Abstract
Vasopressin V1a receptors in the rat brain have been studied for their role in modulating aggression and anxiety. In the current study blood-oxygen-level-dependent (BOLD) functional MRI was used to test whether V1a receptors modulate neural processing in the maternal brain when dams are exposed to a male intruder. Primiparous females were given an intracerebroventricular (ICV) injection of vehicle or V1a receptor antagonist ([deamino-Pen1, O-Me-Try, Arg8]-Vasopressin, 125 ng/10 μL) 90-120 minutes before imaging. During fMRI, awake dams were presented with a male intruder threat to pups using a specialized chamber that contained separate compartments for pups and a male intruder. Our results indicate that the number of activated voxels was reduced in the cortical amygdala with V1a receptor blockade, while an increase was observed in the anterior olfactory nucleus and other areas. Dams treated with V1a antagonist showed significantly greater BOLD responses in the anterior olfactory nucleus, infralimbic prefrontal cortex, gustatory cortex, somatosensory cortex, and substantia innominata when presented with a novel male intruder. BOLD responses were reduced in the cortical amygdala and ventromedial hypothalamus. The V1a receptor sensitive areas play roles in the processing of smell, taste and touch and emotional reactivity. Thus one interpretation of the present fMRI data is that vasopressin, acting through V1a receptors, may modulate sensory processing and perhaps coordinate this effect with changes in visceromotor activity during the initial stages of maternal aggressive motivation and/or anxiogenic responses.
INTRODUCTION
Arginine Vasopressin (AVP) is a member of a family of nonapeptides found in virtually all vertebrates (Goodson, 2008), and is implicated in the regulation of numerous social and behavioral processes such as aggression, social bonding, and maternal behavior (Goodson and Bass, 2001). Within the mammalian central nervous system, the behavioral actions of AVP are predominantly regulated by the V1a receptor subtype (Tribollet et al., 1988), which is found throughout the rodent brain (Ostrowski et al., 1994b). AVP is important for the modulation of aggressive behaviors in males and females. Ferris and Potegal (Ferris and Potegal, 1988) demonstrated that a microinjection of V1a receptor antagonist in the anterior hypothalamus reduced male hamsters' aggression toward a male intruder. Similarly, a microinjection of AVP into the lateral ventricle causes an increased BOLD signal in regions of the brain involved in aggression and known to contain V1a receptors (Ferris et al., 2008). In microtine rodents, AVP also has been observed to promote offensive aggression as well as partner preference (Winslow et al., 1993). Both the aggressive and affiliative responses may be mediated by V1a receptors in this species of rodent (Young et al., 1999; Lim et al., 2004). AVP is also important in maternal behavior, as chronic AVP treatment in lactating rats increases maternal care (Bosch and Neumann, 2008), and V1a antagonists impair maternal memory (Nephew and Bridges, 2008a) and reduce nursing and pup retrieval (Pedersen et al., 1994).
One specific type of maternal behavior is maternal aggression, which is a robust form of aggression most evident within the first two weeks of lactation (Erskine et al., 1978). This aggressive response can be eliminated by bilateral olfactory bulbectomy (Kolunie and Stern, 1995); however, deprivation of auditory and visual inputs has no effect on maternal aggression (Kolunie et al., 1994), indicating that olfaction, but not auditory or visual stimulation, is essential for maternal aggression. While oxytocin is often implicated in maternal aggression (Bosch et al., 2005; Ferris et al., 1992; Giovenardi et al., 1997), AVP appears to be important as well. Primiparous and multiparous rats administered AVP early in lactation are less aggressive, and administration of V1a antagonist increases maternal aggression and alters maternal behaviors such as grooming and retrieval (Nephew and Bridges, 2008b; Nephew et al., 2009), indicating that AVP modulates both maternal aggression and maternal behavior through the V1a receptor.
Several brain regions have been associated with maternal aggression, including regions of the limbic system such as the amygdala, nucleus accumbens and bed nucleus of the stria terminalis (BNST) (Nephew et al., 2009; Numan and Numan, 1996). The hypothalamus is also associated with the onset of maternal behavior, particularly the ventromedial nucleus of the hypothalamus (VMH) (Bridges and Mann, 1994). Lesions to the VMH advance the onset of maternal behavior in primigravid rats (Mann and Babb, 2004), suggesting that this region may be inhibitory toward maternal behavior. Cortical regions involved in maternal aggression are less well understood. Recently, functional MRI was used to investigate the neural circuitry of offensive aggression using an intruder resident model in rats (Ferris et al., 2008). Various cortical regions showed increased BOLD activation in response to an intruder male, including motor, somatosensory and medial prefrontal cortices (Ferris et al., 2008). In the current study, conscious lactating female rats were injected ICV with either a V1a antagonist or saline, and presented with a novel male intruder in the presence of her pups. Brain activation was measured using BOLD fMRI to determine the role of V1a receptors on maternal brain activation during presentation of a novel male intruder.
MATERIALS AND METHODS
Subjects
Timed pregnant Long-Evans rats (gestational days 10-11, 225-275 g) were purchased from Charles River Laboratories (Wilmington, MA). Females were singly housed in a temperature and humidity controlled room and maintained on a 12L: 12D light-dark cycle (lights off at 1900 hr). Home cages consisted of hanging plastic microisolater cages of standard dimensions with woodchip bedding. Water and Purina rat chow were provided ad libitum. All females were primiparous. Rats were acquired and cared for in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 85-23, Revised 1985) and adhere to the National Institutes of Health and the American Association for Laboratory Animal Science guidelines. The Institutional Animal Care and Use Committee at Northeastern University approved the protocols used for this study.
Acclimatization procedures and preparations for imaging
All imaging experiments were done in fully awake, unanesthetized primiparous postpartum day 5 to day 10 (P5 to P10) dams at 90-120 minutes after ICV injections (see below for surgical procedures). Anesthesia (2- 4% isoflurane) was used during rat setup immediately preceding the acclimatization procedures, surgical procedure, and MRI scanning. Rats were imaged while fully awake. In order to minimize physiological response and gross motion during MR scanning, all rats were acclimatized to a head restraining unit and MRI sounds on days P2 to P4 (King et al., 2005). Before MR scanning, dams were again anesthetized with 2-4 % isoflurane. Details of the setup procedure have been previously reported (Ferris et al., 2005). Briefly, a topical anesthetic of 5-10 % lidocaine cream was applied to the skin and soft tissue around the ear canals and over the bridge of the nose before the animal is placed inside a dual coil radiofrequency system under restraint (Ferris et al., 2005). This procedure took 5-6 min, after which gaseous anesthesia flow was turned off and the entire unit was placed through the bore of the magnet for imaging. After the entire unit was placed in the magnet, scanning preparations controlled by Paravision 4.0 typically took 15 minutes and thereafter the entire imaging session including 1 anatomical scan (ca. 6-8 minutes) and 1 functional scan (ca. 10-12 minutes total) lasted about 30-35 minutes. Thus, the entire experiment per animal in an unanesthetized state lasted 40-45 minutes
V1a receptor antagonist administration
Female rats were treated with [deamino-Pen1, O-Me-Tyr, Arg8]-Vasopressin (Manning compound from Sigma-Aldrich, St Louis, MO; Dose: 125 ng in 10 ul of sterile physiological saline, neutral pH) in order to block vasopressin V1a receptors before imaging. The dose of Manning compound has previously been reported to heighten maternal aggression in primiparous and multiparous rats when given 90 minutes before testing (Nephew and Bridges, 2008a) The V1a receptor antagonist does not cross the blood brain barrier and therefore must be given centrally in the lateral cerebral ventricles. We used previous published methods to deliver an acute dose of V1a antagonist before fMRI (Febo et al., 2004; Ferris et al., 2005). Ninety to 120 minutes prior to scanning, rats were anesthetized with 2-4 % isoflurane, ten percent lidocaine cream was applied to the scalp, the skull surface was exposed and the landmark suture Bregma located. A 26-gauge cannula of polyethylene tubing (PE-10: inner diameter 0.28 mm, outer diameter 0.61 mm) was implanted into the lateral cerebral ventricle (1 mm caudal to Bregma, 2 mm lateral to the midsagittal sinus, and 4 mm ventral to dura) and secured to the skull with surgical glue. Following injections, animals were returned to their home cage until MRI experiment preparations.
Imaging the neural response to nest intruder
Twenty primiparous rats were imaged for their neural response to an intruder threat to pups, following ICV injection of V1a antagonist or saline control (10 per group). A custom made clear plastic cylindrical vivarium was used for in vivo stimuli presentation, as previously reported (Ferris et al., 2008). The outer diameter of the vivarium tube is just under 20 cm, thus permitting it to remain stable inside the magnet bore in front of the dual coil imaging setup during functional and anatomical imaging. The front end of the vivarium has a non-magnetic copper wire mesh that permits dams to smell, visualize and hear pups or intruder males. The outer enclosure contains air holes and the inner environment is divided into top and bottom levels. Maternal cage bedding was placed on the vivarium floor to simulate the lactating rat's home environment. During the pre-intruder baseline period and the intruder male presentation, pups were placed in the lower compartment of the vivarium along with their home cage bedding to protect them from any harm during scanning. For the intruder presentation, 100 repetitions (6 seconds per repetition for a total 600 seconds) were collected and the stimulus male was presented in the vivarium at repetition 47-49 (280-294 seconds).
Magnetic resonance imaging scanning parameters
Experiments were conducted in a 300 Mhz Bruker USR 7T/20 cm horizontal magnet (Bruker, Germany) equipped with a Paravision 4.0 console (Bruker, Billerica, MA U.S.A). Studies were performed with a multi-concentric dual-coil, small animal restrainer (Insight MRI, Worcester, MA). Radiofrequency signals are sent and received with dual coil electronics built into the animal restrainer (Ludwig et al., 2004). Functional imaging was performed using a multi-segmented T2-weighted fast spin echo pulse sequence with the following parameters: repetition time TR = 1562 msec, echo time TE = 7.5, effective echo time TEeff= 45 msec and an echo train length ETL = 16. Geometry was setup as follows: 12 slices, field of view of 28 mm, 1.0 mm thick slices with no gaps, data matrix of 642 for functional scans and 2562 for anatomical scans (Thus, the in plane 2D pixel resolution was 438 μm2 for functional and 117 μm2 for anatomical scans). A full set of 12 coronal slices across the brain was collected at each effective repetition time and was completed every 6 seconds 24 msec.
Statistical analysis
Full details for the MRI data analysis using in house software has been previously reported (Ferris et al., 2005). Animals showing an average displacement exceeding 25% of the total in plane (X-Y) pixel resolution (>109 μm out of 437 μm) or slice (Z) direction (>250 μm out of 1000 μm slice thickness) were excluded (n = 3). This cutoff criterion was pre-established by stimulated studies showing false positive BOLD activation with movements corresponding to 6/10 of a single voxel (Ferris et al., 2008). Also, scans with linear baseline drifts over 0.5% were corrected using in house software (Ferris et al., 2008). Following preprocessing steps, groups consisted of 10 V1a antagonist treated and 7 control dams.
ROI-based statistical analysis was done using Medical Image Visualization and Analysis (MIVA) software (Ferris et al., 2005). Each subject was registered to a fully segmented electronic rat brain atlas (Paxinos and Watson, 1997; Swanson, 1999). Statistical t tests were performed on each subject within the original coordinate system. The baseline period used was 20 repetitions immediately preceding stimulus (object, pup, or intruder) presentation and the stimulation window was 20 repetitions. Statistical t tests used a 95 % confidence level, two-tailed distribution, and heteroscedastic variance assumptions. In order to provide a conservative estimate of significance, a false-positive detection-controlling algorithm is introduced into the analysis (Genovese et al., 2002). This ensures that the false-positive detection rate is below our confidence level of 5 % (Ferris et al., 2005). Statistically significant pixels were assigned their percentage change values (stimulus mean minus control mean) and all. Activated voxel numbers were exported to SPSS for statistical comparisons between groups. The number of voxels per region of interest and their corresponding average percent change values were statistically evaluated between groups using a single factor analysis of variance (p < 0.05) with saline and V1a antagonist treatments as independent factors.
RESULTS
Sixty-nine regions of interest spanning the cortex, limbic system, hypothalamus, basal forebrain, and midbrain were analyzed for changes in BOLD signal intensity upon stimulus presentation. A comprehensive summary of the results for both positive and negative BOLD signal changes is provided in Tables 1 and 2. Control and V1a antagonist treated females showed significant BOLD activation in regions of the olfactory system, amygdaloid complex, somatosensory cortical sub-areas, anterior thalamus, dorsal and ventral striatum, subregions of the anterior hypothalamus, limbic prefrontal areas such as the prelimbic, infralimbic and anterior cingulate cortex, with very little or no significant BOLD signal changes in motor cortical areas and anterior hippocampus among other regions (Figure 1).
Table 1.
Positive BOLD signal changes (signal increases) in lactating rats presented with a male intruder in the absence or presence of V1a receptor blockade.
| Number of Positive BOLD voxels | Mean Percent Change in BOLD signal | |||
|---|---|---|---|---|
| Region of Interest | Control ± Std. Error | V1a antagonist ± Std. Error |
Control ± Std. Error | V1a antagonist ± Std. Error |
| Cortical areas | ||||
| Visual Cortex | 6.7 ± 3.1 | 5.0 ± 1.5 | 2.0 ± 0.2 | 2.9 ± 0.5 |
| Posterior Cingulate Cortex | 12.3 ± 5.0 | 7.3 ± 1.0 | 4.9 ± 0.8 | 4.0 ± 0.4 |
| Parietal Cortex | 4.4 ± 2.3 | 4.2 ± 1.2 | 1.4 ± 0.7 | 2.6 ± 0.4 |
| Auditory Cortex | 8.3 ± 5.2 | 11.7 ± 2.9 | 2.4 ± 0.5 | 3.9 ± 0.6 |
| Temporal Cortex | 4.3 ± 2.5 | 3.5 ± 0.9 | 2.8 ± 0.6 | 3.8 ± 0.9 |
| Insular Cortex | 10.0 ± 4.3 | 12.1 ± 2.8 | 3.2 ± 0.4 | 3.6 ± 0.3 |
| Anterior Cingulate Cortex | 5.6 ± 2.3 | 7.2 ± 2.1 | 2.6 ± 0.4 | 2.6 ± 0.2 |
| Gustatory Cortex # | 2.1 ± 1.2 | 4.1 ± 0.8 | 1.7 ± 0.6 | 4.0 ± 0.3 |
| Prelimbic Cortex | 2.9 ± 1.9 | 4.4 ± 1.9 | 1.5 ± 0.6 | 1.8 ± 0.4 |
| Infralimbic Cortex # | 1.0 ± 0.8 | 2.1 ± 0.5 | 0.8 ± 0.5 | 2.4 ± 0.5 |
| Orbital Cortex | 6.6 ± 2.5 | 7.5 ± 2.2 | 2.5 ± 0.2 | 3.0 ± 0.2 |
| Primary Somatosensory Cortex # | 33.7 ± 19.6 | 61.3 ± 12.0 | 2.6 ± 0.3 | 6.0 ± 0.8 |
| Primary Motor Cortex | 15.0 ± 9.8 | 13.3 ± 3.4 | 3.0 ± 0.3 | 3.7 ± 0.5 |
| Supplementary Motor Region | 20.6 ± 9.1 | 23.1 ± 6.5 | 3.1 ± 0.3 | 3.6 ± 0.4 |
| Secondary Somatosensory Cortex # | 5.6 ± 4.1 | 12.2 ± 2.5 | 2.3 ± 0.6 | 5.5 ± 0.5 |
| Somatosensory Cortex, Upper Limb Region |
0.3 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| Somatosensory Cortex, Node Region | 0.4 ± 0.4 | 0.1 ± 0.1 | 0.5 ± 0.5 | 0.2 ± 0.2 |
| Thalamic nuclei | ||||
| Thalamus | 9.3 ± 3.4 | 6.6 ± 1.7 | 3.4 ± 0.2 | 3.5 ± 0.3 |
| Lateral Posterior Thalamus | 1.0 ± 0.4 | 0.4 ± 0.2 | 1.7 ± 0.7 | 0.9 ± 0.5 |
| Ventral Nucleus of Thalamus | 3.7 ± 2.2 | 2.2 ± 0.4 | 2.4 ± 0.5 | 2.7 ± 0.5 |
| Lateral Dorsal Nucleus of Thalamus | 0.9 ± 0.6 | 1.1 ± 0.3 | 1.1 ± 0.8 | 1.6 ± 0.4 |
| Centrolateral Nucleus of Thalamus | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.5 ± 0.5 | 0.2 ± 0.2 |
| Habenula | 1.0 ± 0.6 | 0.9 ± 0.2 | 2.1 ± 1.3 | 2.4 ± 0.4 |
| Mediodorsal Nucleus of Thalamus | 2.0 ± 1.4 | 0.9 ± 0.2 | 1.7 ± 0.7 | 2.0 ± 0.5 |
| Midline Thalamus | 1.1 ± 0.7 | 1.3 ± 0.4 | 1.4 ± 0.7 | 2.1 ± 0.5 |
| Anterior Thalamus | 1.7 ± 0.8 | 1.1 ± 0.4 | 1.9 ± 0.7 | 1.9 ± 0.5 |
| Hippocampal formation and limbic areas | ||||
| Hippocampal CA1 | 10.3 ± 5.2 | 9.0 ± 2.3 | 2.9 ± 0.3 | 2.8 ± 0.2 |
| Subiculum | 4.9 ± 2.7 | 2.0 ± 0.4 | 3.6 ± 0.7 | 3.8 ± 0.8 |
| Dentate Gyrus | 11.6 ± 4.2 | 7.3 ± 1.5 | 3.0 ± 0.3 | 2.8 ± 0.4 |
| Hippocampal CA3 | 5.0 ± 2.4 | 5.6 ± 1.1 | 2.5 ± 0.5 | 2.6 ± 0.4 |
| Hippocampal CA2 | 0.1 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.2 | 1.0 ± 0.4 |
| Perirhinal area | 1.0 ± 0.6 | 1.0 ± 0.2 | 1.6 ± 0.8 | 2.6 ± 0.5 |
| Entorhinal area | 12.7 ± 4.4 | 10.1 ± 1.8 | 5.1 ± 0.4 | 6.8 ± 0.6 |
| Septum | 4.1 ± 2.3 | 4.5 ± 1.0 | 1.8 ± 0.7 | 2.9 ± 0.3 |
| Basal ganglia, hypothalamus and basal forebrain nuclei | ||||
| Dorsal Striatum | 18.4 ± 11.0 | 18.1 ± 3.5 | 2.5 ± 0.2 | 3.0 ± 0.3 |
| Substantia Nigra | 1.6 ± 0.5 | 1.6 ± 0.4 | 2.7 ± 0.8 | 3.8 ± 0.7 |
| Posterior Hypothalamic Nucleus | 0.6 ± 0.3 | 0.7 ± 0.4 | 1.8 ± 1.0 | 1.3 ± 0.6 |
| Mammillary region | 0.6 ± 0.3 | 1.7 ± 0.7 | 1.6 ± 0.7 | 2.7 ± 0.7 |
| Lateral Hypothalamus | 6.0 ± 2.3 | 5.7 ± 1.3 | 4.1 ± 0.5 | 3.5 ± 0.2 |
| Preoptic area | 0.9 ± 0.5 | 0.5 ± 0.3 | 1.4 ± 0.7 | 0.7 ± 0.5 |
| Paraventricular Nucleus of Hypthalamus |
0.4 ± 0.3 | 0.9 ± 0.3 | 1.7 ± 1.2 | 1.9 ± 0.6 |
| Anterior Hypothalamic Area | 1.1 ± 0.4 | 0.7 ± 0.2 | 2.7 ± 0.8 | 2.1 ± 0.6 |
| Periventricular Hypothalamus | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.7 ± 0.4 | 0.4 ± 0.4 |
| Dorsomedial Hypothalamus | 0.4 ± 0.2 | 0.3 ± 0.2 | 1.1 ± 0.5 | 0.6 ± 0.4 |
| Ventromedial Hypothalamus* # | 1.1 ± 0.3 | 0.4 ± 0.2 | 3.2 ± 0.7 | 0.9 ± 0.5 |
| Arcuate Nucleus | 0.1 ± 0.1 | 0.4 ± 0.2 | 0.8 ± 0.8 | 1.4 ± 0.6 |
| Globus Pallidus | 1.4 ± 0.8 | 1.0 ± 0.3 | 1.8 ± 0.6 | 1.9 ± 0.6 |
| Islands of Cajal area | 0.0 ± 0.0 | 0.4 ± 0.2 | 0.0 ± 0.0 | 1.2 ± 0.6 |
| Nucleus Accumbens | 2.9 ± 1.6 | 3.6 ± 0.8 | 2.1 ± 0.6 | 3.0 ± 0.2 |
| Substantia Innominata # | 1.1 ± 0.7 | 1.9 ± 0.5 | 1.4 ± 0.7 | 2.9 ± 0.5 |
| Amygdala and olfactory system | ||||
| Cortical Nucleus of Amygdala* | 7.4 ± 1.7 | 4.0 ± 0.4 | 5.3 ± 0.6 | 6.1 ± 0.4 |
| Posterior Nucleus of Amygdala | 0.3 ± 0.2 | 0.3 ± 0.2 | 1.5 ± 1.1 | 0.7 ± 0.5 |
| Lateral Nucleus of Amygdala | 0.4 ± 0.4 | 0.4 ± 0.2 | 0.5 ± 0.5 | 1.7 ± 0.7 |
| Basal Nucleus of Amygdala | 1.6 ± 0.6 | 1.2 ± 0.2 | 3.3 ± 0.6 | 2.7 ± 0.4 |
| Periform Amygdaloid Nucleus | 0.4 ± 0.3 | 0.4 ± 0.2 | 0.9 ± 0.6 | 4.1 ± 2.6 |
| Central Nucleus of Amygdala | 1.0 ± 0.5 | 0.8 ± 0.2 | 1.3 ± 0.6 | 1.6 ± 0.6 |
| Medial Nucleus of Amygdala | 1.4 ± 0.8 | 0.6 ± 0.3 | 2.3 ± 1.3 | 1.2 ± 0.5 |
| Anterior Amygdala | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.4 |
| Nucleus of the Lateral Olfactory Tract | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.6 ± 0.6 | 1.0 ± 0.5 |
| Olfactory Tubercle | 3.7 ± 1.1 | 4.5 ± 1.3 | 3.1 ± 0.3 | 3.0 ± 0.4 |
| Anterior Olfactory Nucleus* # | 1.6 ± 0.9 | 5.9 ± 1.1 | 1.9 ± 1.0 | 5.0 ± 0.8 |
| Main Olfactory Bulb | 0.3 ± 0.2 | 0.8 ± 0.3 | 1.0 ± 0.6 | 1.6 ± 0.6 |
| Piriform Cortex | 14.3 ± 6.0 | 19.1 ± 3.6 | 4.1 ± 0.5 | 5.8 ± 0.8 |
| Bed Nucleus of Stria Terminalis | 1.0 ± 0.5 | 1.2 ± 0.3 | 1.9 ± 1.0 | 2.1 ± 0.5 |
| Midbrain nuclei | ||||
| Periaqueductal grey | 1.4 ± 1.1 | 0.3 ± 0.2 | 1.7 ± 0.9 | 0.8 ± 0.4 |
| Raphe nucleus | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.0 ± 0.0 |
| Ventral tegmental area | 1.1 ± 0.6 | 1.2 ± 0.4 | 2.3 ± 0.9 | 2.6 ± 0.8 |
| Interpeduncular Nucleus | 0.3 ± 0.3 | 0.2 ± 0.1 | 0.4 ± 0.4 | 1.2 ± 0.9 |
indicates significant differences for number of voxels
indicates differences for BOLD signal changes (ANOVA, p < 0.05).
Table 2.
Negative BOLD signal changes (signal decreases) in lactating rats presented with a male intruder in the absence or presence of V1a receptor blockade.
| Number of Negative BOLD voxels | Mean Percent Change in BOLD signal | |||
|---|---|---|---|---|
| Region of Interest | Control ± Std. Error | V1a antagonist ± Std. Error |
Control ± Std. Error | V1a antagonist ± Std. Error |
| Cortical areas | ||||
| Visual Cortex | 16.7 ± 5.3 | 19.5 ± 5.8 | −2.9 ± 0.6 | −2.7 ± 0.4 |
| Posterior Cingulate Cortex | 16.1 ± 4.4 | 15.6 ± 3.4 | −3.1 ± 0.8 | −5.1 ± 1.2 |
| Parietal Cortex | 13.7 ± 4.0 | 9.0 ± 2.4 | −3.2 ± 0.8 | −2.9 ± 0.3 |
| Auditory Cortex | 17.4 ± 3.2 | 18.4 ± 3.3 | −3.0 ± 0.4 | −3.4 ± 0.3 |
| Temporal Cortex | 4.4 ± 1.4 | 6.9 ± 1.5 | −2.7 ± 0.2 | −4.1 ± 0.7 |
| Insular Cortex | 12.0 ± 1.7 | 10.8 ± 2.9 | −2.9 ± 0.3 | −3.6 ± 0.5 |
| Anterior Cingulate Cortex | 9.0 ± 2.5 | 6.9 ± 2.1 | −3.1 ± 0.3 | −2.9 ± 0.3 |
| Gustatory Cortex | 3.4 ± 0.6 | 2.7 ± 0.8 | −2.5 ± 0.2 | −2.6 ± 0.4 |
| Prelimbic Cortex | 3.7 ± 1.0 | 3.5 ± 1.3 | −2.2 ± 0.4 | −1.7 ± 0.4 |
| Infralimbic Cortex | 1.9 ± 0.9 | 1.7 ± 0.7 | −1.1 ± 0.6 | −1.5 ± 0.5 |
| Orbital Cortex | 7.4 ± 2.2 | 7.2 ± 2.0 | −2.3 ± 0.5 | −2.6 ± 0.2 |
| Primary Somatosensory Cortex | 92.9 ± 24.3 | 92.2 ± 24.4 | −4.2 ± 1.3 | −4.3 ± 0.4 |
| Primary Motor Cortex | 29.9 ± 7.9 | 25.3 ± 7.4 | −2.9 ± 0.5 | −2.7 ± 0.2 |
| Supplementary Motor Region | 35.6 ± 7.6 | 33.4 ± 10.8 | −3.4 ± 0.7 | −3.3 ± 0.5 |
| Secondary Somatosensory Cortex | 13.1 ± 3.3 | 14.7 ± 3.3 | −3.9 ± 0.9 | −4.6 ± 0.5 |
| Somatosensory Cortex, Upper Limb Region |
0.4 ± 0.3 | 0.1 ± 0.1 | −0.6 ± 0.4 | −0.2 ± 0.2 |
| Somatosensory Cortex, Node Region | 0.6 ± 0.3 | 0.2 ± 0.2 | −1.1 ± 0.5 | −0.2 ± 0.2 |
| Thalamic nuclei | ||||
| Thalamus | 9.4 ± 2.2 | 9.7 ± 2.7 | −3.3 ± 0.5 | −3.0 ± 0.3 |
| Lateral Posterior Thalamus | 1.6 ± 0.5 | 1.1 ± 0.5 | −1.8 ± 0.5 | −1.5 ± 0.4 |
| Ventral Nucleus of Thalamus | 6.6 ± 1.2 | 5.3 ± 1.2 | −3.7 ± 0.5 | −3.4 ± 0.2 |
| Lateral Dorsal Nucleus of Thalamus | 0.4 ± 0.3 | 1.5 ± 0.6 | −0.8 ± 0.5 | −1.7 ± 0.5 |
| Centrolateral Nucleus of Thalamus | 0.6 ± 0.2 | 0.3 ± 0.2 | −1.8 ± 0.7 | −0.8 ± 0.4 |
| Habenula | 1.0 ± 0.4 | 1.5 ± 0.6 | −2.0 ± 0.8 | −1.9 ± 0.6 |
| Mediodorsal Nucleus of Thalamus | 2.0 ± 0.8 | 1.2 ± 0.4 | −1.7 ± 0.6 | −1.5 ± 0.4 |
| Midline Thalamus | 0.6 ± 0.3 | 1.6 ± 0.7 | −1.1 ± 0.5 | −1.4 ± 0.5 |
| Anterior Thalamus | 1.3 ± 0.6 | 1.2 ± 0.5 | −1.6 ± 0.6 | −1.3 ± 0.4 |
| Hippocampal formation and limbic areas | ||||
| Hippocampal CA1 | 18.4 ± 5.3 | 15.0 ± 4.0 | −2.6 ± 0.3 | −2.8 ± 0.3 |
| Subiculum # | 4.3 ± 2.0 | 1.1 ± 0.5 | −3.1 ± 0.3 | −1.3 ± 0.5 |
| Dentate Gyrus | 7.9 ± 2.2 | 8.1 ± 2.3 | −2.5 ± 0.2 | −2.3 ± 0.3 |
| Hippocampal CA3 | 7.3 ± 1.9 | 8.2 ± 1.9 | −2.8 ± 0.1 | −2.7 ± 0.2 |
| Hippocampal CA2 | 0.9 ± 0.4 | 0.2 ± 0.1 | −1.1 ± 0.6 | −0.5 ± 0.4 |
| Perirhinal area | 1.9 ± 0.3 | 1.7 ± 0.4 | −3.0 ± 0.2 | −3.1 ± 0.9 |
| Entorhinal area | 9.3 ± 2.5 | 5.0 ± 1.0 | −4.7 ± 0.5 | −6.3 ± 0.9 |
| Septum | 6.0 ± 1.3 | 6.2 ± 1.2 | −3.3 ± 0.9 | −2.9 ± 0.1 |
| Basal ganglia, hypothalamus and basal forebrain nuclei | ||||
| Dorsal Striatum | 30.9 ± 4.1 | 28.2 ± 5.8 | −2.7 ± 0.2 | −2.9 ± 0.2 |
| Substantia Nigra # | 1.1 ± 0.6 | 4.4 ± 1.6 | −1.2 ± 0.6 | −3.2 ± 0.6 |
| Posterior Hypothalamic Nucleus | 1.1 ± 0.3 | 1.2 ± 0.4 | −2.9 ± 0.5 | −2.2 ± 0.6 |
| Mammillary region | 0.3 ± 0.2 | 1.5 ± 0.8 | −0.8 ± 0.5 | −1.5 ± 0.6 |
| Lateral Hypothalamus | 8.9 ± 1.6 | 6.5 ± 1.7 | −3.8 ± 0.3 | −3.3 ± 0.1 |
| Preoptic area # | 1.3 ± 0.3 | 1.4 ± 0.5 | −3.8 ± 0.7 | −1.9 ± 0.5 |
| Paraventricular Nucleus of Hypthalamus |
0.7 ± 0.4 | 0.2 ± 0.1 | −1.6 ± 0.8 | −0.7 ± 0.5 |
| Anterior Hypothalamic Area | 0.4 ± 0.3 | 0.5 ± 0.4 | −1.0 ± 0.7 | −0.6 ± 0.4 |
| Periventricular Hypothalamus | 0.4 ± 0.2 | 0.4 ± 0.2 | −1.5 ± 0.8 | −1.1 ± 0.6 |
| Dorsomedial Hypothalamus | 1.0 ± 0.4 | 0.9 ± 0.3 | −1.9 ± 0.7 | −1.8 ± 0.6 |
| Ventromedial Hypothalamus | 1.1 ± 0.6 | 0.9 ± 0.3 | −1.8 ± 0.7 | −2.7 ± 0.9 |
| Arcuate Nucleus | 0.7 ± 0.4 | 0.3 ± 0.2 | −2.1 ± 1.1 | −1.4 ± 0.7 |
| Globus Pallidus | 1.4 ± 0.2 | 1.7 ± 0.6 | −3.6 ± 0.6 | −2.1 ± 0.5 |
| Islands of Cajal area | 0.1 ± 0.1 | 0.5 ± 0.2 | −0.4 ± 0.4 | −2.0 ± 0.9 |
| Nucleus Accumbens | 5.1 ± 1.1 | 3.8 ± 0.9 | −2.9 ± 0.3 | −3.3 ± 0.2 |
| Substantia Innominata | 2.1 ± 0.4 | 1.2 ± 0.4 | −2.9 ± 0.2 | −2.4 ± 0.6 |
| Amygdala and olfactory system | ||||
| Cortical Nucleus of Amygdala | 6.6 ± 1.6 | 5.0 ± 1.7 | −4.2 ± 0.4 | −3.5 ± 0.9 |
| Posterior Nucleus of Amygdala | 0.4 ± 0.2 | 0.5 ± 0.3 | −2.2 ± 1.2 | −1.6 ± 0.8 |
| Lateral Nucleus of Amygdala | 0.7 ± 0.4 | 0.7 ± 0.4 | −1.7 ± 0.8 | −1.8 ± 0.8 |
| Basal Nucleus of Amygdala | 1.9 ± 0.5 | 0.9 ± 0.3 | −3.3 ± 0.5 | −2.5 ± 0.7 |
| Periform Amygdaloid Nucleus | 0.9 ± 0.3 | 0.6 ± 0.2 | −2.9 ± 0.8 | −4.5 ± 2.0 |
| Central Nucleus of Amygdala | 2.0 ± 0.7 | 0.8 ± 0.3 | −2.3 ± 0.6 | −1.6 ± 0.5 |
| Medial Nucleus of Amygdala | 1.4 ± 0.4 | 1.2 ± 0.4 | −4.5 ± 1.4 | −2.4 ± 0.6 |
| Anterior Amygdala | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Nucleus of the Lateral Olfactory Tract | 0.4 ± 0.2 | 0.2 ± 0.1 | −1.3 ± 0.7 | −0.6 ± 0.4 |
| Olfactory Tubercle # | 3.0 ± 0.8 | 5.3 ± 1.2 | −2.3 ± 0.4 | −3.9 ± 0.3 |
| Anterior Olfactory Nucleus | 2.7 ± 1.1 | 3.9 ± 1.1 | −2.2 ± 0.4 | −2.8 ± 0.6 |
| Main Olfactory Bulb | 0.9 ± 0.6 | 1.1 ± 0.4 | −1.1 ± 0.5 | −2.4 ± 0.7 |
| Piriform Cortex # | 16.9 ± 3.6 | 16.8 ± 2.8 | −4.0 ± 0.2 | −5.9 ± 0.7 |
| Bed Nucleus of Stria Terminalis * | 2.3 ± 0.8 | 0.7 ± 0.3 | −2.2 ± 0.4 | −1.5 ± 0.6 |
| Midbrain nuclei | ||||
| Periaqueductal grey | 2.6 ± 1.2 | 2.1 ± 0.8 | −2.6 ± 0.6 | −2.3 ± 0.6 |
| Raphe nucleus | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | −0.8 ± 0.5 |
| Ventral tegmental area | 0.4 ± 0.3 | 0.6 ± 0.4 | −0.8 ± 0.5 | −0.7 ± 0.5 |
| Interpeduncular Nucleus | 0.4 ± 0.2 | 0.3 ± 0.2 | −1.7 ± 0.9 | −1.1 ± 0.8 |
indicates significant differences for number of voxels
indicates differences for BOLD signal changes (ANOVA, p < 0.05).
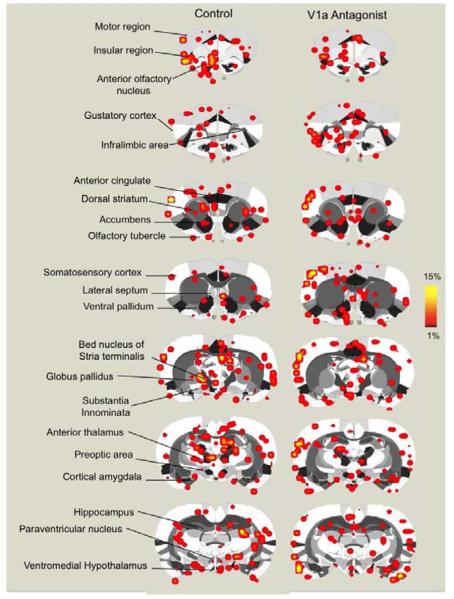
Figure 1.
Composite coronal brain maps showing positive BOLD signal changes in control and V1a antagonist treated dams presented with a threat to pups. Scale bar hue (red-to-yellow) indicates percent increase in BOLD with a lower threshold cut-off of 1%. Various regions of interest are highlighted to the left of the figure.
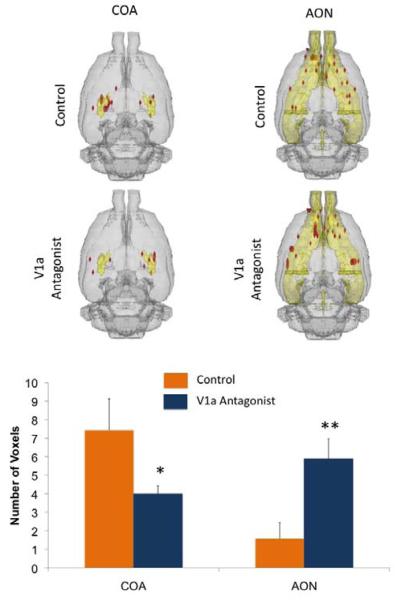
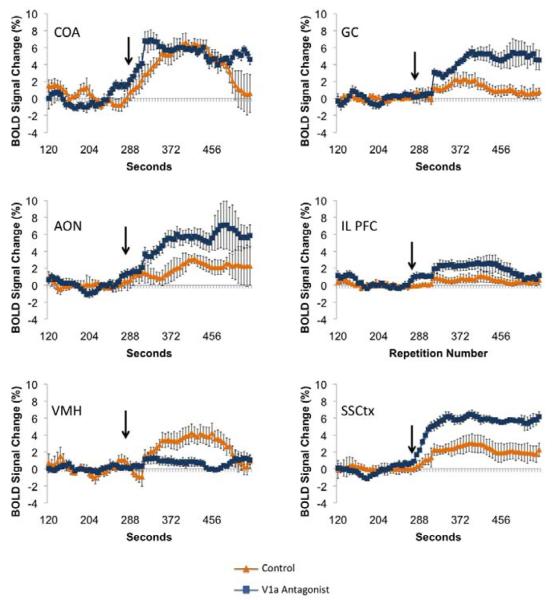
Dams treated with the V1a receptor antagonist showed significantly less positive BOLD voxels (representing volumes of activation) in the cortical amygdala (COA, F1,15 = 5.2, p = 0.03) and ventromedial hypothalamus (data not shown, VMH, F1,15 = 4.7, p = 0.04) (Figure 2). It is important to point out, however, that the VMH showed scant voxel activations in general, the V1a group showing very little or no voxel activation, whereas the control group largely showed a range of 0-2 activated voxels across subjects. Interestingly, there was an increased positive BOLD activation with the V1a antagonist in the anterior olfactory nucleus (AON, F1,15 = 8.6, p = 0.01). Thus, the COA and AON showed opposite responses with antagonist treatment (Figure 2). When viewed in terms of changes in BOLD signal intensity over time (which represents presumed changes in neuronal activity), we observed that the gustatory cortex (GC, F1,15 = 12.1, p = 0.003), infralimbic (IL PFC, F1,15 = 4.5, p =0.05), AON (F1,15 = 6.4, p = 0.02) and somatosensory cortex (SSCtx, F1,15 = 17.1 p = 0.0008) all showed increased BOLD signal intensity with intruder male presentation and V1a receptor blockade, however the opposite response was observed in the VMH (F1,15 = 7.9, p = 0.01)(Figure 3).
Figure 2.
Number of positive BOLD voxels in control and V1a antagonist treated dams presented with a threat to pups. Top: Composite 3D brain volume maps showing positive BOLD signal changes in the amygdaloid complex and the olfactory system. Bottom: Number of positive BOLD voxels (mean ± SEM) in the cortical amygdala (COA) and anterior olfactory system (AON) of control and V1a antagonist treated dams. * F = 5.2, p = 0.03 and ** F = 8.6, p = 0.01, single factor analysis of variance.
Figure 3.
Positive BOLD signal changes over time for several regions of interest for control and V1a antagonist treated dams presented with a threat to pups. Arrow indicates the point of stimulus presentation during the course of the scans.
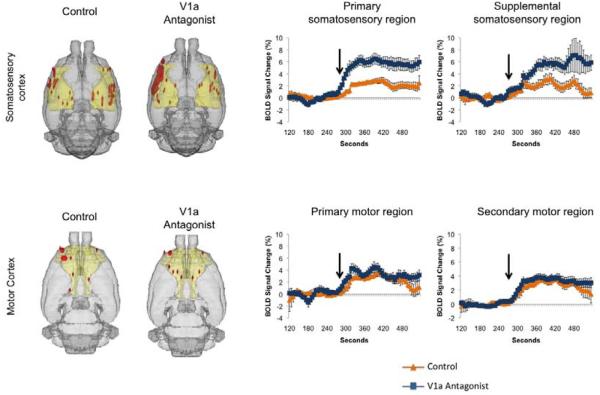
To verify if there were any differences in responsiveness of the sub-areas of the somatosensory cortex modulated by the V1a receptor antagonist, we subdivided the ROI into primary, supplemental, node and upper limb subregions. We observed that both the primary (F1,15 = 13.4, p = 0.002) and supplementary regions (F1,15 = 16.5, p = 0.001 ) showed greater percentage increases in BOLD with the V1a antagonist, but the other 2 sub areas (Figure 4). The differences in percentage BOLD response in these regions, however, were not accompanied by significant differences in volume of activation (Figure 4). To test whether the greater percent changes in BOLD with V1a receptor blockade were generalized across wide regions of the cortex we also subdivided areas of the motor cortex. We did not observe any differences in percent changes in BOLD or volume of activation in the primary or secondary motor regions (Figure 4). No differences in percent change of volume of activation were observed in thalamic nuclei projecting to the somatosensory cortex (data not shown). Wozniak et al. (Wozniak et al., 1989) reported that cholinergic neurons in the basal nucleus of Meynert (nucleus basalis magnocellularis in rats) located within the substantia innominata (SI) area of the basal forebrain also modulate somatosensory processing through direct projections to the cerebral cortex. Although we did not observe significant differences in volumes of activation in the SI, we did find that antagonist pretreatment evoked significantly greater BOLD percent activation during the first 60 seconds after intruder introduction into the vivarium (data not shown, F1,15 = 6.9, p = 0.02). This was not significant when the entire time course was averaged for the full 5 minutes (p = 0.06).
Figure 4.
Cortical somatosensory and motor activity in dams presented with a threat to pups. Composite 3D brain volume maps showing positive BOLD signal changes in somatosensory cortex and motor cortical areas. Positive BOLD signal changes (mean ± SEM) over time for somatosensory and motor cortical subregions in control and V1a antagonist treated dams presented with a threat to pups. Arrow indicates the point of stimulus presentation during the course of the scans.
DISCUSSION
The present study shows that central blockade of V1a receptors modulated BOLD signal responses in primiparous dams presented with an intruder threat to pups. The differential BOLD responses were not generalized across the brain, but were site specific. V1a receptor antagonist enhanced the volume of BOLD activation in the AON and reduced it in the COA and VMH. Greater percentage increases in BOLD signal were observed across several brain areas in response to V1a antagonist treatment, including the AON, GC, IL mPFC, SI and SSCtx. An unexpected finding of the present study was that V1a receptor blockade significantly enhanced BOLD signal in the SSCtx during intruder presentation. The greater BOLD signal response occurred in both primary and supplemental areas, but was not generalized to the overall cortical mantle. The heightened response observed in the SSCtx was not present in the primary or secondary motor cortical areas, or in the parietal or temporal cortices. Thus, the somatosensory modulation by V1a receptors appears to be selective for this cortical area. The only region that showed a lower percentage change in BOLD with V1a receptor blockade was the VMH. Our results indicate that V1a receptors modulate neural processing in specific neural circuits recruited during a timeframe corresponding to the initial phases of maternal aggressive motivation. These V1a sensitive circuits include structures which are involved in sensory processing, the control of visceral responses, and emotional memory (see Figure 5).
Figure 5.
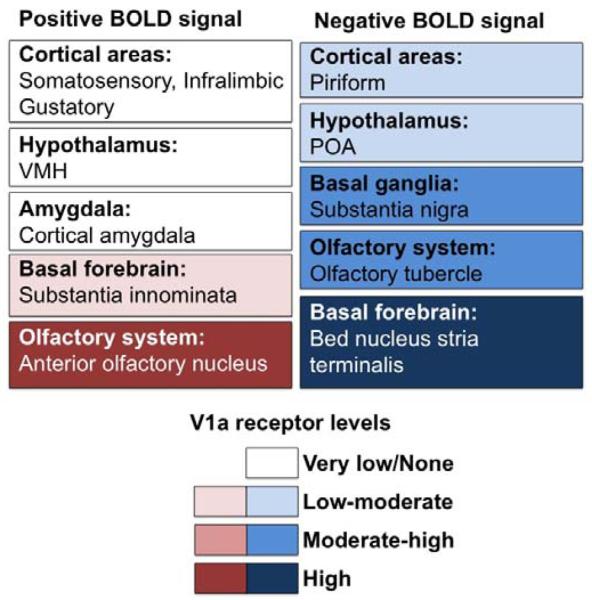
Diagram illustrating maternal brain areas that responded to the presentation of an intruder male rat and that were sensitive to V1a receptor blockade. Both positive and negative BOLD signal changes are shown in relation to brain V1a receptor quantities as reported by Ostrowski et al. (1994). Shown below is a color scale indicating V1a receptor levels from none to high. The directions and magnitudes of the observed differences are not indicated.
Experiments studying the role of V1a receptors in aggressive motivation and anxiety-related behaviors in rodents are well documented. Most studies have focused on male rats, although more recent work has focused on the role of vasopressin in maternal care and aggression. Bosch and Neumann (Bosch and Neumann, 2008) reported that central vasopressin receptors play a direct role in maternal behaviors such as time spent by the dam on pups, retrieval, and kyphotic nursing. Their results point to V1a receptors in the medial preoptic area, and not oxytocin receptors, as key mediators of maternal care and anxiogenic responses (Bosch and Neumann, 2008). Although in the present study we did not observe an effect of blocking V1a receptors on maternal POA or PVN BOLD response to a male intruder, we have previously reported BOLD activation within these brain areas with suckling stimulation (Febo et al., 2005). Preoptic area BOLD activation in response to suckling was responsive to oxytocin receptor blockade and shared similarities with oxytocin receptor stimulation (Febo et al., 2005). The pattern of POA activation observed with the less selective oxytocin receptor antagonist in the Febo et al study may be mediated instead through V1a receptors. It is possible that activation in these regions was not observed in this experiment due to the experimental paradigm. Although the maternal rat is fully awake, she is restrained and is therefore unable to execute maternal aggressive behaviors. These brain regions may be involved in, or a consequence of, the physical component of maternal care and/or aggression as opposed to the aggressive motivation, and therefore BOLD activation was not observed in this particular study.
Cellular expression of V1a protein and mRNA has been detected in distributed areas of the olfactory system, extended amygdala, basal forebrain, and hypothalamus, with scarce quantities observed in cortical areas (Ostrowski et al., 1994a). The pattern of V1a localization throughout the brain partly parallels the sets of brain areas activated in the present study using functional MRI. Attenuated positive BOLD responses of the COA and VMH following V1a antagonist treatment support the notion that these nuclei are involved in the AVP-mediated inhibitory control of maternal aggression and/or anxiety. There is evidence in the literature for a role of vasopressin in these brain areas in maternal aggression. Vasopressinergic activity in the COA has been linked to social behavior in hamsters (Albers et al., 1992; Ferris et al., 1993), and V1a receptors have been located in the VMH of rats (Ostrowski et al., 1994b). Inhibitory roles for the COA and VMH have been reported in studies of male rats. Long Evans males with lesions to the amygdala have decreased fear responses to the presence of a cat (Blanchard and Takahashi, 1988), and the COA has inhibitory control over mouse killing (Karli et al., 1977). In the VMH, lesions advance the onset of maternal behavior in primiparous females, and it is argued that this region has inhibitory control over maternal aggression as well as maternal behavior (Mann and Babb, 2004). This hypothesis is supported by the observation that pup-directed and maternal aggressive responses of mother rats develop virtually simultaneously (Mayer and Rosenblatt, 1984), and vasopressin has been implicated in both behaviors. Previous studies in both primiparous (Nephew and Bridges, 2008b) and multiparous (Nephew and Bridges, 2009) Sprague Dawley rats, as well as recent studies in Long Evans females (unpublished data) support the hypothesis that V1a antagonist treatment increases maternal aggression. It is proposed that the V1a antagonist inhibition of neural activity in the COA and VMH of maternal females exposed to male intruders allows for the enhanced display of maternal aggression. It is important to note, however, that the VMH findings are based on a small amount of voxels and deserve further investigation, perhaps using other invasive techniques.
Elevated BOLD in the AON following ICV V1a antagonist may reflect decreased inhibitory activity by endogenous AVP in this region, where increased olfactory activity may be associated with increased aggression. It is not surprising that the COA and AON would interact given their synaptic connectivity (Haberly and Price, 1978), and the presence of V1a receptors within these areas (Ostrowski et al., 1994b). Both V1a mRNA (Szot et al., 1994) and vasopressin (Hernando et al., 2001) have been located in the rat AON. It is clear that olfaction plays a significant role in maternal aggression, as surgically removing the olfactory bulb (Kolunie and Stern, 1995) or olfactory epithelium (Ferreira et al., 1987) decreases maternal aggression. It is postulated that V1a antagonist treatment facilitates increased stimulatory activity by the odor of a male intruder by decreasing AVP levels in the AON, and this activity may mediate the previously documented increase in maternal aggression following ICV V1a antagonist. It should also be noted that pup suckling stimulates increased BOLD in the AON (Febo et al., 2005). Since recent contact with pups is necessary for the normal display of maternal aggression (Erskine et al., 1978; Stern and Kolunie, 1993), it is possible that increased BOLD in the AON following V1a antagonist treatment represents an enhancement of pup-mediated responses to intruder males.
The imaging experimental design used in the present work simulates previous work by Ferris et al. (Ferris et al., 2008). Using a resident-intruder model, Ferris and co-workers tested the neural response of a resident male to an intruder male in the presence of the resident female. Bench top studies confirmed pilo-erection during the temporal window used to analyze the fMRI data, thus partly substantiating that aggressive motivation occurs under such experimental circumstances. However, it is clear that aggressive displays are not possible during fMRI scanning and other important motivational aspects of aggressive intent, other than pilo-erection, cannot occur. An alternate view of the experimental design used here is that the dams, which are incapable of defending or protecting pups, are experiencing anxiety rather than aggression. Rats selectively bred for high anxiety related behaviors (HAB) show greater basal density of V1a receptors in hypothalamic paraventricular nucleus (PVN) and also show higher basal levels of vasopressin in the same region (Wigger et al., 2004). Anxiety related behaviors were also blocked in these animals with a vasopressin receptor antagonist (Wigger et al., 2004). HAB animals have also been shown to display increased maternal aggression (Bosch et al., 2005). In light of this possibility, V1a receptor modulation of maternal neural processing may correspond to anxiety-related brain circuitry instead of the motivation to attack.
An important feature of the brain areas modulated by V1a receptors is that they share connectivity with the orbital prefrontal cortex in the rats (Ongur and Price, 2000). Gustatory, infralimbic, olfactory, somatosensory and amygdalar networks are processed through this limbic cortical structure that plays an important role in sensory and visceromotor associations (Gabbott et al., 2005; Ongur and Price, 2000; Vertes, 2004). Although V1a receptors are not localized in GC, IL mPFC and SSCtx, the observed enhancement might be achieved through indirect subcortical actions of this receptor antagonist (Figure 5). Activation of the SSCtx and its modulation by V1a receptors in interesting in light of the fact that vasopressin receptors have not been detected in this brain region and that a somatosensory stimulus was not presented to dams. Thalamic sensory relay nuclei were unaffected by antagonist treatment, and do not contain vasopressin V1a receptors. However, the SI, which has been shown to contain as of yet an understudied population of V1a receptors (Ostrowski et al., 1994b), sends major cholinergic projections to the cortical mantle, in particular the SSCtx and prefrontal cortex. Lesions of these cholinergic projections differ dramatically from sensory cortical ablations and alter emotional reactivity in rats (Knox et al., 2008; Wozniak et al., 1989). Indeed, it is possible that V1a receptors in this basal forebrain area and its interaction with the SSCtx may control aspects of behavioral reactivity. Due to the transient nature of the BOLD changes in the SI, it is postulated that this nucleus may mediate the immediate response to the male intruder.
Finally, it is important to point out that the present results in lactating rats are only in partial agreement with previous fMRI work in males (Ferris et al., 2008). Although similar brain areas are shown to be responsive to V1a blockade, the direction of the BOLD responses are opposite in dams versus males. For instance, Ferris et al report that SSCtx BOLD activation was reduced by administration of a peripherally injected antagonist whereas we report here that central administration of the Manning compound evokes the opposite effect. These opposing patterns of BOLD activation to ICV V1a antagonist in males and female are not surprising given that this antagonist has been shown to have opposite effects on male and maternal aggression (Nephew and Bridges, 2008b). The present results underscore the need for additional research on AVP receptors and maternal aggression, anxiety and emotion. Future studies combining behavioral assessments and fMRI will directly investigate the behavioral relevance of these neural changes.
Acknowledgements
The authors thank Ada C. Felix-Ortiz for her excellent technical assistance during portions of this study. This work was supported by NIDA NIH grant R01 DA019946 to Marcelo Febo. Its contents are solely the responsibility of the authors and do not represent the official views of the NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albers HE, Hennessey AC, Whitman DC. Vasopressin and the Regulation of Hamster Social Behavior. Annals of the New York Academy of Sciences. 1992;652:227–242. doi: 10.1111/j.1749-6632.1992.tb34358.x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Takahashi SN. No change in intermale aggression after amygdala lesions which reduce freezing. Physiology & Behavior. 1988;42:613–616. doi: 10.1016/0031-9384(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. J Neuroscience. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. PNAS. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Mann PE. Prolactin-brain interactions in the induction of maternal behavior in rat. Psychoneuroendocrinology. 1994;19:611–622. doi: 10.1016/0306-4530(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behavioral Biology. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J. Neuroscience. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139:167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Dahlof LG, Hansen S. Olfactory mechanisms in the control of maternal aggression, appetite, and fearfulness: effects of lesions to olfactory receptors, mediodorsal thalamic nucleus, and insular prefrontal cortex. Behavioral Neuroscience. 1987;101:709–717. doi: 10.1037//0735-7044.101.5.709. [DOI] [PubMed] [Google Scholar]
- Ferris C, Foote K, Meltser H, Plenby M, Smith K, Insel T. Oxytocin in the Amygdala Facilitates Maternal Aggression. Ann NY Acad Sci. 1992;652:456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Grzonka Z, Luber-Narod J, Insel TR. An iodinated vasopressin (V1) antagonist blocks flank marking and selectively labels neural binding sites in golden hamsters. Physiology and Behavior. 1993;54:737–747. doi: 10.1016/0031-9384(93)90085-t. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr., Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. and Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon a. N. G. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neuroscience. 2008:9. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin M, LP C, AB L. Hypothalamic paraventricular nuclues, oxytocin, and maternal aggression in rats. Ann NY Acad Sci. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the Evolutionary Patterning of Sociality. Progress in Brain Research. 2008:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anotomical characteristics of vasotocin/vasopressin systems in vertibrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Karli P, Vergnes M, Eclancher F, Penot C. Involvement of amygdala in inhibitory control over aggression in the rat: A synopsis. Aggressive Behavior. 1977;3:157–162. [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. Journal of Neuroscience Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Brothers H, Norman GJ, Berntson GG. Nucleus basalis magnocellularis and substantia innominata corticopetal cholinergic lesions attenuate freezing induced by predator odor. Behav Neurosci. 2008;122:601–610. doi: 10.1037/0735-7044.122.3.601. [DOI] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM. Maternal Aggression in Rats: Effects of Olfactory Bulbectomy, ZnSO4-Induced Anosmia, and Vomeronasal Organ Removal. Hormones and Behavior. 1995;29:492–518. doi: 10.1006/hbeh.1995.1285. [DOI] [PubMed] [Google Scholar]
- Kolunie JM, Stern JM, Barfield RJ. Maternal aggression in rats: Effects of visual or auditory deprivation of the mother and dyadic pattern of ultrasnic vocalizations. Behavioral and Neural Biology. 1994;62:41–49. doi: 10.1016/s0163-1047(05)80057-3. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Ludwig R, Bodgdanov G, King J, Allard A, Ferris CF. A dual RF resonator system for high-field functional magnetic resonance imaging of small animals. J Neurosci Methods. 2004;132:125–135. doi: 10.1016/j.jneumeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Mann PE, Babb JA. Disinhibition of maternal behavior following neurotoxic lesions of the hypothalamus in primigravid rats. Brain Research. 2004;1025:51–58. doi: 10.1016/j.brainres.2004.07.064. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Prepartum changes in maternal responsiveness and nest defense in Rattus norvegicus. Journal of Comparative Psychology. 1984;98:177–188. [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology & Behavior. 2008a;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology, Biochemistry and Behavior. 2008b;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Currently under review for Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced Maternal Aggression and Associated Changes in Neuropeptide Gene Expression in Reproductively Experienced Rats. Behavioral Neuroscience submitted. 2009 doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Numan M. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Developmental Psychobiology. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ostrowski N, Lolait S, Young W., 3rd Localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Journal of Endocrinology. 1994a;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994b;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Academic Press; Boston: 1997. [Google Scholar]
- Pedersen C, Caldwell J, Walker C, Ayers G, Mason G. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiology & Behavior. 1993;54:861–868. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier Science; Boston: 1999. [Google Scholar]
- Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Molecular Brain Research. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubios-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Research. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Stewart GR, Finger S, Olney JW. Comparison of behavioral effects of nucleus basalis magnocellularis lesions and somatosensory cortex ablation in the rat. Neuroscience. 1989;32:685–700. doi: 10.1016/0306-4522(89)90290-x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]