Abstract
A simple, robust, single bead-based electrochemical biosensor was fabricated and characterized. The sensor’s working electrode consists of an electrochemically-etched platinum wire, with a nominal diameter of 25 μm, hermetically heat-fusion sealed in a pulled glass capillary (micropipette). The sealing process does not require any epoxy or glue. A commercially available, densely functionalized agarose bead was mounted on the tip of the etched platinum wire. The use of a pre-functionalized bead eliminates the tedious and complicated surface functionalization process that is often the bottleneck in the development of electrochemical biosensors. We report on the use of a biotin agarose bead-based, micropipette, electrochemical (Bio-BMP) biosensor to monitor H2O2 concentration and the use of a streptavidin bead-based, micropipette, electrochemical (SA-BMP) biosensor to detect DNA amplicons. The Bio-BMP biosensor’s response increased linearly as the H2O2 concentration increased in the range from 1×10−6 to 1.2×10−4 M with a detection limit of 5×10−7 M. The SA-BMP was able to detect the amplicons of 1 pg DNA template of B. Cereus bacteria, thus providing better detection sensitivity than conventional gel-based electropherograms.
Keywords: Electrochemical biosensor, agarose beads, hydrogen peroxide detection, B. Cereus bacteria, DNA detection
1. Introduction
Biosensors are used to detect a wide range of analytes in, among other places, the health care industry, food industry, environmental monitoring, and drug development (Ivnitski et al., 2004; Skottrup et al., 2008; Wang, 2002; Wei et al., 2008). Many of the detection modalities require the immobilization of the target analytes to a solid substrate. However, challenges still exist in immobilizing various biorecognition molecules, such as enzymes, oligonucleotides, and proteins, to the biosensors’ surfaces at high density while retaining bioactivity. Numerous approaches have been used for biomolecular immobilization such as physical adsorption (Chen and Pardue, 2000), entrapment in a porous matrix (Yu and Ju, 2003), covalent binding (Yabuki et al., 2000), and copolymerization (Liu et al., 2007; Qu et al., 2007). One of the most commonly used strategies for effective immobilization of biomaterials to different substrates is based on the avidin-biotin affinity reaction (LaGier et al., 2007; Lermo et al., 2008; Luppa et al., 2001; Yean et al., 2008; Zacco et al., 2006).
Microbeads, such as silica beads, magnetic beads, polystyrene beads, and agarose beads are widely used as solid supports for immunoreactions and immunoseparations. The technology for the functionalization of microbeads is highly developed, and microbeads with high density, highly active surface coverage are readily available from various vendors. Hence, in many cases, the use of commercially available microbeads provides improved efficiency and reduces the cost of biosensors. Microbeads have been extensively used in the context of optical detection. For example, Walt and collaborators immobilized functionalized silica micro-spheres in wells etched in the fibers of a high-density fiber optic array to detect thrombin and DNA (Epstein et al., 2003; Lee and Walt, 2000; Tam et al., 2009). McDevitt et al. positioned agarose microbeads with various functionalizations in an array of bottomless wells etched in silicon or perforated in stainless steel (Ali et al., 2003; Christodoulides et al., 2005; Goodey et al., 2001; Li et al., 2005; Sohn et al., 2005). Recently, there has been interest in using microbeads in electrochemical biosensing (Palecek and Fojta, 2007). Heineman’s group proposed electrochemical immunoassays composed of microdrops laden with paramagnetic beads (Boyaci et al., 2005; Farrell et al., 2004; Wijayawardhana et al., 1999). Palecek et al. developed a DNA hybridization biosensor using paramagnetic Dynabeads as a transportable, reactive surface and a hanging mercury drop electrode as a detection electrode (Palecek et al., 2002). Choi et al. constructed a magnetic bead-based electrochemical immunoassay in a lab on chip format (Choi et al., 2002). Centi et al. developed a disposable, electrochemical immunosensor using magnetic beads for detecting polychlorinated biphenyls (PCBs) in food and environmental samples (Centi et al., 2005). In all of the above, multiple beads were used and the electrodes were placed some distance apart from the beads.
In contrast, in this paper, we use a single bead attached to the working electrode. We selected an agarose bead due to its excellent biocompatibility, nontoxicity, resistance to non-specific protein binding, and low cost. Beaded agarose bearing functional groups such as amine (-NH2), carboxylic acid (-COOH), aldehyde (-CHO), thiol (-SH), and hydroxyl (-OH) are the support of choice for many ligands of interest (Punna et al., 2005). Furthermore, agarose beads functionalized, among other things, with streptavidin, biotin, and protein A are available from many vendors. Agarose beads have been widely used as solid phase support for immunoassays (Filipponi et al., 2009; Yang et al., 2008). For example, Christodoulides et al. developed immunoassay chips based on agarose microbead arrays for optical detection of C-reactive protein (Christodoulides et al., 2005), and Gray et al. used protein A-coated agarose beads and Dynal magnetic beads to capture target bacteria from a suspension (Gray and Bhunia, 2005).
Micropipette electrodes are often used for electrochemical measurements. The micropipette electrodes are typically fabricated by sealing a platinum wire or a carbon fiber in a pulled glass capillary with epoxy glue (Kozminski et al., 1998; Wang and Hu, 2006). In this method, the glass/fiber interface contains epoxy, which may adversely affect the characteristics of the electrodes. Often, the epoxy provides a leaky seal, which results in high noise, low sensitivity, and short life of the electrodes. Moreover, the epoxy may contaminate the solution, in particular when working with organic solvents.
In this paper, we describe simple, robust, single agarose bead-based micropipette electrochemical biosensors, which combine micropipette electrodes with commercially available, functionalized agarose beads. An electrochemically etched platinum wire was sealed by flame-fusion in a pulled glass capillary micropipette to form the working electrode. A functionalized agarose bead was mounted on the tip of the etched platinum wire. To demonstrate the utility of these biosensors, we carried out two sequences of experiments. The first sequence of tests utilized the biotin agarose bead-based micropipette (Bio-BMP) biosensor to measure H2O2 concentration. The sensor consisted of a biotinylated agarose bead incubated with streptavidin conjugated to horseradish peroxidase enzyme (HRP). The second set of experiments utilized a streptavidin bead-based micropipette (SA-BMP) biosensor to detect haptenized PCR amplicons of B. Cereus bacteria. The DNA amplicons were labeled with both dig and biotin. Electrochemical detection was achieved by binding enzymes conjugated with an antibody to the antigen (dig) to the labeled DNA. The method that we used to detect DNA does not involve hybridization and is similar to a sandwich immunoassay. The single agarose bead-based micropipette biosensor described herein can, of course, operate with other ligands to detect any analyte of choice.
2. Experiments
2.1. Reagents and materials
Borosilicate glass pipe (OD=1.0mm, ID=0.75, Length=3 inches) was purchased from World Precision Instruments, Inc. (Sarasota, FL, USA). ImmunoPure® Immobilized D-Biotin agarose beads and high capacity streptavidin agarose beads were obtained from Pierce Biotechnology (Rockford, IL, USA). Prior to the beads’ use, the beads’ suspension was vortexed for 1–2 minutes to separate aggregates into individual beads. Bare platinum wire (25 μm in diameter) was obtained from A-M Systems Inc. (Everett, WA, USA). Hydrogen peroxide (30% w/v), potassium ferrocyanide, potassium iodide (KI), hydroquinone, sodium nitrite, and phosphate buffer solution (PBS, pH 7.4) were purchased from Sigma (St. Louis, MO, USA). Streptavidin-horseradish peroxidase conjugate and streptavidin-Alexa Fluor 488 were purchased from Invitrogen (Carlsbad, CA, USA). Anti-dig-HRP conjugate was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). All reagents were of analytical grade and used without further purification. An Ag/AgCl reference electrode (Orion, USA) was used throughout the experiments.
2.2. Fabrication of a single agarose bead-based micropipette biosensor
2.2.1. Electrochemical etching of the platinum wire
We speared the agarose bead with the sharpened platinum electrode to mount the bead onto the probe. Prior to etching, the bare platinum wire was rinsed with ethanol and water. Then, the platinum wire’s tip was gently inserted into the saturated NaNO2 etching solution with a micromanipulator. An AC voltage of 1.3V, which provided a reasonable compromise between etching time and tip smoothness, was applied between the platinum wire and a platinum foil counter electrode submerged in the solution for 15 minutes (Wang and Hu, 2006; Watkins et al., 2003). Bubbles formed at the Pt tip/solution interface during the electrochemical etching. After 15 minutes of etching, the applied voltage was removed, and the etched platinum wire was dipped for 2 s in a 3:1 HCl/HNO3 solution to clean the surface. Then the wire was thoroughly rinsed with DI water.
2.2.2. Fabrication of micropipette electrode
The fabrication process of a micropipette electrode is described in Figure S1, Supporting Information. The end of a borosilicate glass capillary (OD=1.0mm, ID=0.75mm, Length=76mm) was pulled with a micropipette puller (Sutter Instrument, Novato, CA, USA) into a fine, blunt taper. To conveniently insert the etched bare platinum wire (25 μm in diameter) into the glass capillary micropipette, the tip of the pulled glass capillary was cut with a glass pipette cutter (Sutter Instrument, Novato, CA, USA) to obtain a tip diameter of 30μm. The etched platinum wire was cleaned by sonication for 5 min, rinsed with acetone, alcohol, and distilled water, and dried by exposure to room air. Then, the platinum wire was inserted into the glass capillary through the pulled, cut end until 70μm of platinum was left exposed outside the glass. Finally, the tip of the glass capillary was inserted into the flame of a cigarette lighter for one second. The glass softened and fused around the platinum wire to form a tight, hermetic seal. The sealing process was reliable and reproducible. To provide a good electrical contact for electrochemical detection, aluminum foil was wrapped around the distal end of the glass capillary with the end of the platinum wires inserted between the layers of aluminum foil (Figure 1).
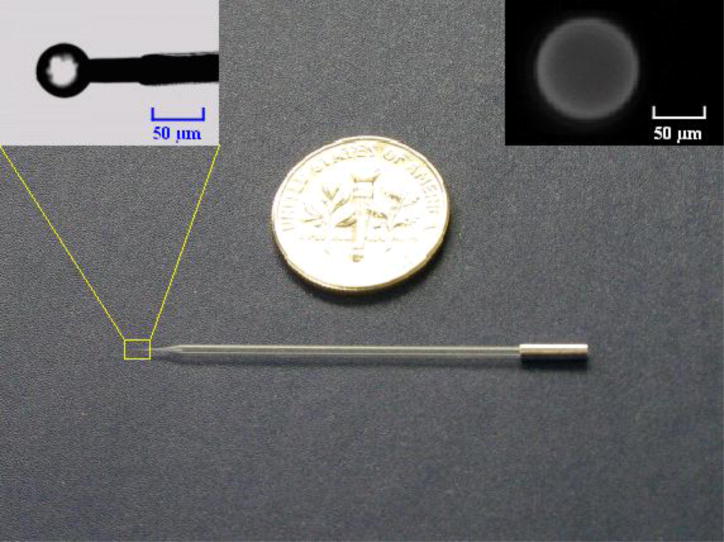
Figure 1.
A photograph of the agarose bead-based micropipette biosensor shown next to a US quarter coin. The left inset is an enlargement of the probe’s tip, showing the dry, functionalized agarose bead mounted on the platinum electrode. The right inset shows a fluorescent image of the hydrated biotin agarose bead labeled with streptavidin-Alexa Fluor 488 and suspended in PBS solution.
2.2.3. Bead mounting to micropipette electrode
To facilitate the spearing of the agarose beads, it is necessary to immobilize them. This can be accomplished by either aspirating agarose beads with a glass micropipette or by immobilizing the beads on a surface. We formed a wedge by placing one glass slide on top of another bigger slide (Figure S2, Supporting Information). A water drop, laden with beads, was placed next to the top glass plate. The suspension flowed into the wedge between the two plates by capillary action, and the agarose beads aligned along the top glass plate’s edge. Then, a glass capillary micropipette electrode with a sharp platinum tip was navigated with a precision XYZ micromanipulator (Eppendorf TransferMan NK 2, Hamburg, Germany) to puncture one of the agaose beads. Once speared, the agarose bead was withdrawn, and the bead-based biosensor was ready for use. Figure 1 shows an image of the complete bead-based electrochemical biosensor.
2.3. Biosensor Characterization
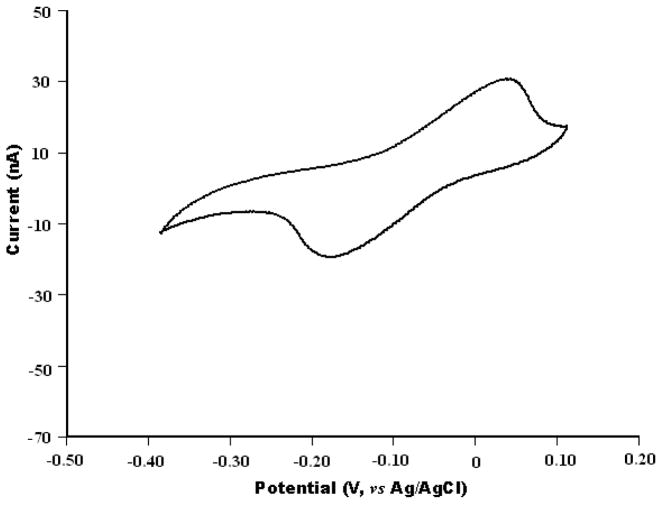
The electrochemical measurements were carried out with a HEKA EPC-10 patch clamp amplifier (HEKA Electronic Lambrecht, Germany) coupled to a desktop computer for data acquisition. Since the electric current associated with the electrochemical experiments is small, a two electrode arrangement was adopted and an Ag/AgCl electrode was used as the reference/counter electrode (Cui et al., 2007; Schwarz et al., 2001). The micropipette biosensor was evaluated by cyclic voltammetry using 1.0 mM hydroquinone in PBS solution as the electroactive agent (Figure 2).
Figure 2.
Cyclic voltammograms of the micropipette biosensor in PBS (pH 7.4) containing 1.0 mM hydroquinone. Scan rate is 100 mV/s.
2.3.1. Electrochemical detection of hydrogen peroxide
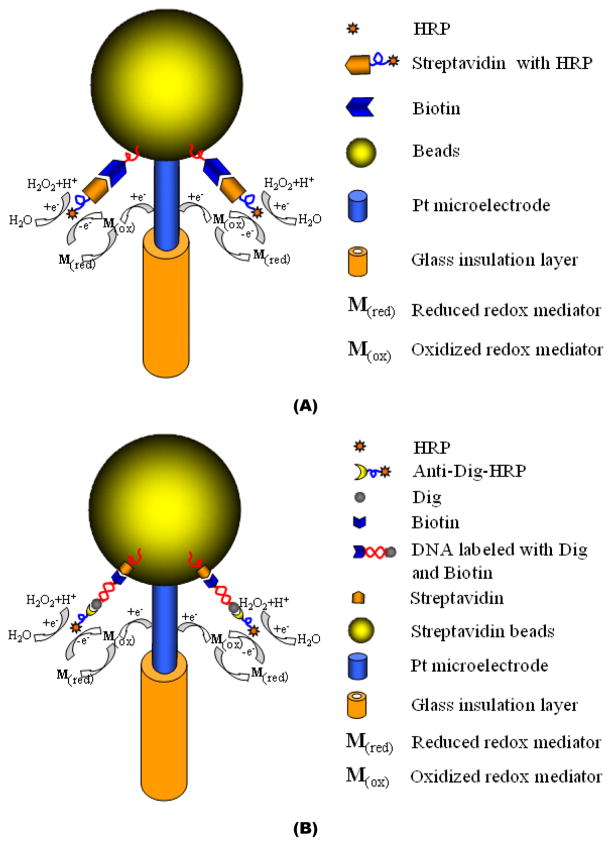
A Bio-BMP biosensor was designed, fabricated, and tested for hydrogen peroxide detection. To immobilize the horseradish peroxidase (HRP) enzyme to the biotin agarose bead’s surface, the tip of the Bio-BMP biosensor was incubated in a solution, consisting of 1.5 μg/mL streptavidin-HRP suspended in PBS, for 30 minutes. Subsequently, the tip of the Bio-BMP biosensor was washed with PBS solution for 10 minutes. Then, the Bio-BMP biosensor was dipped in the reaction solution, which contained 3.0 mM hydroquinone in PBS solution and to which 1% H2O2 portions were added. The H2O2 portions were prepared from a 30 % stock H2O2 solution. Figure 3A depicts the assay schematically.
Figure 3.
A schematic depiction of an agarose bead-based electrochemical assay. (A) A biotin agarose bead-based micropipette (Bio-BMP) biosensor for H2O2 sensing. (B) Streptavidin agarose bead based micropipette (SA-BMP) biosensor for DNA sensing.
2.3.2. Electrochemical detection of PCR products of B. cereus bacteria
The doubled-labeled amplicons of B. cereus genomic DNA templates were detected with the SA-BMP biosensor. The method of labeling the amplicons is described in Supporting Information. PCR amplicons of a 10 fold dilution series of B. cereus genomic DNA templates (ranging from 0.001ng to 10 ng), were used as the analytes in the SA-BMP biosensor experiments. The assay is depicted schematically in Figure 3B. The immobilization of the double labeled amplicons was achieved by submerging the tip of the SA-BMP biosensor in a PCR tube containing amplicons suspended in PBS for 10 minutes at room temperature. The biotin end of the ds-DNA amplicon bonded to the surface of the SA-BMP biosensor’s streptavidin bead. Subsequent to two washing steps with PBS solution (10 minutes each) to remove nonspecifically bound DNA, the tip of the SA-BMP biosensor was submerged in blocking PBS solution (2% BSA and 0.1% Tween 20) for 10 minutes and then in anti-dig-HRP solution (1: 5,000 dilution). The anti-dig-HRP bonded with the 3’ dig end of the ds-DNA amplicon. Two washing steps with PBS buffer solution, lasting 10 minutes each, were then performed to remove unbound anti-Dig-HRP. The SA-BMP biosensor was then transferred into an electrochemical reaction cell containing 1.5 mM H2O2 and 3.0 mM hydroquinone for amperometric measurements. To determine the amplicon concentration, the current was measured at the fixed voltage of −0.30 V (vs. the Ag/AgCl reference electrode). The duration and number of the washing steps and reaction times were not optimized.
3. Results and Discussion
3.1 Fabrication of a single agarose bead-based micropipette biosensor
3.1.1 Sealing of the micropipette electrode
Flame-fused sealing offered a highly efficient method for sealing micropipette electrodes (Huang et al., 2001), averted problems associated with the commonly used epoxy and glue-based sealing, and provided a hermetic seal. We selected borosilicate glass as the pipette material because of its relatively low melting temperature. The flame produced by a common cigarette lighter provided a sufficiently high temperature to fuse the pulled borosilicate glass capillary around the platinum wire. It is important to complete the fusion process within a short period of time (~1s). Prolonged exposure of the glass tip to the flame (>1s) may deform the glass tip and may cause glass beading. Figure S1D, Supporting Information, shows an image of the micropipette electrode after fusion.
3.1.2 Fixing single agarose bead to the tip of a micropipette electrode
Since the agarose bead is soft and porous, it is relatively easy to puncture it with the sharp platinum tip of the micropipette electrode. We speared hydrated agarose beads since they are considerably larger than the dehydrated ones. It is necessary, however, to immobilize the beads, to facilitate spearing. To this end, we took advantage of capillary forces to align the agarose beads along the wedge formed by two glass slides (Figure S2). Figure 1 shows a photograph of an agarose bead-based micropipette biosensor. The left inset shows the mounted, speared agarose bead when the bead is dehydrated. The right inset shows a fluorescent image of a mounted, hydrated biotin agarose bead after incubation with streptavidin-Alexa Fluor 488 for 10 minutes and suspension in PBS solution. The functionalized agarose beads could be hydrated and dehydrated with little or no loss of activity.
3.2 Hydrogen peroxide detection
The detection of hydrogen peroxide (H2O2) is important in clinical diagnostics, the chemical and pharmaceutical industry, and environmental control. Amperometric biosensors based on horseradish peroxidase (HRP) have emerged as the most convenient tools for H2O2 detection due to their simplicity, high sensitivity, and selectivity (Li et al., 2009; Shi et al., 2009; Yao et al., 2006). To improve the performance and long-term stability of the enzyme electrode, effective immobilization of HRP to the transducer surface is of great importance (Cao et al., 2008; Wang et al., 2009).
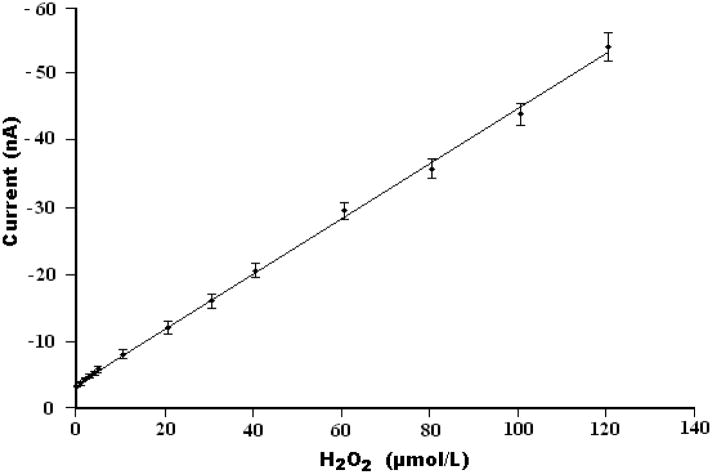
Here, commercial biotin agarose beads, which have high binding affinity to streptavidin-HRP were used as the enzyme immobilization substrate for hydrogen peroxide biosensing. Figure 3A depicts the operating principle of the Bio-BMP biosensor for H2O2 detection. The agarose bead, coated with biotin and mounted on the platinum electrode, was incubated in 1.5 μg/mL streptavidin-HRP PBS solution for 30 minutes. The streptavidin-HRP bonded to the biotin and was immobilized on the agarose bead’s surface through the high affinity biotin-streptavidin HRP conjugate reaction. The immobilized HRP enzyme catalyzed the reaction of hydroquinone with H2O2. The optimization of the experimental parameters is described in Supplementary Information. The amperometric response of the electrode as a function of H2O2 concentration was determined in the presence of 3.0 mM hydroquinone in PBS and a reduction potential of −0.3 V vs. the Ag/AgCl reference electrode. Over 95% of the steady-state current was achieved within 5s, which may be ascribed to the proximity of the Pt electrode to the RedOx mediator. Figure 4 depicts the steady state current as a function of the peroxide concentration. The symbols and solid line correspond, respectively, to experimental data and a best fit line. The Bio-BMP biosensor exhibits a linear response dI/dC~0.4 (nA/μM), where I is the steady state current and C is the concentration of H2O2, in the range from 1.0 × 10−6 to 1.2 × 10−4 M H2O2 with a correlation coefficient of 0.998 (n=3). A detection limit of 5.3×10−7 M is estimated at the signal-to-noise ratio of ~3.
Figure 4.
The steady-state current detected with the Bio-BMP sensor as a function of H2O2 concentration. The measurements were carried out in a 3.0 mM hydroquinone PBS solution at a fixed voltage of −0.30 V (vs. Ag/AgCl).
3.3. Detection of PCR amplicons of B. Cereus genomic DNA
To evaluate the reliability and applicability of the agarose bead-based micropipette biosensor for detecting biomolecules, we carried out a sequence of experiments in which we detected the amplification products of PCR and compared the results of the electrochemical detection with standard agarose gel electrophoresis imaging. DNA diagnosis has enormous applications in various fields including molecular biology, clinical diagnostics, agriculture, forensic science, and pathogen detection. Electrochemical biosensors for DNA detection have received considerable attention because electrochemical transducers offer a simple, sensitive, and inexpensive platform to detect nucleic acids binding (Ahmed et al., 2008; Ariksoysal et al., 2005; Kerman et al., 2006; Meric et al., 2002; Palecek et al., 2002). Here, instead of DNA hybridization, we functionalized the amplicons with dig and biotin (Liu et al., 2009). Thus, our method of DNA detection is similar to protein detection.
In our samples, the concentration of the B. cereus genomic DNA template ranged from 1pg to 10ng. The templates were amplified in a PCR machine. See Supporting Information for details. Figure S6A in Supporting Information depicts the amplification curves of real-time PCR for a 10 fold dilution series of B. cereus DNA template. The real time PCR detected amplicons down to 0.01ng of B. cereus DNA template after 36 cycles. Figure S6B provides images of gel electrophoresis of the various PCR amplicons.
Figure 3B depicts the operating principles of the streptavindin agarose bead-based micropipette (SA-BMP) biosensor used to detect PCR-amplified B. Cereus DNA sequences of 305-bp length. The biotin primer and dig primer used in the PCR amplification produced biotin and dig labeled B. Cereus DNA amplicons, which directly bonded to the streptavidin agarose bead of the SA-BMP biosensor through their biotin functionalization, avoiding the need for an intermediary biotin capture probe. Subsequently, the anti-dig HRP complex bonded to the dig end of the DNA amplicon. The sensor’s current depended on the concentration of the bound HRP. We used hydroquinone as the RedOx mediator (3.0 mM in PBS) and 1.5 mM H2O2 as the substrate for the HRP enzyme.
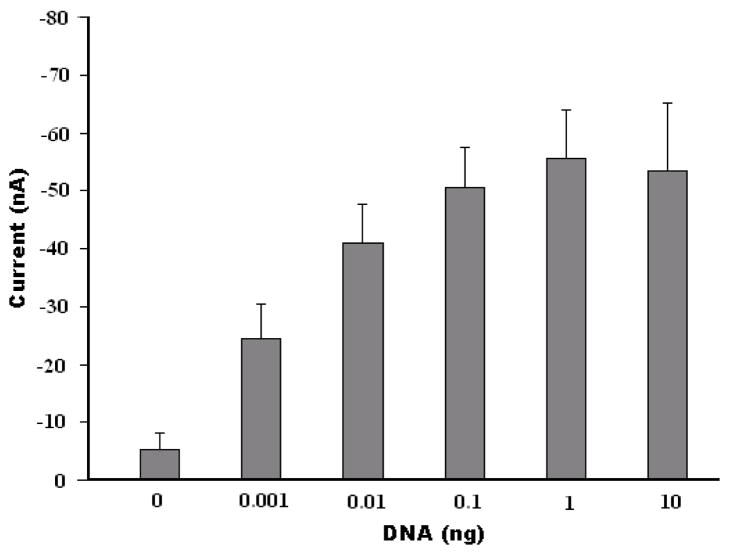
Figure 5 depicts the steady-state current detected with the SA-BMP biosensor as a function of the B. Cereus DNA template concentration (prior to amplification). At low DNA concentrations, the current increased monotonically as the concentration increased. The signal, however, saturated at higher DNA concentrations (template mass of about 10ng). The behavior depicted in Figure 5 is consistent with first order kinetics which predicts the number of bound complexes to be proportional to , where R is the number of binding sites (likely reduced due to steric hindrance), KA is the affinity constant, and C is the analyte concentration. Similar experimental results were reported by Yean et al., who developed a streptavidin-treated, screen-printed carbon electrode for the detection of PCR amplicons (Yean et al., 2008). Figure 5 demonstrates that the SA-BMP biosensor can detect the amplicons of 0.001ng (1 pg) DNA template of B. Cereus, which exceeds the detection ability of the conventional gel electrophoresis by a factor of ~10.
Figure 5.
The current detected with the SA-BMP biosensor as a function of the mass of B. Cereus genomic DNA template. The error bars correspond to the scatter of the data obtained in three experiments. The electrochemical detection was carried out in 1.5 mM H2O2 and 3.0 mM hydroquinone in PBS solution at a fixed voltage of −0.30 V (vs. Ag/AgCl counter electrode).
3.4 Stability and reproducibility of single agarose bead-based micropipette biosensor
The reproducibility of the current response of a particular Bio-BMP biosensor was examined at a H2O2 concentration of 1.0 mM to obtain a relative standard deviation (RSD) of 2.1% (n=6). To ascertain the variability in the current response from biosensor to biosensor, we compared the performance of six similarly fabricated biosensors. The standard deviation of the measurements carried out with the six sensors was 14%. We attribute this relatively large scatter among similar probes primarily to variations in the size of the agarose beads that were supplied by the manufacturer. The beads’ uniformity can be improved by sieving the beads. Non-uniformities in the manufacturing process may have also resulted in variations in the exposed area of the Pt electrodes, which may have contributed to variations in the probes’ performance. These variations can be greatly reduced with an appropriate mounting jig. Biosensors stored in a refrigerator at 4°C for two weeks retained about 95% of their initial current response.
4. Conclusions and Outlook
We describe a new, simple, robust, electrochemical, single bead-based, micropipette biosensor. The biosensor utilizes commercially available functionalized agarose beads and does not require custom functionalization of the electrodes’ surfaces, thus overcoming one of the challenges faced by electrochemical sensor developers and users. In other words, we imported immobilization procedures frequently used in optical detection, where practitioners utilize commercially available beads, into the realm of electrochemistry. The commercially available, functionalized beads offer many advantages over the surface-modified transducers often used in electrochemical sensing. All that is needed is to place the beads in the vicinity of the working electrode. This can be accomplished by mechanical placement (as we have done in this work) or through the use of remote forces such as electrophoresis, dielectrophoresis, or magnetophoresis (when the bead contains magnetically active material).
In this study, the biosensor consisted of an electrochemically sharpened, platinum electrode sealed in a glass capillary. A single agarose bead was mounted on the sharpened tip of the platinum wire. For convenience, the reference/counter electrode can be deposited on the outer surface of the glass capillary. The sensor in its present configuration can be readily used to measure electrochemically the concentration of target analytes in samples inserted in wells of a well plate array or in vials. Other embodiments of the sensor, which are more suitable for microfluidic detection, can also be envisioned. All that is needed is to place the functionalized beads in the vicinity of electrodes.
To demonstrate the utility of the electrochemical biosensor, we described its use in two assays. We successfully employed the single bead-based biosensor to detect hydrogen peroxide (H2O2) and PCR amplicons of B. Cereus bacteria. In the latter case, the single bead-based biosensor exhibited an order of magnitude better resolution than conventional gel imaging. Even better performance can doubtlessly be obtained with further optimization.
Our sensors provided surprisingly reproducible results given their primitive method of assembly. The sensors’ reproducibility can be further enhanced through the use of specialized equipment to mount the beads on the working electrode.
Supplementary Material
Acknowledgments
This work was supported, in part, by the Nanotechnology Institute of the Ben Franklin Technology Partners of Southeastern Pennsylvania and by the NIH/NIDCR Grant U01DE017855. The electrochemistry station was funded by the Nano Bio Interface Center (NBIC) through NSF grant NSEC DMR-0425780. Dr. Michael Mauk provided assistance and guidance with the real time PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed MU, Hossain MM, Tamiya E. Electroanalysis. 2008;20:616–626. [Google Scholar]
- Ali MF, Kirby R, Goodey AP, Rodriguez MD, Ellington AD, Neikirk DP, McDevitt JT. Anal Chem. 2003;75:4732–4739. doi: 10.1021/ac034106z. [DOI] [PubMed] [Google Scholar]
- Ariksoysal DO, Karadeniz H, Erdem A, Sengonul A, Sayiner AA, Ozsoz M. Anal Chem. 2005;77:4908–4917. doi: 10.1021/ac050022+. [DOI] [PubMed] [Google Scholar]
- Boyaci IH, Aguilar ZP, Hossain M, Halsall HB, Seliskar CJ, Heineman WR. Anal Bioanal Chem. 2005;382:1234–1241. doi: 10.1007/s00216-005-3263-8. [DOI] [PubMed] [Google Scholar]
- Cao Z, Jiang X, Xie Q, Yao S. Biosens Bioelectron. 2008;24:222–227. doi: 10.1016/j.bios.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Centi S, Laschi S, Franek M, Mascini M. Anal Chim Acta. 2005;538:205–212. [Google Scholar]
- Chen W, Pardue HL. Anal Chim Acta. 2000;409:123–130. [Google Scholar]
- Choi JW, Oh KW, Thomas JH, Heineman WR, Halsall HB, Nevin JH, Helmicki AJ, Thurman H, Henderson A, Ahn CH. Lab Chip. 2002;2:27–30. doi: 10.1039/b107540n. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Mohanty S, Miller CS, Langub MC, Floriano PN, Dharshan P, Ali MF, Bernard B, Romanovicz D, Anslyn E, Foxf PC, McDevitt JT. Lab Chip. 2005;5:261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- Cui Y, Barford JP, Renneberg R. Biosens Bioelectron. 2007;22:2754–2758. doi: 10.1016/j.bios.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Epstein JR, Leung APK, Lee KH, Walt DR. Biosens Bioelectron. 2003;18:541–546. doi: 10.1016/s0956-5663(03)00021-6. [DOI] [PubMed] [Google Scholar]
- Farrell S, Ronkainen-Matsuno NJ, Halsall HB, Heineman WR. Anal Bioanal Chem. 2004;379:358–367. doi: 10.1007/s00216-004-2632-z. [DOI] [PubMed] [Google Scholar]
- Filipponi L, Sawant PD, Fulga F, Nicolau DV. Biosens Bioelectron. 2009;24:1850–1857. doi: 10.1016/j.bios.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Goodey A, Lavigne JJ, Savoy SM, Rodriguez MD, Curey T, Tsao A, Simmons G, Wright J, Yoo SJ, Sohn Y, Anslyn EV, Shear JB, Neikirk DP, McDevitt JT. J Am Chem Soc. 2001;123:2559–2570. doi: 10.1021/ja003341l. [DOI] [PubMed] [Google Scholar]
- Gray KM, Bhunia AKJ. Microbiol Methods. 2005;60:259–268. doi: 10.1016/j.mimet.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Huang WH, Pang DW, Tong H, Wang ZL, Cheng JK. Anal Chem. 2001;73:1048–1052. doi: 10.1021/ac0008183. [DOI] [PubMed] [Google Scholar]
- Ivnitski D, Sitdykov R, Ivnitski N. Anal Chim Acta. 2004;504:265–269. [Google Scholar]
- Kerman K, Vestergaard M, Nagatani N, Takamura Y, Tamiya E. Anal Chem. 2006;78:2182–2189. doi: 10.1021/ac051526a. [DOI] [PubMed] [Google Scholar]
- Kozminski KD, Gutman DA, Davila V, Sulzer D, Ewing AG. Anal Chem. 1998;70:3123–3130. doi: 10.1021/ac980129f. [DOI] [PubMed] [Google Scholar]
- LaGier MJ, Fell JW, Goodwin KD. Mar Pollut Bull. 2007;54:757–770. doi: 10.1016/j.marpolbul.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Walt DR. Anal Biochem. 2000;282:142–146. doi: 10.1006/abio.2000.4595. [DOI] [PubMed] [Google Scholar]
- Lermo A, Zacco E, Barak J, Delwiche M, Campoy S, Barbe J, Alegret S, Pividori MI. Biosens Bioelectron. 2008;23:1805–1811. doi: 10.1016/j.bios.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Li F, Chen W, Tang C, Zhang S. Talanta. 2009;77:1304–1308. doi: 10.1016/j.talanta.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Li S, Floriano PN, Christodoulides N, Fozdar DY, Shao D, Ali MF, Dharshan P, Mohanty S, Neikirk D, McDevitt JT, Chen S. Biosens Bioelectron. 2005;21:574–580. doi: 10.1016/j.bios.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Liu C, Kuwahara T, Yamazaki R, Shimomura M. Eur Polym J. 2007;43:3264–3276. [Google Scholar]
- Liu C, Qiu X, Ongagna S, Chen D, Chen Z, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. Lab chip. 2009;9:768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppa PB, Sokoll LJ, Chan DW. Clin Chim Acta. 2001;314:1–26. doi: 10.1016/s0009-8981(01)00629-5. [DOI] [PubMed] [Google Scholar]
- Meric B, Kerman K, Ozkan D, Kara P, Erensoy S, Akarca US, Mascini M, Ozsoz M. Talanta. 2002;56:837–846. doi: 10.1016/s0039-9140(01)00650-6. [DOI] [PubMed] [Google Scholar]
- Palecek E, Billova S, Havran L, Kizek R, Miculkova A, Jelen F. Talanta. 2002;56:919–930. doi: 10.1016/s0039-9140(01)00666-x. [DOI] [PubMed] [Google Scholar]
- Palecek E, Fojta M. Talanta. 2007;74:276–290. doi: 10.1016/j.talanta.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Palecek E, Kizek R, Havran L, Billova S, Fojta M. Anal Chim Acta. 2002;469:73–83. [Google Scholar]
- Punna S, Kaltgrad E, Finn MG. Bioconjugate Chem. 2005;16:1536–1541. doi: 10.1021/bc0501496. [DOI] [PubMed] [Google Scholar]
- Qu F, Yang M, Jiang J, Feng K, Shen G, Yu R. Electrochem Commun. 2007;9:2596–2600. [Google Scholar]
- Schwarz MA, Galliker B, Fluri K, Kappes T, Hauser PC. Analyst. 2001;126:147–151. doi: 10.1039/b007383k. [DOI] [PubMed] [Google Scholar]
- Shi L, Liu X, Niu W, Li H, Han S, Chen J, Xu G. Biosens Bioelectron. 2009;24:1159–1163. doi: 10.1016/j.bios.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Skottrup PD, Nicolaisen M, Justesen AF. Biosens Bioelectron. 2008;24:339–348. doi: 10.1016/j.bios.2008.06.045. [DOI] [PubMed] [Google Scholar]
- Sohn YS, Goodey A, Anslyn EV, McDevitt JT, Shear JB, Neikirk DP. Biosens Bioelectron. 2005;21:303–312. doi: 10.1016/j.bios.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Tam JM, Song L, Walt DR. Biosens Bioelectron. 2009;24:2488–2493. doi: 10.1016/j.bios.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Wang C, Hu X. Talanta. 2006;68:1322–1328. doi: 10.1016/j.talanta.2005.07.048. [DOI] [PubMed] [Google Scholar]
- Wang J. Anal Chim Acta. 2002;469:63–71. [Google Scholar]
- Wang Y, Chen X, Zhu J. Electrochem Commun. 2009;11:323–326. [Google Scholar]
- Watkins JJ, Chen J, White HS, Abruna HD, Maisonhaute E, Amatore C. Anal Chem. 2003;75:3962–3971. doi: 10.1021/ac0342931. [DOI] [PubMed] [Google Scholar]
- Wei F, Wang J, Liao W, Zimmermann BG, Wong DT, Ho CM. Nucleic Acids Res. 2008;36:1–7. doi: 10.1093/nar/gkn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayawardhana CA, Halsall HB, Heineman WR. Anal Chim Acta. 1999;399:3–11. [Google Scholar]
- Yabuki S, Mizutani F, Hirata Y. Sens Actuators B. 2000;65:49–51. [Google Scholar]
- Yang Y, Nam SW, Lee NY, Kim YS, Park S. Ultramicroscopy. 2008;108:1384–1389. doi: 10.1016/j.ultramic.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Yao SJ, Xu JH, Wang Y, Chen XX, Xu YX, Hu SS. Anal Chim Acta. 2006;557:78–84. [Google Scholar]
- Yean CY, Kamarudin B, Ozkan DA, Yin LS, Lalitha P, Ismail A, Ozsoz M, Ravichandran M. Anal Chem. 2008;80:2774–2779. doi: 10.1021/ac702333x. [DOI] [PubMed] [Google Scholar]
- Yu J, Ju H. Anal Chim Acta. 2003;486:209–216. [Google Scholar]
- Zacco E, Pividori MI, Alegret S. Biosens Bioelectron. 2006;21:1291–1301. doi: 10.1016/j.bios.2005.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.