Abstract
Visualization of the cerebral vascular tree is important in experimental stroke and cerebral vascular malformation research. We describe a simple method, nuclear contrast angiography, that enables simultaneous visualization of the arterial tree and cerebral endothelial cells in rodent brain whole mounts. A mixture of latex and black ink was injected into the arterial system of rodents, resulting in high contrast demarcation of the arterial tree of the brain. This method clearly differentiates arteries from veins. We applied this method to demonstrate that 14 days of unilateral carotid artery occlusion induces increases in the caliber of (1) bilateral anterior communicating arteries, (2) bilateral anterior cerebral arteries, and (3) ipsilateral proximal middle cerebral artery of the circle of Willis. Unlike other methods, this procedure selectively stains endothelial nuclei of arteries. Thus, cerebral endothelial nuclei can be visualized, quantitated, and morphologically characterized at the same time the cortical arterial tree is delineated. This method should be useful in studies of stroke and cerebral arteriogenesis, which require the accurate assessment of both arterial diameters and endothelial cell density.
Keywords: nuclear contrast angiography, cerebral artery, angiogenesis, arteriogenesis, angiography, endothelial cells, nuclei, latex, ink
1. Introduction
The central nervous system angioarchitecture determines the regional distribution of blood flow to the brain and is a critical determinant of the severity of ischemic injury after arterial occlusion. Development of cerebral arteries and dynamic adaptation to environmental stimuli and have been studied in rodent models. Studies in rats have demonstrated that collateral circulation of the cortical vessels that connect major arterial zones modulate susceptibility to infarction after ligation of the middle cerebral artery [5]. Mouse strain differences in cortical collateral patterning may also determine the susceptibility to infarction after MCA occlusion [9]. Arteriogenesis, the increase in caliber of preexisting arterial trees, occurs after proximal ligation of the cerebral vessels in mice and serves a protective role after MCA occlusion [13].
These and other studies in rodent models have utilized angiographic techniques to map and measure arterial caliber and patterning. The methods for analyzing the angioarchitecture in small animals include injection of the arterial system with low viscosity resin [11], Araldite F [14], gelatin mixed with India ink [7], and latex [4]. Recently, Maeda et al improved the latex technique by including carbon black, which improves the ability to visualize vessels on the pale background of a fixed brain [9].
Endothelial cells play a critical role in the structure and function of cerebral vessels, as a permeability barrier and critical regulator of vascular tone (and hence perfusion). In addition, endothelial cells are the pioneer units that invade avascular tissue in angiogenesis. In spite of a clear role of endothelial cells in brain development, homeostasis, and compensation in response to cerebrovascular disorders, few anatomical studies of endothelial cell properties within the brain in situ have been performed. Such studies have been limited by the difficulty in visualizing significant regions of brain endothelial cells in whole mount.
In this following report, we describe a simple modification of latex angiography. This method was validated in a mouse model of cerebral arterial remodeling. We also show our method enables the simultaneous measurement of arterial diameters, vascular patterning, and visualization of endothelial cell nuclei. This method should be applicable to studies of brain angiogenesis, arteriogenesis, and endothelial biology.
2. Results
The impetus of these experiments was to identify a contrast agent for latex perfusion of the brain. Maeda has noted [9] that non-contrast latex angiography (using white latex) does not permit clear differentiation of cerebral vessels after brain fixation. We tested latex mixed with ink as perfusion agents by combining a series of commercial ink preparations with latex. Most of the latex and ink mixtures did not provide significant contrast when infused into mice; in fact, some of these diffused through the brain parenchyma and were not suitable as a contrast additive to latex. However, two latex and ink mixtures (each containing a black ink) provided effective contrast of the cerebral vasculature. The subsequent experiments described use one of these preparations (Private Selection Ink, Velvet Black), which we call “nuclear contrast angiography.” Figure 1 shows the results of use of nuclear contrast angiography in mouse brain. The procedure provides a striking purple outline of the cerebral vasculature at low magnification. As represented in these photographs, the venous circulation in mice does not fill with significant quantities of latex and remained red. In some mice (15%), latex did fill the cerebral veins, but the veins remained unstained with the contrast agent.
Figure 1.

Nuclear contrast angiography of mouse brain cerebral vasculature. Papaverine perfused mice were injected with a 1:20 mixture of black ink and latex. Ink improves stains the brain arteries, while veins were not stained in these mice. Remaining blood clearly outlines the venous circulation. In some mice, latex enters the veins; however, veins do not stain.
The retina has been commonly studied as a model system for blood vessel development because of its simple organization; restriction of retinal vessels to two-dimensions simplifies analysis, and development of retinal vessels continues for several days after birth in the mouse, facilitating pharmacological studies of angiogenesis. We investigated whether nuclear contrast angiography could be useful in studying retinal vessels. Whole mount preparations of mouse retinas after nuclear contrast angiography revealed excellent delineation of arteries (Figure 2A). As in the brain, veins were either unfilled or were partially filled with latex (marked with a “v” in Figure 2A; about 75% of retinal veins) and were clearly distinguished from arteries. Notably, on higher power examination retinal arteries were clearly stained by the dye, but veins remained unstained despite the presence of latex in some of these vessels (Figure 2B). The staining pattern of the arteries was not homogenous and suggesting that subcellular structures within the artery were targeted by the contrast agent.
Figure 2.

Nuclear contrast angiography selectively labels the arterial circulation of the retina. (A) Whole mount images of the retina. At low magnification, all arteries are filled with latex. In contrast, veins (v) do either do not fill or are only partially filled with latex. At higher power (B), punctuate staining of arteries is clearly observed, while veins do not stain and are incompletely filled with latex.
To validate and exemplify the use of nuclear contrast angiography in a disease model, we applied the technique to cerebral arterial remodeling after common carotid artery occlusion (CCAO) that has been recently used in mice [13]. Wildtype C57 mice underwent CCAO or no surgery. After fourteen days, mice then underwent nuclear contrast angiography to visualize the whole mounted cerebral vessels over 12 positions in the circle of Willis. Statistically significant arterial dilation occurred in bilateral anterior communicating (Acomm) and anterior cerebral arteries (ACA) (Figure 3). Interestingly, there was also a small, but significant, increase in the ipsilateral middle cerebral artery diameter at the takeoff from the circle of Willis (prox MCA). More distal segments of the MCA (mid MCA) did not demonstrate arterial growth. The contralateral Acomm diameter increased by 42%, which was similar to the value reported by Todo et al (who reported a diameter increase of 35% [13]).
Figure 3.

Vascular remodeling within the circle of Willis after carotid occlusion. Two groups of mice (n=14) were analyzed by nuclear contrast angiography: 1) Animals exposed to right CCAO for 14 days and 2) age and sex matched controls. Vessels of the circle of Willis were photographed in 12 regions, and arterial diameters were measured using NIH Image. R and L denote right and left sides of the brain, respectively. Significant (p<0.05) increases in arterial diameter in response to right CCAO were demonstrated in bilateral anterior communicating arteries (Acomm), bilateral anterior cerebral arteries (ACA), and the proximal segment of the middle cerebral artery (prox MCA) ipsilateral (right) of the carotid occlusion. No differences were seen in bilateral middle portions of the MCA (mid MCA), bilateral posterior communicating arteries (Pcomm), or the proximal MCA contralateral (left) to the common carotid occlusion.
In the peripheral circulation, we examined whether nuclear contrast angiography could highlight arteries within neurovascular bundles of the thoracic cage, which each contain the classical (inferior to superior) ordering of intercostal nerve, artery, and vein. As in the brain and the retina, nuclear contrast angiography labeled the artery, while the vein remained red and filled with venous blood (Figure 4A and 4B). Stained arteries were clearly distinguished from the nerve, which retained its natural pale color. The intercostals arteries were difficult to clearly visualize under the pleura, but when dissected out (Figure 4C) demonstrated an inhomogeneous, stippled staining pattern reminiscent of staining in retinal arteries (Figure 2B).
Figure 4.
Selective staining of arteries within neurovascular bundles. (A and B) The thorax was photographed after nuclear contrast angiography to highlight the neurovascular arrangement within the intercostal space. Arteries, but not veins or intercostal nerves, were stained within the classical grouping of nerves (pale color), arteries (stained purple), and veins (filled with blood) in thoracic myotomes paralleling the rib cage. The structures are partially obscured by the pleura, but when carefully dissected, the latex filled arteries display a puncate purple stain (C).
Small arteries in all tissues, on closer inspection, revealed a striking punctuate pattern of staining. High power images of cerebral arteries examined by nuclear contrast angiography demonstrate a fine stippled pattern, indicating that staining was not homogeneous and not targeted to endothelial cytosol (Figure 5). Stained structures had the approximate dimensions of nuclei. In addition, the long axis of the stained structures were orientated parallel to the axis of the vessel, which has been described for endothelial nuclei of blood vessels [8] [3] [10].
Figure 5.

Nuclear contrast angiography labels nuclei of the cerebral arteries. High magnification images of surface cerebral arteries demonstrate staining of endothelial nuclei. Three different calibers of cerebral arteries are shown: a 200 um diameter segment of the MCA (A), a 100 um diameter segment of the mid-MCA (B), and a 40 um terminal artery of the ACA (C).
To confirm that nuclear contrast angiography indeed targets nuclei, we stained cultured cells with the ink used in the nuclear contrast latex. MCF7 cells (Figure 6A–6C) and three other cell lines (not shown) stained strongly with ink only after cells were fixed. Permeabilization strongly enhanced the cell staining. The staining pattern was clearly nuclear. As shown in Figure 6D, nuclei of cultured endothelial cells also avidly stained using this ink.
Figure 6.

Ink used for nuclear contrast angiography selectively stains the nuclei of culture cells. MCF7 cells were exposed to a 1:20 dilution of the nuclear contrast ink in PBS for 30 minutes (A). In addition, MCF7 cells were either fixed (B) or fixed and permeabilized with 0.1% Triton X-100 (C) prior to exposure to nuclear contrast ink. Fixed bEND3 immortalized mouse endothelial cells displayed a similar staining pattern (D). Scale bar represents 100um.
To determine which cell layers within the artery were labeled by nuclear contrast angiography, we examined eosin counterstained sections of brain and kidney from rats examined by nuclear contrast angiography (Figures 7). Purple structures were noted in the lining of arteries but not in the media of blood vessels, suggesting that the stained structures in our experiments are indeed the nuclei of endothelial cells.
Figure 7.
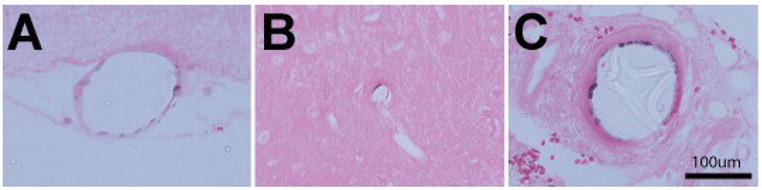
Nuclear contrast angiography preferentially labels the endothelium. Tissues from rats perfused with nuclear contrast latex were fixed and embedded in paraffin; sections were examined for the distribution of staining in vessels. All sections were counterstained with eosin (without hematoxylin). Stained cells were observed in the endothelial layer of cortical vessels of the brain (A) and occasionally small intraparenchymal arteries (B), but not in the media of vessels or the brain parenchyma. An endothelial pattern of staining was also observed in arteries from the kidney from the same animal (C).
We applied this technique to determine size of endothelial cells in the mouse cerebral circulation. We examined whole mount arteries under a dissecting microscope and quantified the number of nuclei of end arteries with diameters ranging from 25–36um. The average cell size was calculated by dividing the surface area of the observed arterial segment by the number of nuclei. The average cerebral endothelial surface area was 865 um2. We did not find significant differences in the endothelial sizes between branches of the anterior, middle, and posterior cerebral arteries (ACA, MCA, and PCA).
3. Discussion
We report a simple technique to simultaneously measure the cerebral arterial diameter and visualize the nuclei of endothelial cells lining the lumen of the arteries. Although other methods have been used to study cerebral artery diameter, nuclear contrast angiography possesses several advantages. Previously, Coyle [4] showed that papaverine dilated vessels could be casted with latex to examine the angioarchitecture of the Circle of Willis and cortical surface vessels. This technique relies on the concept that latex particles fill the arterial circulation but do not cross into the venous system. However, under some conditions, such as overperfusion or in animals with arteriovenous shunts, latex can enter the venous circulation, which may complicate analysis. This was seen commonly in the retina, where the veins frequently filled with latex. However, our method selectively stains the arterial endothelium and thus enables unambiguous arterial identification.
The validity of the method was assessed by quantifying arterial diameters in brain after CCAO. After two weeks of carotid occlusion, we found a significant increase in arterial diameter of the Acomm ipsilateral to the side of the carotid occlusion; the percentage increase in this vessel agreed very well with a previous report [13] which used Coyle’s method in mice.
To capitalize on the ability to measure multiple vessels in whole mount using nuclear contrast angiography, we measured arterial remodeling in the circle of Willis in mice after CCAO. We found that bilateral Acomm and ACA and the proximal segment of the ipsilateral MCA were significantly larger two weeks after CCAO, suggesting that arterial remodeling occurs in multiple vessels of the circle of Willis. This outcome could be explained by an increase in collateral flow across the Acomm and contralateral ACA. However, the vessels which demonstrated arteriogenesis are predicted to have widely divergent changes in flow: the ipsilateral ACA is expected to conduct an increased in flow in a retrograde direction with a significantly lower flow augmentation than the contralateral ACA, which is expected to be exposed to the greatest change in anterograde flow after CCAO; moreover, the proximal ipsilateral MCA is predicted to have decreased flow after CCAO, yet still exhibits arterial growth. These findings indicate that direction of flow and changes in flow within arteries after CCAO are likely not the prime stimuli of arterial remodeling in the circle of Willis. Recent evidence has suggested that monocytes may promote arteriogenesis in which arterioles are converted to arteries [2,12,13], but it remains unclear how the focal arterial segments that we observed to expand in this study could be regulated specifically by a circulating cell type.
The identification of endothelial cells has been performed in histological preparations, using sectioning and conventional staining techniques [6]. However, cross sectional analysis only allows identification of endothelial cell nuclei over a minute section of the tissue. Flaherty et al [8] stained canine arteries en face using Evans Blue; this method required removal of the vessels from the dog and, as such, is difficult to apply to rodents. Additional methods to visualize endothelial cells include genetic models in which marker proteins such as LacZ are selectively expressed using endothelial specific promoters. These methods do not consistently highlight the entire vascular tree and lack cellular resolution in whole mount studies [1,15]. Genetic methods also require the introduction of transgenes into animals under study, a procedure which adds cost and time. Animals have also been perfused with fluorescent nuclear dyes and examined by optical sectioning using confocal microscopy to differentiate endothelial nuclei [3]. Though powerful, this method is technically challenging, requiring specialized equipment; moreover, optical sectioning is impossible to perform over the entire adult brain, even in small rodents. Finally, casting of arteries followed by evaluation of endothelial cell outlines with scanning electron microscopy has been described [10], but this technique is also expensive equipment and ill-suited to whole brain analysis.
In contrast to prior methods, nuclear contrast angiography permits cost-effective examination of endothelial cells which has permitted the calculation of endothelial cell size. An additional advantage of this method is that it allows the simultaneous evaluation of endothelial cell nuclei in other visible vascular beds of the organism. The methodology should permit future studies to evaluate rodent models of both neurological and peripheral vascular disorders. In addition, developmental studies of endothelial orientation and number should be feasible using this technique. We have indeed been able to use this method in mice at postnatal day 7 (not shown).
An additional advantage of the nuclear contrast angiography method is that samples can be archived for long periods of time, which permits that delay of analysis or reanalysis of samples long after perfusion of the animals. We have observed stable appearance of stained vessels in mouse brains for more than eight months without deterioration of the vessels or the nuclear stain. Finally, as with other methods using latex to delineate the arterial tree, our method is capable of detecting tortuosity of arteries, a feature which could be useful in the analysis of mouse models of aberrant arterial morphogenesis and vascular malformations.
There are limitations of this method that should be noted. For instance, there is a faint stain of media cells after perfusion of larger (thicker) arteries. We feel that the latex method works by local permeabilization of endothelial cells, followed by rapid availability of the components of the ink to the endothelial nuclei. However, in larger arteries, the ink may be trapped in the vessel wall and the latex may open smooth muscle cell membranes allowing nuclear staining. Fortunately, in arteries, the smooth muscle cells are oriented orthogonal to the axis of the vessel and are a significant distance from the endothelium and thus out of the focal plane of the region of interest. Therefore, using conventional imaging, it is relatively easy to identify smooth muscle versus endothelial staining in medium sized vessels. However, in larger arteries (such as the circle of Willis), the thickness of the media does interfere with characterization of individual endothelial cells; endothelial visualization is therefore useful optimally for medium to small arteries. An additional limitation of the method is that visualization of arteries and endothelial cells within the brain parenchyma requires sectioning (as in Figure 7). Thus, the technique is best suited to examining accessible vascular beds such as those highlighted in our current study.
In summary, we present a simple method for delineating the cerebral arterial tree which also enables detailed identification of endothelial cells in whole mount. This method should allow easy archival studies of cerebral arteries in rodent experimental models.
4. Experimental procedures
Animals
We used C57B6 mice and Sprague-Dawley rats for this study. All animals were used in accordance with guidelines of the University of Michigan Institutional Animal Care and Use Committee. Common carotid artery occlusion (CCAO) was performed under anesthesia in 8–12 week old C57B6 mice (n=14). There were no differences between genders, so males and females were combined into one group. Angiography was performed on animals 14 days after CCAO. A separate group (n=14) of naïve, age and gender matched animals was analyzed as a control group. Images of the circle of Willis were captured using a Leica dissecting microscope and vessel diameters determined using NIH Image after calibration to a micrometer. The anterior communicating artery (Acomm) was measured at the midpoint of the artery. The proximal anterior and middle cerebral arteries (prox ACA and prox MCA) were defined at the take-off of the ACA and MCA from the circle of Willis. The mid-segment of the anterior cerebral artery (mid MCA) was defined at the bifurcation of the anterior communicating artery and the olfactory artery. The mid-segment of the MCA (mid MCA) was defined as the main branch of the MCA half way between the sagittal sinus and base of the brain (the midpoint superior to inferior).
Nuclear Contrast Angiography
Prior to nuclear contrast angiography, animals were anesthetized with ketamine/xylazine. We modified a procedure used by Coyle and Jokelainen [4] to perfuse the arterial tree with latex. In this procedure, animals were transcardially perfused via a left ventricular puncture with a lethal dose of papaverine (50mg/kg in water), which maximally vasodilated the arterial circulation. This was immediately followed by transcardial perfusion of a 1:20 mixture of black ink (Private Reserve Ink; Velvet Black) and white latex (Chicago Latex Product 563). The right ventricle of animals was incised to allow venous drainage. Approximately 0.5 ml of ink:latex was used for mice, and 5 ml was used for rats. After 5 minutes, tissue were harvested and fixed overnight to six months in 4% paraformaldehyde in PBS. Digital photographs of whole mount tissues were taken using a stereomicroscope. In some experiments, tissues were paraffin embedded and sections were stained with eosin. No hematoxylin was used so that nuclear staining of endothelial nuclei by the nuclear contrast dye could be confirmed.
Cell culture studies
All cultures were grown in DMEM plus 10% FBS. For staining studies, cells were rinsed with PBS, followed by 5 minute incubations of 4% paraformaldehyde in PBS with or without 0.1% Triton X-100. The cells were rinsed with PBS and then stained for 15 minutes with a 1:20 dilution of black ink (Private Reserve Ink; Velvet Black) in PBS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med. 2005;9:113–21. doi: 10.1111/j.1582-4934.2005.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arribas SM, Hillier C, Gonzalez C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–64. doi: 10.1161/01.hyp.30.6.1455. [DOI] [PubMed] [Google Scholar]
- 4.Coyle P, Jokelainen PT. Dorsal cerebral arterial collaterals of the rat. Anat Rec. 1982;203:397–404. doi: 10.1002/ar.1092030309. [DOI] [PubMed] [Google Scholar]
- 5.Coyle P, Jokelainen PT. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke. 1983;14:605–11. doi: 10.1161/01.str.14.4.605. [DOI] [PubMed] [Google Scholar]
- 6.Drixler TA, Borel Rinkes IH, Ritchie ED, Treffers FW, van Vroonhoven TJ, Gebbink MF, Voest EE. Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:3325–30. [PubMed] [Google Scholar]
- 7.Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981;7:519–79. doi: 10.1016/0361-9230(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Maeda K, Hata R, Hossmann KA. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–9. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- 10.Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, Letarte M. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34:783–9. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 11.Sbarbati A, Pietra C, Baldassarri AM, Guerrini U, Ziviani L, Reggiani A, Boicelli A, Osculati F. The microvascular system in ischemic cortical lesions. Acta Neuropathol. 1996;92:56–63. doi: 10.1007/s004010050489. [DOI] [PubMed] [Google Scholar]
- 12.Schneider UC, Schilling L, Schroeck H, Nebe CT, Vajkoczy P, Woitzik J. Granulocyte-macrophage colony-stimulating factor-induced vessel growth restores cerebral blood supply after bilateral carotid artery occlusion. Stroke. 2007;38:1320–8. doi: 10.1161/01.STR.0000259707.43496.71. [DOI] [PubMed] [Google Scholar]
- 13.Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, Yagita Y, Hori M. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–82. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- 14.van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg. 1992;77:927–40. doi: 10.3171/jns.1992.77.6.0927. [DOI] [PubMed] [Google Scholar]
- 15.Wong J, Bennett W, Ferguson MW, McGrouther DA. Microscopic and histological examination of the mouse hindpaw digit and flexor tendon arrangement with 3D reconstruction. J Anat. 2006;209:533–45. doi: 10.1111/j.1469-7580.2006.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



