Abstract
Background:
In conducting clinical high resolution esophageal pressure topography (HREPT) studies we observed that after subjects sat upright between series of supine and upright test swallows, they frequently had a transient lower esophageal sphincter relaxation (tLESR). When achalasia patients were studied in the same protocol, they exhibited a similar HREPT event leading to the hypothesis that achalasics had incomplete tLESRs.
Methods:
We reviewed clinical HREPT studies of 94 consecutive non-achalasics and 25 achalasics. Studies were analyzed for a tLESR-like event during the study and, when observed, that tLESR-like event was characterized for the degree and duration of distal esophageal shortening, the degree of LES relaxation, associated crural diaphragm (CD) inhibition, esophageal pressurization, and upper esophageal sphincter (UES) relaxation.
Results:
64/94 (68%) non-achalasics and 15/24 (63%) of achalasics had a pressure topography event after the posture change characterized by a prolonged period of distal esophageal shortening and/or LES relaxation. Events among the non-achalasics and achalasics were similar in terms of magnitude and duration of shortening and all were associated with CD inhibition. Similar proportions had associated non-deglutitive UES relaxations. The only consistent differences were the absence of associated LES relaxation and the absence of HREPT evidence of reflux among the achalasics leading us to conclude that their events were incomplete tLESRs.
Conclusions:
Achalasic patients exhibit a selective defect in the tLESR response suggesting preservation of all sensory, central, and efferent aspects of the requisite neural substrate with the notable exception of LES relaxation, a function of inhibitory (nitrergic) myenteric plexus neurons.
Keywords: Achalasia, transient lower esophageal sphincter relaxation, high-resolution manometry, esophageal shortening
Transient lower esophageal sphincter relaxation (tLESR) is a reflex to allow gas venting from the stomach that is also one of the key mechanisms underlying both physiological and pathophysiological gastroesophageal reflux.1-4 Gastric distension, particularly of the cardia, is the primary trigger of this neurally mediated event.1,2,5,6 Distention of the proximal stomach stimulates vagal afferents that relay this input to the medulla. Medullary nuclei then orchestrates the efferent limb of the reflex via the vagal and phrenic nerves to elicit: (i) prolonged LES relaxation via vagally activated postganglionic nitrergic myenteric plexus neurons, (ii) crural diaphragm (CD) inhibition via selective (central) phrenic nerve inhibition, and (iii) distal esophageal shortening via vagally activated postganglionic cholinergic myenteric plexus neurons - the three essential components of a complete tLESR. Should reflux occur during a tLESR, esophageal pressurization and upper esophageal sphincter (UES) relaxation may also be observed.7 Each of these tLESR components has a characteristic signature when imaged in high resolution esophageal pressure topography (HREPT).8,9 In our laboratory the current standard clinical HREPT protocol first assesses the patient's esophageal motor function in the supine position after which a subsequent series of test swallows is obtained with the patient sitting upright. In carrying out this protocol, we observed that a spontaneous tLESR frequently occurs almost immediately after the change of body position, presumably a consequence of the patient having swallowed a substantial volume of air and water in the course of intubation and supine swallow testing.
One of the main indications for esophageal motility testing (HREPT in our institution) is to detect achalasia, a disorder characterized by impaired LES relaxation and absent peristalsis.10 Conceptually, achalasia is an autoimmune disease affecting the myenteric plexus within the LES and smooth muscle esophagus. Increasing evidence suggests that the autoimmune process is attributable to a latent infection with human herpes virus-1 in genetically susceptible individuals.11,12 Furthermore, particularly in early disease prior to profound esophageal dilatation and retention, this autoimmune attack leads to selective dysfunction of the nitrergic (inhibitory) myenteric plexus neurons, thereby explaining the hallmark functional defects in achalasia: impaired LES relaxation and loss of propagation of the peristaltic contraction.13 Our experience with HREPT is that many achalasia patients we diagnose meet this definition of early disease with minimal esophageal dilatation.10 Serendipitously, in conducting these clinical studies we noted that when achalasia patients sat upright between the supine and the sitting parts of the study protocol, similar to their non-achalasic counterparts, they often had an esophageal pressure topography event with many similarities to a tLESR; the only notable missing component was the LES relaxation. This lead us to hypothesize that early achalasia is associated with selective dysfunction of the tLESR response; only the component mediated by inhibitory myenteric plexus neurons (LES relaxation) is compromised. Thus, the aim of this study was to analyze these pressure topography events occurring in achalasic patients with similarities to tLESRs to see if, in fact, they are incomplete tLESRs.
MATERIALS AND METHODS
Patients
This study utilized a consecutive series of clinical HREPT studies conducted between January 2008 and March 2008 comprised of 94 non-achalasia patients and 24 achalasia patients (67F; ages 12 - 91). Patients who had been treated for achalasia with Heller myotomy or recent (≤ 1 year) pneumatic dilation were excluded from the analysis. If performed, the endoscopy reports corresponding to the preintervention clinical HREPT studies were evaluated to grade the extent of esophageal dilatation.10 Dilatation was graded as absent if there was no mention of dilatation or retained secretions, mild if the endoscopist described possible dilatation, moderate if the endoscopist described dilatation with retained saliva secretions or liquid, and severe if the edoscopist described obvious dilatation and retained liquid or food.
High Resolution Esophageal Pressure Topography (HREPT) Protocol
High resolution esophageal pressure topography data were obtained using a solid-state manometric assembly (4.2 mm outer diameter) with 36 circumferential sensors spaced at 1-cm intervals (Sierra Scientific Instruments, Los Angeles, CA), the recording characteristics of which have been previously described.14-16 Studies were done after at least a 6-hour fast. Pressure transducers were calibrated at 0 and 100 mmHg using externally applied pressure prior to the study. Patients underwent trans-nasal placement of the manometry assembly, which was positioned to record from the hypopharynx to the stomach with about five intragastric sensors. The assembly was fixed in place by taping it to the nose. The study protocol included at least a 30 s period of baseline recording in a supine position followed by a series of ten 5 ml and two 10 ml test water swallows. Without repositioning the recording assembly or pausing the recording, the subject was then asked to sit upright. Once upright, another 30-60 s baseline recording was obtained followed by another sequence of twelve test swallows.
HREPT Data Analysis
Pressure topography data were analyzed using ManoView™ analysis software (Sierra Scientific Instruments, Los Angeles, CA, USA). The data were firstly corrected for the thermal sensitivity of the pressure-sensing elements using the thermal compensation function. Esophagogastric junction relaxation was then measured using the 4-s integrated relaxation pressure (IRP) tool on the ten 5 ml supine swallows with a mean value ≥ 15 mmHg qualifying as impaired relaxation.16,17 Post-deglutitive contractile function was then characterized in the ten supine water swallows as normal peristalsis, absent peristalsis, hypotensive peristalsis, spastic, elevated intrabolus pressure, or with pan-esophageal pressurization. The achalasia patient population encompassed all three subtypes: classic, achalasia with compression, and spastic achalasia.10 Type I (classic) achalasia was characterized by impaired EGJ relaxation and absent peristalsis with no distal esophageal pressurization to >30 mmHg in > 8 of the 10 test swallows. In Type II (achalasia with compression) there was impaired EGJ relaxation and at least two test swallows were associated with either pan-esophageal pressurization or compartmentalized pressurization of the distal esophagus to greater than 30 mmHg. Type III patients (spastic achalasia) had impaired EGJ relaxation and two or more spastic contractions following swallows with or without periods of compartmentalized pressurization. If patients were found to have more than two swallows with compression, but also had two or more spastic contractions, they were categorized as Type III. All other patients were grouped as non-achalasia patients.
The recording period encompassing and immediately following the body position change between the supine and upright swallow sequences was the major focus of analysis. It was within this interval that spontaneous tLESRs were often observed. Hence this interval was analyzed using the ManoView™ Isobaric Contour and Smart Mouse tools for prolonged periods of esophageal shortening and/or non-deglutitive LES relaxation to ≤4 mmHg. When either or both of these pressure topography events were identified, the concurrent period was analyzed for: (i) the maximal extent of associated distal esophageal shortening, (ii) nadir LES pressure, (iii) associated CD inhibition, (iv) associated esophageal pressurization, and (v) associated non-deglutitive UES relaxation.
Distal esophageal shortening caused by longitudinal muscle contraction was characterized by aboral shift of the pressure band indicative of the LES.9 The onset of esophageal shortening was defined visually as a time point when the LES pressure band began to elevate from its non-deglutitive location immediately after the subject's change of body position, while the end of esophageal shortening was denoted by return of LES pressure band to its approximate pre-event location. In instances of complete LES relaxation (nadir LES pressure near 0 mmHg), the final location of the LES pressure band prior to complete relaxation or the first spatial location at the end of relaxation was used to measure maximal shortening.
Nadir LES pressure was measured by progressively scaling up the Isobaric Contour Tool to the pressure value at which the contour line encompassing the LES pressure band first became discontinuous, indicative of a momentary LES pressure less than that isobaric contour pressure value. Nadir LES pressure was measured: (i) immediately following the change of body position, but prior to the onset of the period of esophageal shortening and (ii) during the period of esophageal shortening. The extent of esophageal shortening was measured using the Smart Mouse tool and given as the distance between the mid-LES prior to shortening and the greatest detectable elevation. When present, the duration of complete LES relaxation was also measured with the Smart Mouse tool.
An abrupt decrease in baseline UES pressure by ≤ 10 mmHg without an accompanying pharyngeal contraction that occurred during the period of esophageal shortening defined a non-deglutitive UES relaxation. These events are indicative of micro-burps in response to esophageal pressurization.8 The pressurization of the proximal and distal esophagus was calculated as the difference in the intraluminal pressure 4s prior to the onset of and during the period of esophageal shortening. Proximal esophageal pressure was taken as the average of three sensors located in the 3-5 cm band below the location of the lower UES margin during the period of esophageal shortening. Distal esophageal pressure was the average of three sensors in the 5-7 cm band above proximal LES margin; if a pressure band consistent with the new LES location during esophageal shortening was visible, the distal esophageal pressure was assessed relative to the new proximal LES margin.8
Statistical Analysis
Nadir LES pressures, the extent of esophageal shortening, duration of tLESR and esophageal pressurization were summarized using medians and 5th – 95th percentile range, unless otherwise indicated. The Mann-Whitney non-parametric test was used to compare the patient groups. A p-value less than 0.05 was considered significant.
RESULTS
Patient groups
Patient demographics are shown in Table 1. Of 118 patients, distal esophageal shortening and/or prolonged LES relaxation was elicited by the change from supine to upright position between series of test swallows in 64/94 (68 %) non-achalasia and 15/24 (63 %) achalasia patients. These 64 non-achalasia patients and 15 achalasics comprised the patient groups subject to further analysis. Among the fifteen achalasics, two (13 %) had Type I (classic), eleven (74 %) had Type II (with compression) and two (13 %) had Type III (spastic) achalasia.10 Upper endoscopy was performed in 12/15 patients on the same day as the HREPT study, reporting no esophageal dilatation in six (50 %), mild dilatation in four (34 %), moderate dilatation in one (8 %) and severe dilatation in one (8 %). None of the achalasia patients had measureable LES-CD separation, the HREPT signature of hiatal hernia18 whereas this was present in 17/64 (27%) of the non-achalasia patients.
Table 1.
Patient group demographics and defining features.
| Patient group | ||
|---|---|---|
| Non-achalasia (n=94) | Achalasia (n=24) | |
| Age (years)a | 52 (24 – 80) | 50 (30 – 89) |
| Gender (F:M) | 57 : 37 | 9 : 15 |
| Distal esophageal shortening and/or prolonged LES relaxationb |
64 (68%) | 15 (63%) |
| Distal esophageal shortening only | 4 | 7 |
| Prolonged LES relaxationb only | 10 | 1 |
Median (5th – 95th percentile)
Defined as any decrease in LES pressure
The group of achalasics in whom no distal esophageal shortening and/or prolonged LES relaxation was elicited by posture change were otherwise similar to the study subjects; they were also predominantly of Type I and II achalasia with minimal esophageal dilatation (4/9 Type I; 4/9 Type II; 1/9 Type III; 1/9 no dilatation, 7/9 mild dilatation, 1/9 severe dilatation).
Distal esophageal shortening
Distal esophageal shortening was elicited in 54/64 (84 %) non-achalasia patients (Figure 1) and 14/15 (93 %) achalasics (Figure 2). Inspecting these Figures, the shortening in achalasia patients appeared more pronounced at least in part because, in the absence of associated LES relaxation, it was possible to track the position of the LES through the entire period of shortening and the maximal degree of shortening occurred at a time when there would be associated LES relaxation, had it occurred. The summary data in Table 2 confirm the trend (p=0.06) that maximal shortening was greater amongst the achalasics but the caveat remains that it was possible to measure this more accurately in that group. Also evident in Table 2, the duration of distal esophageal shortening amongst the achalasics (21s) was comparable to the duration of prolonged LES relaxation and/or shortening in non-achalasia patients (17s). Although this difference was significant, the magnitude of the difference (4s) was at least partly attributable to the fact that it was LES relaxation driving the median in the majority of the non-achalasia patients and LES relaxation tended to occur only after the onset of shortening (compare Figures 1 and 2).
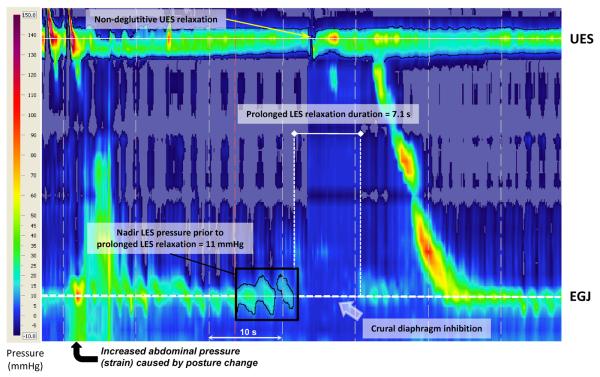
Figure 1.
High resolution esophageal pressure topography (HREPT) plot of a tLESR in a non-achalasic subject triggered shortly after assuming a sitting posture between a series of supine and upright test swallows. The nadir LES pressure prior to the prolonged LES relaxation was 11 mmHg, evident in the black box with the 11 mmHg isobaric contour highlighted in black. Note the pressure topography evidence of reflux immediately prior to the non-deglutitive UES relaxation indicative of a micro-burp. The isobaric contour surrounding the UES pressure band is 5 mmHg, clearly broken at the instant of the micro-burp. In this case, the tLESR ends with a secondary peristaltic contraction.
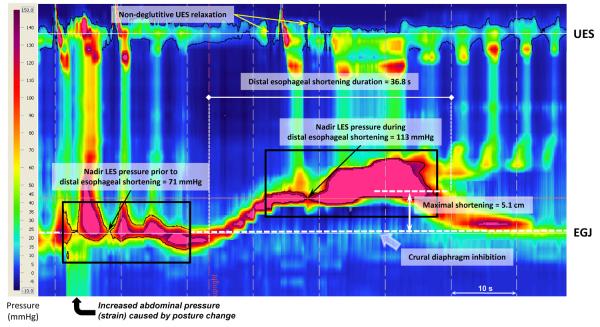
Figure 2.
High resolution esophageal pressure topography (HREPT) event that resembled a tLESR in an achalasic patient triggered shortly after assuming a sitting posture (similar to the scenario in Figure 1). Nadir LES pressure prior to the prolonged LES relaxation was 71 mmHg, evident in the black box with the 71 mmHg isobaric contour highlighted in black. Note that esophageal pressurization occurs in response to esophageal shortening rather than reflux; nadir LES pressure during the period of shortening is 113 mmHg. Again pressurization leads to non-deglutitive UES relaxation. The isobaric contour surrounding the UES pressure band is 5 mmHg, clearly broken at the instants of the micro-burps.
Table 2.
Characteristics of distal esophageal shortening and/or prolonged LES relaxation.
| Patient group | ||
|---|---|---|
| Non-achalasia (n=64) | Achalasia (n=15) | |
| Maximal shortening (cm)a | 1.8 (0 – 7.4) | 4.5 (0 – 5.7)† |
| Duration (s)a | 17 (5 – 27) | 21 (11 – 35)* |
| Nadir LES pressure (mmHg)a | 0 (0 – 8.9) | 32.0 (11.4 – 155.9)* |
Median (5th – 95th percentile)
p = 0.06
p < 0.05
Crural Diaphragm and LES Pressure changes during tLESR or distal shortening events
Distal esophageal shortening and/or prolonged LES relaxation events were accompanied by the inhibition of the crural diaphragm in all 64 (100%) non-achalasia and all 15 (100%) achalasia patients. Corresponding LES relaxation to ≤4 mmHg was seen in 57/64 (89 %) of non-achalasia patients (Table 2, Figure 3A) thereby fully qualifying these to be tLESRs. However, amongst the achalasics, median LES pressure was unchanged during the periods of distal esophageal shortening and, rather, substantially increased in seven of the fifteen individuals (Figure 3B). Similar to the patient illustrated in Figure 2, all seven of these individuals exhibiting a substantial increase in LES pressure during the period of distal shortening were Type II achalasics (with compression). However, whether the LES pressure decreased slightly, remained unchanged, or increased substantially, in no case did it relax to <4 mmHg qualifying the event as a complete tLESR. Hence, given that these distal esophageal shortening events in the achalasics were triggered by the same stimulus as were tLESRs in non-achalasics, exhibited a similar degree of shortening, had similar duration of shortening, and were similarly associated with CD inhibition, we labeled them incomplete tLESRs.
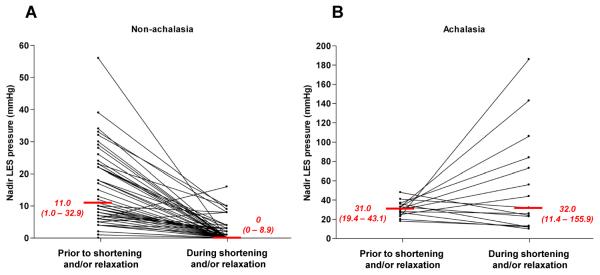
Figure 3.
Nadir LES pressure during periods of distal esophageal shortening and/or prolonged LES relaxation in non-achalasic patients (Panel A) and achalasia patients (Panel B). Median (5th - 95th) percentiles are indicated in red. In each case, nadir LES pressure during the event is compared to the lowest pressure during a comparable time period prior. Most (57/64) non-achalasia patients had LES pressure nadirs to ≤4 mmHg qualifying the event as a complete tLESR. On the other hand none of the fifteen achalasia patients had an LES pressure nadir ≤ 4 mmHg and half of them actually had significantly increased LES pressure during the shortening event. Note that the pressure scales of the two plots are different.
Intra-esophageal pressurization and non-deglutitive upper esophageal sphincter relaxation
Regardless of whether or not accompanying LES relaxation was observed, distal esophageal shortening events were associated with increased proximal and distal esophageal pressure (Table 3). However, as evident in Figure 4 the mechanism of pressurization was distinct depending upon whether the shortening event was indicative of a tLESR (Figure 4A) or and incomplete tLESR (Figure 4B). In the case of a complete tLESR, the associated complete LES relaxation facilitated reflux, presumably mainly gas, from the stomach and this led to near equalization of intragastric, intra-sphincteric, and intra-esophageal pressurization; note the spatial pressure variation plot on the right of Figure 4A. On the other hand, with an incomplete tLESR esophageal pressurization, which was quantitatively greater than with complete tLESR in the distal esophagus (p=0.0008), was not associated with gastroesophageal reflux as there was no permissive LES relaxation; again, note the spatial pressure variation plot to the right of Figure 4B. Rather, with incomplete tLESR, esophageal pressurization was presumably related to the compression of esophageal contents caused by reduced luminal volume. Accompanying non-deglutitive UES relaxation was observed in 37/64 (58 %) non-achalasia patients and 12/15 (80 %) achalasics suggesting this to be a consequence of proximal pressurization, regardless of the cause of that pressurization.
Table 3.
Intra-esophageal pressurization and UES relaxation related to distal esophageal shortening and/or prolonged LES relaxation response.
| Patient group | ||
|---|---|---|
| Non-achalasia (n=64) | Achalasia (n=15 | |
| Distal esophagus (mmHg) | 2.5 (-6.4 – 8.2) | 8.1 (-4.2 – 75.6)* |
| Proximal esophagus (mmHg) | 2.7 (-2.7 – 10.1) | 6.6 (-10.3 – 27.3) |
| Non-deglutitive UES relaxation | 37 (58 %) | 12 (80%) |
Median (5th – 95th percentile)
p < 0.05
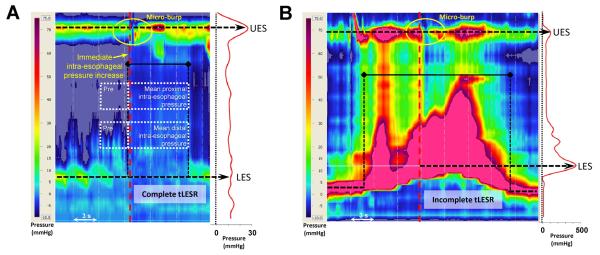
Figure 4.
Esophageal pressurization events in a non-achalasic patient (Panel A) and an achalasic patient (Panel B). In each case, the pressurization occurs during the period of distal esophageal shortening and/or LES relaxation but the mechanism of esophageal pressurization is distinct. In the non-achalasia patients, pressurization occurs in the setting of pressure topography evidence of gastroesophageal reflux with near equalization of pressure from the stomach the UES; note the spatial pressure variation plot to the right displaying the axial pressure profile at the time preceding the micro-burp indicated on the HREPT plot by the vertical red dashed line. On the other hand pressurization in the achalasia patient occurs without LES or UES relaxation and is likely attributable to compression of esophageal contents as a consequence of distal esophageal shortening. Again, note the spatial pressure variation plot to the right illustrating closure at each sphincter and a band of equalized pressure in the esophagus between. The corresponding time of the spatial pressure variation plot is indicated on the HREPT plot by indicated on the HREPT plot by the vertical red dashed line.
DISCUSSION
The central hypothesis of this investigation was that, despite having incurred immune-mediated damage to the myenteric plexus leading to impaired deglutitive LES relaxation and absent peristalsis, achalasia patients can still have tLESRs, albeit incomplete ones. The observation that tLESRs were often triggered by sitting up after performing a series of 12 test swallows during our routine clinical HREPT protocol (Figure 1), along with the serendipitous observation that achalasia patients exhibited a pressure topography response with many similarities to a tLESR (Figure 2) at that same time point in that same protocol, provided the model for the study. The major finding of the study was that achalasia patients do indeed appear to have incomplete tLESRs. The pressure topography responses elicited by the change to a sitting posture occurred in achalasics with similar frequency to tLESRs in non-achalasics, they had similar degree and duration of associated distal esophageal shortening, and they were similarly always associated with concomitant CD inhibition. The only consistent differences between these incomplete tLESRs of the achalasics and the complete tLESRs triggered in the non-achalasics were: i) the absence of associated prolonged LES relaxation and ii) the absence of pressure topography evidence of gastroesophageal reflux.
During the past decade, our understanding of the neurophysiology and mechanics of tLESRs has improved substantially. This is mainly a vago-vagal reflex that can be obliterated by cooling or severing the vagus nerves.19 The primary stimulus to elicit a tLESR is gastric distension, which activates mechano-sensitive vagal afferent nerves with specialized endings known as intraganglionic laminar endings situated in the myenteric plexus between layers of the muscularis propria.20 The central terminus of these afferents is in the nucleus of the solitary tract in the medulla. Within the medulla, there are likely both monosynaptic and polysynaptic pathways, a central pattern generator of sorts, which organizes the efferent pathway from the dorsal motor nucleus of the vagus21 and selective central inhibition of the phrenic nerve innervating the CD, but not the costal diaphragm. The resultant complex motor response elicited by a tLESR is comprised of: i) prolonged, profound LES relaxation, ii) CD inhibition,22 iii) gastric fundus relaxation23, and iv) contraction of the distal esophageal longitudinal muscle resulting in tenting the LES through the diaphragmatic hiatus.9 There is also often a contraction of the circular muscle in the mid esophagus at the termination of a tLESR.24 Relevant to the current work, the only components of this complex response necessarily compromised in achalasia (on the basis of an immunological attack on nitrergic inhibitory myenteric plexus neurons) are LES relaxation and, perhaps, gastric fundic relaxation. Hence, it stands to reason that consistent with the findings of this investigation, the tLESR response may be impaired or incomplete, but not obliterated, in that disease state.
None of the achalasics in this study exhibited a complete tLESR. However, we studied a relatively small number of achalasics and several of them came close with LES pressure diminishing, and both CD relaxation and esophageal shortening demonstrable. Given these observations, it seems likely that the occasional achalasia patient would be able to have a complete tLESR. Certainly, the inhibitory signal to the LES associated with tLESR is more robust in terms of both the completeness and duration of associated relaxation than that associated with swallow and, especially in marginal instances or in early disease, some persistence of tLESRs might be expected. Such speculation is supported by published observations of Holloway et al25 and Hirano et al26 each of whom encountered a single achalasic with preserved tLESR despite impaired deglutitive LES relaxation. That prior investigators did not observe these incomplete tLESRs is attributable to both methodological issues and to the patients studied. Holloway et al described failure to elicit tLESR in response to gastric distension in 16 achalasics, 7 (44%) of whom had severe esophageal dilatation.25 Severe dilatation occurs in advanced achalasia, which often also includes esophageal muscle hypertrophy and myenteric plexus degeneration.27 Consequently, the neurologically mediated distal esophageal shortening and after contraction that are so prominent in Figures 2 and 4 might also be absent. It may well be that in advanced achalasics esophageal shortening is no longer possible. This is unlike our study population in which the majority of achalasics had absent or mild dilatation and clearly were still capable of profound distal esophageal shortening. It is also worth noting that HREPT imaging of contractility allows better appreciation of temporally related pressure changes among sensors. With this technology we could visualize esophageal shortening in early achalasia, without mistaking it for relaxation (pseudorelaxation), as well as incorporate the additional criteria that define tLESR: esophageal shortening9 and non-deglutitive UES relaxation.8
One inescapable conclusion regarding tLESR from the current work is that this is clearly not a passive response triggered by the sphincter being forcibly opened in the setting of elevated intragastric pressure as has been advocated by some surgical investigators.28,29 Rather, these findings emphasize that tLESR is an integrated motor response dependent on an array of sensory, central, and efferent neural pathways. As such, achalasia can be viewed as an “experiment of nature” selectively deleting the neural substrate for a key component of the overall response, but leaving the remainder intact. Our findings are inconsistent with the viewpoint that forcible LES relaxation (opening) is the primary event and all else follows. Massey et al arrived at a similar conclusion during concurrent manometric and endoscopic study of tLESRs elicited by gaseous distention of the stomach.30 That study noted that manometrically evident LES relaxation preceded endoscopically viewed EGJ opening and esophageal shortening, again, arguing that EGJ opening was not the initiating event.
In conclusion, this study utilized a model of tLESR to demonstrate a selective defect in that reflex in patients with (clinically) early achalasia. All sensory, central, and motor components of the tLESR response remained intact with the notable exception of LES relaxation. These findings provide further evidence for a very selective neural defect in achalasia involving only the inhibitory myenteric plexus neurons of the distal esophagus and also emphasize the neurophysiologic complexity of the tLESR response.
Acknowledgments
Grant support: Supported by grant R01 DC00646 (to P.J.K. and J.E.P.) from the Public Health Service.
Abbreviations
- CD
crural diaphragm
- HREPT
high resolution esophageal pressure topography
- LES
lower esophageal sphincter
- tLESR
transient lower esophageal sphincter relaxation
- UES
upper esophageal sphincter
Footnotes
Financial disclosures: No conflicts of interest exist for Monika A. KWIATEK, Jennifer POST, John E. PANDOLFINO and Peter J. KAHRILAS.
References
- 1.Wyman JB, Dent J, Heddle R, Dodds WJ, Toouli J, Downton J. Control of belching by the lower oesophageal sphincter. Gut. 1990;31:639–46. doi: 10.1136/gut.31.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrikios J, Martin CJ, Dent J. Relationship of transient lower esophageal sphincter relaxation to postprandial gastroesophageal reflux and belching in dogs. Gastroenterology. 1986;90:545–51. doi: 10.1016/0016-5085(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 3.Massey BT. Potential control of gastroesophageal reflux by local modulation of transient lower esophageal sphincter relaxations. Am J Med. 2001;111(Suppl 8A):186S–189S. doi: 10.1016/s0002-9343(01)00829-4. [DOI] [PubMed] [Google Scholar]
- 4.Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Physiol. 1995;268:G128–33. doi: 10.1152/ajpgi.1995.268.1.G128. [DOI] [PubMed] [Google Scholar]
- 5.Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779–84. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- 6.Franzi SJ, Martin CJ, Cox MR, Dent J. Response of canine lower esophageal sphincter to gastric distension. Am J Physiol. 1990;259:G380–5. doi: 10.1152/ajpgi.1990.259.3.G380. [DOI] [PubMed] [Google Scholar]
- 7.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–32. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Ghosh SK, Zhang Q, Han A, Kahrilas PJ. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); it is mainly about microburps. Neurogastroenterol Motil. 2007;19:203–10. doi: 10.1111/j.1365-2982.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725–33. doi: 10.1053/j.gastro.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–33. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckxstaens GE. Achalasia: virus-induced euthanasia of neurons? Am J Gastroenterol. 2008;103:1610–2. doi: 10.1111/j.1572-0241.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 12.Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598–609. doi: 10.1111/j.1572-0241.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 13.Sifrim DA, Janssens JP. The ‘artificial high pressure zone’. A non-invasive method to study in man the effect of the inhibitory innervation to the oesophagus. Validation study using a combined manometric--barostat technique. Eur J Gastroenterol Hepatol. 1999;11:165–9. doi: 10.1097/00042737-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988–97. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 15.Pandolfino JE, El-Serag HB, Zhang Q, Shah N, Ghosh SK, Kahrilas PJ. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1033–40. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–63. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 19.Martin CJ, Patrikios J, Dent J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology. 1986;91 doi: 10.1016/0016-5085(86)90691-8. [DOI] [PubMed] [Google Scholar]
- 20.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–68. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackshaw LA, Grundy D. Reflex responses of vagal efferent fibres influenced by gastrointestinal mechanoreceptors to electrical afferent stimulation in the anaesthetized ferret. Q J Exp Physiol. 1988;73:1001–4. doi: 10.1113/expphysiol.1988.sp003209. [DOI] [PubMed] [Google Scholar]
- 22.Mittal RK, Fisher MJ. Electrical and mechanical inhibition of the crural diaphragm during transient relaxation of the lower esophageal sphincter. Gastroenterology. 1990;99:1265–8. doi: 10.1016/0016-5085(90)91148-y. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Noomen C, Wu J, Rigda R, Holloway RH. Transient inhibition of the proximal stomach during transient lower esophageal sphincter relaxations [abstract] Gastroenterology. 2001;120:A630. [Google Scholar]
- 24.Mittal RK, McCallum RW. Characteristics of transient lower esophageal sphincter relaxation in humans. Am J Physiol. 1987;252:G636–41. doi: 10.1152/ajpgi.1987.252.5.G636. [DOI] [PubMed] [Google Scholar]
- 25.Holloway RH, Wyman JB, Dent J. Failure of transient lower oesophageal sphincter relaxation in response to gastric distension in patients with achalasia: evidence for neural mediation of transient lower oesophageal sphincter relaxations. Gut. 1989;30:762–7. doi: 10.1136/gut.30.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano I, Tatum RP, Shi G, Sang Q, Joehl RJ, Kahrilas PJ. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789–98. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]
- 27.Paterson WG. Etiology and pathogenesis of achalasia. Gastrointest Endosc Clin N Am. 2001;11:249–66. vi. [PubMed] [Google Scholar]
- 28.Pettersson GB, Bombeck CT, Nyhus LM. The lower esophageal sphincter: mechanisms of opening and closure. Surgery. 1980;88:307–14. [PubMed] [Google Scholar]
- 29.Mason RJ, DeMeester TR, Lund RJ, et al. Nissen fundoplication prevents shortening of the sphincter during gastric distention. Arch Surg. 1997;132:719–24. doi: 10.1001/archsurg.1997.01430310033006. discussion 724-6. [DOI] [PubMed] [Google Scholar]
- 30.Massey BT, Simuncak C, LeCapitaine-Dana NJ, Pudur S. Transient lower esophageal sphincter relaxations do not result from passive opening of the cardia by gastric distention. Gastroenterology. 2006;130:89–95. doi: 10.1053/j.gastro.2005.11.003. [DOI] [PubMed] [Google Scholar]