Abstract
Vascular endothelial growth factor (VEGF) is a hypoxia-inducible angiogenic peptide with recently identified neurotrophic effects. Because some neurotrophic factors can protect neurons from hypoxic or ischemic injury, we investigated the possibility that VEGF has similar neuroprotective properties. In HN33, an immortalized hippocampal neuronal cell line, VEGF reduced cell death associated with an in vitro model of cerebral ischemia: at a maximally effective concentration of 50 ng/ml, VEGF approximately doubled the number of cells surviving after 24 h of hypoxia and glucose deprivation. To investigate the mechanism of neuroprotection by VEGF, the expression of known target receptors for VEGF was measured by Western blotting, which showed that HN33 cells expressed VEGFR-2 receptors and neuropilin-1, but not VEGFR-1 receptors. The neuropilin-1 ligand placenta growth factor-2 failed to reproduce the protective effect of VEGF, pointing to VEGFR-2 as the site of VEGF's neuroprotective action. Two phosphatidylinositol 3′-kinase inhibitors, wortmannin and LY294002, reversed the neuroprotective effect of VEGF, implicating the phosphatidylinositol 3′-kinase/Akt signal transduction system in VEGF-mediated neuroprotection. VEGF also protected primary cultures of rat cerebral cortical neurons from hypoxia and glucose deprivation. We conclude that in addition to its known role as an angiogenic factor, VEGF may exert a direct neuroprotective effect in hypoxic-ischemic injury.
Vascular endothelial growth factor (VEGF) is an angiogenic peptide that is released in response to hypoxia in developing or neoplastic tissue; it acts on endothelial cells to promote the sprouting of blood vessels (1, 2). The angiogenic action of VEGF involves an antiapoptotic effect that promotes endothelial cell survival and is mediated through the VEGFR-2 receptor and the phosphatidylinositol 3′-kinase (PI3-K)/Akt signaling pathway (3). This pathway has also been implicated in the cell survival-promoting effects of insulin-like growth factor 1 on cerebellar neurons (4) and of glial cell line-derived neurotrophic factor on motor neurons (5).
VEGF itself appears to have direct neurotrophic effects, as it stimulates axonal outgrowth and increases the survival of mouse superior cervical and dorsal root ganglion neurons (6) and promotes the survival of rat mesencephalic neurons (7) in culture. We reported recently that VEGF also rescues HN33 hippocampal cells from death induced by serum withdrawal (8).
These observations and current interest in VEGF as a potential treatment for stroke based on its angiogenic action (9, 10) led us to investigate the possibility that VEGF, like certain other trophic factors (11, 12), has a direct neuroprotective effect in an in vitro cell culture model of cerebral ischemia. Our results indicate that VEGF acts through VEGFR-2 receptors and PI3-K to reduce cell death from hypoxia and glucose deprivation in cultured HN33 cells (13) and that the protective effect of VEGF also occurs in primary cultures of cerebral cortical neurons.
Materials and Methods
HN33 Cell Culture.
HN33 is an immortalized cell line derived from somatic cell fusion of mouse hippocampal neurons and N18TG2 neuroblastoma cells (13). HN33 cells express a broad range of neuronal signaling properties (13–18) and have been used to investigate pathophysiological features of neuronal injury states, including meningitis (19), polyglutamine disease (20), oxidative stress (21), and ischemia (22). HN33 cells were cultured as described by Shi et al. (22), with modifications. Briefly, cells at passage ≤20 were plated at a density of 1 × 105 cells per well onto uncoated, 24-well plastic culture dishes in DMEM containing 10% (vol/vol) FBS (Life Technologies, Rockville, MD) and maintained at 37°C in humidified 95% air/5% CO2 until ∼90% confluent.
Hypoxia and Glucose Deprivation (HGD).
To induce HGD, cultures were placed in modular incubator chambers (Billups–Rothenberg, Del Mar, CA) for 0–24 h at 37°C, in humidified 95% air/5% CO2 and serum-free medium with 30 mM glucose (control) or humidified 95% N2/5% CO2 and serum-free medium without glucose (HGD) (23). Cultures were then returned to normoxic conditions for the remainder, if any, of 24 h. The effects of VEGF (Sigma), placenta growth factor-2 (PlGF-2) (R & D Systems), wortmannin (Sigma), and 2-[4-morpholinyl]-8-phenyl-1[4H]-benzopyran-4-one (LY294002) (Sigma) were evaluated in cultures exposed to these agents for the entire 24 h.
Cell Survival Assay.
Cell survival was assessed by adding 0.08% trypan blue dye to culture wells for 5 min at 25°C, substituting dye-free buffer, and counting dye-containing (injured) and dye-excluding (viable) cells in five 40× microscope fields per well.
Western Blotting.
Western blotting was used to determine which receptors for VEGF were expressed by HN33 cells in culture. Cell lysates were extracted in 0.1 M NaCl, 0.01 M Tris⋅HCl (pH 7.6), 1 mM EDTA (pH 8.0), 1 μg/ml aprotinin, and 100 μg/ml PMSF, and protein concentration was determined by a Bio-Rad protein assay. Protein (100 μg) was boiled at 100°C in SDS sample buffer for 5 min, electrophoresed on 7–12% SDS-PAGE gels, and transferred to polyvinyldifluoridine membranes. These were then incubated overnight at 4°C with (i) mouse monoclonal antibody against amino acids 1–140 of human VEGF (Santa Cruz Biotechnology; 1:500), (ii) affinity-purified rabbit polyclonal antibody against the carboxy terminus of human Flt-1/VEGFR-1 (Santa Cruz Biotechnology; 1:500), (iii) mouse monoclonal antibody against an epitope corresponding to amino acids 1158–1345 and mapping at the carboxyl terminus of mouse Flk-1/VEGFR-2 (Santa Cruz Biotechnology; 1:500), or (iv) affinity-purified rabbit polyclonal anti-neuropilin-1, raised against an epitope corresponding to amino acids 813–827 and mapping at the amino terminus of human neuropilin-1 (Oncogene Research Products, Cambridge, MA; 1:200). Membranes were washed with PBS containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated anti-mouse (for monoclonal primary) or anti-rabbit (for polyclonal primary) secondary antibody (both Santa Cruz Biotechnology; 1:3,000) at room temperature for 60 min, and washed three times for 15 min with PBS/Tween. Peroxidase activity was visualized with a chemiluminescence substrate system (NEN Life Science Products). Controls for nonspecific binding included omission of the primary antibody or preabsorption of the primary antibody with antigen peptides for 2 h at 37°C at a dilution of 1:5.
Immunodetection of Phosphorylated VEGFR-2.
HN33 cells were maintained under control conditions or exposed to HGD as described above for 24 h, in the absence or presence of 100 ng/ml of VEGF. Cell lysates were extracted in 0.1 M NaCl, 0.01 M Tris⋅HCl (pH 7.6), 1 mM EDTA (pH 8.0), 1 μg/ml aprotinin, 100 μg/ml PMSF, and 100 μM Na3VO4 and centrifuged at 10,000 × g for 15 min. The supernatants were used for immunoprecipitation with the anti-Flk-1/VEGFR-2 antibody described above. After incubation for 2 h at 4°C and centrifugation at 10,000 × g for 15 min, the pellet was washed three times with lysis buffer and resuspended in 1× loading buffer containing 50 mM Tris⋅Cl (pH 6.8), 100 mM DTT, 2% SDS, 0.1% bromophenol blue, and 10% glycerol. The immunocomplex was separated by 7% SDS-PAGE and transferred to poly(vinylidene difluoride) membranes. Membranes were blocked in 5% nonfat milk and 0.2% Tween-20 in PBS for 1 h at room temperature and incubated overnight at 4°C with a mouse monoclonal antibody against p-Tyr, which specifically detects phosphotyrosine-containing proteins (Santa Cruz Biotechnology; 1:200). Horseradish peroxidase-conjugated anti-mouse secondary antibody was visualized with a chemiluminescence substrate system (NEN).
Primary Neuronal Culture.
Neuronal cultures were prepared from 16- to 17-day-old Charles River CD1 mouse embryos (24). Cerebral hemispheres were removed aseptically; freed of meninges, olfactory bulbs, basal ganglia, and hippocampi; and incubated at 37°C in Ca2+- and Mg2+-free Earle's balanced salt solution containing 0.01% trypsin 1:250. After 30 min, 10% horse serum (HS) was added. Cells were placed in 2 ml of fresh MEM and triturated. They were resuspended in Eagle's MEM prepared without glutamine and with twice the usual concentration of other amino acids and four times the usual concentration of vitamins (MEM-Pak; Cell Culture Facility, University of California, San Francisco, CA) and supplemented on the day of plating with glucose (final concentration, 30 mM), 2 mM glutamine, and 15 mM Hepes (pH 7.4). Cell suspensions were filtered through a 70-μm Falcon nylon cell strainer, supplemented with 10% horse serum and 10% FBS, and seeded at a density of 3 × 105 cells per well on 24-well Corning tissue culture dishes coated with 100 μg/ml of poly-d-lysine. Cultures were incubated for 20 min at 37°C in humidified 95% air/5% CO2, and one-half of the medium was replaced with medium containing 5% horse serum and 5% FBS. Cytosine arabinoside (10 μM) was added on the sixth day in vitro. On the next day, two-thirds of the medium was replaced with medium lacking cytosine arabinoside; thereafter, one-half of the medium was replaced with fresh medium twice weekly. Experiments were conducted at 12 days in vitro, when >90% of cells were microtubule-associated protein 2-immunoreactive.
Results
VEGF Protects HN33 Cells from HGD.
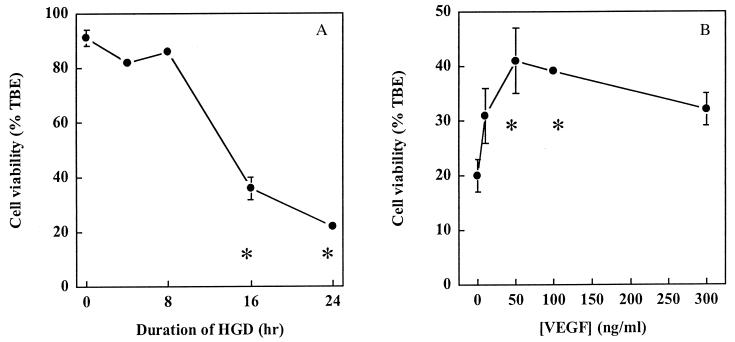
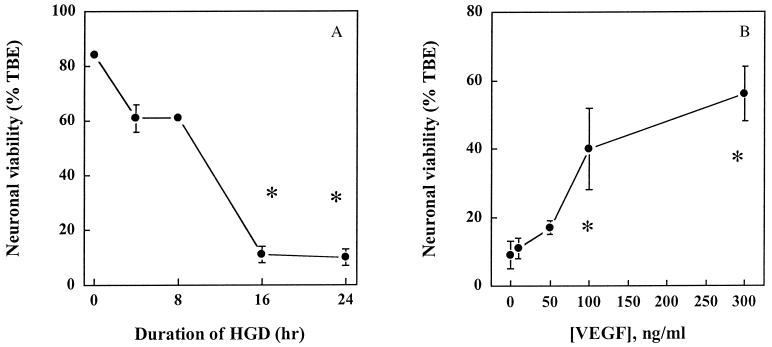
Fig. 1 shows that HN33 cell viability decreased progressively as the duration of HGD increased, with only ∼20% of cells still able to exclude trypan blue dye at 24 h. This progression resembles the time course and magnitude of HGD-induced cell death in a prior study of HN33 cells (22), as well as our own previous results for primary cultures of cerebral cortical neurons (23). The addition of VEGF to cultures reduced cell death from HGD, leading to an approximately 2-fold increase in cell viability after 24 h of HGD at a maximally effective concentration (50–100 ng/ml) of VEGF.
Figure 1.
Protection of HN33 cells from hypoxia and glucose deprivation (HGD) by VEGF. (A) Cultures were maintained in oxygen- and glucose-free medium for the indicated times, and cell viability at 24 h was determined by counting cells excluding trypan blue dye (TBE) as a percentage of all cells. Cell viability after 16 and 24 h of HGD was significantly different from that at 0 h (P < 0.05 by ANOVA and Student–Newman–Keuls tests). (B) VEGF was added to cultures at the indicated concentrations at the onset of exposure to HGD for 24 h. Data shown in A and B are mean values ± SEM from representative experiments, performed in triplicate, which were repeated three times with similar results. Asterisks indicate P < 0.05 by ANOVA and Student—Newman–Keuls tests relative to 0 h of HGD (A) or 0 ng/ml of VEGF (B).
HN33 Cells Express VEGF, VEGFR-2 Receptors, and Neuropilin-1.
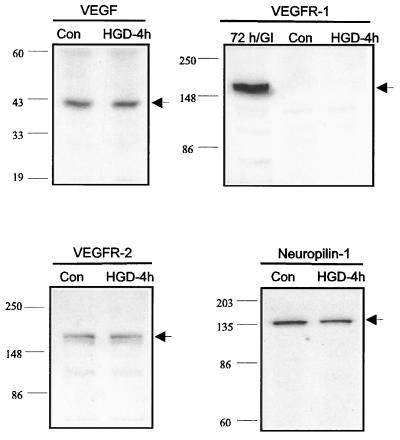
To determine if HN33 cells produce VEGF, and if VEGF expression in these cells is induced by HGD, control and HGD-treated cultures were probed by Western blotting with an anti-VEGF antibody. Fig. 2 shows that VEGF immunoreactivity was detected in control cultures but was not induced further by HGD. The protective effect of VEGF in HGD could be mediated through a variety of signaling pathways, because VEGF activates not only the tyrosine kinase receptors VEGFR-1/Flt-1 and VEGFR-2/Flk-1, but also the neuropilin family of class 3 semaphorin receptors (25), which are involved in axonal pathfinding, retraction, and collapse during development and neuronal death (26–28). Moreover, a role for neuropilins in the regulation of HGD-induced neuronal cell death would be consistent with findings that class 3 semaphorins mediate the apoptotic effect of dopamine on sympathetic neurons (29) and that semaphorin 3 and neuropilin-1 are induced at sites of neural injury (30). To determine which receptor system is involved in the protective effect of VEGF, we first used Western blotting to determine which candidate receptors were expressed in our HN33 cultures. To detect both constitutively expressed and induced receptors, either of which could be involved, both control and HGD-treated cultures were examined. Fig. 2 shows that control and HGD-treated cultures both expressed VEGFR-2 receptors and neuropilin-1, but not VEGFR-1 receptors. No bands were seen at the relevant Mr when the primary antibody was omitted or preabsorbed for 2 h at 37°C with peptide antigen.
Figure 2.
Western analysis of VEGF and VEGF receptor expression in HN33 cells. Protein from control cultures (Con) or from cultures exposed to HGD for 4 h was probed for candidate VEGF receptors with monoclonal (anti-VEGF and anti-VEGFR-2/Flk-1) or affinity-purified polyclonal (anti-VEGFR-1/Flt-1 and anti-neuropilin-1) antibodies, as described in Materials and Methods. Hippocampal tissue taken 72 h after 15 min of global cerebral ischemia (72 h/GI) was used as a positive control for VEGFR-1. The experiment was repeated three times with similar results.
Placenta Growth Factor-2, a Neuropilin-1 Ligand, Is Not Neuroprotective.
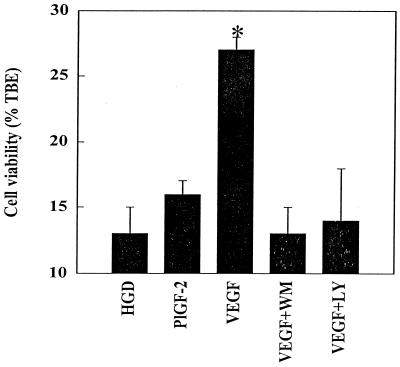
Because VEGFR-2, neuropilin-1, or both could be responsible for the protective effect of VEGF in our cultures, we next sought to distinguish among these possibilities. To this end, we took advantage of the fact that whereas VEGF is a ligand for VEGFR-1, VEGFR-2, and neuropilin-1, a related angiogenic peptide, PlGF-2, binds only to VEGFR-1 and neuropilin-1 (31). Thus, if PlGF-2 were to reproduce the protective effect of VEGF, it would suggest that neuropilin-1 is the responsible receptor, whereas failure of PlGF-2 to protect would point to VEGFR-2. As illustrated in Fig. 3, PlGF-2 had no effect on cell viability, arguing against the involvement of neuropilin-1 (or VEGFR-1) and for the involvement of VEGFR-2 in cytoprotection.
Figure 3.
Effects of VEGF family members (PlGF-2 and VEGF) and of PI3-K inhibitors (wortmannin and LY294002) on HN33 cell viability after HGD. Cultures were exposed to control conditions (cell viability = 90 ± 2%, n = 3; not shown) or to HGD for 24 h, in the absence of added factors (HGD), or in the presence of 300 ng/ml of PlGF-2, 100 ng/ml of VEGF, 100 ng/ml of VEGF + 30 nM wortmannin (WM), or 100 ng/ml of VEGF + 10 μM LY294002 (LY). Cell viability was measured as described in the legend to Fig. 1. Data shown are mean values ± SEM from a representative experiment, performed in triplicate, which was repeated three times with similar results. PlGF-2 also failed to increase viability at 10, 50, or 100 ng/ml, and neither wortmannin nor LY294002, added alone, altered the viability of control or HGD-treated cells (not shown). The asterisk indicates P < 0.05 by ANOVA and Student–Newman–Keuls tests relative to all other conditions. The experiment was repeated three times with similar results.
VEGF Stimulates Phosphorylation of VEGFR-2 in HN33 Cells.
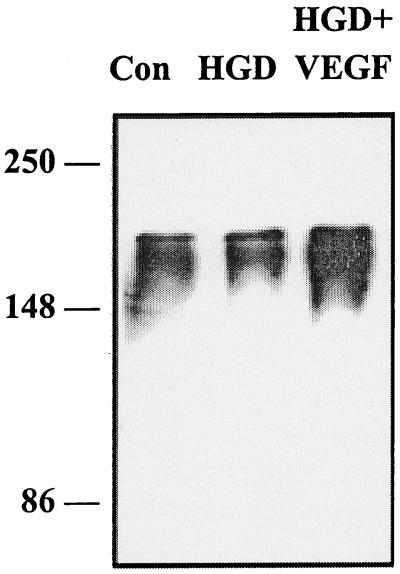
Physiological activation of VEGFR-2 by VEGF is associated with tyrosine phosphorylation of the receptor (32). If VEGF protects HN33 cells through an interaction with functional VEGFR-2 receptors, it should be possible to demonstrate VEGF-induced receptor autophosphorylation in this system. Fig. 4 shows that when HN33 cells were exposed to HGD in the presence of a maximally protective concentration of VEGF, phosphotyrosine immunoreactivity coprecipitating with VEGFR-2 was increased.
Figure 4.
VEGF-stimulated phosphorylation of VEGFR-2 in HN33 cells. Cultures were exposed to control conditions (Con), HGD for 24 h (HGD), or HGD for 24 h in the presence of 100 ng/ml of VEGF (HGD + VEGF). Protein was immunoprecipitated with a mouse monoclonal antibody against amino acids 1158–1345 of mouse Flk-1/VEGFR-2, and Western blotting was performed using a mouse monoclonal antibody against p-Tyr, which specifically detects phosphotyrosine-containing proteins. Horseradish peroxidase-conjugated anti-mouse secondary antibody was visualized as described in Materials and Methods.
PI3-K Inhibitors Block Neuroprotection by VEGF.
Because most evidence favors the participation of the PI3-K/Akt signaling cascade in antiapoptotic and related effects of VEGFR-2 activation (3), we examined the effects of two PI3-K inhibitors, wortmannin and LY294002, on the cytoprotection by VEGF. Neither inhibitor, added alone, altered cell viability in control or HGD-treated cultures (not shown). However, both reversed the protective effect of VEGF (Fig. 3), suggesting that VEGF promotes cell survival in our model of HGD by interacting with VEGFR-2 and activating PI3-K.
VEGF Protects Primary Neuronal Cultures from HGD.
To assess whether the protective effect of VEGF that we observed in HN33 cultures extends to non-immortalized central neurons, primary cultures of cerebral cortical neurons were exposed to HGD under the same conditions used for studies with HN33 cells. Fig. 5 illustrates that HGD decreased neuronal viability with a time course similar to that observed for HN33 cells. In addition, VEGF reduced neuronal death from HGD, at optimal concentrations and to a maximal extent that resembled findings in HN33 cell cultures.
Figure 5.
Protection of cultured cortical neurons from hypoxia and glucose deprivation (HGD) by VEGF. (A) Cultures were maintained in oxygen- and glucose-free medium for the indicated times, and cell viability at 24 h was determined by counting cells excluding trypan blue dye (TBE) as a percentage of all cells. (B) VEGF was added to cultures at the indicated concentrations at the onset of exposure to HGD for 24 h. Data shown in A and B are mean values ± SEM from representative experiments, performed in triplicate, which were repeated three times with similar results. Asterisks indicate P < 0.05 by ANOVA and Student–Newman–Keuls tests relative to 0 h of HGD (A) or 0 ng/ml of VEGF (B).
Discussion
Although VEGF was identified as an angiogenic and vessel-permeability factor (33, 34), our results document an additional, direct neuroprotective effect. Recent studies have shown neurotrophic functions of VEGF (6, 7) as well as induction of VEGF in pathological states affecting the central (35–39) or peripheral (40) nervous system. In cultured superior cervical and dorsal route ganglion neurons, VEGF stimulates axonal outgrowth and improves cell survival (6). These cells express VEGFR-2, and axonal outgrowth induced by VEGF can be blocked by a mitogen-activated protein kinase inhibitor (6). Thus, VEGFR-2 and mitogen-activated protein kinase appear to be involved in trophic effects of VEGF on these neurons, as also proposed for endothelial cells (41). VEGF also increases the survival of dopaminergic neurons in organotypic midbrain explant cultures (7), but the presence of vascular and glial elements in this preparation and the apparent absence of [3H]VEGF binding sites on the affected neurons make it more likely that their improved survival is mediated through effects of VEGF on nonneuronal cells.
We found that, at concentrations comparable to those with neurotrophic effects on ganglion neurons, VEGF produced a roughly 2-fold increase in the number of HN33 cells that survived 24 h of HGD. This increase in viability, from ∼10–20 to ∼25–40% of cells in different cultures, reflects only partial protection, which could be explained by the severity of the insult, the expression of VEGF receptors on only a subpopulation of cells, or the ability of VEGF to affect only a portion of the signaling pathways that lead to cell death. Interestingly, VEGF produced partial protection of a comparable magnitude against HN33 cell death induced by serum withdrawal (8).
Although our HN33 cultures expressed VEGF, as detected by Western blotting, we saw no evidence for the induction of VEGF expression by HGD. Hypoxic induction of VEGF in other cell types, including astrocytes (42), depends on mRNA and protein synthesis and may not occur if the injurious stimulus is too severe. If this result of injury is also true for HN33 cells, it could explain the absence of VEGF induction by HGD. Alternatively, the time course of VEGF induction could be too brief to be detected in our studies. If VEGF is indeed not subject to hypoxic induction in neural cells, and because it is a secreted protein, it might be released from other cell types, such as astroglia (42), to interact with neuronal VEGF receptors.
Western blots showing the pattern of VEGF receptor expression in HN33 cells, the demonstration that VEGF stimulates autophosphorylation of VEGFR-2 receptors in these cells, and studies of other VEGF receptor ligands (PlGF-2) and PI3-K inhibitors (wortmannin and LY294002) strongly suggest that the neuroprotective effect of VEGF against HGD is mediated through VEGFR-2 receptors and PI3-K. Neuropilin-1, which serves as a receptor for both semaphorins and VEGF, was also expressed in our cultures, but did not appear to be involved in neuroprotection by VEGF, because the neuropilin-1 ligand PlGF-2 was not protective. Thus, although neuropilin-1 might bind VEGF in our cultures, this binding does not appear to activate the same cell survival pathways mobilized through VEGFR-2.
Western analysis of VEGF and VEGF receptor expression (Fig. 2) showed that VEGF and VEGFR-2 were both expressed for at least 4 h after the onset of HGD, when cells are presumably still viable, because restoring them to a normoxic environment at this point prevents cell death (Fig. 1A). At longer intervals up to 24 h, expression of VEGF and VEGFR-2, as well as neuropilin-1, declined (data not shown). This decline in expression indicates that VEGF and VEGFR-2 are available to elicit a neuroprotective effect at a time when cells can still be salvaged, even though expression may be lost as cells become irreversibly injured. Thus, the ability of VEGF to rescue cells from HGD-induced death at 24 h (Fig. 1B) is most likely due to an effect that is initiated within the first 4–8 h of VEGF exposure, although VEGF-activated downstream signaling events that are involved in cell salvage may unfold over a longer period.
PI3-K has also been implicated in the ability of VEGF to enhance the survival of endothelial cells (3) and of insulin-like growth factor 1 (4) and glial cell line-derived neurotrophic factor (5) to promote the survival of cerebellar and spinal neurons. PI3-K is thought to regulate cell death by activating the serine-threonine protein kinase Akt (3), which enhances the activity of antiapoptotic proteins through the transcription factor NF-κB and inhibits proapoptotic signaling by Bad, caspase-9, and other effectors (43). The fact that several of these proteins are induced or activated in ischemic brain (44–48) is consistent with a role for VEGFR-2/PI3-K signaling in the regulation of hypoxic or ischemic neuronal cell death.
Because HN33 cells are not normal neurons, we tested further the hypothesis that VEGF is neuroprotective by examining its effect on cell death from HGD in primary cultures of cerebral cortical neurons. The potency and magnitude of VEGF's protective effect in these cultures were similar to those in our HN33 cultures. Thus, VEGF was maximally protective at 50–100 ng/ml in HN33 and at 100–300 ng/ml in neurons and increased HN33 cell viability by about 2-fold and neuronal viability by about 5-fold after HGD. These findings indicate that the protective effect of VEGF that we observed in HN33 cells also occurs in bona fide neurons. Whether it occurs in vivo as well must now be explored.
How might endogenous VEGF act to prevent or limit ischemic neuronal injury under known pathological conditions? Cerebral ischemia triggers hypoxia-sensing mechanisms that activate hypoxia-inducible factor-1, a transcription factor that induces VEGF expression (49). Hypoxic induction of VEGF has been demonstrated in astroglia (42), but may also occur in neurons, depending on the severity of the insult. For example, VEGF is induced in neurons of the ischemic border zone, but not those in the more severely compromised ischemic core, after focal cerebral ischemia from occlusion of the middle cerebral artery (50). Our findings suggest that VEGF released from any of a number of possible cellular sources might target VEGFR-2 receptors on the surface of neurons. This targeting could stimulate survival-promoting signaling pathways that involve PI3-K, Akt, and NF-κB and ultimately operate through Bcl-2 family and other antiapoptotic proteins, as observed in nonneural cells (51–53).
A perceived limitation to the potential usefulness of VEGF as a therapeutic agent in cerebral ischemia has been the fact that its angiogenic effect is delayed in onset, beginning days to weeks after the ischemic insult (35), and therefore presumably is too late to rescue many vulnerable neurons (54). Our results raise the possibility that VEGF may also exert direct neuroprotective effects in ischemic tissue in the interval that precedes angiogenesis, which might help prolong cell survival until angiogenesis can occur.
Acknowledgments
This study was supported by National Institutes of Health Grant NS37695.
Abbreviations
- HGD
hypoxia and glucose deprivation
- PI3-K
phosphatidylinositol 3′-kinase
- PlGF-2
placenta growth factor-2
- VEGF
vascular endothelial growth factor
- LY294002
2-[4-morpholinyl]-8-phenyl-1[4H]-benzopyran-4-one
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 2.Petrova T V, Makinen T, Alitalo K. Exp Cell Res. 1999;253:117–130. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 3.Gerber H P, McMurtrey A, Kowalski J, Yan M, Keyt B A, Dixit V, Ferrara N. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 4.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 5.Soler R M, Dolcet X, Encinas M, Egea J, Bayascas J R, Comella J X. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sondell M, Lundborg G, Kanje M. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman W F, Krum J M, Mani N, Rosenstein J M. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- 8.Jin K L, Mao X O, Greenberg D A. J Mol Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Abe K, Itoyama Y. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Bao W L, Lu S D, Wang H, Sun F Y. Chung Kuo Yao Li Hsueh Pao. 1999;20:313–318. [PubMed] [Google Scholar]
- 11.Maiese K, Boniece I, DeMeo D, Wagner J A. J Neurosci. 1993;13:3034–3040. doi: 10.1523/JNEUROSCI.13-07-03034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattson M P, Kumar K N, Wang H, Cheng B, Michaelis E K. J Neurosci. 1993;13:4575–4588. doi: 10.1523/JNEUROSCI.13-11-04575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H J, Hammond D N, Large T H, Roback J D, Sim J A, Brown D A, Otten U H, Wainer B H. J Neurosci. 1990;10:1779–1787. doi: 10.1523/JNEUROSCI.10-06-01779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenox R H, McNamara R K, Watterson J M, Watson D G. J Clin Psychiatr. 1996;57:23–31. [PubMed] [Google Scholar]
- 15.Petitto J M, Huang Z, Raizada M K, Rinker C M, McCarthy D B. Brain Res Mol Brain Res. 1998;53:152–162. doi: 10.1016/s0169-328x(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 16.Shi L C, Wang H Y, Horwitz J, Friedman E. J Neurochem. 1996;67:1478–1484. doi: 10.1046/j.1471-4159.1996.67041478.x. [DOI] [PubMed] [Google Scholar]
- 17.Watson D G, Wainer B H, Lenox R H. J Neurochem. 1994;63:1666–1674. doi: 10.1046/j.1471-4159.1994.63051666.x. [DOI] [PubMed] [Google Scholar]
- 18.Watson D G, Lenox R H. J Neurochem. 1996;67:767–777. doi: 10.1046/j.1471-4159.1996.67020767.x. [DOI] [PubMed] [Google Scholar]
- 19.Tauber M G, Sachdeva M, Kennedy S L, Loetscher H, Lesslauer W. J Infect Dis. 1992;166:1045–1050. doi: 10.1093/infdis/166.5.1045. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y F. J Biol Chem. 1998;273:28873–28877. doi: 10.1074/jbc.273.44.28873. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham T J, Hodge L, Speicher D, Reim D, Tyler-Polsz C, Levitt P, Eagleson K, Kennedy S, Wang Y. J Neurosci. 1998;18:7047–7060. doi: 10.1523/JNEUROSCI.18-18-07047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi L C, Wang H Y, Friedman E. J Neurochem. 1998;70:1035–1044. doi: 10.1046/j.1471-4159.1998.70031035.x. [DOI] [PubMed] [Google Scholar]
- 23.Koretz B, Ahern K v B, Lustig H S, Greenberg D A. Brain Res. 1994;643:334–337. doi: 10.1016/0006-8993(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 24.Yu A C H, Chan P H, Fishman R A. J Neurochem. 1986;47:1181–1189. doi: 10.1111/j.1471-4159.1986.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 25.Soker S, Takashima S, Miao H Q, Neufeld G, Klagsbrun M. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 26.Polleux F, Giger R J, Ginty D D, Kolodkin A L, Ghosh A. Science. 1998;282:1904–1906. doi: 10.1126/science.282.5395.1904. [DOI] [PubMed] [Google Scholar]
- 27.Giger R J, Urquhart E R, Gillespie S K, Levengood D V, Ginty D D, Kolodkin A L. Neuron. 1998;21:1079–1092. doi: 10.1016/s0896-6273(00)80625-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Bagri A, Zupicich J A, Zou Y, Stoeckli E, Pleasure S J, Lowenstein D H, Skarnes W C, Chédotal A, Tessier-Lavigne M. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 29.Shirvan A, Ziv I, Fleminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A. J Neurochem. 1999;73:961–971. doi: 10.1046/j.1471-4159.1999.0730961.x. [DOI] [PubMed] [Google Scholar]
- 30.Pasterkamp R J, Giger R J, Ruitenberg M J, Holtmaat A J, De Wit J, De Winter F, Verhaagen J. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 31.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 32.Dougher M, Terman B I. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 33.Keck P J, Hauser S D, Krivi G, Sanzo K, Warren T, Feder J, Connolly D T. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 34.Leung D W, Cachianes G, Kuang W-J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 35.Kovács Z, Ikezaki K, Samoto K, Inamura T, Fukui M. Stroke (Dallas) 1996;27:1865–1873. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T, Abe K, Suzuki H, Itomaya Y. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 37.Kalaria R N, Cohen D L, Premkumar D R, Nag S, LaManna J C, Lust W D. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 38.Cobbs C S, Chen J, Greenberg D A, Graham S H. Neurosci Lett. 1998;249:79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 39.Issa R, Krupinski J, Bujny T, Kumar S, Kaluza J, Kumar P. Lab Invest. 1999;79:417–425. [PubMed] [Google Scholar]
- 40.Samii A, Unger J, Lange W. Neurosci Lett. 1999;262:159–162. doi: 10.1016/s0304-3940(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 41.Kroll J, Waltenberger J. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 42.Sinor A D, Irvin S, Cobbs C S, Chen J, Graham S H, Greenberg D A. Brain Res. 1998;812:289–291. doi: 10.1016/s0006-8993(98)00976-7. [DOI] [PubMed] [Google Scholar]
- 43.Romashkova J A, Makarov S S. Nature (London) 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 44.Krajewski S, Krajewska M, Ellerby L M, Welsh K, Xie Z, Deveraux Q L, Salvesen G S, Bredesen D E, Rosenthal R E, Fiskum G, et al. Proc Natl Acad Sci USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabriel C, Justicia C, Camins A, Planas A M. Brain Res Mol Brain Res. 1999;65:61–69. doi: 10.1016/s0169-328x(98)00330-1. [DOI] [PubMed] [Google Scholar]
- 46.Clemens J A, Stephenson D T, Smalstig E B, Dixon E P, Little S P. Stroke. 1997;28:1073–1080. doi: 10.1161/01.str.28.5.1073. [DOI] [PubMed] [Google Scholar]
- 47.Kitagawa H, Warita H, Sasaki C, Zhang W R, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K. Neurosci Lett. 1999;274:45–48. doi: 10.1016/s0304-3940(99)00676-x. [DOI] [PubMed] [Google Scholar]
- 48.Ouyang Y B, Tan Y, Comb M, Liu C L, Martone M E, Siesjo B K, Hu B R. J Cereb Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Bergeron M, Yu A Y, Solway K E, Semenza G L, Sharp F R. Eur J Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 50.Lennmyr F, Ata K A, Funa K, Olsson Y, Terent A. J Neuropathol Exp Neurol. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Gerber H P, Dixit V, Ferrara N. J Biol Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 52.Nor J E, Christensen J, Mooney D J, Polverini P J. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran J, Rak J, Sheehan C, Saibil S D, LaCasse E, Korneluk R G, Kerbel R S. Biochem Biophys Res Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 54.Zivin J A. Neurology. 1998;50:599–603. doi: 10.1212/wnl.50.3.599. [DOI] [PubMed] [Google Scholar]