Abstract
Maternal hormones can dramatically modify offspring phenotypes via organizational actions on morphological and behavioral development. In placental mammals, there is the possibility that some portion of hormones in maternal circulation may be derived from fetal origin. We tested the possibility that maternal androgens in pregnant female marmosets reflected, in part, contributions from male fetuses by comparing levels of urinary androgens across pregnancy in females carrying varying numbers of male offspring. We monitored urinary androgen excretion in 18 pregnancies from five female white-faced marmosets (Callithrix geoffroyi). Androgen levels rose significantly in the first trimester of pregnancy, reached a peak in the middle of the second trimester, and then declined gradually until parturition. At no point in pregnancy were levels of urinary androgens higher in females carrying litters that had 50% or more males than females carrying litters that were less than 50% male. Levels of maternal androgens were not associated with litter size, the number of males in the litter, or with the proportion of the litter that was male. The high levels of androgen in pregnant females are therefore likely of strictly maternal origin, and any modification of fetal growth and development can be considered a ‘maternal effect’.
1. Introduction
Steroid hormone production during pregnancy in female mammals is typified by dramatic increases in estrogens and progestagens, derived from ovarian, luteal, and placental sources (Albrecht & Pepe, 1990; Ojeda, 2004). The onset of pregnancy in a number of species is also associated with concomitant increases in the production of androgens (dog: Concannon & Castracane, 1985; baboon: Castracane & Goldzieher, 1983; human females: Castracane & Asch, 1995; Castracane et al., 1998; rhesus macaques: Challis et al., 1975; marmoset: Chambers & Hearn, 1978; Fite et al., 2005; rat: Gibori & Sridaran, 1981; Legrand et al., 1984). There is a growing interest in the possibility that maternally-derived androgens can significantly impact embryonic and fetal development in vertebrates (Groothuis et al., 2005; Dloniak et al., 2006; Hines, 2006). Developing male fetuses also produce androgens (Parker, 2004) that may move from the fetal compartment to the maternal compartment via placental vasculature. In order to adequately assess strictly ‘maternal effects’ of gestational androgens, therefore, it is critical to determine whether the measurement of androgens from maternal biological samples reflect steroids of solely maternal origin, or of maternal plus fetal origin.
There is some controversy whether androgens of fetal origin enter maternal circulation in concentrations sufficient to alter maternal levels. There are a number of studies that show that maternal androgens are higher in human mothers when she is carrying a male fetus than when she is carrying a female fetus (Muelenberg & Hofman, 1992; Nagamani et al., 1979), with significant differences emerging as early as five to seven weeks post-conception (Klinga et al., 1978; Harrison & Mansfield, 1980). Similar differences in maternal androgens have also been reported in baboons (Altman et al., 2004), elephants (Duer et al., 2002), and lemurs (Ostner et al., 2003). However, there is also evidence that maternal androgen concentrations are not affected by the sex of the fetus the mother is carrying (Glass & Klein, 1981; Steler et al., 2002; Triosi et al., 2003). Several recent studies have also demonstrated no effect of fetal sex on circulating maternal androgens. Two studies contrasted androgen concentrations in amniotic fluid or cord blood, as well as in general maternal circulation, in human mothers carrying males or females. As expected, androgens were higher in both amniotic fluid and cord blood for male fetuses than female fetuses, but there was no evidence that fetally-derived androgens altered maternal endocrine states, since circulating androgens did not differ as a function of fetal sex (Triosi et al., 2003; Van de Beek et al., 2004). Finally, Cohen-Bendahan et al. (2005) evaluated maternal testosterone levels in women carrying same-sex or opposite-sex twins, and found that maternal androgens did not vary significantly as a function of the sex composition of the twins. In a litter-bearing rodent (rat), levels of maternal androgen do not vary systematically with the sex ratio of the litter (Castracane & dela Cruz, 1990; Houtsmuller et al., 1995). In rats, normal variation in litter size is not associated with variation in circulating maternal androgens (Houtsmuller et al., 1995), but experimental reduction of litters early in gestation to four or one implanted embryos is associated with a significant reduction in maternal androgen (Castracane & dela Cruz, 1990).
Marmosets of the genus Callithrix provide a useful model for assessing the potential influence of maternal physiology on developing offspring. First, marmosets are the only simian primates that produces a litter. The modal litter size is two, but litter sizes can range from one to five (Smucny et al., 2004; Ross et al., 2007). Second, since the offspring are not identical twins, the sex ratios of litters can vary from 0% to 100% males. Finally, it has already been demonstrated that marmoset females, like other primates, exhibit elevated levels of circulating androgens during gestation (both testosterone (T) and androstenedione (A4)), with peak concentrations occurring during the second trimester (Chambers & Hearn, 1979). However, it is important to determine whether measures of maternal androgen concentrations during gestation reflect solely maternal sources, via ovarian or placental steroidogenesis, or reflect both maternal and fetal contributions.
In the present study, we evaluated androgen profiles in female marmosets throughout pregnancy, and the impact of litter sex ratio on levels of excreted urinary androgens in female marmosets. Urine was collected from female marmosets throughout pregnancy, and we examined the resulting endocrine profiles as a function of the sex ratio of the litter carried by the mother. To the extent that male fetuses constitute a source of androgen for their mothers, we predicted that mothers with male fetuses, or a greater proportion of fetuses that were male, would have higher levels of excreted urinary androgens. We also evaluated the impact of overall litter size (regardless of sex ratio) and maternal age on androgen levels.
2. Methods
2.1 Subjects
Five female marmosets (Callithrix geoffroyi) provided samples for this study. The marmosets were housed in the Callitrichid Research Center at the University of Nebraska at Omaha. All females were housed with an adult male pairmate, and from zero to eight offspring. Table 1 provides demographic information on the females in the study, including female ages and the sex ratios of their litters. Pairs and family groups were housed in large enclosures (minimum: 0.6 × 0.6 × 2.2 m; maximum: 3.0 × 3.0 × 2.5 m) that varied depending upon groups size, and contained natural branches, nest boxes, and feeding stations. Rooms were maintained on a 12h:12h light:dark cycle, with light onset occurring between 0700 and 0800 h. Marmosets had ad lib access to water, and were fed a varied diet of commercial marmoset chow, fresh fruits and vegetables, and animal protein once each day. The routine research and husbandry procedures in our facility were designed to minimize disruptions and disturbances to the day-to-day activities of the marmosets. Details of colony management and husbandry can be found in Schaffner et al., (1995). All of the procedures were evaluated and approved by the University of Nebraska at Omaha/University of Nebraska Medical Center Institutional Animal Care and Use Committee: Protocols 01-022-03 and 07-033-05.
Table 1.
Subjects and Demographic Characteristics
| Female | Age Range (years) | Sex Ratios of Litters in Studya |
|---|---|---|
| Bes | 4.6 – 6.6 | 1.1, 1.0 |
| Dar | 4.8 – 5.7 | 1.1, 0.2, 1.1 |
| Lor | 5.7 – 6.6 | 1.2, 0.2, 1.0 |
| Pop | 4.7 – 8.7 | 1.1, 4.0, 2.1, 2.0, 2.1 |
| Swe | 4.5 – 6.7 | 1.1, 1.2, 0.2, 1.1, 1.3 |
Numbers represent males.females in litter
2.2 Selection of pregnancies
We used the following criteria to identify candidate pregnancies for this study. First, we selected only those pregnancies that went to full-term, either on the basis of the estimated length of gestation as determined from hormone profiles or on the presence of full-term infants (perinatal weights > 30 g). Second, we eliminated any pregnancies that were known or suspected to have had early fetal loss. Finally, we eliminated any pregnancies in which the sex of all offspring in the litter could not be unambiguously determined. The application of these criteria yielded 18 pregnancies from the five females.
2.3 Urine collection
We collected urine samples from the breeding females two to five times per week continuously throughout the study period. A non-invasive, stress-free procedure was used as described previously (French et al., 1996). Briefly, animals were trained, using reward conditioning, to provide a urine sample. Samples were collected in disposable aluminum pans, immediately after light onset in the colony rooms, and hence represented first-void samples. After collection, the samples were centrifuged at 2000 rpm for two minutes to separate solid detritus, and the clean supernatant was then transferred to a clean 1.5 ml minivial for storage. All samples were cataloged in our database and kept frozen at −20° C until assayed.
2.4 Androgen assay
Concentrations of maternal androgen were determined by assaying urine samples via a testosterone enzyme immunoassay that has previously been characterized and validated for marmosets (Nunes et al., 2000; Fite et al., 2005). Briefly, urine samples (10 µl) were extracted in 5 ml freshly-opened diethyl ether after enzyme hydrolysis with β-glucuronidase (Sigma Chemical, St. Louis MO). The ether was evaporated in a warm water bath under a gentle stream of air, and samples were reconstituted in 1.0 ml phosphate buffered saline. Procedural losses of androgen during the extraction procedure were monitored by the recovery of radiolabelled testosterone, and sample concentrations were corrected for loss. Each microtiter plate contained a standard curve (1000 – 7.8 pg testosterone), high and low concentration quality controls consisting of pooled marmoset urine, and samples. All standards, quality controls, and samples were assayed in duplicate. The testosterone antibody used in the assay system cross-reacts with androstenedione and dihydrotestosterone (Dloniak et al., 2004), hence the results are described as urinary androgen concentrations, rather than urinary testosterone. We used two sets of quality control pools, and interassay coefficients of variation averaged 17.9% and 7.4% for the high and low concentration pools, respectively. Intra-assay coefficients of variation for the same pools averaged 7.9% and 10.4% for the high and low concentration pools, respectively. In order to control for variable fluid intake and urinary output, all androgen concentrations were corrected by urinary creatinine concentrations. We used a modified Jaffé end-point assay (Tietz, 1976) which has been previously described and validated for marmosets (French et al., 1996).
2.5 Statistical analyses
We conducted several analyses to assess whether the sex ratio of the litter was associated with levels of excreted androgens in pregnant female marmosets. First, we divided pregnancies into those in which 50% or more of the litter were male fetuses (n = 12) and those in which less than 50% of the litter were male fetuses (n = 6). For each pregnancy, we calculated pre-pregnancy levels of androgen excretion immediately prior to conception, and for 10-day blocks during the 146 – 149 day gestation period. The values for each 10-day block represented the androgen levels averaged from one to five samples collected during each block. A mixed-model ANOVA was used to assess differences across gestation as a function of the sex ratio of the litter. “Female” was included as an independent effect, and multiple pregnancies from the same female were nested under the Female effect. In this way, we could also assess individual differences among females in androgen excretion profiles throughout pregnancy. We also conducted a similar mixed-model ANOVA across 10-day blocks of gestation but contrasted excreted androgens in litters that only contained males (n = 4) versus litters that only contained females (n = 3).
Second, we conducted correlational analyses to assess whether continuous variation in litter sex ratio was predictive of maternal androgen excretion. Toward this end, we calculated nonpregnant androgen levels for each pregnancy and a mean concentration for each trimester of pregnancy. Means for the trimesters derived from androgen concentrations from more than 10 – 20 samples that were distributed evenly throughout each trimester. These values were then correlated with two estimates of sex ratio: (1) the number of males in the litter (range: 0 – 4) and the proportion of the litter that was male (range: 0 – 1.0). To the correlational analyses we also added female age and litter size, to see if any variation in androgen concentrations could be explained by these variables.
3. Results
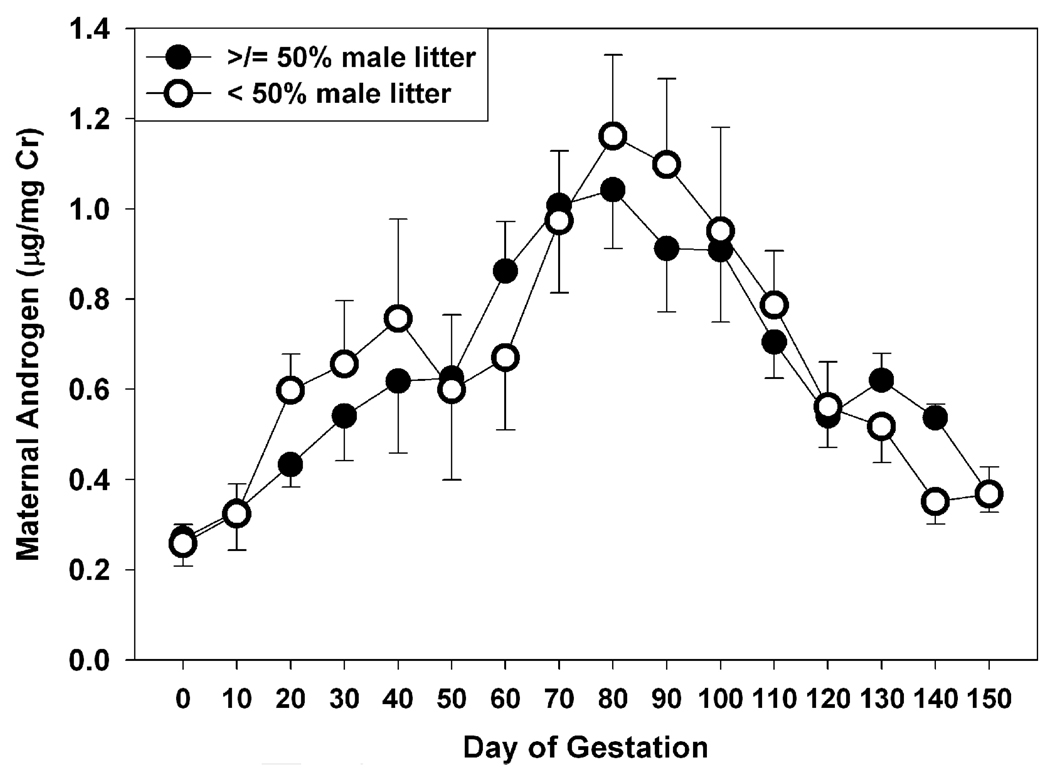
Gestational status was associated with significant variation in excreted maternal androgens (Fig. 1). Concentrations were low prior to conception (Day 0), then rose early in pregnancy, reaching maximal concentrations during the second trimester (days 50 – 100). Excreted androgens dropped rapidly during the third trimester, returning to near pre-pregnancy levels in the final 10 days of gestation. The differences in androgen excretion across gestation were highly significant (F(15,150) = 13.93, p < 0.001), and post-hoc comparisons revealed that relative to preconception levels, excreted androgens were significantly higher at all times during gestation, including the period just prior to parturition (p’s < 0.005). Patterns of androgen excretion did not vary as a function of the sex composition of the litter, as the interaction between gestational stage and litter sex composition was not significant (F(15, 150) = 1.21, n.s.; see Fig. 1). When contrasts were limited to those pregnancies in which marmoset females were carrying male-only versus female-only litters, an identical pattern was noted. That is, there were significant changes across gestation (F(14,70) = 3.68, p < 0.001), but androgen levels did not vary at any time point based on litter sex composition. However, females differed from each other in the pattern of androgen excretion throughout gestation (F(60,150) = 2.46, p < 0.001). Some females (e.g., Bes, Swe) showed moderate elevations in androgen during gestation, while other females (e.g., Dar, Pop) exhibited five- to eight-fold increases in androgen excretion, relative to nonpregnant concentrations.
Fig. 1.
Patterns of urinary androgen excretion across gestation in female white-faced marmosets. Values indicate mean ± s.e.m. Filled circles represent females that were carrying litters that were 50% or more males (n =12); open circles represent females whose litters were less than 50% male fetuses (n = 6). Day 0 reflects mean androgen excretion in nonpregnant females immediately prior to conception. See Table 1 for additional details.
Our correlational analyses also indicated that litter sex ratio has no effect on maternal androgen. As seen in Table 2, neither the number of male fetuses carried by the female, nor the proportion of the litter that was male, was significantly associated with maternal androgen concentrations at any stage of gestation. In fact, androgen levels in the third trimester tended to be lower in females whose litters had a higher proportion of male fetuses (r = −0.23, n.s.), but the relationship did not reach conventional levels of significance. Variation in litter size (range: 1 – 4; M ± sem: 2.24 ± 0.21) was not associated with maternal androgen excretion. Finally, maternal age (and parity, since both variables covary) did not predict maternal androgen excretion during gestation. However, baseline androgen concentrations in nonpregnant females were significantly associated with age (r = 0.56, p < 0.02), with older females exhibiting higher concentrations than younger females. Levels of androgen excretion during nonconceptive periods were not associated with subsequent levels during pregnancy, but levels in adjacent trimesters (1st – 2nd, 2nd – 3rd) were significantly related to each other. However, levels in the 1st trimester were only weakly correlated with levels in the 3rd trimester (r = 0.41, p < 0.10).
Table 2.
Correlations Among Maternal Gestational Androgens and Litter Characteristics
| Gestational Phasea |
Tri1 | Tri2 | Tri3 | # Males | Prop. Male | Lit Size | Ageb |
|---|---|---|---|---|---|---|---|
| NonPreg | 0.37 | 0.09 | 0.17 | 0.02 | 0.10 | 0.02 | 0.56d |
| Tri1 | - | 0.70c | 0.41 | 0.28 | 0.11 | 0.34 | 0.30 |
| Tri2 | - | - | 0.74 c | 0.16 | −0.08 | 0.17 | 0.04 |
| Tri3 | - | - | - | −0.11 | −0.23 | −0.17 | 0.22 |
Maternal androgens were averaged for periods in which the female was not pregnant, and in each trimester of pregnancy (Tri1, Tri2, and Tri3)
Maternal age is also a proxy variable for female parity
p < 0.001
p < 0.02
4. Discussion
Maternally-derived hormones have the potential to dramatically organize and modify behavioral and morphological trajectories in developing organisms. This study revealed that pregnant female marmosets have elevated levels of excreted androgens. Androgen levels rose during the first trimester, reached peak concentrations in the second trimester (~ 80 days post-conception), and gradually declined during the third trimester. There were no differences in maternal androgen as a function of the sex composition of the female’s litter, as analyzed either by levels associated with the presence or absence of a male fetus, or as a function of the number or proportion of males in the litter. Litter size was not related to variation in gestational androgen concentrations, nor was female age and parity. However, nonconceptive androgen levels were higher in older females than in younger females. Since excreted androgens correlate highly with circulating androgens in marmosets (Nunes et al., 2002), these results suggest that maternally derived androgens could exert a masculinizing influence on offspring in utero.
The finding that maternal androgen levels throughout pregnancy did not differ as a function of litter sex ratio is in contrast with early studies on human females (e.g., Muelenberg & Hofman, 1991; Klinga et al., 1978), but is consistent with more recent reports (Van de Beek et al., 2004). Our study parallels the study of human mothers carrying same-sex or opposite-sex twins (Cohen-Bendahan et al., 2005). While levels of maternal testosterone and estradiol rose throughout the mothers’ pregnancies, mothers carrying twin males or mixed sex twins did not have higher circulating androgens at 24 or 32 weeks of gestation than mothers carrying twin females. In humans and Old World monkeys, fetal testes begin synthesizing and secreting androgens in the first half of pregnancy (35 – 50 days in macaques: Resko & Ellingwood, 1981; 40 – 60 days in humans: Parker, 2004). There are no published data on the timing of testicular production of androgens in male marmoset fetuses, but some androgen-driven sexual differentiation clearly occurs during fetal development, since neonatal males have differentiated primary sexual characteristics (McKinnell et al., 2001). There may be some postnatal behavioral and morphological plasticity, however, since treating female marmosets with perinatal androgen masculinizes external genitalia and sexual and aggressive behavior (Abbott & Hearn, 1979).
While the present study did not explicitly examine the origin of urinary androgens in pregnant female marmosets, it is likely that they derive from multiple sources, including ovarian and/or corpora luteal, placental, and adrenal tissue. There are increases in plasma T and A4 and urinary androgen excretion in pregnant female marmosets within one to two weeks of conception, prior to implantation and the elaboration of the fetoplacental unit (Chambers & Hearn, 1979; Fite et al., 2005). These patterns suggest that early gestational androgen is of ovarian origin. Ovarian steroidogenesis in the immediate post-conceptive period in a number of mammalian species is characterized by elevated circulating maternal testosterone levels (dog: Concannon & Castracane, 1985; baboon: Castracane & Goldzieher, 1983; human females: Castracane & Asch, 1995; Castracane et al., 1998). In the baboon, elevated androgen production by the ovary appears to be under the regulation of chorionic gonadotropin (CG) stimulation, since administering hCG to nonpregnant females late in the luteal phase elevates androgen concentrations to levels that are characteristic of pregnant baboons (Castracane & Goldzieher, 1983). Gonadotropin levels in pregnant marmosets begin to rise 13 – 17 days after conception, coincident with increasing androgen production, and peak in early to mid second trimester, when androgen levels are also peaking (Chambers & Hearn, 1979; French et al., 1996; present study), suggesting that gonadotropins are important for regulating androgen production in marmosets, as well.
Direct evidence for placental production of androgens is not available, but the production of other steroids suggests a shift from gonadal to placental hormone production well before we documented peak levels of urinary androgen in pregnant marmosets. By comparing circulating levels of progesterone and estradiol with levels sampled from the utero-ovarian vein, Hodges et al. (1983) noted that both the corpus luteum and placenta were contributing to circulating progesterone at 40 days post-conception, but that luteal production of progesterone has ceased by day 60. To the extent that androgen production follows a similar pattern, the peak levels of urinary androgens we noted at day 80 of gestation is well past the shift from luteal to placental steroid production.
Molecular evidence for the role of the placenta in androgen steroidogenesis is also provided by the timing and distribution of mRNA for a critical steroid enzyme, 17β-hydroxysteroid dehydrogenase (HSD) in reproductive tissues. This enzyme converts androstenedione (A4) to T, and estrone (E1) to estradiol (E2). mRNA for one variant of the enzyme (HSD1) is found extensively in marmoset placenta, and mRNA for aromatase is co-localised with HSD1 mRNA. A second variant of HSD (HSD7) is widespread in corpora lutea (Husen et al., 2003). Conception status also affects the presence and distribution of mRNA for HSD. HSD7 mRNA is not expressed in the uteri of marmosets in the luteal phase of nonconceptive cycles, but is massively up-regulated in the uterus of female marmosets 13 to 15 days after conception, especially in the uterine endometrium near the implantation sites (Einspanier et al., 2009). The marmoset ovary, uterus, and placenta are active sites for both androgen and estrogen biosynthesis.
We noted in the present study that baseline, nonpregnant levels of androgen excreted by female marmosets increased with a female’s age. In many species, aging in females is associated with reduced, not elevated, circulating androgen concentrations. In human females, testosterone and especially androstenedione decline with age (Elmlinger et al., 2005; Davison et al. 2005), but the decline in circulating androgens is gradual, and there is no discontinuity at the time of menopause (Davison et al., 2005). Androgen excretion is also reported to decline in wild female baboons, but only when exceedingly old females are included in the sample (Beehner et al., 2005). Androgens become elevated in older female rats (25 months of age), but declines again when exceedingly old females experience reproductive senescence (Lu et al., 1979; Goya et al., 1990), but this effect is seen only in very old rats (25 – 30 months of age). Female marmosets exhibit signs of follicular depletion and hence a cessation of ovulation at or near the expected lifespan, so they do not exhibit significant age-related declines in reproduction (Tardif et al., 2008). Unlike Old World and hominid primates, however, the steroidogenic capacity of the aging callitrichine primate (marmosets and tamarins) ovary does not show a decline, since levels of estrogens and progestagens are similar to or higher in older females than in younger females (Tardif et al., 2008; Tardif & Ziegler, 1992). Our results suggest that the maintenance and/or enhancement of steroidogenic capacity in older females holds for androgens as well as other ovarian and placental hormones. That being said, however, the age range of female marmosets in our study was relatively restricted (4.5 – 8.7 years of age). These ages are not exceptionally old for a species in a genus (Callithrix) that can live to more than 16 years of age (Ross et al., 2007).
In summary, our results show that excreted urinary androgen concentrations in pregnant female marmosets are not altered by the presence of one or more male fetuses in utero. The elevated levels of androgens in pregnant females appear to be of maternal origin only, and are likely to be of ovarian and placental origin. The uterine endocrine environment can have profound impacts on morphological and behavioral phenotypes in mammals, and the role of prenatal androgens in shaping sex-typical traits is well established (Morris et al., 2004; Wallen, 2005). Given that there is considerable variation both within and among females in levels of androgen during gestation, the marmoset represents a potent model in which to examine maternal effects on offspring development that results from prenatal androgen exposure.
Acknowledgments
Jeff Fite initiated the research program from which these data derive. We thank Heather Jensen and Danny Revers for excellence in animal care and husbandry. The Psychobiology Journal Club provided useful comments on previous versions of this manuscript, and we especially thank Shelton Hendricks. The research was supported by funds from the National Institutes of Health (HD 42882) and the National Science Foundation (IBN 00-91030) awarded to JAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott DH, Hearn JP. The effects of neonatal exposure to testosterone on the development of behaviour in female marmoset monkeys. CIBA Found. Symp. 1979;62:299–327. doi: 10.1002/9780470720448.ch14. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Placental steroid hormone biosythesis in primate pregnancy. Endo. Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. Life-history correlates of steroid concentrations in wild peripartum baboons. Am. J. Primatol. 2004;64:95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Phillips-Conroy JE, Whitten PL. Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am. J. Primatol. 2005;67:101–119. doi: 10.1002/ajp.20172. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Asch RH. Testosterone and androstenedione in premature ovarian failure pregnancies: evidence for an ovarian source of androgens in early pregnancy. Hum. Reprod. 1995;10:677–680. doi: 10.1093/oxfordjournals.humrep.a136010. [DOI] [PubMed] [Google Scholar]
- Castracane VD, dela Cruz JL. The relationship of conceptus number and fetal sex to maternal serum testosterone concentration in the rat. Proc. Soc. Exp. Biol. Med. 1990;195:109–113. doi: 10.3181/00379727-195-43126. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Goldzieher JW. Plasma androgens during early pregnancy in the baboon (Papio cynocephalus) Fertil. Steril. 1983;39:553–559. doi: 10.1016/s0015-0282(16)46950-5. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum. Reprod. 1998;13:460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- Challis JR, Davies IJ, Benirschke K, Hendrickx AG, Ryan KJ. The effects of dexamethasone on the peripheral plasma concentrations of androstenedione, testosterone and cortisol in the pregnant rhesus monkey. Endocrin. 1975;96:185–192. doi: 10.1210/endo-96-1-185. [DOI] [PubMed] [Google Scholar]
- Chambers PL, Hearn JP. Peripheral plasma levels of progesterone, oestradiol-17β, oestrone, testosterone, androstenedione and chorionic gonadotrophin during pregnancy in the marmoset monkey Callithrix jacchus. J. Reprod. Fert. 1979;56:23–32. doi: 10.1530/jrf.0.0560023. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, van Goozen HM, Buitelaar JK, Cohen-Kettenis PT. Maternal serum steroid levels are unrelated to fetal sex: a study in twin pregnancies. Twin Res. Hum. Genet. 2005;8:173–177. doi: 10.1375/1832427053738764. [DOI] [PubMed] [Google Scholar]
- Concannon PW, Castracane VD. Serum androstenedione and testosterone concentrations during pregnancy and nonpregnant cycles in dogs. Biol. Reprod. 1985;33:1078–1083. doi: 10.1095/biolreprod33.5.1078. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- Dloniak SM, French JA, Place NJ, Weldele ML, Glickman SE, Holekamp KE. Non-invasive monitoring of fecal androgens in spotted hyenas (Crocuta crocuta) Gen. Comp. Endocrinol. 2004;135:51–61. doi: 10.1016/j.ygcen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Duer C, Carden M, Schmitt D, Tomasi T. Utility of maternal serum total testosterone analysis for fetal gender determination in Asian elephants (Elephas maximus) Anim. Reprod. Sci. 2002;69:47–52. doi: 10.1016/s0378-4320(01)00147-6. [DOI] [PubMed] [Google Scholar]
- Einspanier A, Lieder K, Husen B, Ebert K, Lier S, Enspanier R, Unemori E, Kemper M. Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann. N. Y. Acad. Sci. 2009;1160:140–146. doi: 10.1111/j.1749-6632.2009.03947.x. [DOI] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Wormstall H, Doller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin. Lab. 2005;51:625–632. [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Ross CN. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Horm. Behav. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Shaffner CM, Schalley J, Hightower-Merritt DL, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied’s black tufted-ear marmosets (Callithrix kuhli) Am. J. Primatol. 1996;40:231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gibori G, Sridaran R. Sites of androgen and estradiol production in the second half of pregnancy in the rat. Biol. Reprod. 1981;24:249–256. doi: 10.1095/biolreprod24.2.249. [DOI] [PubMed] [Google Scholar]
- Glass AR, Klein T. Changes in maternal serum total and free androgen levels in early pregnancy: lack of correlation with fetal sex. Am. J. Obstet. Gynecol. 1981;140:656–660. doi: 10.1016/0002-9378(81)90199-x. [DOI] [PubMed] [Google Scholar]
- Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech. Ageing Dev. 1990;56:77–88. doi: 10.1016/0047-6374(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Groothuis TG, Muller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Harrison RF, Mansfield MD. Maternal plasma androgens in early human pregnancy. Br. J. Obstet. Gynaecol. 1980;87:695–704. doi: 10.1111/j.1471-0528.1980.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Hines M. Prenatal testosterone and gender-related behaviour. Eur. J. Endocrinol. 2006;155:115–121. doi: 10.1530/eje.1.02236. [DOI] [PubMed] [Google Scholar]
- Hodges JK, Henderson C, Hearn JP. Relationship between ovarian and placental steroid production during early pregnancy in the marmoset monkey (Callithrix jacchus) J. Reprod. Fert. 1983;69:613–621. doi: 10.1530/jrf.0.0690613. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, de Jong FH, Rowland DL, Slob AK. Plasma testosterone in fetal rats and their mothers on day 19 of gestation. Physiol. Behav. 1995;57:495–499. doi: 10.1016/0031-9384(94)00291-c. [DOI] [PubMed] [Google Scholar]
- Husen B, Adamski J, Bruns A, Deluca D, Fuhrmann K, Moller G, Schwabe I, Einspanier A. Characterization of 17b-hydroxysteroid dehydrogenase type 7 in reproductive tissues of the marmoset monkey. Biol. Reprod. 2003;68:2092–2099. doi: 10.1095/biolreprod.102.012476. [DOI] [PubMed] [Google Scholar]
- Klinga K, Bek E, Runnebaum B. Maternal peripheral testosterone levels during the first half of pregnancy. Am. J. Obstet. Gynecol. 1978;131:60–62. doi: 10.1016/0002-9378(78)90474-x. [DOI] [PubMed] [Google Scholar]
- Legrand C, Marie J, Maltier JP. Testosterone, dihydrotestosterone, androstenedione and dehydroepiandrosterone concentrations in placentae, ovaries and plasma of the rat in late pregnancy. Acta Endocrinol. 1984;105:119–125. doi: 10.1530/acta.0.1050119. [DOI] [PubMed] [Google Scholar]
- Lu JK, Hopper BR, Vargo TM, Yen SSC. Chronological changes in sex steroid, gonadotropin and prolactin secretion in aging female rats displaying different reproductive states. Biol. Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PTK, Fraser HM, Kelnar CJH, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor b immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reprod. 2001;122:419–429. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- Meulenberg PMM, Hofman JA. Maternal testosterone and fetal sex. J. Ster. Biochem. Molec. Biol. 1991;39:51–54. doi: 10.1016/0960-0760(91)90012-t. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nagamini M, McDonough PG, Ellegood JO, Mahesh VB. Maternal and amniotic fluid steroid throughout human pregnancy. Am. J. Obstet. Gynecol. 1979;134:674–680. doi: 10.1016/0002-9378(79)90649-5. [DOI] [PubMed] [Google Scholar]
- Nunes S, Brown CM, French JA. Variation in circulating and excreted estradiol associated with testicular activity in male marmosets. Am. J. Primatol. 2002;55:27–42. doi: 10.1002/ajp.1061. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant-care behaviour and experience in male marmosets (Callithrix kuhlii) Anim. Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Ojeda SJ. Female reproductive function. In: Griffin JE, Ojeda SR, editors. Textbook of Endocrine Physiology. Oxford: Oxford University Press; 2004. pp. 186–225. [Google Scholar]
- Ostner J, Heistermann M, Kappeler PM. Intersexual dominance, masculinized genitals and prenatal steroids: comparative data from lemurid primates. Naturwissenschaffen. 2003;90:141–144. doi: 10.1007/s00114-003-0404-9. [DOI] [PubMed] [Google Scholar]
- Parker KL. Sexual differentiation. In: Griffin JE, Ojeda SR, editors. Textbook of Endocrine Physiology. Oxford: Oxford University Press; 2004. pp. 167–185. [Google Scholar]
- Resko JA, Ellinwood WE. Testicular hormone production in fetal rhesus macaques. In: Novy MJ, Resko JA, editors. Fetal endocrinology. New York: Academic Press; 1981. pp. 253–269. [Google Scholar]
- Ross CN, Jensen H, Fite JE, French JA. A demographic review of a captive colony of callitrichids (Callithrix kuhlii) Am. J. Primatol. 2007;68:812–824. doi: 10.1002/ajp.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual social relationships in wied’s black tufted-ear marmoset (Callithrix kuhli) Am. J. Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Smucny DA, Abbott DH, Mansfield KG, Schultz-Darken NJ, Yamamoto ME, Alencar AI, Tardif SD. Reproductive output, maternal age, and survivorship in captive common marmoset females (Callithrix jacchus) Am. J. Primatol. 2004;64:107–121. doi: 10.1002/ajp.20065. [DOI] [PubMed] [Google Scholar]
- Steler JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstet. Gynecol. 2002;100:552–556. doi: 10.1016/s0029-7844(02)02088-4. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Ziegler TE. Features of female reproductive senescence in tamarins (Saguinus spp.), a New World primate. J. Reprod. Fertil. 1992;94:411–421. doi: 10.1530/jrf.0.0940411. [DOI] [PubMed] [Google Scholar]
- Tietz NW. Fundamentals of Clinical Chemistry. Philadelphia PA: W. B. Saunders; 1976. [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidimiol. Biomarkers Prev. 2003;12:452–456. [PubMed] [Google Scholar]
- Van de Beek C, Thijssen JHH, Cohen-Ketternis PT, van Goozen SHM, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm. Behav. 2004;46:663–669. doi: 10.1016/j.yhbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]