Abstract
We propose a model in which cell loss in the aging brain is seen as a root cause of behavioral changes that compromise quality of life, including the onset of generalized anxiety disorder, in elderly individuals. According to this model, as stem cells in neurogenic regions of the adult brain lose regenerative capacity, worn-out, dead, or damaged neurons fail to be replaced, leaving gaps in function. As most replacement involves inhibitory interneurons, the net result is the acquisition over time of a hyper-excitable state. The stress axis is subserved by all three neurogenic regions in the adult brain, making it particularly susceptible to these age-dependent changes. We outline a molecular mechanism by which hyper-excitation of the stress axis in turn activates the tumor suppressor p53. This reinforces the loss of stem cell proliferative capacity and interferes with the feedback mechanism by which the glucocorticoid receptor turns off neuroendocrine pathways and resets the axis.
Anxiety and old age
Anxiety is an emotion characterized by activity in the hypothalamic-pituitary-adrenal (HPA) axis and psychological discomfort ranging from uneasiness to immobilizing terror. Common to many mammals, feelings of anxiety are considered normal when expressed under stressful circumstances and for relatively short periods of time. However, a subset of the population experiences HPA activation and emotional anxiety for abnormally long periods of time and/or under inappropriate circumstances. In humans, this is referred to as Generalized Anxiety Disorder (GAD) and is seen in 3.7% - 7.4% of the general population [1-4]. In the elderly, however, studies have estimated the prevalence of anxiety symptoms to be as high as 20% [5, 6]. In fact, as indicated in Fig. 1, age is a significant risk factor for anxiety disorders requiring hospitalization (Source: Public Health Agency of Canada, A Report on Mental Illnesses in Canada, www.phac-aspc.gc.ca/publicat/miic-mmac/sum_e.html).
Fig. 1. Effect of age on anxiety.
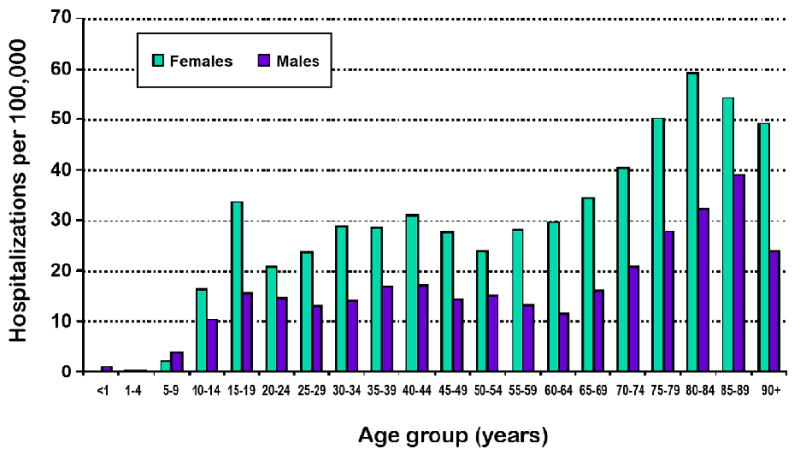
Number of hospitalizations per 100,000 patients in each half-decade of life between birth and 95 years of age. Source: Public Health Agency of Canada, A Report on Mental Illnesses in Canada, www.phac-aspc.gc.ca/publicat/miic-mmac/sum_e.html
The actual percentage of elderly persons suffering from anxiety may be even higher than estimated due to several factors. For instance, the elderly have a stronger stigmatization associated with mental health issues and are less likely to seek out professional help than younger members of the population [7]. When the elderly do seek consultation for a mental health problem, they tend to use the general health sector [8]. Unfortunately, several studies showed that primary care physicians did not detect most psychiatric disorders among their patients, particularly anxiety and affective disorders [9-11]. This is likely due to the fact that elderly persons may tend to somatize anxiety symptoms in the form of general aches and pains, masking the psychological nature of the problem [12]. Furthermore, anxiety and other psychiatric symptoms commonly overlap in older persons, leading to underestimated prevalence and diagnosis rates [12]. This suggests that clinically important symptoms may be occurring in the elderly that are not necessarily recognized and treated.
Abnormally high anxiety in elderly individuals leads to diminishing physical functioning and increasing social isolation. Physically, anxiety disorders are associated with low compliance with medical treatment, potentially worsening the effect of concurrent physical conditions [13], and poorer functional recovery after disabling medical events such as a stroke [14, 15]. Social life also suffers in those with high anxiety. A significant correlation between anxiety and instrumental activities suggests that anxiety may contribute to disabilities in social functioning and to decreased independence [16]. It is not surprising, then, that anxiety symptoms have been associated with higher admission rates to nursing homes [17], where it then interferes with social integration into the new community.
Several researchers argue that age-associated anxiety can be partially attributed to psychological distress that occurs with the recognition of declining cognitive [18] and physical [19] functioning. This suggests a circular relationship between anxiety and physical/cognitive function. The psychological stress of declining physical and mental faculties increases anxiety. Heightened anxiety, in turn, further compromises cognitive [20] and physical [21-23] function.
While anxiety clearly has psychological influences, we argue here that normal age-associated changes in the nervous system, independent of cognition and disease, also contribute to high incidence of anxiety disorders in the elderly. Specifically, we review the evidence that anxiety in old age can be partially explained as a physiological rather than a psychological entity that accompanies normal aging and results from the gradual breakdown of regenerative processes that occur in many tissues, including the brain.
Cellular and Molecular Substrates of Anxiety (the HPA axis)
Anxiety is a physiological response to stress that is coordinated by a hormonal pathway initiated by the release of corticotropin releasing hormone (CRH) by the paraventricular nucleus (PVN) of the hypothalamus (Fig. 2). Axons from parvocellular neurons in the PVN project through the median eminence to the anterior pituitary, where the release of CRH stimulates secretion of adrenocorticotropin hormone (ACTH). ACTH reaches the adrenal glands via the bloodstream, where it stimulates the synthesis and secretion of stress hormones (glucocorticoids), such as cortisol. Once inside the cell, glucocorticoids bind to the glucocorticoid receptor (GR), translocating it to the nucleus where it functions as a transcription factor (Fig. 2) that can activate or suppress gene expression (reviewed in [24].
Fig. 2. The HPA axis seen from the cellular and molecular levels.
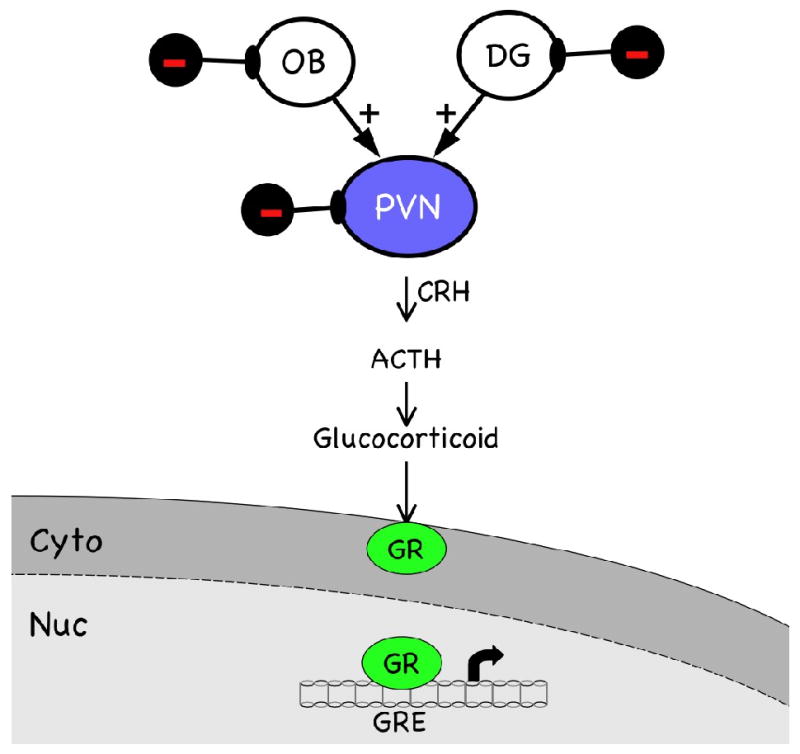
The hypothalamic—pituitary—adrenal (HPA) axis is activated by multiple inputs to the paraventricular nucleus (PVN) of the hypothalamus, including excitatory pathways originating in the olfactory bulb (OB) and dentate gyrus (DG) of the hippocampus. Inhibitory interneurons (black symbols) modulate (dampen) neural activity in all three brain regions. Corticotropin releasing hormone (CRH) produced in the PVN stimulates secretion of adrenocorticotropin hormone (ACTH) by the anterior pituitary. ACTH reaches the adrenal glands via the bloodstream, where it stimulates the synthesis and secretion of glucocorticoids. Once inside the cell, glucocorticoids bind to the glucocorticoid receptor (GR, green symbol), translocating it to the nucleus where it binds to the glucocorticoid response element (GRE) in the promoters of target genes, such as CRH. This feeds back and turns off the stress response.
This hypothalamic—pituitary—adrenal (HPA) axis is activated by multiple inputs to hypothalamic nuclei, both neural and humoral. Humoral inputs include hormones, blood sugar, and sodium, which enter the brain via the fenestrated capillaries of the circumventricular organs. The primary neural inputs arise in limbic structures such as the amygdala, hippocampus, and dentate gyrus (DG) and in sensory structures such as the retina and olfactory bulb (OB). Projection neurons in each of these regions form reciprocal synapses with inhibitory interneurons (Fig. 2, black symbols), which serve to modulate the total output to the hypothalamus and elsewhere. For example, inhibitory interneurons in the OB integrate excitatory signals from mitral and tufted cells with excitatory signals from more central sources, such as noradrenergic projections from the locus ceruleus and serotonergic projections from the midbrain and pons, and convey this integrated information back to the projection neurons via GABAergic synapses. When the activity of these interneurons is blocked, there are effects on olfactory discrimination and higher order cognitive functions involving emotion and behavior. Obviously, loss of modulatory inputs to projection neurons into and out of hypothalamic centers such as the PVN would be expected to have profound effects on the functions these centers perform. As discussed in the following section, ongoing neurogenesis specifically maintains population of interneurons in the OB, DG, and PVN, a process that is subject to deterioration as the organism ages.
Adult neurogenesis in regions of the brain subserving the HPA axis
In mammals, the nervous system develops from neural stem cells that have the capacity to self-renew and differentiate into neurons and glial cells. Although most neurons are generated before birth, the ability of the brain to produce new neurons extends into adulthood. A widespread phenomenon, adult neurogenesis occurs in many species of invertebrates and vertebrates, including humans [25]. Neurogenesis persists in defined areas of the brain suggesting that production of new neurons may be limited to a few neuronal phenotypes affecting very specific functions. In addition, neurogenesis may also be part of a regenerative process in response to injury and disease [26, 27].
Olfactory bulb
The subventricular zone (SVZ) of the lateral ventricles is the largest neurogenic area of the adult brain [28-30]. This region contains four different types of cells distinguishable by their ultrastructure and molecular markers [31, 32]. Of these, the SVZ astrocytes are stem cells with the capacity to generate a population of transit-amplifying progenitor cells. These progenitor cells then undergo rapid cell division to produce neuroblasts, which are the immediate precursors to neurons. Neuroblasts migrate along the rostral migratory stream (RMS) to the OB, and differentiate only into interneurons, either periglomerular cells or granule cells [28-30]. Newly generated neurons integrate into existing neuronal circuits in the olfactory bulb, a process important for odor discrimination [33]. The importance of cell turnover in the granule cell layer of the OB is obvious for species of mammals that rely heavily on odor cues for successfully managing their interactions with the environment. However, OB neurons also make direct connections to neurons in neuroendocrine regions of the brain, such as those controlling reproduction and behavior, reinforcing the idea that maintaining olfaction may not be the only or even the primary reason for granule cell turnover in the adult mammal. This provides an explanation for why neurogenesis continues in the OB even in species such as humans, which are less obviously dependent on olfactory processing for survival.
Hippocampus
The subgranular zone of the DG of the hippocampus is the second largest neurogenic area in the adult brain [34]. Extensive evidence suggests that newly generated neurons in this area are important in learning and memory functions [35-38]. Stem cells in this region have not been fully characterized, but there is evidence that astrocyte-like cells in the SGZ give rise to a population of intermediate fast dividing precursors [39]. In the DG, these cells migrate only a short distance and differentiate locally into granule cell neurons [34, 40-42]. Adult-generated granule cells extend axons into the CA3 region [43], and form axosomatic, axodendritic, and axospinous synapses with the target cells [44]. Moreover, new granule cells are more likely than existing cells to be recruited into circuits supporting spatial memory in rats [45]. Overall, this evidence suggests that adult born granule cells become morphologically and functionally integrated in the hippocampal circuitry. A recent study indicates that adult hippocampal neurogenesis plays a role in regulating mood-related behavior. Mice with deficient TrkB expression in adult DG progenitors, exhibit reduced dendrite and spine growth in newborn neurons, impaired neurogenesis-dependent long-term potentiation and compromised cell survival, accompanied by a remarkably increased anxietylike behavior [46].
Hypothalamus
Recent studies suggest that there are also stem cells residing in the region surrounding the third ventricle that can generate new neurons in the adult brain. The identification of neurogenic precursors that could provide ongoing neurogenesis in the hypothalamus is important because of the role this brain region plays in regulating several neuroendocrine axes, including that of the stress response. Cells in the ependymal layer of the 3rd ventricle of the rodent can proliferate in response to basic fibroblast growth factor (bFGF) and give rise to neurons in the parenchyma of the adult hypothalamus [47], but even in the absence of external manipulation, new neurons are born in large numbers [48]. These new hypothalamic neurons are capable of forming synapses and producing neuropeptides.
Cells present along the dorsoventral extent of the third ventricle and into the hypothalamic parenchyma also respond to CNTF (ciliary neurotrophic factor). CNTF induces proliferation in the arcuate nucleus, and in the ventromedial and dorsomedial nuclei, areas known to be involved in energy balance and feeding behavior. Newborn cells express neuronal markers and exhibit relevant functional phenotypes, including expression of NPY (neuropeptide-Y) and POMC (proopiomelanocortin) and a capacity to respond to the metabolic hormone leptin. Hypothalamic neurogenesis in response to CNTF is physiologically relevant, as blocking proliferation by administration of the mitosis inhibitor cytosine-β-D-arabinofuranoside (Ara-C) blocked the effect of CNTF on body weight [49].
Some additional evidence suggests that adult neurogenesis may extend to other hypothalamic areas. For example, sexual maturation of the female pig is associated with the generation of new oxytocin-producing neurons in the PVN [50]. Moreover, when hypothalamic precursors are cultured in vitro, they can generate a range of neuronal phenotypes characteristic of the neuroendocrine system, including cells that produce GHRH (growth hormone-releasing hormone), GnRH (gonadotropin-releasing hormone), oxytocin, somatostatin, TRH (thyrotropin-releasing hormone), and vasopressin, and, most importantly for our model, CRH [51]. Widespread neurogenesis in the adult hypothalamus could potentially alter regulation of a variety of neuroendocrine functions, including that of the HPA axis.
Deterioration of adult neurogenesis in the healthy old
The effects of age on hypothalamic functions such as reproduction, fat and glucose utilization, and the capacity to deal with stress, among many others, are well known. One clinically significant question is whether or not anxiety in the elderly may be due, at least in part, to loss of interneurons in the OB, DG, or hypothalamus as a consequence of declining neurogenesis.
A number of factors have been shown to affect the process of adult neurogenesis, including hormones such as glucocorticoid, estrogen, testosterone and prolactin, and growth factors such as EGF, FGF2, IGF1, and BDNF (reviewed in [25]). Olfactory stimulation, environmental enrichment, and exercise, and associated neurotransmitters such as nitric oxide, glutamate, and serotonin, can also modulate the production of new neurons. However, the most important risk factor for impaired neurogenesis is the age of the organism. A progressive reduction in the number of newly generated neurons from the SVZ and in the DG during the lifespan of the organism has been demonstrated in several species of mammals [52-56], including humans. Moreover, evidence shows that a decline in adult neurogenesis parallels the gradual loss of sensory and cognitive functions that occurs as healthy adults age [52-58].
Two recent reports highlight the effect declining neurogenesis has on the function of the HPA axis. First of all, selective obliteration of neural precursors in the hippocampus by inducible over-expression of the pro-apoptotic protein Bax increased anxiety-related behavior [59]. Loss of proliferating progenitors and of newly generated hippocampal neurons was accompanied by increased avoidance of potentially threatening situations, such as exposure to open field and brightly lit environments. The effect did not depend on non-specific changes in locomotor activity or differences in exploratory behavior, and specifically affected anxiety, without altering other affective behaviors such as depression. These results provide strong evidence of a casual relationship between reduced neurogenesis and increased anxiety.
In the second instance, suppression of adult neurogenesis by selectively expressing HSV-tk in neural stem and progenitor cells increased the HPA axis stress response [60]. When mice in which neurogenesis was suppressed were exposed to mild stress, circulating corticosterone levels increased to a much higher level than in mice in which neurogenesis was not impaired. This increased stress response was not due to lower expression of hippocampal glucocorticoid receptors, which would lead to reduced sensitivity to negative feedback by glucocorticoids. Instead, reduced neurogenesis had a direct and causative effect on increased stress response.
These data clearly indicate that reduced neurogenesis in the adult brain can have deleterious consequences by increasing both the level of HPA axis activity and the appearance of anxiety-like behavior. Neurons replaced by neurogenesis in the adult appear to be mostly inhibitory interneurons, which are responsible for modulating and coordinating the excitatory output of large projection neurons that carry information from one brain region to another. Within the HPA axis, inhibitory interneurons in the OB and DG modulate sensory and limbic information carried on projection neurons to various nuclei in the hypothalamus, principally the PVN. Declining neurogenesis in very old animals, by compromising regeneration of inhibitory interneurons, therefore, would have the effect of increasing the excitatory drive on the HPA axis (Fig. 3).
Fig. 3. The aging HPA axis.
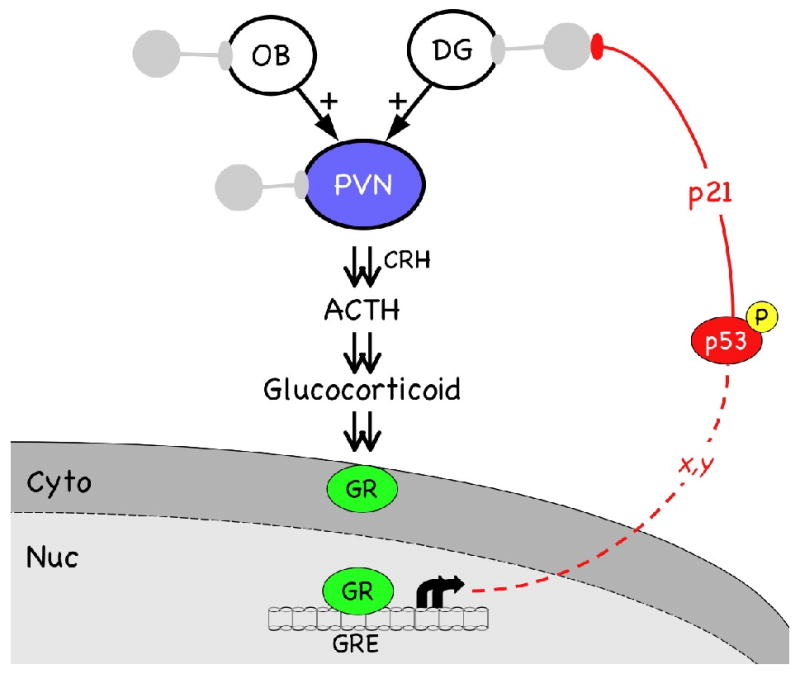
Increased drive on the HPA axis is the result of several factors, including loss of inhibitory interneurons (grey symbols) that modulate the output of the OB, DG, and PVN. Increased HPA drive hyperactivates GR, resulting in phosphorylation of p53 (red symbol) on serine15. The pathway by which activated GR converts p53 from a latent to an active transcription factor is not known, but does not involve transcription of p53 itself (x, y, unknown steps in pathway). Transcription of p53 target genes, such as p21, leads to a block of cellular proliferation, including that of neural stem cells. Symbols are as for Fig. 2, except interneurons are now shown in grey to represent the loss of inhibitory activity as their numbers decline with age.
How p53 and GR mutually antagonize HPA function
What is the molecular mechanism underlying the decline in neurogenesis in older healthy individuals? As outlined in Fig. 3, increased inputs to the PVN from disinhibited projection neurons in the OB and DG, and from the loss of GABAergic modulation of the parvocellular neurons within the PVN, would be expected to increase the release of CRH, hyperactivating pituitary adrenocorticotrophs and increasing the level of ACTH and glucocorticoids in the bloodstream. In fact, corticosteroid levels are known to increase with age in humans [61] and rodents [62]. Increased availability of corticosteroids, furthermore, would be predicted to lead to increased activity of the GR, which translocates to the nucleus upon binding the ligand. Consistent with this prediction, we have seen a significant increase in the level of phosphorylated (activated) GR in the hypothalamus of 17 month-old mice compared to 4 month-old mice, with further increases at 24 months of age (unpublished results).
One of the targets of activated GR is the tumor suppressor p53. p53 is a transcription factor that regulates cell cycle progression and can induce cell cycle arrest or cell death in response to a variety of cellular stressors. The activity of p53 depends on an extensive repertoire of post-translational modifications, in particular to the activation domain at the amino terminus of the protein (reviewed in [63, 64]). Dexamethasone specifically induces phosphorylation of serine15, which stimulates expression of the cell cycle inhibitor p21Cip1/Waf1 and causes proliferation arrest in glucocorticoid responisive cells [65] (see Fig 3). In neural cells, the mechanism by which dexamethasone induces proliferation arrest by p53 is known to act through the GR, but the effect is indirect and not due to GR-induced transcription of p53 itself [66]. Thus, the changes in cellular composition and hormone levels that occur in old age, by hyperactivating GR signaling pathways, could provoke p53 activity and induce intracellular stress responses mediated by p53. This, in turn, would reinforce proliferation arrest of neural progenitor cells, dampen cellular proliferation, and further impair regeneration of inhibitory interneurons in neurogenic regions. These effects of hyperactivated GR acting through p53 to inhibit neural cell proliferation can explain why corticosterone is a potent suppressor of neurogenesis in the hippocampus [67, 68] and why blocking the age-associated increase in corticosterone by adrenalectomy restored hippocampal neurogenesis and prevented the accompanying loss of hippocampal function in older animals (rats) [68].
In neural stem cells (NSCs), p53 is required for self-renewal and differentiation [69, 70]. Constitutive activation of p53, however, has deleterious consequences, specifically on the ability of NSCs to proliferate. In mice, hyperactivated p53 led to a generalized cell cycle arrest, which depended on p21Cip1/Waf1 [71] and included neural stem and progenitor cells in the adult SVZ [72]. The impaired ability of SVZ NSCs to proliferate limited the supply of newly generated neurons in the adult OB and had significant consequences on the ability of the mice to maintain normal olfactory acuity [72]. Because the OB makes direct connections to the amygdala, it is possible that neurological malfunctions that originate in the OB could affect the emotional behavior as well as olfactory sensitivity of these mice. It will be interesting to determine if impaired neurogenesis in the hypothalamus or in associated structures such as the hippocampus that are responsible for normal HPA function could have direct consequences on complex behaviors such as anxiety that manifest themselves as the organism ages, and experiments to test this idea are currently underway.
In summary, molecular mechanisms controlling the proliferation of neural stem cells are compromised as the individual ages. We propose that this has direct consequences on the HPA axis by causing the loss of inhibitory interneurons in the aging brain, thereby reducing inhibitory modulation of large projection neurons that control neuroendocrine function. As a result, increased secretion of releasing hormones, such as CRH, elevates the levels of stress hormones in the bloodstream, hyperactivating the GR and inducing a stress response mediated by the tumor suppressor p53. Potent anti-proliferative activity of this transcription factor would dampen neural stem cell proliferation, exacerbating the loss of inhibitory interneurons already compromised by age. Experimental evidence from mice suggests that this model could explain the age-associated increase in anxiety and GAD in mammals, including in humans, but more work must be done to validate the model fully.
Acknowledgments
We thank the many members of our laboratories past and present, who have contributed so much to the collective ideas and activities represented in this Review. Our work is supported by grants from the National Institutes of Health and the Ellison Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindesay J, Briggs K, Murphy E. The Guy's/Age concern survey: prevalence rates of cognitive impairment, depression, and anxiety in an urban elderly community. British Journal of Psychiatry. 1989;155:317–329. [PubMed] [Google Scholar]
- 2.Manela M, Katona C, Livingston G. How common are the anxiety disorders in old age? International Journal of Geriatric Psychiatry. 1996;11:65–75. [Google Scholar]
- 3.Beekman A, Bremmer MA, Deeg D, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amerstdam. International Journal of Geriatric Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Uhlenhuth EH, Balter MB, Mellinger GD, Disin IH, Clinthorne J. Symptom checklist syndromes in the general population: correlations with psychotherapeutic drug use. Archives of General Psychiatry. 1983;40:1167–1173. doi: 10.1001/archpsyc.1983.01790100013001. [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb S, Murrell SA. The prevalence and correlates of anxiety symptoms in older adults. Journal of Psychology. 1984;116:159–167. doi: 10.1080/00223980.1984.9923632. [DOI] [PubMed] [Google Scholar]
- 6.Zung WW. Prevalence of clinically significant anxiety in a family practice setting. American Journal of Psychiatry. 1986;143:1471–1472. doi: 10.1176/ajp.143.11.1471. [DOI] [PubMed] [Google Scholar]
- 7.Lauderdale SA, Sheikh JI. Anxiety disorders in older adults. Clin Geriatr Med. 2003;19:721–741. doi: 10.1016/s0749-0690(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 8.Blazer DG. Depression in late life. 2nd. Mosby; St. Louis: 1993. [Google Scholar]
- 9.Baca Baldomero E, Saiz Ruiz J, Aguera Ortiz LF, Caballero Martinez L, Fernandez-Liria A, Ramos Brieva JA. Prevalence of psychiatric disorders in primary care using the PRIME-MD questionnaire. Aten Primaria. 1999;23:275–279. 31. [PubMed] [Google Scholar]
- 10.German PS, Schapiro S, Skinner EA. Mental health of the elderly: use of health and mental health services. Journal of American Geriatrics Society. 1985;33:246–252. doi: 10.1111/j.1532-5415.1985.tb07111.x. [DOI] [PubMed] [Google Scholar]
- 11.Waxman HM, Carner EA, Klein M. Under-utilization of mental health professionals by community elderly. The Gerontolotist. 1984;24:23–30. doi: 10.1093/geront/24.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Fuentes K, Cox BJ. Prevalence of anxiety disorders in elderly adults: a critical analysis. Journal of Behavioral Therapy and Experimental Psychiatry. 1997;28:269–279. doi: 10.1016/s0005-7916(97)00025-6. [DOI] [PubMed] [Google Scholar]
- 13.Rabins PV. Prevention of mental disorder in the elderly: current perspectives and future prospects. Journal of American Geriatrics Society. 1992;40:723–733. doi: 10.1111/j.1532-5415.1992.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 14.de Beurs E, Beekman ATF, Van Balkom A, Deeg D, Van Dyck R, Van Tilburg W. Consequences of anxiety in older persons: its effects on disability, well-being and use of health services. Psychological Medicine. 1999;29:583–593. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- 15.Astrom M. Generalized anxiety disorder in stroke patients: a 3-year longitudinal study. Stroke. 1996;27:270–275. doi: 10.1161/01.str.27.2.270. [DOI] [PubMed] [Google Scholar]
- 16.Porter VR, Buxton WG, Fairbanks LA, et al. Frequency and characteristics of anxiety among patients with Alzheimer's disease and related dementias. Journal of Neurpsychiatry and Clinical Neuroscience. 2003;15:180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JM, Monson RR, Oliver DC, Sobol AM, L AH. Affective disorders and mortality: a general population study. Archives of General Psychiatry. 1987;44:473–480. doi: 10.1001/archpsyc.1987.01800170095012. [DOI] [PubMed] [Google Scholar]
- 18.Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychological Medicine. 1997;27:91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- 19.Preville M, Herbert R, Bravo G, Boyer R. Predisposing and facilitating factors of severe psychological distress among frail elderly. Canadian Journal on Aging. 2002;21:559–582. [Google Scholar]
- 20.Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int J Geriatr Psychiatry. 2003;18:951–959. doi: 10.1002/gps.1004. [DOI] [PubMed] [Google Scholar]
- 21.Fava M, Abraham M, Pava J, Shuster J, Rosenbaum J. Cardiovascular risk factors in depression. The role of anxiety and anger. Psychosomatics. 1996;37:31–37. doi: 10.1016/S0033-3182(96)71595-5. [DOI] [PubMed] [Google Scholar]
- 22.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Archives of Family Medicine. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95:818–824. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- 24.Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680:114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Cayre M, Malaterre J, Scotto-Lomassese S, Strambi C, Strambi A. The common properties of neurogenesis in the adult brain: from invertebrates to vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- 26.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 27.Curtis MA, Penney EB, Pearson AG, et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci U S A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- 29.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 31.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 32.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 35.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 36.Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 37.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 39.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 42.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Toni N, Teng EM, Bushong EA, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 45.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 46.Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Tamamaki N, Noda T, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 49.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 50.Raymond AD, Kucherepa NN, Fisher KR, Halina WG, Partlow GD. Neurogenesis of oxytocin-containing neurons in the paraventricular nucleus (PVN) of the female pig in 3 reproductive states: puberty gilts, adult gilts and lactating sows. Brain Res. 2006;1102:44–51. doi: 10.1016/j.brainres.2006.04.113. [DOI] [PubMed] [Google Scholar]
- 51.Markakis EA, Palmer TD, Randolph-Moore L, Rakic P, Gage FH. Novel neuronal phenotypes from neural progenitor cells. J Neurosci. 2004;24:2886–2897. doi: 10.1523/JNEUROSCI.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 54.Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Revest JM, Dupret D, Koehl M, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 60.Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 62.Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appella E, Anderson CW. Post-translational modifications and activation of p53 by genotoxic stresses. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 64.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 65.Urban G, Golden T, Aragon IV, et al. Identification of a functional link for the p53 tumor suppressor protein in dexamethasone-induced growth suppression. J Biol Chem. 2003;278:9747–9753. doi: 10.1074/jbc.M210993200. [DOI] [PubMed] [Google Scholar]
- 66.Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation. FASEB J. 2002;16:761–770. doi: 10.1096/fj.01-0577com. [DOI] [PubMed] [Google Scholar]
- 67.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 68.Montaron MF, Drapeau E, Dupret D, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Armesilla-Diaz A, Bragado P, Del Valle I, et al. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience. 2009;158:1378–1389. doi: 10.1016/j.neuroscience.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 70.Meletis K, Frisen J. Blood on the tracks: a simple twist of fate? Trends Neurosci. 2003;26:292–296. doi: 10.1016/S0166-2236(03)00125-5. [DOI] [PubMed] [Google Scholar]
- 71.Maier B, Gluba W, Bernier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medrano S, Burns-Cusato M, Atienza M, Rahimi D, Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiology of Aging. 2007 doi: 10.1016/neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


