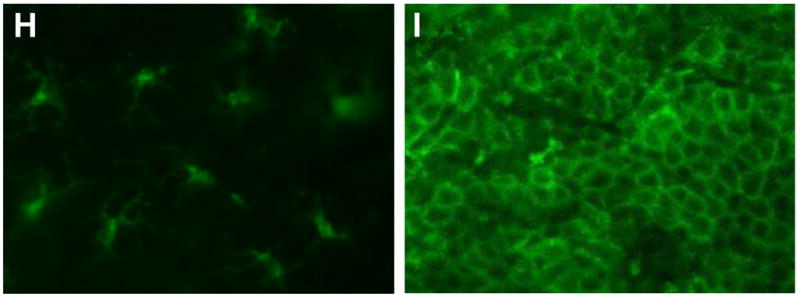
Epidermal sheets were separated from mouse ears and stained for MHC II molecules to visualize LCs (200×) on Day 2. Representative data from C57BL/6 and K14-mOVA(6) Tg mouse ears: (a) untreated controls, (b) following injection of 2 × 106 OT-I cells in 20 μl PBS, and (c) following injection of 20 μl PBS. Both mice injected with OT-I cells and those injected with PBS alone demonstrated enlarged LCs with elongated dendrites, suggestive of maturation. H&E histology (200×) of the ears from K14-mOVA(6) Tg mice after injection of 2 × 106 OT-I cells on (d) day 1, (e) day 2, (f) day 3, and (g) day 4. Characteristic basal vacuolar changes, apoptotic keratinocytes, and lymphocytic infiltration were clearly evident by day 3. Epidermal sheets were separated from mouse ears and stained for MHC II molecules (400×) on day 4 after i.d. injection of 2 × 106 OT-I cells into (h) C57BL/6 mice and (i) K14-mOVA(6) Tg mice. The K14-mOVA(6) Tg mice exhibited widespread MHC II molecule expression on keratinocytes in a manner not observed in control C57BL/6 mice. Epidermal sheets were separated from mouse ears and stained for MHC II (400×) on day 3 in C57BL/6 mice i.d. injected with (j) 20 μl of PBS or (k) 100,000 U of mIFN-γ in 20 μl of PBS for 2 d. The mIFN-γ-injected mice (j) exhibited widespread keratinocyte MHC II expression in a manner similar to K14-mOVA(6) mice after i.d. injection of OT-I cells (i) but not observed in control C57BL/6 mice (H).
A time-course experiment revealed that i.d. injection of 2 × 106 OT-I cells consistently resulted in vacuolar changes on day 3 with occasional apoptosis of keratinocytes (Fig 3D-G). By day 4, a complete GvHD reaction could be consistently identified (Fig 3G). Thus, the onset of GvHD occurred soon after the activation of LCs within the epidermis. The sequence and kinetics of events suggests that the maturation of LCs could provide the requisite co-stimulatory signals that induce early priming of OT-I cells that elicit rapid and severe local GvHD.
I.d. injection of OT-I cells induces expression of MHC II molecules by keratinocytes
As previously described for acute GvHD reactions in skin, we examined whether MHC II molecules were expressed on keratinocytes following i.d. injection of OT-I cells (Breathnach and Katz, 1983; Breathnach et al., 1986). After injection of OT-I cells, epidermal sheets stained for MHC II on day 4 showed widespread keratinocyte MHC II expression (Fig 3I). This was not yet obvious on day 2 (Fig 3B). It has been previously reported that systemic administration of IFN-γ to normal mice caused similar MHC II expression by keratinocytes (Gaspari and Katz, 1988). Whether the response of keratinocytes in the localized GvHD reaction is due to IFN-γ secretion by effector OT-I cells is uncertain. However, i.d. injection of 100,000 U of murine IFN-γ (mIFN-γ) into C57BL/6 mice daily for two days showed similar keratinocyte MHC II expression on day 3 (Fig 3K). These results indicate that development of GvHD reactions in skin coincided with keratinocyte MHC II expression, as reported in other mouse models of GvHD (Breathnach and Katz, 1983; Breathnach et al., 1986).
OT-I cells proliferate and become activated within the dermis of the K14-mOVA(6) Tg mouse ears
Upon maturation, LCs emigrate from the epidermis with the help of inflammatory cytokines such as tumor necrosis factor-α and interleukin-1β (Stoitzner et al., 1999; Cumberbatch et al., 1997). Given the activation of LCs on day 2, OT-I cells were extracted directly from the dermis of the K14-mOVA(6) Tg mouse ears on day 2 following i.d. injection. CFSE-labeled OT-I cells proliferated (Fig 4A-D) and the OT-I cells exhibited upregulation of the CD69 activation marker (Fig 4E). Interestingly, the draining LN of the injected K14-mOVA(6) Tg mouse ear did not contain significant numbers of CFSE+ OT-I cells on day 2 in contrast to the draining LN of the control C57BL/6 mouse ear (Fig 4C,D). Thus it appears that OT-I cells are activated locally, presumably by resident skin APC, rather than in draining lymph nodes. To additionally test this hypothesis, we utilized GFP+ OT-I cells. I.d. injection of 2 × 106 GFP+ OT-I cells into the K14-mOVA(6) mouse ears demonstrated that few GFP+ OT-I cells traveled to the draining LN in the first 72 h, while most appear on day 14 (Fig 4F). In contrast, GFP+ OT-I cells readily appeared in control C57BL/6 mice in the draining LN at 6, 24, and 72 h, and disappeared by day 14 in the absence of Ag stimulation. These results demonstrate that the GFP+ OT-I cells do not migrate to the draining LN in significant numbers in the K14-mOVA(6) Tg mice during the expected time period (∼48 h) required for T-cell priming in the LN (Mempel et al., 2004). This is likely due to the proliferation of OT-I cells as they directly encounter an activating signal within the skin, and not in the draining LN, and thereby acquire effector function(s).
Figure 4. OT-I cells proliferate, are activated and remain in the dermis of K14-mOVA(6) Tg mice on day 2 following i.d. injection.
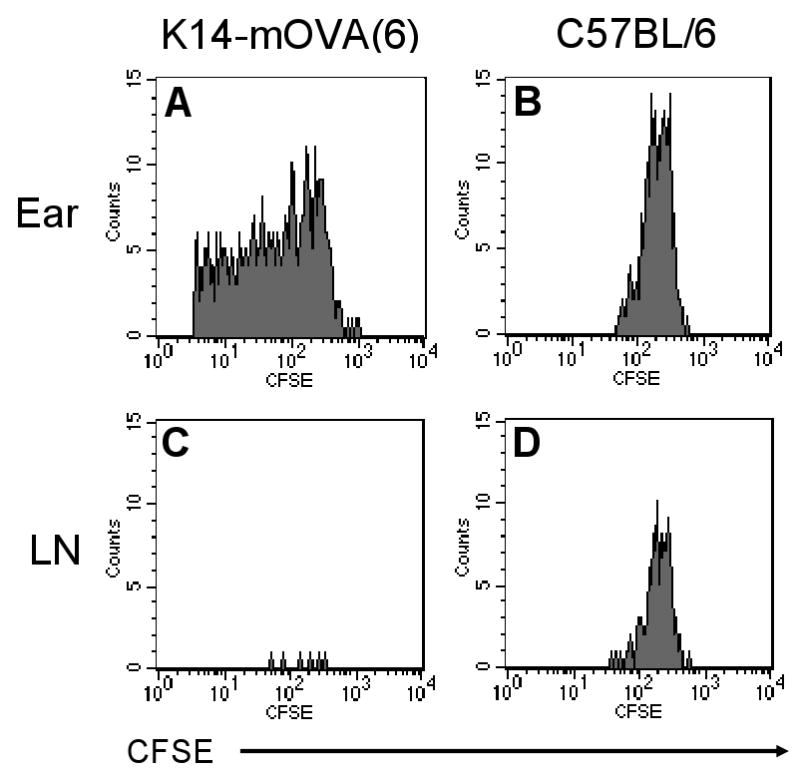
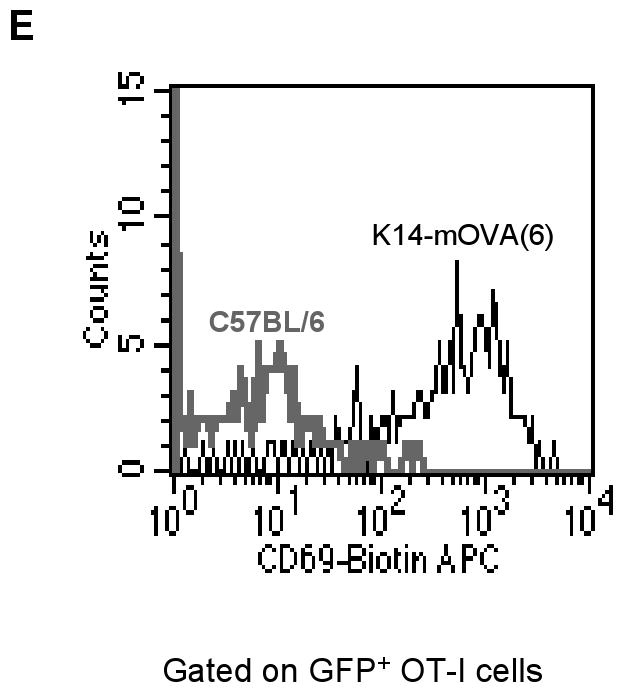
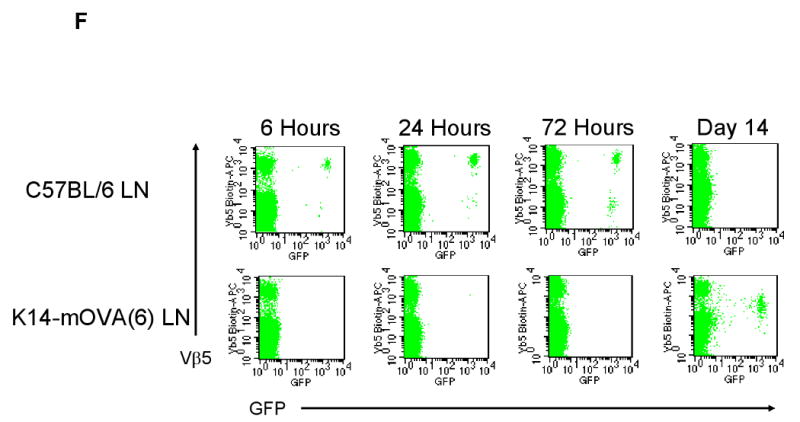
Naïve OT-I cells were labeled with CFSE dye and i.d. injected at a dose of 2 × 106 cells into the ears of K14-mOVA(6) Tg mice and control C57BL/6 mice. The dermal OT-I cells were extracted on day 2 for FACS analysis and proliferated in the (a) K14-mOVA(6) Tg mice but not in the (b) control C57BL/6 mice. The draining LNs were also harvested on day 2 for FACS analysis and the CFSE+ nonproliferating OT-I cells were readily detectable in the LN of (d) control C57BL/6 mice but few cells were present in LNs of (c) K14-mOVA(6) Tg mice. Cells were gated on CFSE+/CD8+ cells. GFP+ OT-I cells were also i.d. injected at a dose of 2 × 106 cells into K14-mOVA(6) Tg mice and control C57BL/6 mice, and the dermal OT-I cells were extracted on day 2 for FACS analysis. (e) The GFP+ OT-I cells in the K14-mOVA(6) Tg mice expressed enhanced amounts of CD69 on day 2 compared to those injected into C57BL/6 mice. (f) GFP+ OT-I cells were i.d. injected at a dose of 2 × 106 cells into K14-mOVA(6) Tg and control C57BL/6 mice and draining LN OT-I cells were harvested at 6, 24, and 72 h, and on day 14. Few GFP+ OT-I cells were detected in the LNs of K14-mOVA(6) Tg mice in contrast to control C57BL/6 mice at 6, 24, and 72 h. On day 14, GFP+ OT-I cells were readily detected in the LNs of K14-mOVA(6) Tg mice but not in control C57BL/6 mice.
Conditional ablation of epidermal LCs does not abrogate local GvHD in K14-mOVA(6) Tg mice
To begin to assess the contributions of resident skin APC, K14-mOVA(6) Tg mice were crossed with Langerin-DTR Tg mice that express diphtheria toxin receptor (DTR) under control of a Langerin promoter (12). K14-mOVA(6) × Langerin-DTR double Tg mice were injected i.p. with 500 ng of DT to ablate LCs in the epidermis after 48 h (Fig 5A,B), as previously reported (Bennett et al., 2005). OT-I cells were then i.d. injected 4 d after LC ablation, and treated ears were harvested for H&E staining, while untreated ears were stained with anti-MHC II antibody to verify complete ablation of LCs 5 d after OT-I injection (Fig 5C-E). On day 5 after i.d. injection of OT-I cells, LC-ablated K14-mOVA × Langerin-DTR Tg mice developed local GvHD similar to DT-treated normal K14-mOVA(6) Tg controls (Fig 5G,H). These results indicate that conditional ablation of LCs does not abrogate local GvHD reactions.
Figure 5. Local GvHD developed in DT-treated K14-mOVA(6) × Langerin-DTR double Tg mice following i.d. injection of OT-I cells.
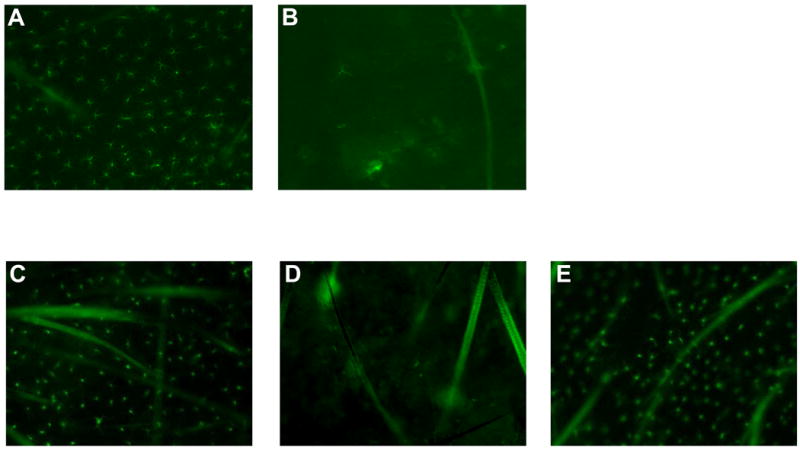
LC ablation was confirmed at 48 h after DT treatment in (b) K14-mOVA(6) × Langerin-DTR Tg mice but not in (a) control C57BL/6 mice by staining epidermal sheets for MHC II molecules. On day 4 after DT treatment, K14-mOVA(6) × Langerin-DTR Tg mice were i.d. injected with 2 × 106 OT-I cells and treated ears were harvested on day 5 after OT-I treatment. Untreated ears were harvested on the same day (day 9 after DT treatment) and LC ablation was confirmed in (d) K14-mOVA(6) × Langerin-DTR Tg mice but not in control (c) C57BL/6 and (e) K14-mOVA(6) Tg mice. H&E histology (400×) demonstrated local GvHD-like changes in DT-treated (g) K14-mOVA(6) × Langerin-DTR Tg mice on day 5 after i.d. injection of OT-I cells as in DT-treated normal (h) K14-mOVA(6) Tg mice but not in DT-treated (f) control C57BL/6 mice.
Impairment of dDCs and ablation of epidermal LCs does not abrogate local GvHD in K14-mOVA(6) Tg mice
LC populations in the epidermis are radioresistant, in contrast to other DC subsets in mice (Merad et al., 2002). Therefore, irradiation followed by transfer of CD45 congenic β2-microglobulin deficient (b2m) bone marrow cells results in hematopoietic chimeras with recipient-derived LCs and donor-derived DCs in the LNs, spleen, and dermis. Because b2m cells do not express MHC I molecules and therefore cannot present OVA peptide to OT-I cells, antigen presentation by dermal and lymphoid APC are impaired (Koller et al., 1990). To determine the contribution of dDCs to the local GvHD reaction, hematopoietic chimeras of the K14-mOVA(6) Tg mice were established. After reconstitution with b2m bone marrow cells, mice were assessed for chimerization by FACS analysis of epidermal, dermal, LN, and spleen suspensions. The vast majority of the LCs from epidermal cell suspensions were recipient-derived (CD45.2) (Fig 6A), while >90% of MHC IIhigh cells from dermal cell suspensions (Fig 6B) and >98% of MHC IIhigh cells in the LNs and spleen were donor-derived (CD45.1) (Fig 6C,D). Though not entirely depleted (approximately 10% of the dermal MHC IIhigh cells remained recipient-derived as previously reported), the vast majority were replaced by donor-derived b2m cells (Bogunovic et al., 2006) I.d. injection of OT-I cells into K14-mOVA(6) Tg mice whose OVA-presenting capacity was limited to LCs (b2m→K14-mOVA(6)) resulted in a local GvHD reaction (Fig 7D) similar to that seen in K14-mOVA(6) Tg mice chimerized with wild-type littermate (B6.SJL) bone marrow cells (BJ.SJL→K14-mOVA(6)) (Fig 7B). Therefore b2m chimeras of K14-mOVA(6) × Langerin-DTR Tg mice were generated to eliminate antigen-presentation by all skin DCs. Surprisingly, the K14-mOVA(6) × Langerin-DTR Tg mice that had been chimerized with b2m bone marrow and then treated with DT (LC-depleted) also developed local GvHD after OT-I injection (Fig 7C). These results indicate that skin DCs (LCs and dDCs) are not critical for in the induction of local GvHD in the K14-mOVA(6) Tg mice.
Figure 6. K14-mOVA(6) Tg mice were reconstituted with b2m (CD45.1+) MHC IIhigh cells in the dermis, LN, and spleen but not in the epidermis.
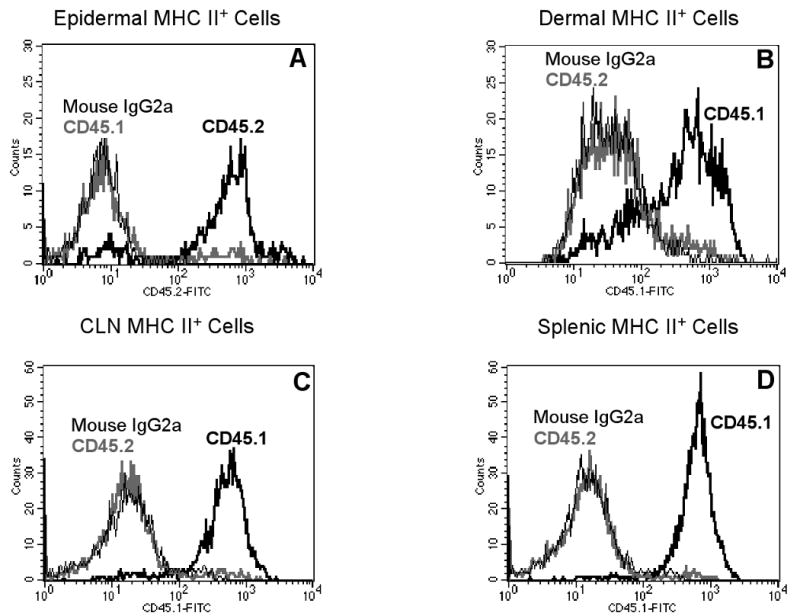
(a) MHC IIhigh cells in the epidermis of b2m→K14-mOVA(6) Tg bone marrow chimeras remained recipient-derived (CD45.2+) 8 weeks after transplantation. MHC IIhigh cells in the (b) dermis, (c) CLN, and (d) spleen were mostly donor-derived (CD45.1+) 8 weeks after transplantation.
Figure 7. GvHD developed in b2m→K14-mOVA(6) Tg and b2m→K14-mOVA(6) × Langerin-DTR Tg bone marrow chimeras following both i.d. injection of OT-I cells and treatment with DT.
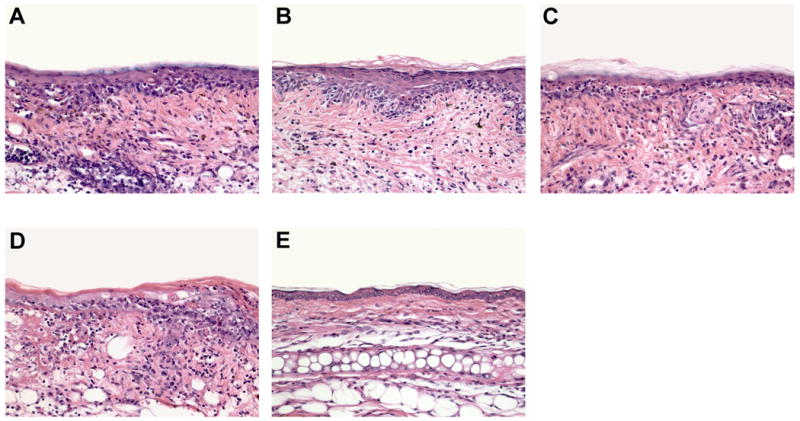
H&E histology (200×) of the ears from (a) B6.SJL→K14-mOVA(6) × Langerin-DTR Tg, (b) b2m→K14-mOVA(6) × Langerin-DTR Tg, (c) B6.SJL→K14-mOVA(6) Tg, (d) b2m→K14-mOVA(6) Tg, and (e) b2m→C57BL/6 X-irradiated, bone marrow-reconstituted chimeras after i.d. injection of 2 × 106 OT-I cells. Skin biopsies from all chimeras (a-d) except control b2m→C57BL/6 chimeras (e) demonstrated GvHD-like skin lesions.
Depletion of Langerhans cells from K14-mOVA(6) × Langerin-DTR Tg epidermal cells abrogates the induction of a mixed epidermal lymphocyte reaction (MELR) in the presence of OT-I cell stimulation
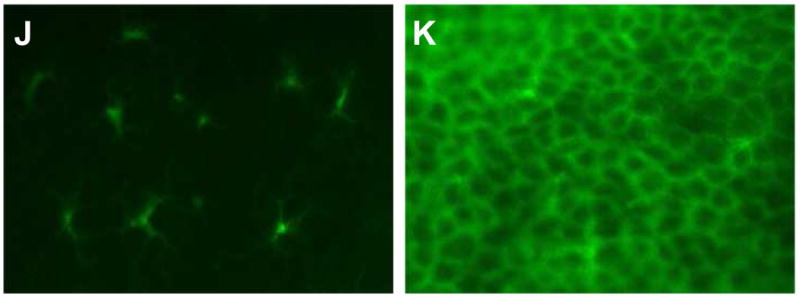
Given the unexpected result that the functional elimination of all skin DCs did not abrogate the GvHD reaction in vivo, activation of OT-I cells was further investigated in vitro. K14-mOVA(6) × Langerin-DTR Tg mice and littermate K14-mOVA(6) Tg mice were treated with 500 ng of DT i.p. After 48 h, epidermal cell suspensions were prepared and LC-depletion was verified. DT treatment resulted in >50-fold reduction in the percentage of MHC II+ LCs recovered from the epidermal cell suspensions after enrichment by gradient centrifugation (Fig 8J,K).
Figure 8. Langerhans cell-depleted K14-mOVA(6) × Langerin-DTR Tg epidermal cells induced proliferation, acquisition of effector function, and activation of OT-I cells.
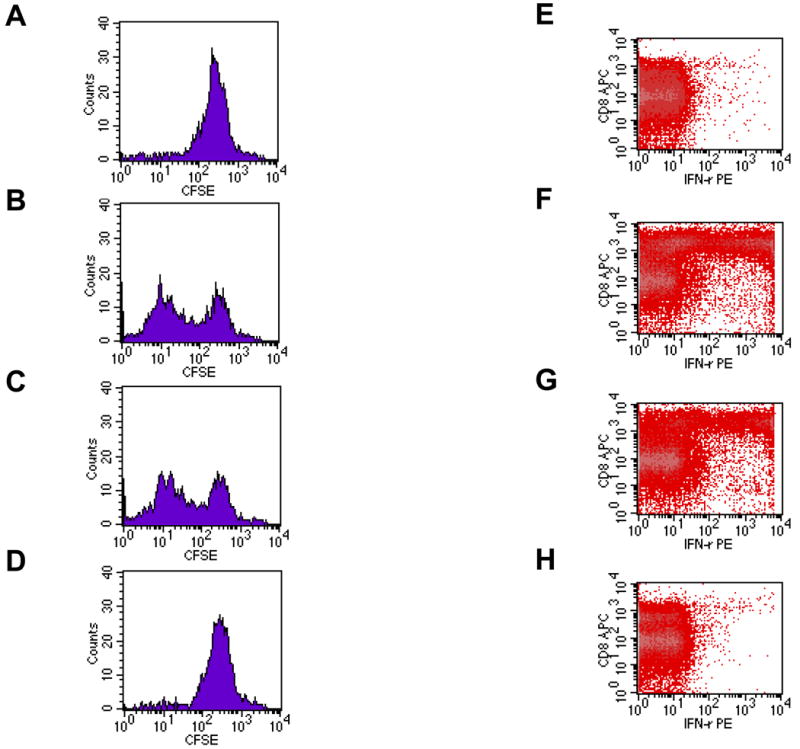
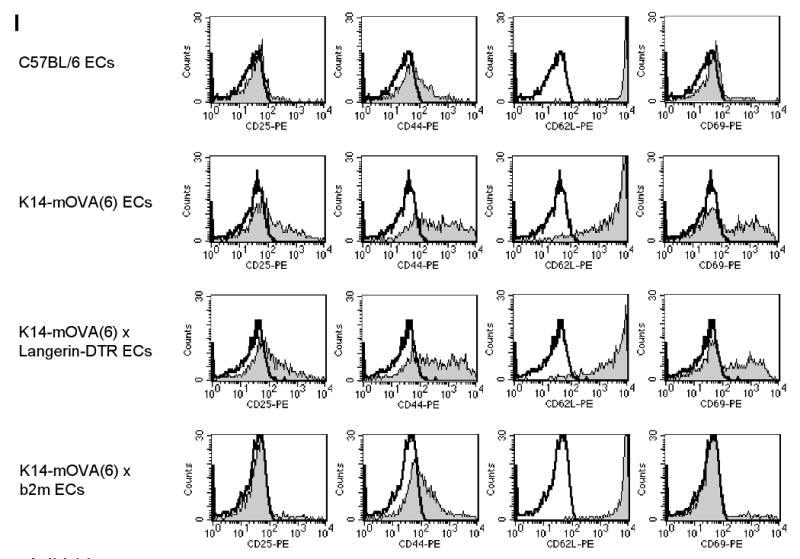
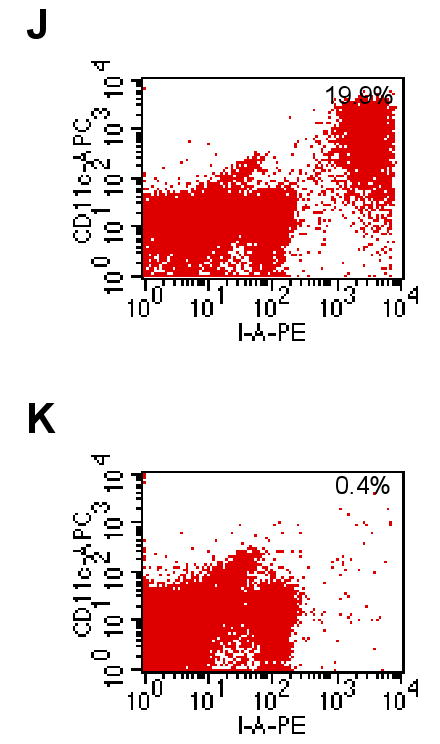
Day 3 of CFSE-labeled OT-I cells cultured with crude epidermal cells from DT-treated (a) C57BL/6, (b) littermate K14-mOVA(6) Tg, (c) K14-mOVA(6) × Langerin-DTR Tg, and (d) K14-mOVA(6) × b2m Tg mice. Day 3 intracellular IFN-γ staining of OT-I cells cultured with crude epidermal cells from DT-treated (e) C57BL/6, (f) littermate K14-mOVA(6) Tg, (g) K14-mOVA(6) × Langerin-DTR Tg, and (h) K14-mOVA(6) × b2m Tg mice. (i) Day 3 staining of OT-I cells cultured with crude epidermal cells from DT-treated C57BL/6, littermate K14-mOVA(6) Tg, K14-mOVA(6) × Langerin-DTR Tg, and K14-mOVA(6) × b2m Tg mice for CD25, CD44, CD62L, and CD69 activation markers. Cells were gated on Vα2+/Vβ5+ (OT-I) cells. Lympholyte-M gradient centrifuge-enriched epidermal cells from (j) K14-mOVA(6) × Langerin-DTR Tg and (k) littermate K14-mOvA(6) Tg mice at 48 h after treatment with DT.
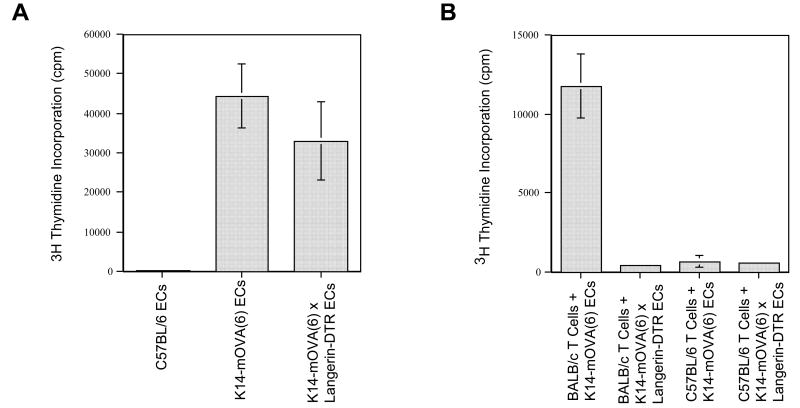
When either K14-mOVA(6) Tg or K14-mOVA(6) × Langerin-DTR Tg epidermal cell suspensions, not enriched for LCs, were cultured with CFSE-labeled OT-I cells, the OT-I cells proliferated (Fig 8B,C). However, proliferation was not observed when OT-I cells were cultured with either control C57BL/6 or K14-mOVA(6) × b2m Tg epidermal cells (Fig 8A,D). The latter control was used to rule out the possibility that contaminating DCs in the OT-I cell suspension could be responsible for cross-presentation of OVA antigen directly to the OT-I cells. Furthermore, both K14-mOVA(6) Tg and K14-mOVA(6) × Langerin-DTR Tg epidermal cells (Fig 8F,G) induced interferon-γ (IFN-γ) production by the OT-I cells and upregulation of CD25, CD44, and CD69 activation markers in contrast to that induced by control C57BL/6 and K14-mOVA(6) × b2m Tg epidermal cells (Fig 8E,H-I). However, these same LC-depleted K14-mOVA(6) × Langerin-DTR Tg epidermal cells that induced OT-I proliferation (Fig 9A) did not induce a MELR when cultured with allogeneic BALB/c T cells (Fig 9B). These results indicate that OVA-expressing keratinocyte suspensions, in the absence of skin DCs, can directly prime OT-I cells.
Figure 9. Diphtheria toxin-mediated depletion of Langerhans cells from K14-mOVA(6) × Langerin-DTR Tg epidermal cells simultaneously abrogated allogeneic mixed epidermal lymphocyte reactions while inducing OT-I cell proliferation.
(a) Day 3 of [3H]thymidine proliferation assay of OT-I cells cultured with crude epidermal cells from DT-treated C57BL/6, littermate K14-mOVA(6), and K14-mOVA(6) × Langerin-DTR Tg mice. (b) Day 5 of [3H]thymidine proliferation assay of allogeneic BALB/c and syngeneic C57BL/6 T cells cultured with crude epidermal cells from DT-treated littermate K14-mOVA(6) and K14-mOVA(6) × Langerin-DTR Tg mice. All measurements were performed in triplicates. Standard error bars are included.
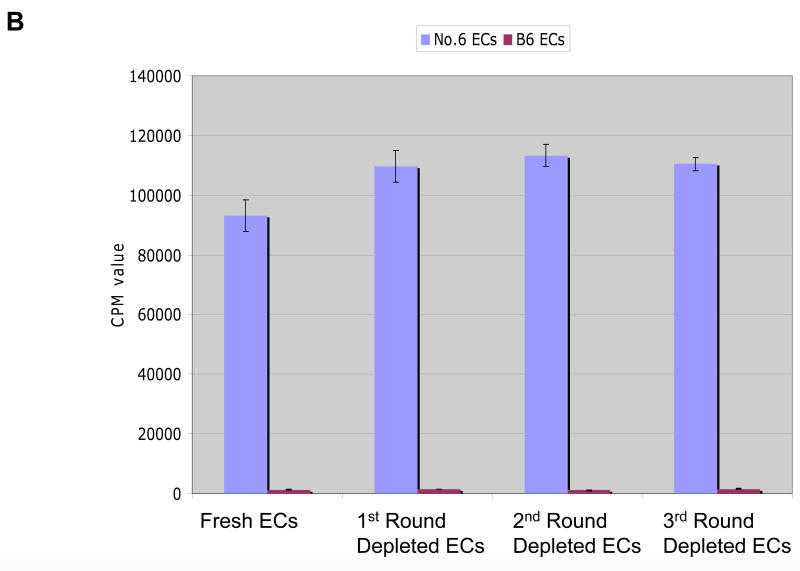
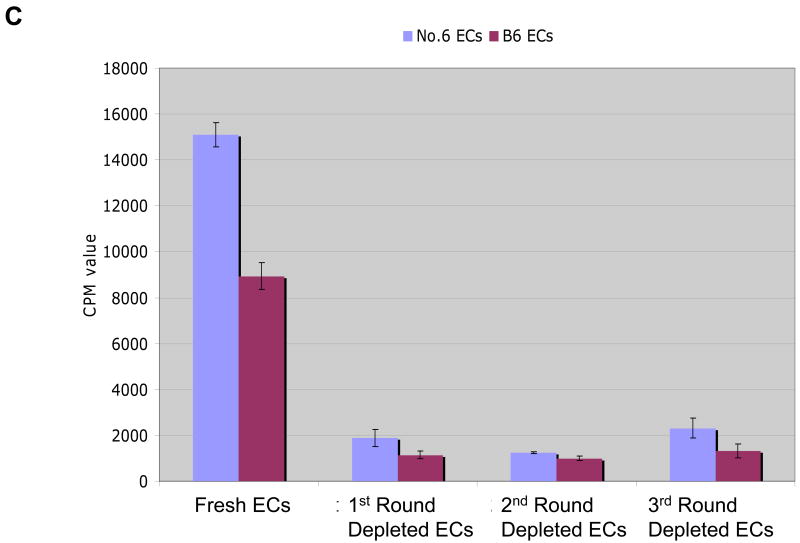
We used two other methods to verify that skin DCs were dispensable for antigen-presentation. The first method involved harvesting keratinocytes from neonatal K14-mOVA(6) Tg mouse epidermis and eliminating the vast majority of LCs by repeated depletion using an AutoMACS separator after incubating with MHC II microbeads. Using this method, the percentage of LCs in the epidermal cell suspensions was reduced to approximately 0.001% following three rounds of depletion via the AutoMACS separator (Fig 10A). Despite this marked depletion of MHC II+ cells from the neonatal K14-mOVA(6) Tg epidermal cell suspensions, OT-I cell proliferation ensued when incubated with these cells (Fig 10B). As demonstrated with the K14-mOVA(6) × Langerin-DTR depleted of LCs using diphtheria toxin, the LC-depleted neonatal K14-mOVA(6) Tg epidermal cells also did not elicit a MELR when cultured with allogeneic BALB/c T cells after depletion through the AutoMACS separator (Fig 10C).
Figure 10. Depletion of Langerhans cells using MHC II microbeads from neonatal K14-mOVA(6) epidermal cells simultaneously abrogated allogeneic mixed epidermal lymphocyte reactions while inducing OT-I cell proliferation.
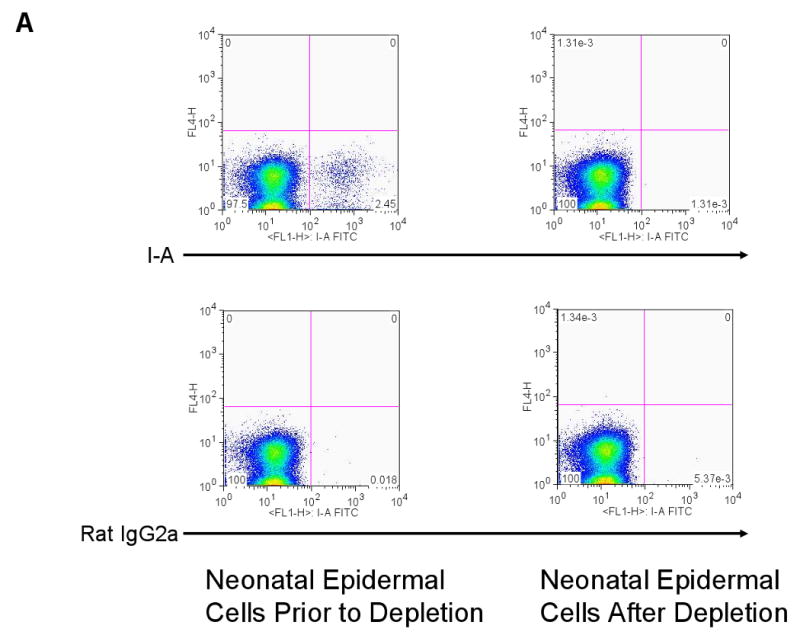
(a) Depletion of LCs using MHC II microbeads resulted in the reduction of the percentage of LCs in the neonatal K14-mOVA(6) Tg epidermal cell suspensions from 2.45% to 0.001% of the total population. (b) Neonatal K14-mOVA(6) Tg epidermal cell suspensions after LC-depletion via the AutoMACS separator resulted in the same level of OT-I cell proliferation as in non-depleted control epidermal cell suspensions. Negative control neonatal C57BL/6 epidermal cell suspensions did not induce OT-I cell proliferation. (c) Both neonatal K14-mOVA(6) and C57BL/6 epidermal cells did not induce allogeneic BALB/c T cell proliferation after depletion of LCs using MHC II microbeads.
The second method involved culturing primary neonatal keratinocytes from the K14-mOVA(6) mice in low calcium medium and selectively removing the supernatant to reduce the number of LCs in the suspension. This method allows for the proliferating keratinocytes to remain adherent to the Petri dish for retrieval while the LCs in suspension are eliminated. By this method, the percentage of LCs in the epidermal cell suspensions was reduced to approximately 0.26% following 3 days of culture (data not shown). Whether the initial keratinocyte suspension placed into culture was non-depleted (crude) or depleted of LCs using the AutoMACS separator, the reduction in the number of LCs was sufficient to abrogate a MELR when cultured with allogeneic BALB/c T cells in the presence of OT-I cell stimulation (data not shown). Furthermore, these keratinocyte suspensions induced an activated cell-surface phenotype on the OT-I cells with upregulation of CD25, CD44, and CD69, and downregulation of CD62L (data not shown). These additional results, using different methods, further support the conclusion that keratinocytes can directly present OVA peptide and induce an OT-I cell response.
Discussion
In this localized GvHD model, autoreactive T cells (OT-I cells) elicit skin GvHD reactions after i.d. injection of OVA-reactive CD8 cells (OT-I) into transgenic mice that express OVA in epidermal keratinocytes. We used this model to investigate the in vivo and in vitro contribution of skin DCs (LCs and dDCs) to present epidermal Ag by conditional ablation of epidermal LCs and impairment of MHC I presentation on dDCs. Unexpectedly, the elimination of LCs and the impairment of dDCs did not abrogate localized GvHD reactions. Additionally, using two different methods of cell preparation, a >99% reduction in the number of LCs in vitro completely abrogated the induction of MELRs without attenuating priming of OT-I cells by the same OVA-expressing epidermal cells. These results strongly suggest that keratinocytes expressing endogenous tissue antigens (OVA) can directly prime naïve T cells (OT-I cells).
LCs have traditionally been considered the primary APCs involved in the initiation of T cell responses in the skin (Silberberg-Sinakin and Thorbecke, 1980; Silberberg-Sinakin et al., 1976; Macatonia et al., 1987). Previous studies have implicated LCs as critical mediators of allogeneic GvHD and CD8+ T cell responses to epidermal self-Ag (Mayerova et al., 2004; Merad et al., 2004). More complex models using murine Herpes Simplex Virus (HSV) and Leishmania have suggested that LCs do not play a significant role in Ag presentation (Allan et al., 2003; Zhao et al., 2003; Ritter et al., 2004). More recently, classical CHS experiments using LC-deficient mice have demonstrated that LCs are dispensable for the elicitation of CHS responses (Kissenpfennig et al., 2005; Bennett et al., 2005; Kaplan et al., 2005). Collectively, these studies have supported the notion that dDCs are the critical mediators of T cell responses in the skin. It has also been reported that under inflammatory conditions, such as CHS reactions, Gr1+ circulating DCs may be responsible for the crosspriming of CD8+ T cells in a CCR6-dependent manner in the epidermis (Le Borgne et al., 2006). As such, the precise contribution of DCs (or other cells) to immune responses in the skin remains unclear. A recent study demonstrated that NK cells mediate CHS responses independent of T and B cells in the skin (O'Leary et al., 2006). It has also been reported that other cell populations in the epidermis such as keratinocytes under the influence of IFN-γ can directly stimulate T cells with a variety of stimuli including HSV antigens and superantigens (Cunningham and Noble, 1989; Nickoloff et al., 1993). However, these studies involved a mixed population of responder CD4+ and CD8+ T cells and the authors postulated that the immune response was initiated by the priming of CD4+ T cells. It is more commonly believed that due to the absence of costimulatory molecules the presentation of Ag by keratinocytes, particularly in the steady-state, induces CD4+ T cell anergy rather than priming (Bal et al., 1990; Gaspari et al., 1988). In this study we sought to investigate these questions using an in vivo model with a defined self-Ag (OVA) expressed in the epidermis and using a specified monoclonal population of CD8+ T cells (OT-I cells).
To examine the role of LCs in cross-presentation of epidermal OVA we crossed the K14-mOVA(6) Tg mice onto a Langerin-DTR Tg background (Bennett et al., 2005). Interestingly, following ablation of LCs with DT the K14-mOVA(6) × Langerin-DTR Tg mice still developed local GvHD with i.d. injection of OT-I cells. These results are consistent with the lack of abrogation of CHS observed in previous studies using DT-based technology to ablate LCs (Kissenpfennig et al., 2005; Bennett et al., 2005; Kaplan et al., 2005). However, these studies did not examine the effects of impairing dDCs in CHS.
To identify the role of dDCs in the cross-presentation of epidermal OVA we generated bone marrow chimeras that have MHC I-deficient (b2m) dDCs in the K14-mOVA(6) mice. This was done because of the striking systemic GvHD phenotype we previously reported in this particular strain (Shibaki et al., 2004). Lethally irradiated mice reconstituted with donor b2m bone marrow retain recipient LCs while allowing for the impairment of MHC I-dependent Ag presentation by dDCs (Merad et al., 2002). Despite the impairment of cross-presentation by MHC I molecules in the dermis, LN, and the spleen, these mice developed local GvHD following i.d. injection of OT-I cells. Surprisingly, even when K14-mOVA(6) × Langerin-DTR Tg mice were chimerized with b2m bone marrow and depleted of LCs with DT, local GvHD ensued after OT-I injection. In contrast to our results, Azukizawa et al. reported complete abrogation of OT-I cell proliferation in vivo via similar reconstitution studies in their K5-mOVA Tg mice (Azukizawa et al., 2003). This would suggest that LCs are not the primary APCs involved in OT-I cell responses in their model but that either dermal or other lymphoid-resident DCs may subserve this role. However, in our model, neither epidermal LCs nor dDCs appear to be absolutely critical for the induction of local GvHD.
To better assess the mechanism by which GvHD occurred in vivo despite the functional elimination of skin DCs, epidermal cells from LC-depleted K14-mOVA(6) × Langerin-DTR Tg mice were cultured with OT-I cells in vitro. Interestingly, despite the drastic reduction of LCs (>50-fold) in the epidermal cells, OT-I cells were still stimulated (proliferated, activated, and acquired effector function) to a similar degree as when mixed with littermate control K14-mOVA(6) Tg epidermal cells. Furthermore the LC-depleted epidermal cells could not induce a mixed epidermal lymphocyte reaction, suggesting that the reduction in LCs was sufficient to abrogate other modes of physiologically relevant APC-dependent responses. These striking in vitro results corroborate the in vivo findings that despite the impairment or elimination of almost all skin DCs, GvHD ensues, due to the underlying accessory cell function of keratinocytes. These results are further reinforced by a recent study showing that LCs in the skin are not required for skin graft rejection in mice mismatched for major histocompatability antigens. In fact, it appears that the lack of LCs may even enhance graft rejection in mice mismatched for minor histocompability antigens (Kaplan et al., 2008).
Accessory cell function of keratinocytes has recently been addressed specifically with regard to MHC I-specific presentation to a population of CD8+ T cells. In contrast to the findings reported in our study, Stoitzner et al. demonstrated that LCs rather than keratinocytes present endogenous epidermal OVA to OT-I cells using a similar K14-OVA Tg mouse model (Stoitzner et al., 2006). Indeed, we also found that purified LC can induce CD8 T cell (OT-I) proliferation. In their study Stoitzner et al. used three-day cultured cells that were sorted according to the presence or absence of MHC class II positivity. The MHC class II- cells (keratinocytes) from their K14-OVA peptide Tg mice did not induce T cell proliferation. However, data for this experiment was “not shown” so no comparison can be made between their experimental procedures as well as data and the current study. Furthermore, the data to which they refer relate only to the K14-OVA SIINFEKL peptide mice that differ from our Tg mice that are Tg for a large segment of the OVA protein (9). In our study we report that keratinocytes directly present self-Ag to CD8+ T cells to induce priming. This was demonstrated using two markedly different systems – diphtheria toxin-mediated depletion of DTR-expressing LCs and depletion of LCs from neonatal keratinocyte suspensions using MHC II microbeads. Whether this process is dependent on costimulatory molecules or is simply due to the high density of self-peptide on MHC I complexes remains to be elucidated. Furthermore, these findings do not preclude the role of LCs or dDCs in these reactions under normal physiologic conditions.
Keratinocytes have been reported to directly activate autoreactive CD4 T cells in the context of tissue inflammation (Fan et al., 2003). In this study, Fan et al. propose that an inflammatory stimulus mobilizes IL-1 and/or TNF-α to induce the upregulation of MHC molecules on the surface of keratinocytes to allow them to act as primary APCs. It is possible that the inflammation associated with i.d. injection creates a similar context in which keratinocytes can act as APCs to present OVA antigen directly to OT-I cells. Nonetheless, our studies demonstrate, for the first time, that naïve autoreactive CD8 T cells can be directly primed by keratinocytes in an antigen-specific manner.
There remains the possibility that very small numbers of dermal DCs are sufficient to induce the priming of OT-I cells even in the absence of detectable allostimulation. However, given the fact that T cells respond in a dose-dependent manner to the number of stimulator DCs, if such were the case, we would still expect a drastic reduction in the OT-I cell response after depletion of LCs and this is not observed. We previously reported that approximately 1,000 migratory LCs from skin explants of K14-mOVA(6) Tg mouse ears were required to induce a minimal detectable level of OT-I cell proliferation in vitro and only at 10,000 stimulator LCs was a significant level of proliferation observed (Shibaki et al., 2004). A generous approximation would suggest that the LC-depleted epidermal cell suspensions contain ∼100 remaining stimulator LCs after DT treatment in contrast to ∼5,000 stimulator LCs in the undepleted epidermal cell suspensions. For the neonatal epidermal cell suspension, the number of remaining stimulator LCs would be even lower on the order of ∼1-2 cells after depletion with MHC II microbeads and the AutoMACS separator. Thus it seems highly unlikely that such small numbers of OVA-bearing LCs could induce such robust priming of naïve OT-I cells.
In summary, we have developed an efficient i.d. injection model whereby a local GvHD reaction can be elicited in multiple strains of K14-OVA Tg mice that express both soluble and membrane-bound forms of OVA at different levels of expression. This model provides a novel approach to study the mechanisms underlying presentation of epidermally-expressed self-Ag by specific populations of skin-resident cells. We have demonstrated both in vivo and in vitro, by the elimination of skin DCs, that keratinocytes can function as accessory cells even in the absence of functional APCs. In addition to gaining mechanistic insights, we believe that this is an effective method by which to test potential therapies, both topical and systemic, that modulate cell-mediated skin diseases.
Materials and Methods
Mice
All K14-OVA Tg mouse lines were obtained from the National Cancer Institute Animal Production Program (Frederick, Maryland), housed in a clean (not pathogen-free) facility that does not allow MHV or pinworms, and were bred and used in accordance with institutional guidelines. OT-I × RAG-1-deficient mice were purchased from Taconic (Germantown, New York) and UBI-GFP/BL6 mice were purchased from The Jackson Laboratory (Bar Habor, Maine).
Generation of Tg mice
The generation of the K14-mOVA(6) Tg mouse expressing membrane-associated OVA under a K14 promoter was previously described (Shibaki et al., 2004). The K14-mOVA(3) Tg mouse was generated in the same manner. The Tg mice (K14-sOVA(5), K14-sOVA(15), and K14-sOVA(17 Tg) expressing soluble OVA (sOVA) were prepared by excising the OVA fragment (100-1167) from the TC-OVA plasmid (Lim et al., 1998) with BgIII and BamHI. The vector sequence encoding β galactosidase was excised from a K14-βgal plasmid (Shibaki et al., 2004) with NotI and subsequently replaced with the OVA fragment by blunt end ligation with T4 polymerase to make the pK14-sOVA plasmid. The sOVA plasmids do not contain the PDGFR, HA, or Myc sequences. All subsequent steps were the same as for the generation of the K14-mOVA Tg mouse lines.
Quantitative real-time RT-PCR
Total RNA was extracted from all strains of K14-OVA Tg mice using the RNeasy Mini Kit (QIAGEN) and reverse transcribed with the StrataScript First-Strand Synthesis System (Stratagene). The resulting cDNAs were used for real-time PCR using SYBR-Green PCR Master Mix (Applied Biosystems) in triplicates (OVA-1, 5′-GGCATCAATGGCTTCTGAGAA-3′; OVA-2, 5′-CCAACATGCTCATTGTCCCA-3′). All quantitations were normalized to endogenous control β-actin (β-actin-1, 5′-TGACAGGATGCAGAAGGAGA-3′; β-actin-2, 5′-GTACTTGCGCTCAGGAGGAG-3′). PCR and data collection were performed on ABIS PRISM 7700 Sequence Detector (Perkin Elmer).
I.d. injection of OT-I cells
Single cell suspensions were prepared from LNs of OT-I × RAG-1-deficient or F1 (GFP+ × OT-I) mice, further purified for CD8+ cells using CD8 columns (R&D systems), and resuspended in RPMI with 5% FCS. Prior to injection, 2 × 106 OT-I cells were resuspended in 20 μl of PBS and injected intradermally into the left ears of K14-OVA Tg, K14-OVA × Langerin-DTR Tg, control C57BL/6, or x-irradiated bone marrow-reconstituted chimeric mice. The mice were anesthetized by i.p. injection of 50 μg xylazine (Phoenix Pharmaceuticals) and 2.5 mg of ketamine (Fort Dodge) in 250 μl of PBS just prior to i.d. injection.
Preparation of epidermal sheets and fluorescent microscopy
Epidermal sheets were prepared as previously described (Caughman et al., 1986) and stained with mouse-anti-I-Ab followed by secondary fluorescein-conjugated IgG Fab'2 fragments of goat-anti-mouse IgG (Biosource). Images were captured using a Nikon Eclipse TE300 camera and IPLab software. I.d. injection of mIFN-γ (Peprotech) was performed at a dose of 100,000 U in 20 μl of PBS once a day for 2 d and epidermal sheets were prepared on day 3.
Histological analyses
Ear samples were prepared from K14-OVA Tg mice at various times after i.d. injection of OT-I cells and fixed in 10% neutral-buffered formalin. Paraffin embedded tissues were sectioned and stained with hematoxylin and eosin using standard techniques (American Histolabs, Rockville, Maryland).
Flow cytometry
For monitoring T cell proliferation, 2 × 106 CFSE-labeled OT-I cells were i.d. injected into the left ears of recipient K14-OVA Tg mice. On day 2 after i.d. injection, CFSE-labeled OT-I cells were harvested from the left cervical lymph nodes and from the dermis (see below) and stained with APC-conjugated anti-CD8 monoclonal antibodies for FACS analysis. For monitoring of T cell migration, 2 × 106 OT-I cells isolated from F1 (GFP+ × OT-I) mice were i.d. injected into the left ears of recipient K14-OVA Tg mice. At 6 h, 12 h, 24 h, 48 h, 72 h, and 14 d, the left cervical lymph nodes were harvested and stained with biotin-conjugated anti-Vβ5 monoclonal antibodies. APC-conjugated streptavidin was used for visualization of the biotin-conjugated monoclonal antibody. For staining of activation markers PE-conjugated and APC-conjugated anti-CD25, -CD44, -CD62L, -CD69, and isotype control antibodies were used. Experiments were done on a FACSCalibur and analyzed using CellQuest software (Becton Dickinson).
Dermal extraction of OT-I cells
OT-I cells were extracted from the dermis by mechanically splitting the dorsal and ventral halves of mouse ears on day 2 after i.d. injection. The split ears were than floated on a solution of 0.50 mg/ml Liberase CI (Roche Applied Science) in RPMI for 1 h at 37°C and the dermis was mechanically scraped into the Liberase CI suspension. The suspension was diluted in RPMI with 5% FCS and 0.05% DNase and then filtered through a 70 μm filter. The recovered cells were then washed once in PBS with 5% FCS and stained with antibodies for FACS analysis.
Generation of x-irradiated bone marrow-reconstituted chimeras
Seven- to eight-week-old recipient CD45.2+ C57BL/6, K14-mOVA(6), or K14-mOVA(6) × Langerin-DTR mice were lethally irradiated (9 Gy) and reconstituted by i.v. injection of 1 × 107 bone marrow cells from CD45.1+ B6.SJL or b2m mice. Eights weeks after transplantation, ears, cutaneous lymph nodes (CLNs), and spleens were harvested. Ears were split into dorsal and ventral halves and incubated in 0.5% trypsin and EDTA (5mM) in PBS (Gibco) for 60-90 min at 37°C to allow for separation of epidermal and dermal sheets. Epidermal sheets were made into a single cell suspension and isolated by Lympholyte M (Cedarlane) gradient. Dermal sheets were further incubated in 0.5 mg/ml Collagenase IA (Sigma) and 0.05% DNase (Sigma) and made into a single cell suspension. LNs and spleens were made into single cell suspensions. All cells were stained with various combinations of the following antibodies (BD Pharmingen): FITC-conjugated anti-CD45.1, CD45.2, and isotype mouse IgG2a; PE-conjugated or APC-conjugated anti-I-A/I-E.
Depletion of Langerhans cells from K14-mOVA(6) × Langerin-DTR Tg mice
Diphtheria toxin (DT) (BIOMOL) was reconstituted with sterile distilled water at a concentration of 2.5 ng/μl and injected at a dose of 500 ng i.p. Depletion of LCs was verified by immunofluorescence staining of epidermal sheets as described above.
Depletion of Langerhans cells from neonatal K14-mOVA(6) Tg mice
Primary keratinocytes were isolated from neonatal (day 1 to 3) K14-mOVA(6) Tg and C57BL/6 whole mouse skin as previously described (37). The neonatal keratinocyte suspensions were then incubated with anti-MHC II microbeads (clone M5/114.15.2) at 4°C for 15 min and then run through an AutoMACS separator (mode: DEPLETES) (Miltenyi Biotech). The neonatal keratinocyte suspensions were re-incubated with anti-MHC II microbeads (clone M5/114.15.2) at 4°C for 15 min between runs and serially depleted through two more rounds via the AutoMACS separator. For verification and quantification of depletion of MHC II+ cells, the cell suspensions were stained with a FITC-conjugated anti-I-A/I-E (clone 2G9) (BD Pharmingen) that binds a different epitope of MHC II than the MHC II microbeads (clone M5/114.15.2).
Primary keratinocytes from neonatal (day 1 to 3) K14-mOVA(6) Tg and C57BL/6 mice were cultured in Eagle's minimal essential medium supplemented with 0.05 mM calcium chloride and 10% Chelex (Sigma)-treated fetal bovine serum (Hyclone) in flat-bottom 96-well plates (1 × 105 cells/well) for 3 days as previously published (37). The supernatants were removed and the adherent keratinocytes were harvested by trypsinization and mixed with OT-I and BALB/c T cells for proliferation assays. The OT-I and BALB/c T cell suspensions were also depleted of MHC II+ cells using MHC II microbeads and the AutoMACS separator and quantified by FACS analysis.
In vitro proliferation assays
Adult epidermal cells were prepared from littermate K14-mOVA(6) Tg, K14-mOVA(6) × Langerin-DTR Tg, K14-mOVA(6) × b2m Tg, and C57BL/6 mice as previously described (Sauder et al., 1982). Neonatal epidermal cells were prepared from K14-mOVA(6) Tg and C57BL/6 mice as previously described (37). 1 × 105 crude epidermal cells were added per well to a 96-well flat-bottom plate along with 5 × 104 CFSE-labeled OT-I cells. On day 3 the cells were harvested and stained with APC-conjugated anti-CD8 antibody and analyzed by FACS for proliferation. The same conditions were used to stain for activation markers of OT-I cells using the antibodies described above. Alternatively, 1 × 105 crude epidermal cells were added per well to a 96-well flat-bottom plate along with 5 × 104 unlabeled OT-I cells. During the last 18 h of the culture period 1 μCi of [3H]TdR was added. On day 3 cells were harvested and cell-associated radioactivity was counted by direct β counting using a gas ionization counter (Packard). For the mixed epidermal lymphocyte reactions 4 × 105 crude epidermal cells were added per well to a 96-well flat-bottom plate along with 2 × 105 allogeneic BALB/c T cells or syngeneic C57BL/6 T cells isolated by a mouse T cell enrichment column (R&D Systems). During the last 18 h of the culture period 1 μCi of [3H]TdR was added. On day 5 cells were harvested and cell-associated radioactivity was counted by direct β counting using a gas ionization counter (Packard). All cell cultures were performed in complete RPMI media containing 1 μg/ml indomethacin (Sigma) at 37°C under 5% CO2. To verify ablation, epidermal cell suspensions were enriched by centrifugation over a Lympholyte-M (Cedarlane) gradient.
Intracellular cytokine staining
1 × 106 OT-I cells were cultured along with 2 × 106 epidermal cells from K14-mOVA(6) Tg littermate, K14-mOVA(6) × Langerin-DTR Tg, K14-mOVA × b2m Tg, and C57BL/6 mice on 24-well flat-bottom plates in complete RPMI containing 1 μg/ml indomethacin (Sigma) at 37°C under 5% CO2. After 3 d cells were cultured on anti-CD3 antibody-coated (BD Pharmingen) 24-well flat-bottom plates and Golgiplug (Pharmingen) was added at a concentration of 5 μg/ml. After 5 h cells were harvested and stained with APC-conjugated anti-CD8 antibody (Pharmingen) and fixed and permeabilized by incubation with cytofix/cytoperm solution (Pharmingen). The permeabilized cells were then stained with PE-conjugated anti-IFN-γ antibody (BD Pharmingen) and analyzed by FACS for IFN-γ production.
Acknowledgments
The authors thank Mr. Jay Linton for his excellent technical expertise, Dr. Xu Feng and Dr. Takashi Kakinuma for helpful suggestions, and Dr. Mark Udey for critical discussions and review of the manuscript. This study was supported in part by the Center for Cancer Research Intramural Research Program, NCI, NIH and by the Landsteiner Foundation for Blood Transfusion Research (LSBR) (BEC) and a VIDI fellow of The Netherlands Organization for Scientific Research (NWO) (BEC)
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, Sumikawa Y, Okabe M, Yoshikawa K, Itami S. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- Bal V, McIndoe A, Denton G, Hudson D, Lombardi G, Lamb J, Lechler R. Antigen presentation by keratinocytes induces tolerance in human T cells. Eur J Immunol. 1990;20:1893–1897. doi: 10.1002/eji.1830200904. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, Najfeld V, Phelps RG, Grosskreutz C, Scigliano E, Frenette PS, Merad M. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach SM, Katz SI. Keratinocytes synthesize Ia antigen in acute cutaneous graft-vs-host disease. J Immunol. 1983;131:2741–2745. [PubMed] [Google Scholar]
- Breathnach SM, Shimada S, Kovac Z, Katz SI. Immunologic aspects of acute cutaneous graft-versus-host disease: decreased density and antigen-presenting function of Ia+ Langerhans cells and absent antigen-presenting capacity of Ia+ keratinocytes. J Invest Dermatol. 1986;86:226–234. doi: 10.1111/1523-1747.ep12285176. [DOI] [PubMed] [Google Scholar]
- Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Caughman SW, Sharrow SO, Shimada S, Stephany D, Mizuochi T, Rosenberg AS, Katz SI, Singer A. Ia+ murine epidermal Langerhans cells are deficient in surface expression of the class I major histocompatibility complex. Proc Natl Acad Sci U S A. 1986;83:7438–7442. doi: 10.1073/pnas.83.19.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Noble JR. Role of keratinocytes in human recurrent herpetic lesions. Ability to present herpes simplex virus antigen and act as targets for T lymphocyte cytotoxicity in vitro. J Clin Invest. 1989;83:490–496. doi: 10.1172/JCI113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Busser BW, Lifsted TQ, Oukka M, Lo D, Laufer TM. Antigen presentation by keratinocytes directs autoimmune skin disease. Proc Natl Acad Sci U S A. 2003;100:3386–3391. doi: 10.1073/pnas.0437899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari AA, Katz SI. Induction and functional characterization of class II MHC (Ia) antigens on murine keratinocytes. J Immunol. 1988;140:2956–2963. [PubMed] [Google Scholar]
- Gaspari AA, Jenkins MK, Katz SI. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988;141:2216–2220. [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, Shimoyama S, Letterio JJ, Nakabayashi T, Tagaya H, Yamane T, Ogawa M, Nishikawa S, Ryoke K, Inaba K, Hayashi S, Kunisada T. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lim YS, Kang BY, Kim EJ, Kim SH, Hwang SY, Kim TS. Potentiation of antigen-specific, Th1 immune responses by multiple DNA vaccination with an ovalbumin/interferon-gamma hybrid construct. Immunology. 1998;94:135–141. doi: 10.1046/j.1365-2567.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Mitra RS, Green J, Zheng XG, Shimizu Y, Thompson C, Turka LA. Accessory cell function of keratinocytes for superantigens. Dependence on lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. J Immunol. 1993;150:2148–2159. [PubMed] [Google Scholar]
- Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- Sauder DN, Carter CS, Katz SI, Oppenheim JJ. Epidermal cell production of thymocyte activating factor (ETAF) J Invest Dermatol. 1982;79:34–39. doi: 10.1111/1523-1747.ep12510569. [DOI] [PubMed] [Google Scholar]
- Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976;25:137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I, Thorbecke GJ. Contact hypersensitivity and Langerhans cells. J Invest Dermatol. 1980;75:61–67. doi: 10.1111/1523-1747.ep12521144. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, Lutz MB, Schuler G, Echtenacher B, Ryffel B, Koch F, Romani N. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]