Abstract
Objective
Inadequate vitamin D status is related to increased adiposity, risk of falls, and muscle weakness, particularly in the elderly. We hypothesized that serum 25-hydroxyvitamin D (25(OH)D) is related to physical fitness indices (androidal fat, whole body lean mass, balance, strength) in healthy postmenopausal women.
Design
Covariates for fitness indices included: age or years since menopause; weight; 25(OH)D; energy expenditure; calcium intake. Overall and regional (androidal fat mass=waist+hip fat) body composition was assessed (N=242) via dual-energy X-ray absorptiometry.
Results
Regression analyses revealed that 71% of variability (P≤0.0001) in androidal fat mass was accounted for by weight (53.0%, P≤0.0001), white blood cell (WBC) count (2.0%, P≤0.0001), supplemental calcium (1.7%, P=0.0004), years since menopause (1.1%, P=0.0034), 25(OH)D (1.0%, P=0.0051), and vegetable servings (0.6%, P=0.027); 64% of variability (P≤0.0001) in lean mass was accounted for by weight (63.1.%, P≤0.0001), WBC count (1.4%, P=0.0038), and 25(OH)D (1.0%, P=0.013); 12% of variability (P≤0.0001) in balance (right+left leg) was accounted for by age (3.8%, P=0.0019), 25(OH)D (2.0%, P=0.025), and WBC count (1.8%, P=0.032); 14% of variability (P≤0.0001) in hand grip strength (right+left) was accounted for by weight (9.3%, P≤0.0001), 25(OH)D (2.4%, P=0.013), WBC count (2.1%, P=0.019), and age (1.6%, P=0.044); 22% of variability (P≤0.0001) in torso strength was accounted for by site (15.0%, P≤0.0001) and weight (4.6%, P=0.0003).
Conclusion
Serum 25(OH)D was the common contributor to physical fitness indices (androidal fat mass, lean mass, balance, hand grip strength) in healthy postmenopausal women.
Keywords: Vitamin D, hand grip strength, balance, androidal fat mass, lean body mass
Introduction
Vitamin D deficiency can cause life-long sequelae, ranging from skeletal deformation and growth retardation in children, to osteopenia, osteoporosis, and osteomalacia in adults.1 Based upon a large study that included 18 countries worldwide, 64% of postmenopausal women with osteoporosis had vitamin D inadequacy.2 Vitamin D deficiency can be exacerbated during the winter months due to lack of exposure to UVB rays,3,4 although location does not always dictate vitamin D status. Vitamin D status is based upon serum 25(OH)D (25(OH)D2 plus 25(OH)D3) concentration (nmol/L) [ng/mL]: deficient = ≤50 [≤20], insufficient = 51-74 [21-29], sufficient = 75-100 [30-40], or intoxication = >374 [150].1,5,6
Higher serum 25(OH)D is associated with better physical performance.7,8 Vitamin D deficiency among older men and women (65+ years) was associated with slower performance for chair stands and tandem stands (serum <25 nmol/L [10 ng/mL]) and slower walking time (serum <49.2 nmol/L [20 ng/mL]). Further, physical performance improved with increasing 25(OH)D, but performance leveled off at >50 nmol/L (20 ng/mL).8 The health benefits of physical activity include improved muscle fitness and reduction in falls, but these benefits may also be related to 25(OH)D concentration.9 Whereas vitamin D deficiency causes muscle weakness,10 greater serum 25(OH)D concentration has been associated with improved muscle function,9,11,12 improved balance,9,12 reduced risk of falling,7,9,12 and decreased fracture rate.13 NHANES III data indicated that among 4,100 elderly (60+ years of age), those with lower status (<40 nmol/L) had slower performances, whereas those with better status (>40 nmol/L) had faster performances.7 Although performance speed was faster in the range of 40-94 nmol/L compared with lower status (<40 nmol/L), the performance speed slope was much less dramatic in this range. These analyses were adjusted for demographics, comorbidities, calcium intake, and physical activity. Serum 25(OH)D (ranging from 24.5 to 58.9 nmol/L) has been shown to be related to functional performance (walking, rising from a chair, ascent and decent of 13 stairs), choice reaction time,1,4 and balance (bipedal postural sway and balance on one leg), but not to quadriceps strength.15 A meta-analysis9 stated that an explanation for the improvement in muscle function may be that 1,25(OH)2 vitamin D (1,25(OH)2D derived from 25(OH)D) binds to a highly specific nuclear receptor in muscle tissue, which leads to de novo protein synthesis and hence muscle cell growth.
Moderate-to-high cardiorespiratory exercise has been associated in both men and women with reduced total body and abdominal fat mass (particularly visceral fat), independent of change in BMI16. 25(OH)D concentration has been shown to be inversely related to weight, BMI, fat mass, and waist-to-hip ratio.17-20 The purpose of this cross-sectional analysis was to examine the relationship between serum 25(OH)D, taking into account weight, age or years since menopause, serum high sensitivity C-reactive protein (CRP) concentration, white blood cell count, energy expenditure (kJ/wk), and various dietary intake factors, and indicators of physical fitness (androidal fat mass, lean mass, strength, and balance) in healthy postmenopausal women who were selected as part of a longitudinal study on bone mineral density.
Methods
Study design
Healthy postmenopausal women 45.8-65.0 years of age were enrolled as part of a randomized, double-blind, placebo-controlled multi-center (Iowa State University [ISU], Ames, IA and University of California at Davis [UCD], Davis, CA) NIH-funded clinical trial that began in 2003. The parent study (Soy Isoflavones for Reducing Bone Loss; SIRBL) was designed to examine the effect of two doses of isoflavones extracted from soybeans on bone loss during three years in at-risk postmenopausal women who upon entry were non-osteoporotic, without diseases or conditions, and not taking hormones or medications. This ancillary project focused on the relationship between serum 25(OH)D concentration and overall physical fitness at baseline for 242 women. We excluded 13 women at UCD from this analysis because they did not meet the entry criteria (11 had thickened endometrium, 1 had breast cancer, 1 could not provide a blood sample).
Subject screening, selection, and characteristics
For the parent SIRBL project, we recruited subjects throughout the state of Iowa and the Sacramento region in California primarily through direct mailing lists, stories in local newspapers, local/regional radio advertisements, as well as other recruiting avenues. Women who responded (N=5,255) to outreach materials were initially screened via a telephone questionnaire to identify healthy women who underwent natural menopause (cessation of menses nine months to 10 years), were not experiencing excessive vasomotor symptoms, were ≤ 65 years of age, nonsmokers, and had a body mass index (BMI, kg/m2) ranging from 18.5 through 29.9 (inclusive) to exclude women at the extremes of adiposity. We excluded vegans and high alcohol consumers (>7 servings/week) because alcohol interferes with isoflavone metabolism. The parent SIRBL project established the inclusion/exclusion criteria; thus, we also excluded women diagnosed with chronic disease and those who had a first-degree relative with breast cancer. We also excluded women based on the following criteria: chronic use of medications (cholesterol-lowering or anti-hypertensive medications, use of oral hormone or estrogen therapy, selective estrogen receptor modulators, or other hormones within the last 12 months, use of estrogen or progestogen creams or calcitonin within the last six months, use of antibiotics within the last three months, and/or any previous use of bisphosphonates).
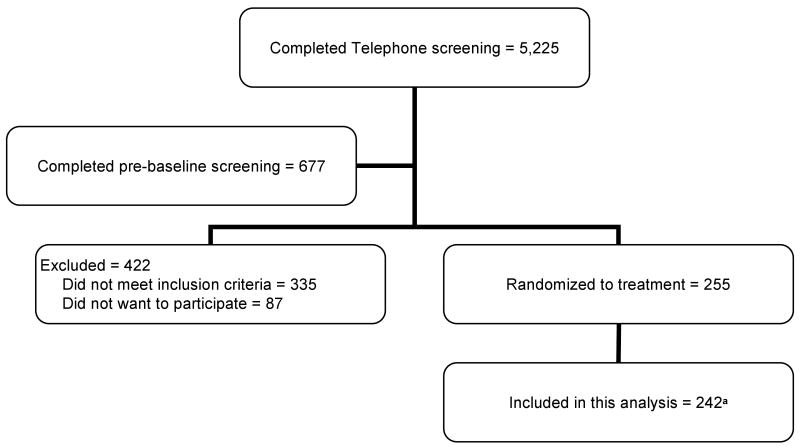
Women who met the initial criteria via telephone (N=677) attended a pre-baseline appointment to determine eligibility for additional entry criteria. We measured height and weight to confirm BMI status and used a Delphi-W QDR dual-energy x-ray absorptiometry (DXA) bone densitometer (Hologic, Bedford, MA) to assess bone mineral density (BMD) to establish eligibility. The SIRBL project focused on prevention rather than treatment of disease; thus, we excluded women based on lumbar spine and/or proximal femur BMD (using >1.5 SD below the young adult mean as cut-off), with evidence of previous or existing spinal fractures, or with a high BMD (>1.0 SD above the mean). Once a woman qualified based on her BMD, our phlebotomist drew blood for a chemistry profile. We excluded women if their fasted blood values indicated diabetes mellitus (fasting blood glucose ≥126 mg/dl); abnormal renal (elevated creatinine), liver (elevated enzymes), and/or thyroid function; or elevated lipid profile (low density lipoprotein-cholesterol >160 mg/dl; triacylglycerol >200 mg/dl). For this ancillary project, we included 242 women who met our entry criteria (Fig. 1) for the cross sectional analysis.
Fig. 1.
Subject screening and enrollment flow chart
a We excluded 13 women at UCD from this analysis because they did not meet entry criteria (11 had elevated endometrial thickness, 1 had breast cancer, 1 could not provide blood sample)
The respective Institutional Review Boards (IRB) at ISU (ID# 02-199) and at UCD (ID# 200210884-2) approved our study protocol, consent form, and subject-related materials. Approvals for the DXA procedures were obtained from each institution's IRB and State Department of Public Health in Iowa and California. We obtained informed consent from all women at the start of pre-baseline screening.
Data collection
Questionnaires
At the pre-baseline visit, trained interviewers administered three questionnaires to ensure the health status of participants: health and medical history,21 reproductive history,22 and nutrition history.21 Subjects were asked to cease taking herbal therapies and/or dietary supplements prior to baseline testing. At baseline, we assessed dietary intake using a semi-quantitative food frequency questionnaire from Block Dietary Data Systems (Berkeley, CA). We assessed physical activity using the Paffenbarger physical activity recall23 to obtain information from the previous year's activity, including walking, climbing stairs, sport and recreational activity, and time spent engaged in activities ranging from light to heavy activity. Each reported recreational or work-related activity was summed using metabolic equivalents of 4, 6, or 8 for activities classified as light, moderate, or heavy, respectively. The summation of activities provided an estimate of weekly energy expenditure for these physical activities.
Body size and composition measurements
A trained anthropometrist measured standing and sitting heights (Model S100; Ayrton Corp., Prior Lake, MN) and weight (ABCO Health-o-meter; Bridgeview, IL). Body composition measurements were obtained using matching DXA instruments (Delphi W Hologic Inc; Waltham, MA) at each site and daily calibration to ensure that the instruments provided comparable results. One certified DXA operator at ISU and one at UCD performed all DXA scans, with cross-training between sites to ensure comparable quality control. We standardized subject placement for the scans and adhered to the manufacturer's recommendations. One operator assessed overall adiposity from the whole body DXA scans. To assess central adiposity, one evaluator sectioned each whole body DXA scan into waist, hip, and thigh regions based on bone landmarks24,25 and these regions were analyzed using special software (Discovery Version 12.3:7). The waist region included the first lumbar through the fourth lumbar vertebrae. The hip region began below the fourth lumbar vertebrae and extended to the tip of the greater trochanter of the femur. Lastly, the thigh region extended superiorly from the greater trochanter to the approximate midpoint between the edge of the thigh region and lateral condyle of the femur. The lateral edge of each region was extended distally to encompass all tissue. This analysis provided an estimate of the fat and lean mass within each of these three regions. The androidal-to-gynoidal fat mass ratio was calculated for each subject: waist + hip fat mass / thigh fat mass.
Laboratory measurements
Phlebotomists collected fasted (9 hours) blood samples between 0700 h and 0800 h. Serum samples were allowed to clot for 30 minutes prior to centrifugation. We separated serum and plasma from whole blood by centrifuging for 15 minutes (4°C) at 1300 × g and stored aliquots at -80°C until analyses. Certified clinical laboratories (LabCorp, Kansas City, KS for ISU, and UCD Medical Center, Sacramento, CA for UCD) analyzed our blood samples, including a complete blood count (CBC) with differential, general chemistry panel, and thyroid screen. Serum 25(OH)D concentration was determined for each individual in duplicate with radioimmunoassay (RIA) kits (Diasorin 25(OH)D 125I RIA kit; Stillwater MN) using a Cobra II series auto-gamma counting system (PerkinElmer Life and Analytical Sciences; Meriden, CT). We report the total circulating serum 25(OH)D (25(OH)D2 plus 25(OH)D3) because clinical decisions are based upon these total values.26 According to Hollis,26 the only assays that quantitatively detect total circulating 25(OH)D are the HPLC and LC-MS methods and the DiaSorin assay. During specimen collection and preparation, we were careful and only exposed serum and plasma samples minimally to light. According to the manufacturer's instructions, we used single serum aliquots for extraction; duplicate samples were then taken from the supernatant for subsequent analysis. We used quality control samples included in the Diasorin kit, with low to normal values ranging from 26.0 to 55.9 nmol/L and normal to high values ranging from 95.4 to 203.2 nmol/L. We used these ranges as the basis for accepting sample data veracity for each batch/run, which included 6 standards in duplicate. We collected sufficient in-house serum as quality control (frozen at -80°C) to run with each kit for calculating the inter-assay coefficient of variation (CV). To determine the intra-assay CV, we used duplicate serum sample CV based upon all data points. The intra-assay and inter-assay CVs (%), respectively, were 2.71 and 2.69.
Statistical analyses
Statistical analyses were performed using SAS (version 9.1; Cary, NC) with results considered statistically significant at P≤0.05. Descriptive statistics included means ± standard deviation (SD) for all data, since the majority were normally distributed data, with range provided for each variable. Classes of variables in modeling the outcomes of interest included independent variables that were biologically plausible and/or significantly related using Pearson correlation analysis. We used stepwise regression analyses to assess the combined contribution of independent variables to androidal fat mass, whole body lean mass, single leg balance, and strength (hand, torso, and leg). Classes of variables in modeling each of these six outcomes included: weight, age or years since menopause, circulating 25(OH)D, serum CRP concentration, white blood cell count, energy expenditure (kJ/wk), and various dietary intake factors (calcium intake [total, supplemental, or dairy], energy, protein, saturated fat, and/or vegetable servings). Each model included site as an obligatory variable to account for potential study site differences, with the ISU site folded into the model intercept and the UCD site indicated in the table. In modeling each outcome, we removed variables that exhibited multicollinearity as indicated by the variance inflation factor. The variance inflation factor measures the impact of collinearity among the independent variables in a regression model and the degree to which multicollinearity degrades the precision estimate. A value exceeding 10 is of concern, but in weaker regression models, a value exceeding 2.5 may be cause for concern.27
Results
Subject characteristics
These analyses included 242 healthy postmenopausal women, with baseline characteristics presented in Table 1. Women ranged from 45.8-65.1 years of age and were from 0.8-10.0 years since menopause. We enrolled three African American (1%), one Native Hawaiian (<1%), one Native American (<1%), three Asians (1%), seven women of more than one race (3%), two of unknown race (<1%), and two who chose not to report race (<1%); the remaining women were Caucasian (92%). BMI values ranged from 17.8-32.7; UCD enrolled women beyond our inclusion criteria (two women below 18.5 and seven women above 29.9). Overall and regional body composition as assessed by DXA (Table 1) indicated wide variability in both overall and regional body fat measures among these women. Approximately half of the women had a BMI <25.0 kg/m2. Values for circulating analytes are presented in Table 1, demonstrating that mean values were within the range reported in the literature. The mean values for dietary and supplement (calcium and vitamin D) intake (Table 2) illustrated wide variability in intake.
Table 1.
Characteristics of subjectsa at baseline
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 54.3 ± 3.3 | 45.8-65.0 |
| Years since menopause (years) | 3.5 ± 2.0 | 0.8-10.0 |
| Body size | ||
| Weight (kg) | 67.7 ± 9.3 | 43.7-94.5 |
| Height (cm) | 164.7 ± 6.3 | 146.3-182.2 |
| BMI (kg/m2) | 24.9 ± 3.1 | 17.8-32.7 |
| Body compositionb | ||
| Fat mass (kg) | 23.3 ± 6.4 | 8.1-47.8 |
| Lean mass (kg) | 43.2 ± 4.6 | 29.5-55.6 |
| Androidal (waist + hip) fat (kg) | 5.8 ± 2.1 | 1.1-11.8 |
| Strength and balance measurements | ||
| Hand grip strength (right + left, kg) | 56.8 ± 8.7 | 33.0-82.5 |
| Torso strength (kg) | 72.3 ± 18.9 | 15.0-127.0 |
| Leg strength (kg) | 96.3 ± 28.9 | 10.0-182.0 |
| Balance (right + left, sec) | 2.8 ± 0.7 | 1.4-4.5 |
| Energy expenditure | ||
| Energy expenditurea (kJ/wk) | 12,569 ± 8534 | 0-51,077 |
| Circulating analytes | ||
| Serum 25(OH)vitamin Dc (nmol/L) | 69.6 ± 22.7 | 21.6 – 157.6 |
| White blood cell count (×109/L) | 5.1 ± 1.0 | 2.3-8.4 |
Number of subjects = 242 for most variables, except N = 241 for energy expenditure, N = 240 for balance, N = 239 for torso and leg strength, N = 237 for hand grip strength and white blood cell count.
Assessed by DXA (dual-energy x-ray absorptiometry).
Reference range for serum 25(OH)vitamin D (nmol/L): deficient = ≤50, insufficient = 51-74, sufficient = 75-100, intoxication = >374.
Table 2.
Dietary intake of subjectsa from food at baseline
| Daily Intakeb | Mean ± SD | Range |
|---|---|---|
| Total energy (kcal), (kJ) | 1593 ± 600 (6667 ± 2511) | 423-4564 (1770-19,100) |
| Carbohydrate (g) | 181 ± 74 | 27-476 |
| Protein (g) | 63 ± 25 | 15-168 |
| Total fat (g) | 69 ± 31 | 17-247 |
| Vegetable servings/day | 3.8 ± 2.4 | 0.5-18.7 |
| Dairy servings/day | 1.4 ± 1.0 | 0-5.9 |
| Vitamin D (IU) | 138 ± 106 | 12-478 |
| Dairy calcium (mg) | 484 ± 363 | 405-2409 |
| Supplemental calcium (mg) | 462 ± 455 | 0-1130 |
| Supplemental vitamin Dc (IU) | 313 ± 111 | 114-400 |
Number of subjects = 242 for all variables
Reported from Semi-Quantitative Food Frequency Questionnaire
Reflects vitamin D supplement intake prior to baseline among the 130 women (70 at ISU, 60 at UCD) who took these supplements
Serum 25(OH)D indicated that 19.4% of participants were deficient (<50 nmol/L), 44.2% were insufficient (50-74.9 nmol/L), and 36.4% were sufficient (>75 nmol/L). Among the 130 women (70 at ISU, 60 at UCD) who had taken vitamin D supplements prior to baseline, their mean 25(OH)D concentration was 72.9 nmol/L, whereas those who had not taken supplements had a mean value of 65.9 nmol/L. Women from the UCD site (71.1 ± 22.7 nmol/L) had a 6% higher mean serum 25(OH)D concentration than those from the ISU site (67.7 ± 21.1 nmol/L), but this difference was not significant (P=0.18). Serum 25(OH)D ranged from 65.0 in winter to 79.6 in summer, with intermediate spring (68.3) and fall (70.3) values (nmol/L). The only season in which there was a statistically significant difference in serum 25(OH)D (nmol/L) between sites (UCD = 79.7 ± 21.3 [n=26]; ISU = 66.7 ± 17.0 [n= 67]; P=0.0084) was for women who were enrolled during the fall (Sept 21-Dec 20).
Regression analyses
We performed regression analyses to examine the independent factors contributing to the variability in androidal fat mass, whole body lean mass, balance, strength (hand, torso, and leg) as our primary outcomes (Table 3). No notable multicollinearities emerged among the independent variables, as indicated by the low (<2) variance inflation factors in all regression models. Residual analyses indicated that the model assumptions of normality of error terms and homogeneity of error variance were satisfied for the final regression models. Geographic site was significant for torso strength (P≤0.0001) and leg strength (P=0.0007), but not for androidal fat mass, whole body lean mass, hand grip strength, and balance. Nevertheless, we retained site as obligatory in each model to account for the potential influence of site. We explored the relationship between serum 25(OH)D and each outcome variable graphically, with these scatterplots showing a continuously linear relationship between 25(OH)D and each outcome variable. Based upon our data, we found no firm indication of a threshold effect of 25(OH)D on the outcomes of interest. After stepwise variable selection was completed, multiple regression analyses revealed that weight (53%), white blood cell count (2.0%), supplemental calcium (1.7%), years since menopause (1.1%), 25(OH)D (1.0%), and vegetable servings/day (0.6%) accounted for 71% of the variability in androidal fat mass (F=78.8, P≤0.0001). Likewise, weight (63%), white blood cell count (1.4%), and 25(OH)D (1.0%) accounted for 64% of the variability in whole body lean mass (F=100.9, P≤0.0001). Age (3.8%), 25(OH)D (2.0%), and white blood cell count (1.8%) accounted for 12% of the variability in balance (F=6.2, P≤0.0001). Multiple regression analyses revealed that weight (9.3%), 25(OH)D (2.4%), white blood cell count (2.1%), and age (1.6%) accounted for 14% of the variability in hand grip strength (F=7.2, P≤0.0001), whereas site (15.0%), weight (4.6%), and energy expenditure (1.2%) accounted for 22% of the variability in torso strength (F=22.0, P≤0.0001), and weight (5.0%), site (4.5%), white blood cell count (2.0%), energy expenditure (1.8%), and age (1.3%) accounted for 14% of the variability in leg strength (F=7.4, P≤0.0001).
Table 3.
Regression Analyses: Contributors to androidal fat mass, whole body lean mass, balance, hand strength, torso strength, and leg strength
| Androidal Fat Mass (kg)a | |||||
|---|---|---|---|---|---|
| Overall Model R2 = 70.7% (Adj R2= 69.8%) [F=78.79; df=(7, 229)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | -6.190 | -7.553 to -4.827 | ≤0.0001 | ||
| Site (UCD)f | 0.187 | -0.111 to +0.484 | 0.20 | 0.22 | 1.08 |
| Total body weight (kg) | 0.165 | 0.149 to 0.181 | 52.68 | ≤0.0001 | 1.06 |
| WBC count (× 109/L)g | 0.289 | 0.144 to 0.435 | 1.96 | ≤0.0001 | 1.10 |
| Calcium supplement (mg/day) | -5.833 × 10-4 | -9.028 ×10-4 to -2.638 × 10-4 | 1.66 | 0.0004 | 1.03 |
| Years since menopause | 0.111 | 0.0370 to 0.184 | 1.12 | 0.0034 | 1.04 |
| 25(OH) vitamin D (nmol/L) | -0.00946 | -0.0161 to -0.00287 | 1.03 | 0.0051 | 1.02 |
| Vegetable servings/day | -0.0679 | -0.128 to -0.00765 | 0.63 | 0.027 | 1.04 |
| Whole Body Lean Mass (kg)a | |||||
| Overall Model R2= 63.5% (Adj R2= 62.9%) [F=100.92; df=(4, 232)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | 17.196 | 13.837 to 20.556 | ≤0.0001 | ||
| Site (UCD)f | -0.320 | -1.0552 to 0.416 | 0.12 | 0.39 | 1.05 |
| Total body weight (kg) | 0.406 | 0.366 to 0.446 | 63.08 | ≤0.0001 | 1.05 |
| WBC count (× 109/L)g | -0.542 | -0.906 to -0.177 | 1.35 | 0.0038 | 1.09 |
| 25(OH) vitamin D (nmol/L) | 0.0210 | 0.00443 to 0.0375 | 0.98 | 0.013 | 1.02 |
| Balance (seconds)a,h | |||||
| Overall Model R2= 11.9% (Adj R2= 9.9%) [F=6.16; df=(5, 229)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95 % CL | Percentage Varianceb | P valuec | VIFd |
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | 6.0719 | 4.459 to 7.685 | ≤0.0001 | ||
| Site (UCD)f | -0.0708 | -0.255 to 0.113 | 0.22 | 0.45 | 1.06 |
| Age (years) | -0.0442 | -0.0720 to -0.0165 | 3.80 | 0.0019 | 1.03 |
| 25(OH) vitamin D (nmol/L) | 4.700 × 10-3 | 6.0333 × 10-4 to 8.810 × 10-3 | 1.97 | 0.025 | 1.02 |
| WBC count (× 109/L)g | -0.100 | -0.191 to -0.00885 | 1.80 | 0.032 | 1.11 |
| Total body weight (kg) | -9.990 × 10-3 | -2.003 × 10-2 to 5.508 × 10-5 | 1.48 | 0.051 | 1.06 |
| Hand Grip Strength (kg)a | |||||
| Overall Model R2= 13.7% (Adj R2= 11.8%) [F=7.16; df=(5, 226)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | 58.271 | 39.382 to 77.161 | ≤0.0001 | ||
| Site (UCD)f | -1.0837 | -3.256 to 1.0890 | 0.37 | 0.33 | 1.07 |
| Total body weight (kg) | 0.292 | 0.175 to 0.409 | 9.29 | ≤0.0001 | 1.06 |
| 25(OH) vitamin D (nmol/L) | 0.0613 | 0.0132 to 0.109 | 2.41 | 0.013 | 1.02 |
| WBC count (× 109/L)g | -1.310 | -2.400 to -0.220 | 2.14 | 0.019 | 1.12 |
| Age (years) | -0.333 | -0.657 to -0.00836 | 1.56 | 0.044 | 1.04 |
| Torso Strength (kg)a | |||||
| Overall Model R2= 22.0% (Adj R2= 21.0%) [F=22.01; df=(3, 234)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | 47.161 | 31.0938 to 63.228 | ≤0.0001 | ||
| Site (UCD)f | -14.504 | -18.768 to -10.240 | 14.97 | ≤0.0001 | 1.01 |
| Total body weight (kg) | 0.429 | 0.202 to 0.657 | 4.60 | 0.0003 | 1.00 |
| Energy expenditure (kJ/wk) | 2.420 × 10-4 | -8.340 × 10-6 to 4.231 × 10-4 | 1.21 | 0.058 | 1.00 |
| Leg Strength (kg)a | |||||
| Overall Model R2= 14.0% (Adj R2= 12.1%) [F=7.37; df=(5, 227)] P≤0.0001 | |||||
| Parameter | Parameter Estimate | 95% CIb | % Variancec | P valued | VIFe |
| Interceptf | 123.828 | 62.398 to 185.258 | ≤0.0001 | ||
| Site (UCD)f | -12.464 | -19.585 to -5.343 | 4.51 | 0.0007 | 1.07 |
| Total body weight (kg) | 0.705 | 0.322 to 1.0879 | 4.98 | 0.0004 | 1.06 |
| WBC count (× 109/L)g | -4.144 | -7.672 to -0.616 | 2.03 | 0.022 | 1.11 |
| Energy expenditure (kJ/wk) | 4.615 × 10-4 | 3.852 × 10-5 to 8.844 × 10-4 | 1.75 | 0.033 | 1.01 |
| Age (years) | -0.987 | -2.0604 to 0.0856 | 1.25 | 0.071 | 1.03 |
N = 242 Number of subjects = 242 for most variables, except N = 241 for energy expenditure, N = 240 for balance, N = 239 for torso and leg strength, N = 237 for hand grip strength and white blood cell count
95 % Confidence Interval (CI)
Squared semi-partial Type II correlation coefficient; accounts for shared variance among variables.
Variables left in the model were significant at P ≤0.10 level.
Variance inflation factor (VIF) measures the impact of collinearity among the independent variables in a regression equation and the degree to which multicollinearity degrades the precision estimate.
Each model included site as an obligatory variable to account for potential study site differences, with the ISU site folded into the model intercept and the UCD site indicated separately.
White blood cell (WBC) count (× 109/L)
Balance was log transformed because it was not normally distributed.
Discussion
Only 36.4% of our subjects were vitamin D sufficient (>75 nmol/L), whereas 63.6% were either vitamin D insufficient (44.2%) or deficient (19.4%). Vitamin D is a hormone synthesized from cutaneous 7-dehydrocholesterol in response to UVB exposure,28 but vitamin D can also be obtained from dietary sources or supplements. The women in this study were asked to refrain from supplement use prior to baseline, but we did not control their dietary sources or cutaneous UVB exposure. Although assessment of vitamin D using a questionnaire is a very rough estimate at best, dietary intake of vitamin D was 162 IU/day and 114 IU/day for the ISU and UCD women, respectively. Although increased skin pigmentation, use of sun screen, clothing, aging, and latitude decrease the cutaneous synthesis of previtamin D3,28 we did not take these factors into account. Instead, we assessed circulating 25(OH)D concentration to account for vitamin D status among individuals and between sites. Between sites, the distributions had a similar shape and range (ISU and UCD: 26.0-146.6 and 21.6-157.6), illustrating that such typically low dietary intake of vitamin D does not contribute appreciably to serum 25(OH)D.
Serum 25(OH)D was the common contributor to physical fitness indices (androidal fat mass, whole body lean mass, balance, and hand grip strength) in healthy postmenopausal women in our cross-sectional study. Indeed, women with insufficient and deficient 25(OH)D, respectively, had 8.5% and 12.3% higher mean fat mass than those with sufficient status, suggesting that vitamin D status may contribute to adiposity, as previously indicated in a randomized controlled trial of 90 young women.29 In a study with healthy older adults provided supplemental calcium and vitamin D3, the change in plasma 25(OH)D was negatively associated with waist circumference and central but not peripheral fat.30 Similarly, in healthy women 20 to 80 years of age, serum 25(OH)D was negatively related to total body fat percentage after adjusting for race, season, age, and dietary vitamin D.20 Accordingly, we noted an inverse relationship between serum 25(OH)D and androidal fat mass, although this does not demonstrate causality. The literature is not in complete agreement as to why obese individuals have compromised vitamin D status, although serum 25(OH)D is typically inversely related to parathyroid hormone concentration.31 One prevailing thought is that 25(OH)D is sequestered in fat cells32, thereby lowering circulating 25(OH)D. This does not preclude other potential mechanisms, such as an enhanced metabolic clearance of 25(OH)D by hepatocytes in obese individuals.33
We also noted a positive link between years since menopause and androidal fat, similar to a study34 in 29 healthy postmenopausal women who had significantly (P=0.04) higher visceral adipose tissue compared with premenopausal women. We noted a statistically significant inverse relationship between supplemental but not dairy-derived calcium with androidal fat mass, perhaps because ∼1/3 of the women did not take calcium supplements at baseline. In contrast, data from clinical trials35-37 have indicated a two-fold greater effect of dairy versus supplemental sources of calcium in attenuating adiposity. Interestingly, vegetable servings/day also contributed to androidal fat mass, somewhat similar to a study38 reporting that dietary fiber and fruit servings per day were inversely related to percentage body fat in 52 overweight/obese versus 52 normal-weight adults.
As expected, 25(OH)D was directly related to lean body mass in our study, which has not been fully explored by researchers, perhaps because the adiposity story has overshadowed the relationship between vitamin D status and lean mass. A study in the elderly39 demonstrated that vitamin D osteopathy was largely mediated through lean mass in men, with serum 25(OH)D related (r=0.24, P=0.002) to lean body mass in men, but not in women. Furthermore, Wortsman and colleagues (2000)32 noted an inverse relationship between serum 25(OH)D and BMI, a combination of adiposity and lean body mass, in a relatively small sample (N=38) of medical school personnel (age and sex unstated). In a group of post-pubescent adolescent Chinese girls (N=323)39, not only was serum 25(OH)D related positively to BMI, but also to whole body lean mass, yet not to percentage body fat (based upon DXA measurements). A study in young females (16-22 years)40 demonstrated an inverse relationship between serum 25(OH)D versus weight (r=-0.28, P=0.007) or versus BMI (r=-0.35, P=0.001), but they did not report lean body mass. Depending upon the overall level of adiposity, BMI is more reflective of lean mass at the lower end of the adiposity spectrum. The significance of the relationship between vitamin D status and body composition needs further investigation, but it is important to note that we observed this link in “healthy” postmenopausal women.
The women in our study with insufficient and deficient vitamin D status had 5.7% and 10.6%, respectively, poorer mean balance than those with sufficient status, suggesting that vitamin D status may contribute to better balance as supported by previous studies.41,42 A cross-sectional study8 in men and women ≥65 y showed that decreased serum 25(OH)D (≤50 nmol/L [≤20 ng/mL]) was associated with decreased physical performance. Further, during the three year longitudinal portion of this study,8 researchers showed that continued insufficient vitamin D status was associated with higher odds of decline in physical performance when compared to controls (≥75 nmol/L [≥30 ng/mL]). A cross-sectional study7 with 4,100 subjects from the third National Health & Nutrition Examination Survey (NHANES III) assessed lower-extremity function in a subset of ambulatory men and women 60-90+ years of age. The data were adjusted for other factors that predicted lower-extremity function: age, sex, ethnicity, BMI, comorbid conditions (such as arthritis), level of activity, use of walking device, and poverty-income ratio. The researchers observed significant negative associations (P≤0.0001) between serum 25(OH)D and walking ability and the sit-to-stand test; as serum 25(OH)D increased, it took less time to perform these tasks. Most of the improvement in performance speed was evidenced as serum 25(OH)D increased from 22.5 to ∼40 nmol/L, whereas smaller improvements were made in the range from 40 to 94 nmol/L.7 Serum 25(OH)D was identified as a significant independent variable for functional performance-aggregate time (P<0.05), muscle strength adjusted for age (P=0.02), postural stability (sway and balance) (P=0.03), and psychomotor function-choice reaction time (P<0.05) in a group of elderly subjects prone to falling.15 Similarly, low 25(OH)D (<25 nmol/L) was independently associated with a higher risk of recurrent falling,43 mediated by physical performance (impairment of gait, postural balance, and muscle strength) among men and women aged 65-75 years of age, suggesting that vitamin D status affects muscle function. Certainly, age is an important contributor to balance, as we observed in our study. This was clearly illustrated in women 40-60 years of age who participated in a 12 week balance-strategy training program44 that either preserved or reversed the decline in balance associated with age, as well as improved muscle strength. Interestingly, in our study, body weight was inversely related to balance, but was positively associated with each of the other indicators of fitness.
The women in our study with insufficient and deficient vitamin D status had 3.1% and 7.3%, respectively, lower mean hand grip strength than those with sufficient status, with hand grip strength similar between geographic sites. Correspondingly, vitamin D insufficiency and deficiency have been linked to increased risk of muscle weakness.10 However, we also found that women with insufficient and deficient vitamin D status had 9.5% and 2.6%, respectively, higher mean torso strength than those with sufficient status. Overall body strength, reflected by torso and leg strength, is determined by many factors (i.e., strength training, genetic predisposition, activities of daily living, etc.), most of which we did not include in our analyses, with the exception of body weight and energy expenditure assessed by the Paffenbarger Questionnaire. Strength training increased muscle mass and strength, balance, and overall levels of physical activity in postmenopausal women,41 but our study did not involve exercise intervention. It may be that we did not capture additional influences on overall body strength, but that hand grip strength was more strongly impacted by 25(OH)D. Another explanation for these results might have been that torso and leg strength differed according to site, but had nothing to do with vitamin D status. Indeed, site contributed significantly to torso and leg strength (P≤0.0001, P=0.0007); ISU subjects had 24% greater mean torso strength and 16% greater mean leg strength than those at UCD. Perhaps the greater strength in ISU women was related to their involvement in farm chores and lifting heavy objects (data not shown) or that the age range was narrower for ISU women and age was inversely related to strength.
In our cross-sectional study, WBC count was a significant contributor to most of the fitness outcomes: positively associated with androidal fat mass, but negatively associated with whole body lean mass, balance, and strength measures (hand grip and leg). These results suggested that low WBC count may be a good indicator of overall health as indicated by others;45 it is important to note that none of the women in our study had elevated WBC count (reference range 4.5-10.5 × 109/L).46 Elevated WBC count is also an indicator of inflammation and has been associated with obesity and morbid obesity in women47 and also in both men and women.48 A two year study49 in 477 men and women who had undergone lap-band surgery reported that WBC count declined by 14% (P<0.001) as BMI decreased by 24% (P<0.001), providing further evidence for the link between weight and WBC count.
The primary limitation of this study is that it was cross-sectional and thus we cannot imply cause and effect. In addition, we cannot apply these results to groups other than postmenopausal women. However, the value of this study is that it illustrates the importance of vitamin D status in a group of healthy postmenopausal women who were not chosen based upon their vitamin D status, yet only ∼1/3 of these women were sufficient at baseline. This study provides some basis for suggesting that many healthy women do not have adequate vitamin D status, which is important for functional indices of health, providing evidence to test serum 25(OH)D clinically.
Acknowledgments
We would like to thank each participant who volunteered for this study. We would also like to thank our project coordinator, Laura Hanson, our phlebotomists, Cindy Kruckenberg, Marilyn Chrusciel, and Shirley Nelson, as well as our undergraduate and graduate research assistants who assisted with many aspects of this project.
The overall project described was supported by a grant (RO1 AR046922 A2) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The project was also supported by a grant (P01 ES012020) from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), and by a grant (95P50AT004155) from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS of the National Institutes of Health. The project was supported by USDA, ARS, Western Human Nutrition Research Center, and the CTSC Clinical Research Center at the University of California (1M01RR19975-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
Supported by: NIAMS, NIEHS, NCCAM, and ODS of NIH; WHNRC of USDA
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Fagi-eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 3.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 4.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF. High prevalence of vit D inadequacy and implications for health. Mayo Clinic Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. J Steroid Biochem Mol Biol. 2007;103:614–619. doi: 10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥ 60 y. Am J of Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 8.Wicherts IS, van Schoor NM, Boeke AJP, Visser M, Deeg DJH, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB. Effect of Vitamin D on falls: A meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. Erratum. [DOI] [PubMed] [Google Scholar]; Am J Clin Nutr. 2006;84:1253. [Google Scholar]
- 11.Bischoff-Ferrari HA, Borchers M, Gudat F, Duermueller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 12.Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469–474. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frewer LJ, Hindmarch I. The effects of time of day, age, and anxiety on a choice reaction task. In: Hindmarch I, Aufdembrinke B, Ott H, editors. Psychopharmacology and Reaction Time. Chickester, UK: Wiley; 1988. pp. 103–114. [Google Scholar]
- 15.Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SHD, Sift CG, Allain TJ. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 16.Ross R, Katzmarzyk PT. Cardiorespiratory fitness is associated with diminished total and abdominal obesity independent of body mass index. Int J Obesity. 2003;27:204–210. doi: 10.1038/sj.ijo.802222. [DOI] [PubMed] [Google Scholar]
- 17.Vilarrasa N, Maravall J, Estepa A, Sanchez R, Masdevall C, Navarro MA, Alia P, Soler J, Gomez JM. Low 25-hydroxyvitamin D concentrations in obese women: their clinical significance and relationship with anthropometric and body composition variables. J Endocrinol Invest. 2007;30:653–8. doi: 10.1007/BF03347445. [DOI] [PubMed] [Google Scholar]
- 18.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–3157. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 19.Snijder MB, van Dam RM, Visser M, Deeg DJH, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 20.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hyddroxyvitamin D levels in healthy women. J Clin Endocrinology Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 21.Alekel DL, St Germain A, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 22.Morabia A, Costanza MC. International variability in ages at menarche, first live birth, and menopause: World Health Organization collaborative study of neoplasia and steroid contraceptives. Am J Epidemiol. 1998;148:1195–1205. doi: 10.1093/oxfordjournals.aje.a009609. [DOI] [PubMed] [Google Scholar]
- 23.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 24.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Applied Physiol. 2004;97:509–514. doi: 10.1152/japplphysiol.01234.2003. [DOI] [PubMed] [Google Scholar]
- 25.Moeller LE, Peterson CT, Hanson KB, Dent SB, Lewis DS, King DS, Alekel DL. Isoflavone-rich soy protein prevents loss of hip lean mass but does not prevent the shift in regional fat distribution in perimenopausal women. Menopause. 2003;10:322–331. doi: 10.1097/01.GME.0000054763.94658.FD. [DOI] [PubMed] [Google Scholar]
- 26.Hollis BW. Measuring 25(OH)vitamin D in a clinical environment: Challenges and needs. Am J Clin Nutr. 2008;88:507S–510S. doi: 10.1093/ajcn/88.2.507S. [DOI] [PubMed] [Google Scholar]
- 27.Allison PD. Logistic Regression Using the SAS System: Theory and Application. SAS Institute Inc.; Cary, NC: 1999. Multicollinearity. [Google Scholar]
- 28.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: A D-lightful story. J Bone Miner Res. 2007;22(2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 29.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrin Metab. 2008 Nov 4; doi: 10.1210/jc.2008-1575. First published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am College Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arabi A, Baddoura R, Awada H, Salamoun M, Ayoub G, El-Hajj Fuleihan G. Hypovitaminosis D osteopathy: Is it mediated through PTH, lean mass, or is it a direct effect? Bone. 2006;39(2):268–275. doi: 10.1016/j.bone.2006.01.140. [DOI] [PubMed] [Google Scholar]
- 32.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 33.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence of alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rheaume C, Dupont P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 35.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. 2002;21:146S–151S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 36.Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr. 2003;133:252S–256S. doi: 10.1093/jn/133.1.252S. [DOI] [PubMed] [Google Scholar]
- 37.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24:537S–546S. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 38.Davis JN, Hodges VA, Gillham MB. Normal-weight adults consume more fiber and fruit than their age-and height-matched overweight/obese counterparts. J Am Diet Assoc. 2006;106:833–840. doi: 10.1016/j.jada.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Foo LH, Zhang Q, Zhu K, Ma G, Trube A. Relationship between vitamin D status, body composition, and physical exercise of adolescent girls in Beijing. Osteoporos Int. 2009;20:417–425. doi: 10.1007/s00198-008-0667-2. [DOI] [PubMed] [Google Scholar]
- 40.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94:67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. JAMA. 1994;272:1909–1914. doi: 10.1001/jama.1994.03520240037038. [DOI] [PubMed] [Google Scholar]
- 42.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 43.Snijder MB, van Schoor NM, Pluijm SMF, van Dam RM, Visser M, Lips P. Vitamin D status in relation to one-year risk of recurrent falling in older man and women. J Clin Endocrin Metab. 2006;91:2980–85. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 44.Fu S, Low Choy N, Nitz J. Controlling balance decline across the menopause using a balance-strategy training program: A randomized, controlled trial. Climacteric. 2008 Dec 4;:1–12. doi: 10.1080/13697130802506614. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RH, Jr, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- 46.Fischbach FT, Dunning MB., III . A Manual of Laboratory and Diagnostic Tests. Seventh. Philadelphia, PA: Lippincott Williams and Wilkins; 2004. p. 48. [Google Scholar]
- 47.Womack J, Tien PC, Feldman J, Shin JH, Fennie K, Anastos K, Cohen MH, Bacon MC, Minkoff H. Obesity and immune cell counts in women. Metab Clin Exper. 2007;56:998–1004. doi: 10.1016/j.metabol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white bold cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 49.Dixon JB, O'Brien PE. Obesity and the white blood cell count: Changes with sustained weight loss. Obesity Surgery. 2006;16:251–257. doi: 10.1381/096089206776116453. [DOI] [PubMed] [Google Scholar]