Abstract
OBJECTIVE
Evaluate the expression and function of Catechol-O-methyltransferase (COMT) in human fetal membranes at term.
METHODS
Fetal membranes obtained from women between 38 and 42 weeks of gestation, after 1) vaginal delivery with spontaneous labor and 2) pre-labor elective cesarean section (no-labor), were assayed for COMT expression using quantitative real time PCR, immuno-histochemistry and western blot analysis. Prostaglandin E2 secretion from amnion and choriodecidua explants treated with or without COMT inhibitor was assayed by ELISA.
RESULTS
Amnion layer of fetal membranes from laboring women expressed significantly higher levels of COMT, compared to those from women with no-labor. COMT was higher in the amnion layer than in choriodecidua. Selective COMT inhibition significantly decreased prostaglandin E2 production from fetal membranes.
CONCLUSION
Labor increasesCOMT expression in the amnion of human fetal membranes. Selective COMT inhibition decreased prostaglandinE2 secretion in fetal explant cultures, suggesting a role for COMT in human labor and delivery.
Keywords: Catechol-O-methyltrasferase, Fetal Membranes, PGE2, Term labor
INTRODUCTION
In most mammalian species progesterone helps in maintaining uterine quiescence while estrogen stimulates changes that increase myometrial contractility.1 Onset of labor is preceded by an increase in estrogen/progesterone ratio in the maternal circulation. These changes are seen in species that depend on the placenta for sex steroid synthesis. But in women no such changes in circulating estrogen and progesterone ratios are seen during late pregnancy. 2 Thus, it has been suggested that localized changes in estrogen and progesterone levels or their hormonally active metabolites, not reflected in maternal circulation, may influence the initiation and progression of human labor.
Estrogen and progesterone are synthesized by all the layers of human fetal membranes: Amnion, Chorion, and Deciduas.3 However, each hormone is metabolized differently within these tissues. Progesterone is converted completely to an inactive metabolite, 20α-dihydroprogesterone,4 whereas, estrone the major estrogen formed by human fetal membranes, is converted to the more potent estradiol.5 Estrogen secreted in these membranes is known to stimulate the expression of oxytocin receptors, myometrial gap junctions and secretion of prostaglandins from fetal membranes.1,6–8 Therefore, local estrogenic milieu in the fetal membranes has an important role in inducing the changes which stimulate labor in women.
Catechol-O-methyl transferase (COMT) is a crucial enzyme in the pathway of estrogen metabolism. The activity of COMT determines the local estrogenic milieu in target tissues.9, 10 The initial step in estrogen metabolism is an oxidation reaction by cytochrome P450 (CYP450) to produce catecholestrogens, mainly 2- and 4-hydroxyestrogens. COMT catalyzes the methylation of these hydroxyestradiols converting them to 2- and 4-methoxyestrogens. 11,12 It has been demonstrated that these estrogen metabolites have distinct estrogenic or anti-estrogenic functions in the ovary,13 placenta,14 human myometrium15 and cancer.16 Since COMT regulates the levels of estrogen metabolites, it can be an important factor in regulating estrogen dependant changes in contractile associated factors like prostaglandin E2 (PGE2). We found that the inhibition of COMT enzyme activity significantly diminishes RU486 induced preterm delivery rate in rats.17 Therefore our objectives in this study were to, i) measure COMT expression in fetal membranes obtained from two groups of pregnant women at term; one group delivering via elective cesarean sections (no labor) and other group delivering vaginally (labor) and, ii) assess the effect of COMT inhibition on PGE2 production by fetal membranes in vitro.
Materials and Methods
Preparation of human fetal membranes
Institutional Review Board approval and informed consents from participants were obtained prior to this study. Fetal membranes were collected at term (between 38 and 42 weeks of gestation) from eight elective cesarean sections before labor and eight vaginal deliveries after spontaneous onset of labor. There was no significant difference between the two groups with respect to mean gestational age, parity, or sex of the infants. All pregnancies, labors, and newborn infants were without complications or evidence of infection. Immediately after delivery, placenta and fetal membranes were collected in sterile containers and transferred to the laboratory. Fetal membranes were cut around the placental plate under aseptic conditions in Ca2+ and Mg2+ free Hank’s buffered saline solution (HBSS) supplemented with Pencillin 100 units/mL, and Streptomycin 100 μg/mL (Sigma Aldrich, WI), Amphotericin 0.25 μg/mL (Invitrogen Corporation, CA) and Gentamicin sulfate 50 μg/mL (Cellgro, VA). From the middle zone of each of the fetal membranes, five full thickness (2 × 2 cm) segments were randomly sampled. The first segment was fixed in 10% formalin-PBS for at least 24 hours at 4°C for immunohistochemistry. The second segment was used for qRTPCR and western blot analyses. Amnion and choriodecidua layers were snap frozen separately in liquid nitrogen and stored at −70°C until further analyses. The remaining three fetal membrane segments were used for in vitro explants experiments.
Immunohistochemistry
The full thickness fixed fetal membranes were rolled up with amnion remaining the innermost, embedded in paraffin and sectioned at five micron thickness. After deparaffinization and rehydration of tissue sections with xylene and graded alcohol, antigen retrieval was performed in Target Retrieval Solution (Dako Corporation, Carpinteria, CA). Endogenous peroxidase was quenched by immersion in 3% hydrogen peroxide. Sections were blocked first with 10% goat serum and then by Avidin and Biotin. Following overnight incubation at 4°C with rabbit anti-human COMT polyclonal antibody (1:10000; Chemicon, Temecula, CA) sections were washed with 0.5% saponin buffer and incubated with biotinylated anti-rabbit IgG; (1:10000 Sigma Aldrich, St. Louis, MO). Color was developed by incubating the sections in Avidin-Biotin peroxidase solution followed by Diamniobenzidine (Vector, Burlington, CA) in PBS with 0.0003% H202. Sections were counterstained with hematoxylin (Sigma Chemicals, St. Louis, MO), mounted with DPX medium (Sigma), and visualized under light microscope at 400x magnification. Human liver specimens obtained from pathology archives served as positive controls and in negative control, the primary antibody was replaced with buffer solution.
RNA extraction
Total RNA was extracted from frozen specimens of amnion and corresponding choriodecidua using the acid guanidine thiocyanate phenol-chloroform method. All RNA samples were treated with a recombinant DNase I (Ambion; Austin, TX) to remove contaminating DNA. The amount of RNA was calculated using the A260 value.
Reverse Transcription (RT) and Polymerase chain reaction (PCR)
Two-step quantitative PCR assays were conducted to measure COMT mRNA expression using Applied Biosystems’ primers and supplies, (Foster City, CA). For RT, 200 ng of total RNA was added to a master mix containing random primers, dNTPs and RTase and incubated at 42°C for 30 minutes. Equivalent amounts of cDNA were used and amplification was performed for both COMT and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, and a 60°C annealing/extension for 60 seconds using ABI Prism 7700 Sequence Detection system. Two negative controls were used: one with water, substituted for RNA to check for contamination: the other was devoid of RTase to confirm that amplification was not due to genomic DNA. The threshold cycle (Ct) values of COMT and GAPDH, derived from the ABI Sequence Detector software (Foster City, CA), were used to calculate the relative mRNA levels. Each Ct value of COMT was normalized with the corresponding Ct value of GAPDH. Fold changes in COMT mRNA expression in laboring versus non laboring fetal membrane tissues were calculated using the delta/delta Ct method as described in User Bulletin #2, Applied Biosystems, Foster City, CA.
Protein extraction
Whole cell lysates were prepared by grinding frozen tissues and incubating in Tris HCl buffer (50 mM NaCl, 50 mM Tris, 1% NP-40, 0.5% DOC, 0.1% SDS) at 4°C for 30 minutes. After a brief sonication at 4°C, the insoluble materials were removed by a brief centrifugation at 10,000 g and 4°C for 10 minutes. Samples were aliquoted and stored at −70°C until further analysis.
Western blot analysis
Equal amounts of protein (10 μg) were resolved on polyacrylamide gel and transferred onto a nitrocellulose membrane by electroblotting. Membranes were blocked with 5% non fat milk in 25 mM Tris-Cl, 150 mM NaCl, 0.1% Tween-20 and incubated for 16h at 4°C with COMT rabbit polyclonal antibody (1:75,000; Chemicon; Temecula, CA). This was followed by a brief wash and incubation with secondary antibody for 1 hour at room temperature. Protein signals were captured on a Hyperfilm ECL after incubation of membranes in enhanced chemiluminiscence reagent (Amersham Biosciences; Piscataway, NJ). The intensity of immunoreactive bands was quantified by image analyzer (Bio-Rad Laboratories Hercules, CA). Membranes were re-probed for β-actin (1:10,000; Sigma Aldrich; St. Louis, MO) as the internal loading control. COMT protein levels were expressed as a ratio of respective β-actin signal.
In-vitro explants of human fetal membranes
Three 2 × 2 cm circular full thickness segments of fetal membranes were randomly sampled from the mid zone of fetal membranes obtained from pregnant women who delivered via elective cesarean at term and kept in a fresh sterile HBBS buffer. The amnion layer was carefully separated from the underlying choriodecidua and wet weight of each amnion and choriodecidua segment was determined. Tissue explants were subjected to preconditioning in Dulbecco’s minimum essential medium (DMEM; Invitrogen Corporation CA) for 4h at 37°C to revive the tissues from the effect of mechanical manipulation. Amnion and choriodecidua segments were cultured separately in explants media (DMEM supplemented with FBS) in duplicates and three groups for each time interval, at 37°C for 24 hrs before the treatments. Selective COMT inhibitor RO 41-0960 (Sigma Chemicals, St. Louis, MO) dissolved in ethanol at 10 and 50 μM concentrations were added to the two treatment groups while exact volume of ethanol was added to the explants media of the control group. The explants media were sampled at 6- and 24-hour intervals and stored at −70°C until further analyses.
Measurement of prostaglandinE2 secretion
PGE2 concentrations were measured in explant media obtained from control and treated wells using an ELISA kit (Cayman Chemical, Ann Arbor, MI) following Manufacturer’s protocol. To 50 μl explants media taken in duplicates into 96-well plate, 50 μl of PGE2 antiserum and 50 μl of tracer was added. After overnight incubation at 4° C, the wells were washed five times with wash buffer. Ellman’s reagent was added to each well and incubated in dark for 90 min and read at 405 nm. Standards ranging from 0–500 μg/ml were run in duplicates along with the samples. For assessing the non specific binding, a sample was run in two wells without antiserum. Concentrations of PGE2 in the explants media were normalized to the corresponding tissue weights (ng/g tissue) to eliminate the effect of the slight variation in sample weights. Paired student t-test was used for statistical analysis. Data were expressed as the means ± SD.
RESULTS
Immunolocalization of COMT
Hematoxylin and Eosin staining of cross sections of human fetal membranes showed no evidence of inflammatory infiltrate or chorioamnionitis. Histological examination of fetal membrane used in various experiments showed integrity of tissues and absence of any necrotic changes (data not shown). Figure 1 depicts immunohistochemical staining for COMT in cross sections of human fetal membranes (A–C). There was weak staining of COMT protein in the amnion and chorion but not in the decidua of fetal membranes obtained from elective cesarean section (no labor-panel B). However, fetal membranes obtained from women who delivered vaginally after spontaneous labor showed intense staining, particularly in the amnion layer, demonstrating elevated expression of COMT, (labor-panel C).
FIGURE 1. Immunohistochemical staining of COMT in term fetal membranes.
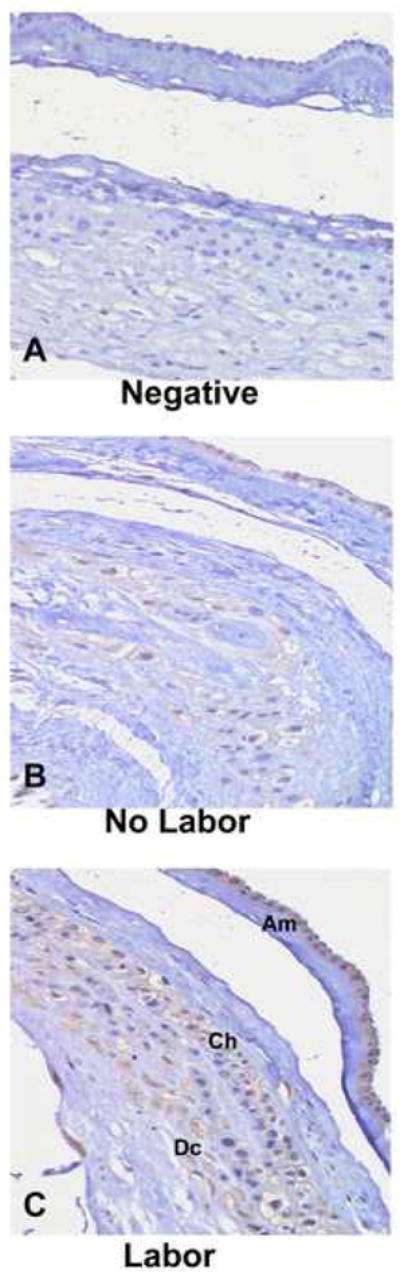
Panels show immune-staining of COMT in representative tissue sections from elective cesarean delivery before labor (Panels B- no labor) and from vaginal delivery after spontaneous onset of labor (Panel C- Labor). Brown staining indicates a positive reaction to COMT expression. The three layers of the fetal membranes the Amnion (Am), Chorion (Ch) and the Decidua (Dc) are labeled. Primary antibody was omitted for negative control (Panel A).
Messenger RNA expression of COMT fetal membranes
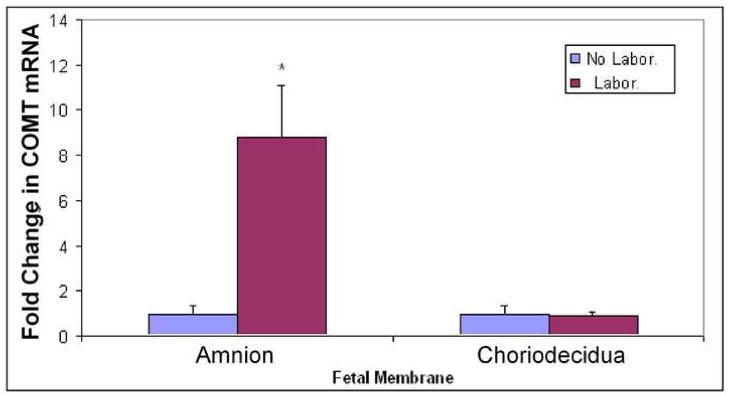
COMT mRNA is expressed both in the amnion and choriodecidua of fetal membranes. COMT expression increased nine fold (p < .05) in the amnion layers obtained from vaginal delivery after spontaneous labor (Laboring) compared to non laboring tissues obtained from elective cesarean section (Figure 2). In choriodecidua layers, COMT mRNA expression was not different between laboring versus non-laboring pregnant women.
FIGURE 2. Expression of COMT mRNA in term fetal membranes.
Amnion and Choriodeciduaobtained from pregnant women who delivered either via elective cesarean before labor (no labor) or vaginally after spontaneous onset of labor (labor) were subjected to quantitative RTPCR. The bars represent the fold change in COMT density (mean ± SD) relative to GAPDH from eight women in each group. Group with asterisk (*, P < 0.05) at the top of the bar is significantly different.
Protein expression of COMT in amnion and choriodecidua fetal membrane layers from laboring versus non laboring women
Western blot analysis demonstrated COMT protein expression both in the amnion and choriodecidua. COMT showed differential expression in the amnion, with levels being markedly elevated (p < 0.01) in tissues from laboring women than in the tissues from non laboring women (Figure 3A). On the other hand expression of COMT did not show any significant difference in choriodecidua obtained fromlaboring versus non laboring women (Figure 3B).
FIGURE 3. Levels of COMT protein at delivery.
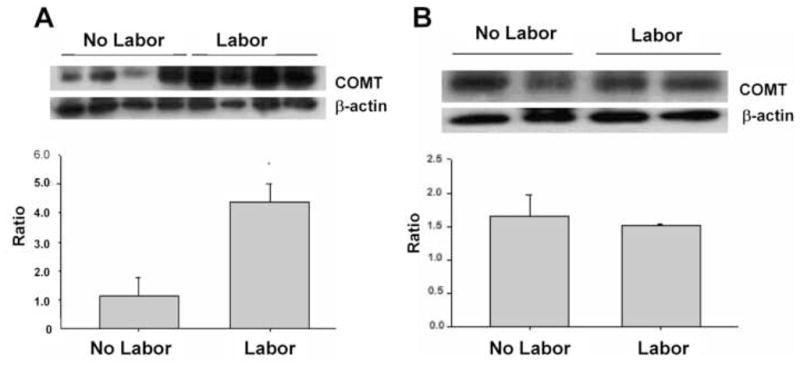
COMT was measured in homogenates prepared either from amnion (panel A) or choriodecidua (panel B), obtained from pregnant women who delivered either via elective cesarean before labor (no labor) or delivered vaginally after spontaneous onset of labor (labor). Top) Western blot analysis for COMT and β-actin. Bottom) Densitometric analysis of COMT levels normalized to β-actin. Data are presented as the mean ±SEM from eight replicates in each group.
In-vitro explants of human fetal membranes
The levels of PGE2 secreted by the amnion layer in culture were 20–40 folds higher than those of choriodecidua both at 6 and 24h (Figure 4). Although there was a gradual increase in PGE2 secretion with time (at 24h compared to 6h) in both amnion and choriodecidua explants, the levels were not statistically significant.
FIGURE 4. PGE2 levels secreted into the explant media by fetal membranes.
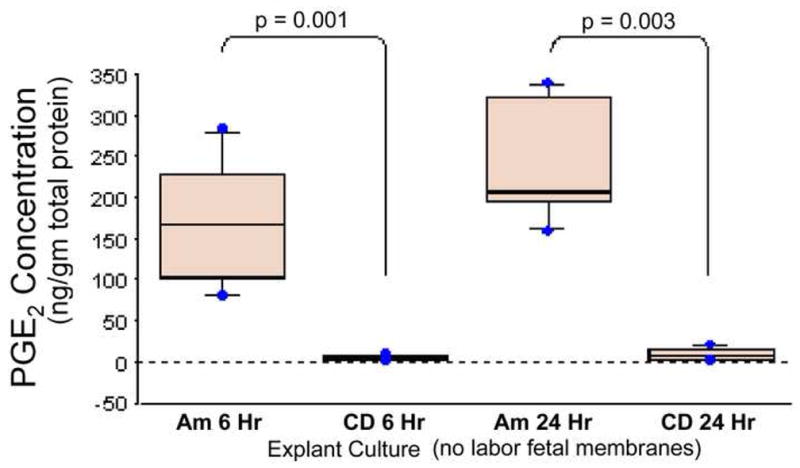
Amnion and choriodecidua were separated carefully from the fetal membranes obtained from women who delivered via elective cesarean before labor and cultured in explant media for 6 and 24h. ProstaglandinE2 secreted into the media was measured. The bars represent the mean ±SD of eight samples.
Incubation of human fetal membranes with COMT inhibitor resulted in a significant decrease in PGE2 production. The reduction was more evident in the amnion explants compared to the choriodecidua explants (Figure 5). COMT inhibition significantly decreased PGE2 levels in amnion explants at 6h, from 172±77 ng/g in untreated control, to 16 ± 15 ng/g (p = .005) in 10 μM and 37 ± 28 ng/g (p=. 012) in 50 μM. Similarly at 24-hour PGE2 levels decreased from 239±74 ng/g in untreated control, to 26 ± 23 ng/g (p = .001) at10 μM and 14 ± 11 ng/g (p=. 001) at 50 μM. In choriodecidua explants, PGE2 levels were not different between the treatments (1.5 ± .7 ng/g and 1.1 ± .6 ng/g for 10uM and 50uM COMT inhibitor respectively) and controls (4.3 ± 3 ng/g) at 6h. However, at 24-hour interval, PGE2 levels decreased significantly (Figure 6) both with 10 and 50 μM COMT inhibitor (0.5 ± 0.2 ng/g; p = .04 and 0.2 ± 0.1 ng/g; p = .03 respectively) compared to the levels in controls (9.6 ± 8.3 ng/g).
FIGURE 5. PGE2 levels in the media of amnion tissue explants treated with specific COMT inhibitor RO41-0960.
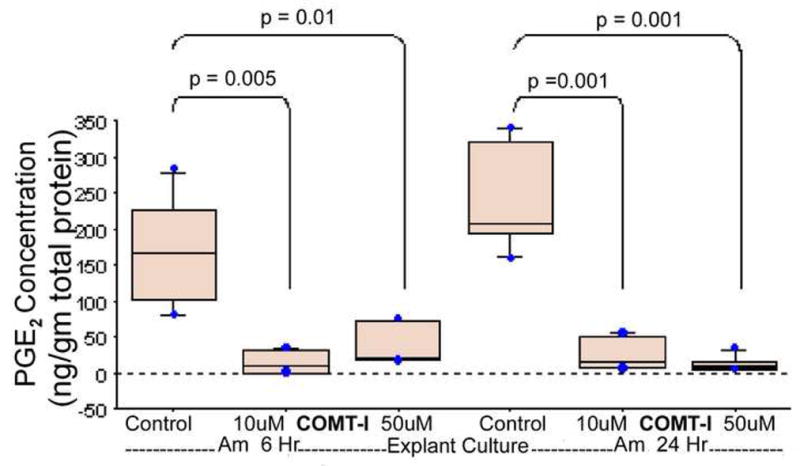
Amnion explants were treated with 10 and 50uM of selective COMT inhibitor RO 41-0960 dissolved in ethanol. The explants media were sampled at 6- and 24-hour intervals and PGE2 was measured by ELISA. The values obtained were normalized against total protein concentration. The bars represent the mean ± SD of eight samples. p≤.05 is statistically significant.
FIGURE 6. PGE2 levels in the media of the choriodecidua tissue explants treated with COMT inhibitor RO41-0960.
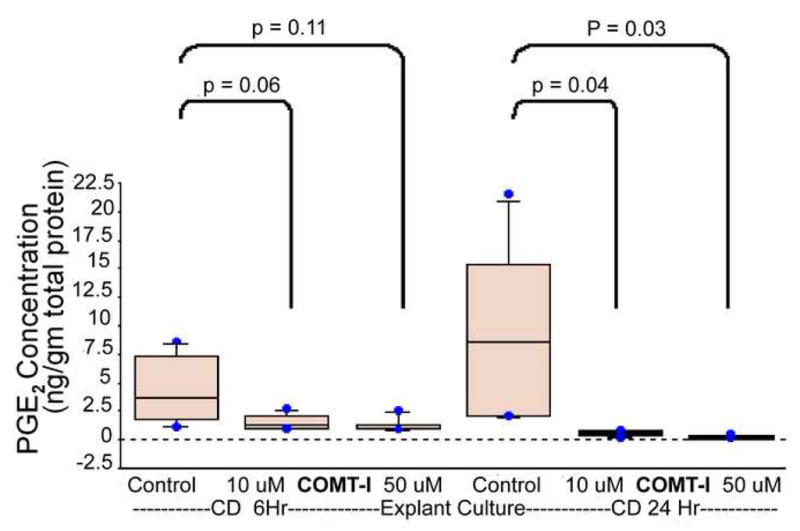
Choriodecidua explants were treated with 10 and 50uM of selective COMT inhibitor RO 41-0960 dissolved in ethanol. The explants media were sampled at 6- and 24-hour intervals and PGE2 was measured by ELISA. The values obtained were normalized against total protein concentration. The bars represent the mean ± SD of eight samples. p≤.05 is statistically significant.
COMMENT
We report for the first time, that COMT is expressed in the layers of human fetal membranes. Immunostaining showed increased expression of COMT in the amnion layer obtained from laboring women compared to the expression observed in membranes from women not in labor. RT-PCR analysis also revealed a nine fold increase in COMT mRNA expression in the amnion layer obtained from laboring women compared to the membranes from women not in labor. Results from western blot analysis confirmed the increase in COMT expression in amnion from laboring women suggesting a strong role for COMT in labor at term. Further, selective inhibition of COMT in amnion explants using RO 41-0960, resulted in a significant decrease in prostaglandin production. We have demonstrated COMT immunolocalization in amnion, chorion and decidua of human fetal membranes. Other studies have reported COMT in myometrium,15 ovaries,18 endometrium,19 decidua vera20 and placenta.21 Our studies showed that COMT levels are higher in amnion layer obtained from laboring compared to those from non laboring women. COMT is a crucial enzyme in the estrogen metabolism. It catalyzes the methylation reaction that converts the hydroxy estrogens to methoxy estrogens. Hydroxy estrogens have weak estrogenic effect and in fact act as estrogen antagonists15,16, 22 while methoxy estrogens have potent estrogenic properties.23, 24 Therefore, an increase in the activity of COMT will enhance the conversion of hydroxy estrogens to methoxy estrogens leading to more estrogenic milieu. RT-PCR and western analysis of fetal membranes in the present study further confirms the increase in COMT expression levels observed in the amnion from laboring women. COMT activity measured in placenta did not show any difference between spontaneous vaginal and elective cesarean delivery. 21
We observed similar results in choriodecidua tissues. COMT mRNA and protein expression measured in choriodecidua tissues was not different between normal spontaneous vaginal delivery and elective cesarean section at term. Results reported in decidua vera20 a component of the choriodecidua layer of fetal membranes showed an increase in COMT at term delivery compared to the levels early in gestation. In our study, expression of COMT was evaluated only at term pregnancy as it is not possible to conduct longitudinal studies with fetal membranes. The difference is that we found higher expression of COMT mRNA and protein in amnion layers obtained after spontaneous vaginal compared with amnion obtained after elective cesarean section. It was reported that COMT expression increase during pregnancy in the uterus17 and placenta25 and that inhibition of COMT in late pregnancy leads to pre eclampsia like symptoms14 suggesting that COMT plays an important role in growth and maintenance of placenta. These are further supported by our studies on the COMT expression in myometrium and the levels of 2-hydroxyestradiol in urine during pregnancy in rats,17 indicating that COMT starts increasing from early third trimester and peaks at the time of labor and delivery. Since estrogens are synthesized in human fetal membranes, we believe that the function of COMT is to regulate the local estrogenic milieu and estrogen dependent functions including uterine contractility related pathways. Therefore, the elevated COMT expression observed in the amnion from laboring (versus non-laboring) women in this study, strongly suggests an important role for COMT in labor.
COMT is expressed in the amnion layer which is also the main site for prostaglandin synthesis. 26, 27, 28 Therefore, we used an in vitro explants model of human fetal membranes to study the effect of COMT inhibition on PGE2 production. In this study, COMT inhibitor dramatically reduced the secretion of PGE2, especially in the amnion explants. In the present study, decreases in pro estrogenic methoxy estrogen as a result of COMT inhibition might have decreased PGE2 in fetal membranes. In our studies, 29 using term human myometrial cells, selective COMT inhibitor, RO 41-0960 decreased both COX-2 expression and PGE2 levels. This along with the increases observed in COX-2 after 2-methoxy treatment30 and decreases in the rate of RU486 induced preterm births in rats treated with COMT inhibitor17 supports a role for COMT in the initiation of labor. In summary, our study demonstrated a significant increase in COMT expression in fetal membranes from laboring women, especially, in the amnion layer. Further, these results suggest that COMT regulates PGE2 production in term human fetal membranes. However, mechanisms involved in the regulation of prostaglandin secretion by COMT are not known and need further investigation.
Acknowledgments
We also would like to thank Dr. Veera Rajaratnam, Director of Scientific Publications and Grant Support Core at the Center for Women’s Health Research, Meharry Medical College, for the excellent editing and proof reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Challis JRG, Mitchell BF. Steroid production by the fetal membranes in relation to the onset of parturition. In: McNillis D, Challis JRG, McDonald PC, Nathanielsz PW, Roberts JM, editors. The onset of labour: cellular and integrative mechanisms. Ithaca, New York: Perinatology Press; 1988. pp. 233–53. [Google Scholar]
- 2.Boroditsky Richard S, Reyes Francisco I, Winter Jeremy SD, Faiman Charles. Maternal Serum Estrogen and Progesterone Concentrations Preceding Normal Labor. Obstetrics and Gynecology. 1978;51:686–91. [PubMed] [Google Scholar]
- 3.Mitchell BF, challis JRG, Lukash L. Progesterone synthesis by human amnion, chorion, and deciduas at term. Am J Obstet Gynecol. 1987;157:349–53. doi: 10.1016/s0002-9378(87)80169-2. [DOI] [PubMed] [Google Scholar]
- 4.Strickler RC, Tobias B. Estradiol 17 beta-dehydrogenase and 20 alpha-hydroxysteroid dehydrogenase from human placental cytosol: one enzyme with two activities? Steroids. 1980;36:243–53. doi: 10.1016/0039-128x(80)90022-7. [DOI] [PubMed] [Google Scholar]
- 5.Romano WM, Lukash LA, Challis JRG, Mitchell BF. Substrate utilization for estrogen synthesis by human fetal membranes and deciduas. Am J Obstet Gynecol. 1986;155:1170–5. doi: 10.1016/0002-9378(86)90139-0. [DOI] [PubMed] [Google Scholar]
- 6.Dong YL, Yallampalli C. Pregnancy and exogenous steroid treatments modulate the expression of relaxant EP2 and contractile FP receptors in the rat uterus. Biol of Reprod. 2000;62:533–9. doi: 10.1095/biolreprod62.3.533. [DOI] [PubMed] [Google Scholar]
- 7.Fang X, Wong S, Mitchell BF. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and parturition. Endocinol. 1996;137:3213–9. doi: 10.1210/endo.137.8.8754742. [DOI] [PubMed] [Google Scholar]
- 8.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Reviews 2000. 2000;21:514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 9.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the new Selective COMT Inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 11.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Tekpetey FR, Armstrong DT. Catecholestrogen modulation of steroid production by rat luteal cells: mechanism of action. Mol Cell Endocrinol. 1994;101:49–57. doi: 10.1016/0303-7207(94)90218-6. [DOI] [PubMed] [Google Scholar]
- 13.Creveling CR. The role of catechol-O-methyltransferase in the inactivation of catecholestrogen. Cell Mol Neurobiol. 2003;23:289–91. doi: 10.1023/A:1023680302975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanasaki Keizo, Palmsten Kristin, Sugimato Hikaru, ahmad Shakil, Hamano Yuki, Xie liang, Parry Samuel, Augustin Hellmut G, Gattone Vincent H, Folkman Judah, Strauss Jerome F, Kalluri Raghu. Defeciency in catechol-O-methyltransferase and 2-methoxyestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 15.Wentz MJ, Jamaluddin M, Garfield RE, Al Hendy A. Regulation of catechol-O-methyltransferase expression in human myometrial cells. Obstetrics and Gynecology. 2006;108:1439–47. doi: 10.1097/01.AOG.0000243775.73788.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider Jill, Huh Martin M, Leon Bradlow H, Fishman Jack. Antiestrogen action of 2-Hydrosyestrone on MCF-7 Human Breast cancer cells. J Biol Chem. 1984;259:480–5. [PubMed] [Google Scholar]
- 17.Melissa Wentz, Shi Shao-Qing, Shi Leili, Salama Salama A, Harirah Hassan M, Fouad Hala, Garfield Robert E, Al-Hendy Ayman. Treatment with an inhibitor of catechol-O-methyltransferase activity reduces preterm birth and impedes cervical resistance to stretch in pregnant rats. Reproduction. 2007;134:831–9. doi: 10.1530/REP-07-0245. [DOI] [PubMed] [Google Scholar]
- 18.Salih SM, Jamaluddin M, Salama SA, Fadl AA, Nagamani M, Al-Hendy A. Regulation of catechol O-methyltransferase expression in granulosa cells: a potential role for follicular arrest in polycystic ovary syndrome. Fertil Steril. 2008;89:1414–21. doi: 10.1016/j.fertnstert.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Salih SM, Salama SA, Fadl AA, Nagamani M, Al-Hendy A. Expression and cyclic variations of catechol-O-methyl transferase in human endometrial stroma. Fert Steril. 2008;90:789–97. doi: 10.1016/j.fertnstert.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey ML, MacDonald PC. Characterization of catechol-O-methyltransferase activity in human uterine decidua vera tissue. Am J Obstet and Gynecol. 1983;145:453–7. doi: 10.1016/0002-9378(83)90316-2. [DOI] [PubMed] [Google Scholar]
- 21.Barnea ER, MacLusky NJ, DeCherney AH, Naftolin F. Catechol-O-methyl transferase activity in the human term placenta. Am J Perinatol. 1988;5:121–7. doi: 10.1055/s-2007-999669. [DOI] [PubMed] [Google Scholar]
- 22.Bradlow LH. 2-Hydroxyesterone: the “good” estrogen. Journal of Endocrinology. 1996;150:S259–S65. [PubMed] [Google Scholar]
- 23.Vandewalle B. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrionol. 1989;61:239–46. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland TE, Schuliga M, Harris T, Eckhardt BL, Anderson RL, Quan L, et al. 2-methoxyestradiol is an estrogen receptor agonist that supports tumor growth in murine xenograft models of breast cancer. Clin Cancer Res. 2005;11:1722–32. doi: 10.1158/1078-0432.CCR-04-1789. [DOI] [PubMed] [Google Scholar]
- 25.Castren O, Saarikoski S. The simultaneous function of catechol-O-methyltransferase and monoamine oxidase in human placenta. Acta Obstet Gynecol Scand. 1974;53:41–7. doi: 10.3109/00016347409156887. [DOI] [PubMed] [Google Scholar]
- 26.Hirst JJ, Teixeira FJ, Zakar T, Olson DM. Prostaglandin H synthase-2 expression increases in human gestational tissues with spontaneous labour onset. Reproduction, Fertility and Development. 1995;7:633–7. doi: 10.1071/rd9950633. [DOI] [PubMed] [Google Scholar]
- 27.Gibb W, Sun M. Localization of prostaglandin H synthase type 2 protein and mRNA in term human fetal membranes and decidua. J Endocrinol. 1996;50:497–503. doi: 10.1677/joe.0.1500497. [DOI] [PubMed] [Google Scholar]
- 28.Olson DM, Smieja Z, Zakar T, MacLeod EA, Walton J, Milne K. Regulation of prostaglandin synthesis in the human amnion. Reproduction, Fertility and Development. 1991;3:413–9. doi: 10.1071/rd9910413. [DOI] [PubMed] [Google Scholar]
- 29.Thota C, Pullman S, Al-Hendy A. Catechol-O-Methyltransferase regulates the expression of contractile associated proteins in term human myometrial cells. 11th RCMI International Symposium on Health Disparities; Honolulu. Dec. 1–4, 2008.p. 120. [Google Scholar]
- 30.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–74. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]