Abstract
It is well-established that following a toxic dose of acetaminophen (APAP), nitrotyrosine protein adducts (3-NT), a hallmark of peroxynitrite production, were colocalized with necrotic hepatic centrilobular regions where cytochrome P450 2E1 (CYP2E1) is highly expressed, suggesting that 3-NT formation may be essential in APAP-mediated toxicity. This study was aimed at investigating the relationship between CYP2E1 and nitration (3-NT formation) followed by ubiquitin-mediated degradation of proteins in wild-type and Cyp2e1-null mice exposed to APAP (200 and 400 mg/kg) for 4 and 24 h. Markedly increased centrilobular liver necrosis and 3-NT formation were only observed in APAP-exposed wild-type mice in a dose- and time-dependent manner, confirming an important role for CYP2E1 in APAP biotransformation and toxicity. However, the pattern of 3-NT protein adducts, not accompanied by concurrent activation of nitric oxide synthase (NOS), was similar to that of protein ubiquitination. Immunoblot analysis further revealed that immunoprecipitated nitrated proteins were ubiquitinated in APAP-exposed wild-type mice, confirming the fact that nitrated proteins are more susceptible than the native proteins for ubiquitin-dependent degradation, resulting in shorter half-lives. For instance, cytosolic superoxide dismutase (SOD1) levels were clearly decreased and immunoprecipitated SOD1 was nitrated and ubiquitinated, likely leading to its accelerated degradation in APAP-exposed wild-type mice. These data suggest that CYP2E1 appears to play a key role in 3-NT formation, protein degradation, and liver damage, which is independent of NOS, and that decreased levels of many proteins in the wild-type mice (compared with Cyp2e1-null mice) likely contribute to APAP-related toxicity.
Keywords: Liver toxicity, acetaminophen, CYP2E1, nitrotyrosine, ubiquitin, superoxide dismutase
1. Introduction
Acetaminophen (APAP) is a commonly used analgesic/antipyretic drug which can cause hepatoxicity in cases of high overdose, and in severe cases, centrilobular necrosis [1] and acute liver failure [2,3]. It is well-established that the toxicity of APAP is initiated by the formation of the reactive electrophile, N-acetyl-p-benzoquinone imine (NAPQI), through a cytochrome P450-mediated reaction, causing covalent binding to proteins, depletion of glutathione, and massive apoptosis and necrosis of liver cells [4,5]. Despite the remarkable correlation between the covalent binding of NAPQI to proteins and APAP-related liver damage [6,7], none of the proteins identified that form adducts with NAPQI was inactivated to an extent sufficient to cause the massive liver necrosis mediated by APAP, and thus the mechanism(s) through which APAP induces hepatic necrosis is still not clear [8-11].
Peroxynitrite, which is formed by a reaction between superoxide and nitric oxide (NO) and can damage protein, DNA, and lipids [12], has been suggested as a critical mediator of APAP-related organ toxicity based on strong immunohistochemical staining of the 3-nitrotyrosine (3-NT) adducts in the centrilobular necrotic areas following APAP treatment [11,13]. However, previous studies using either inducible nitric oxide synthase (iNOS)-null mice treated with APAP or iNOS inhibitors yielded opposing results with respect to the role of iNOS in the development of liver necrosis, thus raising the possibility that iNOS may not be the only source for NO in APAP toxicity [14]. The role of 3-NT, however, in mediating APAP liver necrosis was further supported by data from the delayed treatment with glutathione (GSH) after APAP treatment, which prevents nitrotyrosine staining and attenuates liver injury despite the persistence of oxidative stress in the mitochondria [15].
Many cytochrome P450 enzymes are involved in APAP metabolism. The P450 enzymes include cytochrome (CYP) 1A2, CYP2A6, CYP3A, and CYP2E1, the latter of which is considered the main enzyme responsible for the bioactivation of APAP as evident by enzyme kinetics and properties of APAP intoxication [16,17]. Furthermore, Cyp2e1-null mice were highly resistant to toxic doses of APAP [18]. When human CYP2E1 gene was reintroduced into Cyp2e1-null mice (humanized CYP2E1 transgenic mice), APAP-mediated hepatotoxicity was restored [19]. CYP2E1, compared to other P450 enzymes, exhibits a higher NADPH oxidase activity, resulting in increased production of hydrogen peroxide (H2O2), hydroxyl radical, as well as superoxide [20,21], which may play a role in peroxynitrite formation through reaction with nitric oxide, albeit low levels. However, this possibility (i.e., the role of CYP2E1 in peroxynitrite and 3-NT formation) was not evaluated systematically. Thus, using Cyp2e1-null mice as a negative control in APAP-mediated liver necrosis protocol would be of great advantage in characterizing the potential role of CYP2E1 in nitrotyrosine production and the implication of 3-NT in APAP-mediated liver necrosis. Surprisingly, pharmacokinetics and metabolite profiling studies of urine and serum from wild-type and Cyp2e1-null mice exposed to different doses of APAP revealed that the differences in the generation of NAPQI, thiol conjugates, and detoxification products became minimal when higher doses of APAP were administered despite the dramatic differences in liver damage where Cyp2e1-null mice seem to be totally protected [22,23]. Furthermore, NAPQI protein adducts were still observed when hepatotoxicity was prevented by pretreatment with gadolinium chloride [24]. These studies further support the notion that NAPQI production may not be the main cause of APAP-induced liver necrosis and that CYP2E1 in higher doses may have a limited role in the formation of NAPQI [22].
It is thus reasonable to hypothesize that CYP2E1 may play a central role in APAP-mediated liver damage through other mechanism(s), such as oxidative/nitrative stress formation, based on its ability to form oxidative radicals. The main aim of this study was to evaluate whether CYP2E1 is involved in the development of protein nitration following exposure to toxic doses of APAP in Cyp2e1-null mice compared with wild-type mice. We also evaluated the rates of protein ubiquitination following APAP treatment, since nitrated proteins are degraded by proteasomes as a part of cell defense against toxicity [25,26]. Finally, the role of lipid peroxidation in APAP-mediated hepatoxicity in both WT and Cyp2e1-null mice was re-evaluated since lipid peroxidation was suggested to play a role in mediating liver toxicity in iNOS-null mice [27].
2. Materials and methods)
2.1. Materials
APAP, 3-[(3-cholamidopropyl)-1-dimethylammonio]-propanesulfonic acid (CHAPS), dithiothreitol (DTT), iodoacetamide, primary antibody for β-actin, and all other chemicals used in this study were obtained from Sigma Chemical (St. Louis, MO), unless indicated otherwise. Protease inhibitor and phosphatase inhibitor cocktails were obtained from Calbiochem (San Diego, CA). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies were purchased from Bio-Rad (Hercules, CA). Specific antibodies to 3-NT, cytosolic superoxide dismutase 1 (SOD1), and ubiquitin were from Cell Signaling Technology, Inc. (Boston, MA). Horseradish peroxidase-conjugated Protein A/G agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence reagents were obtained from Thermo Scientific (Rockford, IL).
2.2. Animals
Age- and gender-matched (n=4) inbred Cyp2e1-null mice on a 129/Svj background [18] and wild-type mice were used in this study. All mice were housed in a temperature-controlled room (23-25 °C) on a 12 h light/12 h dark cycle at the National Cancer Institute, National Institutes of Health, and were fed standard rodent chow ad libitum. Handling and treatment procedures were in accordance with the animal study protocol approved by the NCI Animal Care and Use Committee.
2.3. Animal treatment and histopathology analysis
All experiments were performed with 2- to 3-month-old female mice (200 mg/kg) or male mice (400 mg/kg). APAP was dissolved in warm isotonic saline solution and administered by intraperitoneal injection to female or male mice for 4 or 24 h as reported [22,23]. Control mice were treated with sterile saline solution. The whole liver was harvested immediately at the indicated time points and a small liver section from the largest lobe of each mouse was fixed in 10% formalin in PBS and subjected to hematoxylin and eosin (H&E) staining by American HistoLabs, Inc. (Gaithersburg, MD). Histological examination was performed under a light microscope (200x). The rest of the liver samples were frozen immediately at −80 °C until use.
2.4. Sample preparation and Immunoblot (IB) analysis
Cytosolic fractions were prepared from pooled mouse livers from different groups (n=4 per group) by using differential centrifugation followed by two separate washing steps, as described [28]. The buffer used in this study was freshly pre-equilibrated with nitrogen gas for 1 h to remove dissolved oxygen [29]. Cytosolic proteins (10-40 μg) were separated by 12 or 15% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to nitrocellulose membranes. Upon completion of electrophoretic transfer of the proteins, membranes were blocked for 1 h in 5% milk powder in Tris-HCl buffered saline containing 0.01% Tween 20 (TBS-T). Membranes were then probed with specific primary antibodies (1:1,000 dilution) for 3-NT, ubiquitin, SOD1, or β-actin (usually 1:2,000 dilution in 5% milk powder in TBS-T) and incubated overnight at 4 °C. After the washing steps to remove the primary antibodies, the membranes were either incubated with goat anti-mouse (anti-3-NT and β-actin detection) or goat anti-rabbit (anti-ubiquitin, anti-SOD1) horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution in 5% milk powder in TBS-T). Protein bands were detected by enhanced chemiluminescence with Kodak X-OMAT film and their densities quantified using UN-SCAN-IT gel version 6.1 from Silk Scientific Corporation (Orem, UTAH) [30]. Band densities of the target proteins in each figure, when appropriate, were normalized to those of the corresponding β-actin band and the relative ratios between the target protein bands and the corresponding β-actin bands of APAP-treated samples and control (set at 1) were described in the top of each figure.
2.5. 2D-PAGE and immunoblot analysis for nitrotyrosine detection
Two-dimensional gel electrophoresis was performed with the IPGphor/IsoDalt systems from Amersham Biosciences (Pittsburgh, PA) as previously described [31], using 300 μg liver cytosolic proteins pooled from 4 mice per group. For the first dimension electrophoresis, IEF was performed with the IPGphor system using nonlinear pH 3–10 immobilized pH gradient strips (13 cm) and a programmed voltage gradient. Following the first dimension IEF, the strips were incubated in 15 ml of reducing solution (50 mM Tris-HCl, pH 8.8, 6 M urea, 1% DTT, 30% glycerol, 2% SDS) for 15 min at room temperature and then in 10 ml of alkylation solution (50 mM Tris-HCl, pH 8.8, 6 M urea, 1% iodoacetamide, 30% glycerol, 2% SDS) for 15 min at room temperature. For the second dimension electrophoresis, the immobilized pH gradient strips were sealed in 0.5% (w/v) agarose on the top of 12% SDS-PAGE. Immunoblotting was performed with or without pre-incubating the membrane-bound proteins with 10 mM sodium dithionite in 50 mM pyridine-acetate buffer, pH 5.0, for 1 h at room temperature to reduce nitrotyrosine to aminotyrosine [31]. Membranes were blocked for 1 h in 5% milk powder in 0.01% TBS-T and probed with specific primary antibodies (1:1,000 dilution) to 3-NT as described above. Specific 3-NT protein spots should not be detected following the treatment with sodium dithionite and hence the specificity of the 3-NT spots could be confirmed.
2.6. Immunoprecipitation and immunoblot analyses
The immunoprecipitation was performed as described [32]. A separate aliquot of liver cytosolic proteins pooled from 4 mouse livers per group (1 mg protein) was incubated with 3 μg anti-3NT or 3 μg of anti-SOD1 antibody overnight at 4 °C with constant head-to-tail rotation followed by addition of protein A/G-agarose for another 1 h. Proteins bound to the protein A/G-agarose were washed 4 times with PBS containing 1% CHAPS to remove nonspecifically bound proteins. After centrifugation, agarose-bound proteins were dissolved in Laemmli buffer and separated on 15% SDS-PAGE gels for immunoblot analysis using the specific antibody against ubiquitin for the immunoprecipitated 3-NT proteins (Fig. 5) or with the specific antibody against 3-NT, ubiquitin, or SOD1 for the immunoprecipitated SOD1 (Fig. 6).
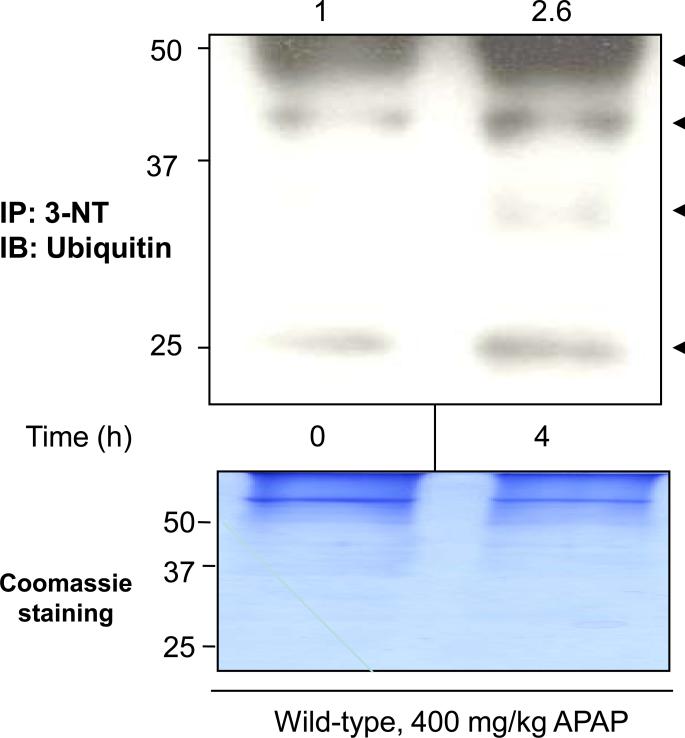
Fig. 5. Ubiquitin conjugation of nitrated proteins in APAP-exposed wild-type mice.
Equal amounts of cytosolic proteins (1 mg/sample) in saline control (left lane) and wild-type mice treated with 400 mg/kg APAP for 4 h (right lane) were immunoprecipitated with the monoclonal antibody against 3-NT. The immunoprecipitated proteins were then subjected to immunoblot analysis using the specific antibody against ubiquitin (arrows in top panel). A Coomassie-stained gel is also presented to demonstrate equal protein loading (bottom panel). The relative ratio of the ubiquitin immunoreactivity detected between the control (set as 1) and APAP-exposed samples is shown in the top.
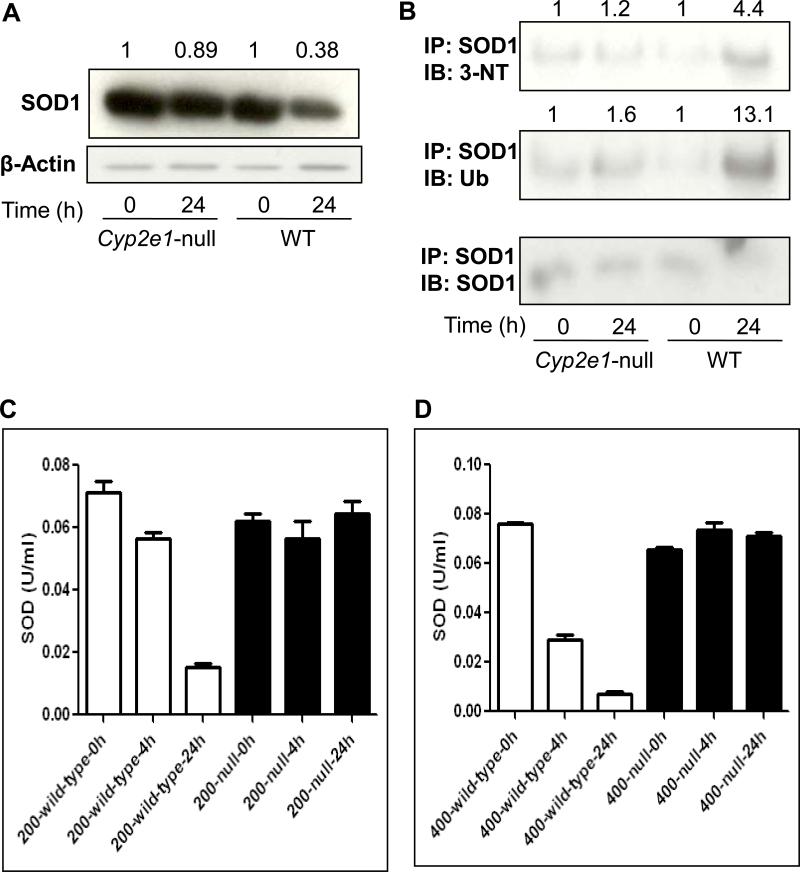
Fig. 6. Levels of cytosolic SOD1, nitrated SOD1, ubiquitinated SOD1, and activity in wild-type and Cyp2e1-null mice treated with APAP.
Equal amounts of or cytosolic proteins (20 μg protein/well) from different groups were separated on 15% SDS–PAGE, transferred to nitrocellulose membrane, and subjected to immunoblot analysis by using the specific anti-SOD1 antibody (A, upper panel) or β-actin (A, lower panel). Density of SOD1 band in each lane was calculated by quantitative densitometry, normalized to that of the corresponding β-actin bands. The relative ratio is presented for the SOD1 level detected in the corresponding control group, which was set at 1, and APAP-exposed groups. Immunoprecipitated proteins from each group were then subjected to immunoblot analysis with the anti-3-NT (B, top panel), anti-ubiquitin (Ub) (B, middle panel), or anti-SOD1 antibody (B, bottom panel). Density of 3-NT or ubiquitin bands was normalized to that of the corresponding SOD1 band. The relative ratio of the immunoreactivity against each target protein detected between the control (set as 1) and APAP-exposed samples is shown in the top. The enzyme activity of SOD was measured using commercially available kit following the manufacturer's protocol in both wild-type and Cyp2e1-null mice treated for 4 or 24 h with 200 mg/kg (C) or 400 mg/kg APAP (D).
2.7. Determination of NOS activity
NOS activity (50 μg cytosolic proteins) was determined using the commercially available kit from Oxford Biomedical research Inc. (Oxford, MI) by following the manufacturer's instruction. One unit of NOS activity was defined as the amount of NO (nM) produced per 1 mg (milligram) protein/min.
2.8. Determination of SOD activity
The enzyme activity of SOD (250 μg of liver cytosolic proteins) was measured using the commercial kit from Calbiochem (San Diego, CA) by following the manufacturer's protocol. One unit of SOD enzyme activity corresponds to the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
2.9. Determination of MDA concentrations
The amounts of MDA were measured using the commercially available kit from Oxford Biomedical research Inc. (Oxford, MI) following the manufacturer's protocol. MDA concentration (μM) in each sample was measured with using 20 μg liver cytosol proteins.
2.10. Data processing
All data were obtained with at least two or four separate experiments and three different measurements for enzyme activities. Data are presented as mean ± S.E.M. Other materials and methods not described here were the same as before [29,30,32,33].
3. Results
3.1. Differential effects of APAP on liver necrosis in wild-type and Cyp2e1-null mice
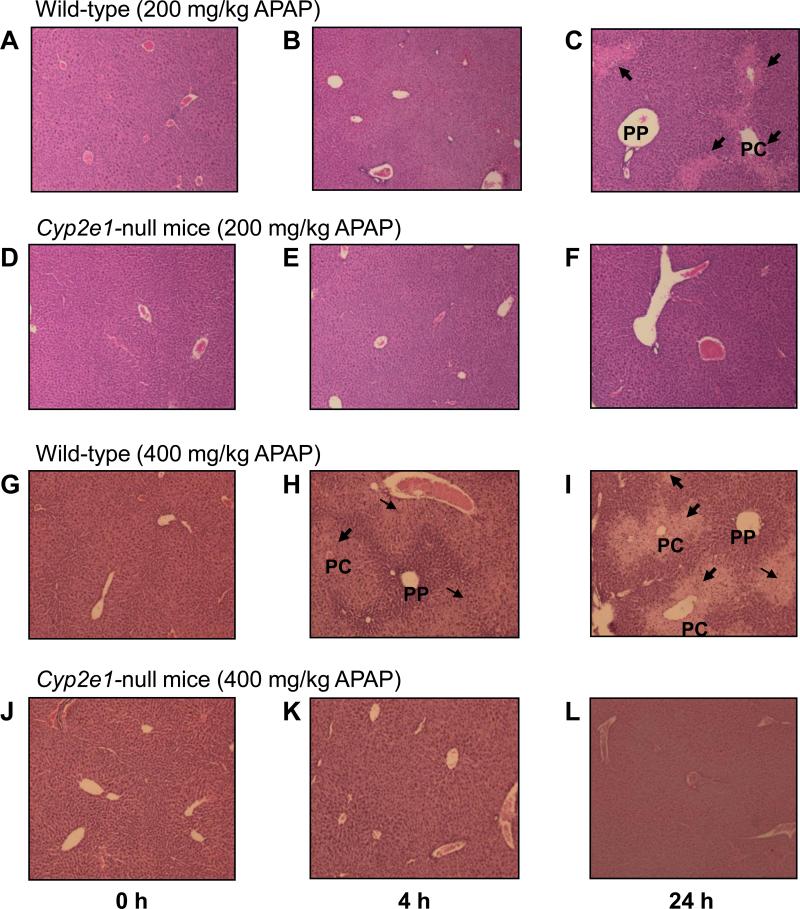
We have previously shown that APAP (200 and 400 mg/kg) caused a significant increase of serum ALT and AST levels in wild-type, but not in Cyp2e1-null mice [22,23], implying that hepatic injury occurred only in wild-type mice. Consistent with these reports, histological analyses revealed that hepatic necrosis, mainly observed in centrilobular regions, was slightly increased in wild-type mice treated with 200 mg/kg for 4 h, and necrosis was markedly increased following 24 h treatment, compared with the controls (Fig. 1A-C). In contrast, Cyp2e1-null mice were unaffected in response to 200 mg/kg APAP for 4 or 24 h, compared with their saline-treated control (Fig. 1D-F). The increase in liver necrosis was more prominent with a higher dose of APAP (400 mg/kg) in wild-type mice as evident by the remarkable increase in necrosis detected following 4 and 24 h treatment, where necrosis was massive, compared with their saline control (Fig. 1G-I). Similar to the response to 200 mg/kg APAP, liver necrosis could not be detected in Cyp2e1-null mice even at 400 mg/kg APAP exposed for 4 h and 24 h (Fig. 1J-L). Together, these data underscore the importance of CYP2E1 in mediating liver toxicity in response to APAP at the doses used in this study.
Fig. 1. Effect of APAP on hepatic necrosis in wild-type and Cyp2e1-null mice.
Photomicrographs (200×) after H&E staining from the left hepatic lobes from the indicated mouse livers are presented. Liver histology was performed for: wild-type mice (A-C) or Cyp2e1-null mice (D-F) exposed to 200 mg/kg APAP for 0, 4, and 24 h, respectively. Liver histology was also performed for wild-type mice (G-I) or Cyp2e1-null mice (J-L) exposed to 400 mg/kg APAP for 0, 4, and 24 h, respectively. PC, pericentral regions; PP, periportal regions. Arrows indicate severe necrosis in the PC regions.
3.2. Increased 3-NT formation on wild-type mice subjected to APAP treatment
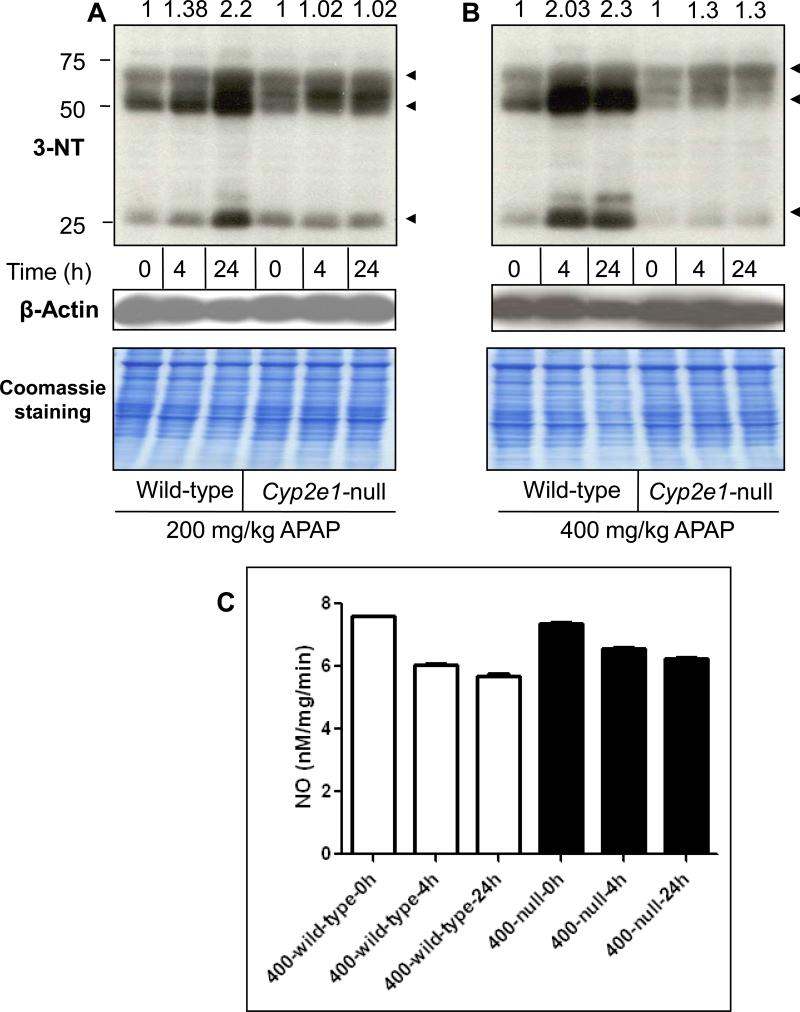
Peroxynitrite and formation of 3-NT adducts have recently gained much interest as potential new markers of APAP hepatotoxicity since 3-NT formation was predominantly observed in the centrilobular regions which correspond to the necrotic areas following APAP treatment [11,13]. Our previous data suggested that the development of oxidative stress in wild-type mice treated with 400 mg/kg APAP was prolonged as evident by the persistent elevation of H2O2 for 24 h and the delayed restoration of GSH levels, compared with Cyp2e1-null mice treated with the same dose of APAP [22,23], suggesting a critical role for CYP2E1 in the induction of this oxidative/nitrative stress. Since CYP2E1 is predominantly expressed in the centrilobular areas where 3-NT formation and necrosis occur, we evaluated the levels of 3-NT formation in both wild-type mice and Cyp2e1-null mice treated with toxic doses of APAP (Fig. 2). Protein nitration was slightly increased (by ~1.4-fold) in wild-type mice treated with 200 mg/kg for 4 h, and markedly increased (by 2.2-fold) when treated for 24 h, compared with their control (Fig. 2A, lanes 1-3). In contrast, there was little change in the 3-NT levels in Cyp2e1-null mice treated with 200 mg/kg APAP for 4 or 24 h compared with their control (Fig. 2A, lanes 4-6). The increased 3-NT formation was also more prominent in wild-type mice treated with 400 mg/kg APAP since 3-NT levels were increased by ~2.0 and 2.3-fold at 4 and 24 h, respectively, following APAP treatment, compared with their control (Fig. 2B, lanes 1-3). The 3-NT level was slightly increased (~1.3-fold) in Cyp2e1-null mice treated with 400 mg/kg for 4 and 24 h, compared with their controls (Fig. 2B, lanes 4-6). The marked increase in the 3-NT levels in APAP-exposed wild-type mice, however, was not accompanied by the concurrent activation of NOS. In fact, the total NOS activity levels were decreased in APAP-exposed wild-type by ~21% and 25% and by ~10% and 16% in Cyp2e1-null mice for 4 and 24 h, respectively, compared with their controls (Fig. 2C), similar to the earlier results [11]. Taken together, these data suggest that the formation of 3-NT protein adducts produced after APAP exposure is dependent on CYP2E1 but independent from NOS activity level under our experimental conditions.
Fig. 2. Levels of hepatic protein nitration and NOS activity in wild-type and Cyp2e1-null mice following APAP treatment.
Equal amounts of liver cytosolic proteins (40 μg/well) from different groups were separated on 12% SDS–PAGE, transferred to nitrocellulose membranes, and subjected to immunoblot analysis by using anti-3-NT antibody (A and B, upper panels) and anti-β-actin antibody (A and B, middle panels) for normalization. Coomassie-stained gels are also presented (A and B, lower panels) to further demonstrate equal protein loading. The relative ratio of the 3-NT level detected between the APAP-exposed samples and the corresponding control, which was set at 1, is shown in the top. Equal amounts of liver cytosolic proteins were used to measure total NOS activity (C) according to the manufacturer's instructions.
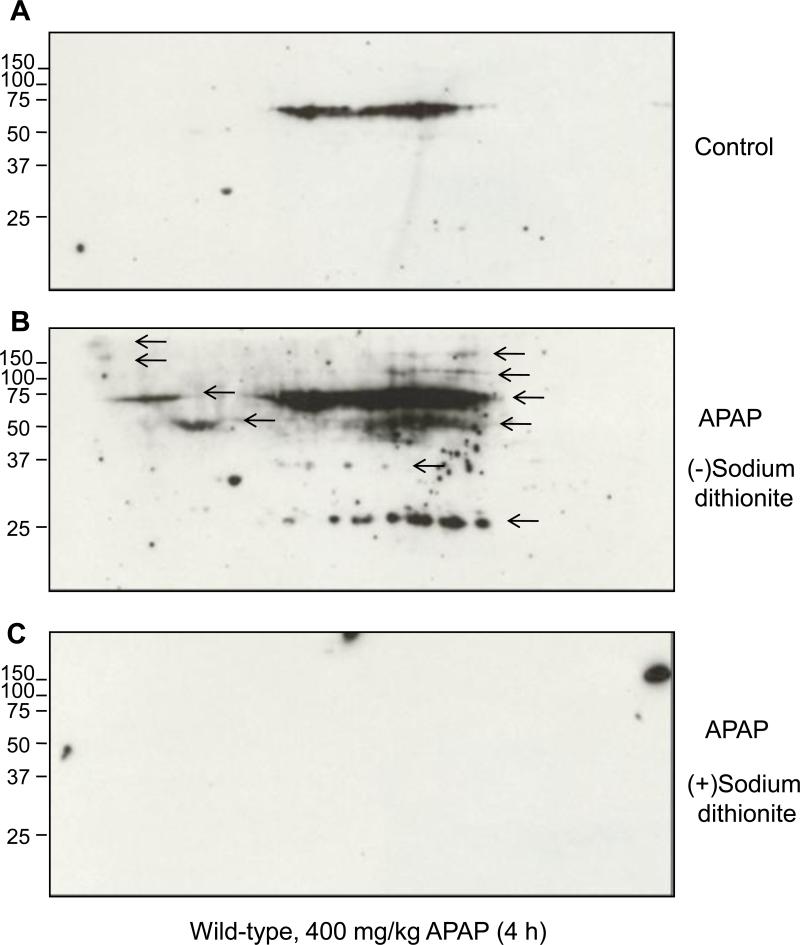
To ensure the specificity of the antibody used to detect 3-NT adduct formation and to better show the molecular weight distribution of nitrated proteins in wild-type mice treated with APAP (400 mg/kg) for 4 h, we used the previously described method [31] of 2-D gel electrophoresis followed by immunoblot analysis for the immunoreactivity of 3-NT before and after the conversion of nitrated proteins with dithionite (from nitrotyrosine to aminotyrosine) (Fig. 3). Only a few proteins were nitrated in saline-treated tissues (Fig. 3A). However, the number and intensity of nitrated proteins detected by the anti-3-NT antibody were markedly increased after APAP exposure (Fig. 3B). Without dithionite treatment, the antibody to 3-NT recognized immunoreactive proteins with the apparent molecular weights of ~250, 150, 100, 60, 50, 37, and 25 kDa (arrows in Fig. 3B), with the strongest bands at around ~60, 50 and 25 kDa (Fig. 3B). After dithionite treatment, these proteins were not detected by the same antibody (Fig. 3C). These data confirm the specificity of the nitrotyrosine immunoreactivity and further support the results in Fig. 2.
Fig. 3. 2D-PAGE and immunoblot analysis for 3-nitrotyrosine detection in hepatic proteins.
Immunoreactive nitrated proteins in cytosolic proteins (300 μg/gel) are shown from untreated wild-type mice (A) or treated (B) with 400 mg/kg APAP for 4 h. Immunoblot results (A and B) are before reduction, while (C) represents the result after reduction in the presence of sodium dithionite.
3.3. Increased ubiquitination of nitrated proteins in wild-type mice exposed to APAP
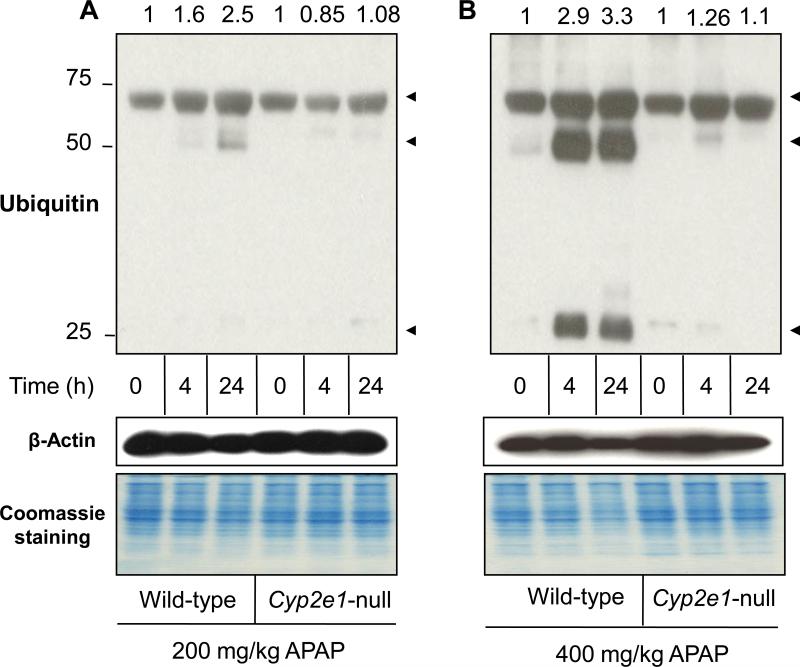
Protein ubiquitination is a vital pathway in maintaining cellular homeostasis through degradation of the improperly folded and/or damaged proteins [34,35]. It is well-established that nitrated proteins are degraded as part of the cellular defense against oxidative stress-mediated effects, suggesting that the degraded proteins, that may be essential for cell survival, would be replaced with newly synthesized proteins [26,36]. Because of the elevated levels of nitrated proteins following APAP exposure, we examined the effects of APAP treatment on protein ubiquitination in both wild-type and Cyp2e1-null mice. The data (Fig. 4) showed a protein ubiquitination pattern similar to those of protein nitration (Fig. 2). Protein ubiquitination was increased by 1.6-fold in wild-type mice treated with 200 mg/kg for 4 h and markedly increased by 2.5-fold when treated for 24 h, compared with their control (Fig. 4A, lanes 1-3). In contrast, there was little change in the levels of ubiquitinated proteins in Cyp2e1-null mice treated with 200 mg/kg APAP for 4 or 24 h, compared with their control (Fig. 4A, lanes 4-6). The increase level of protein ubiquitination was more evident in wild-type mice treated with 400 mg/kg APAP since it was increased by 2.9- and 3.3-fold at 4 and 24 h, respectively, following APAP treatment compared with their controls (Fig. 4B, lanes 1-3). Protein ubiquitination was slightly increased by ~1.2-fold in Cyp2e1-null mice treated with 400 mg/kg for 4 and 24 h, compared with their control (Fig. 4B, lanes 4-6). It is noteworthy to mention that the apparent molecular weights of ubiquitinated proteins (Fig. 4) were very similar to those of nitrated proteins (Fig. 2).
Fig. 4. Levels of hepatic protein ubiquitination in wild-type and Cyp2e1-null mice following APAP exposure.
Equal amounts of liver cytosolic proteins (40 μg/well) from different groups were separated on 12% SDS–PAGE, transferred to nitrocellulose membranes, and subjected to immunoblot analysis by using anti-ubiquitin (A and B, upper panels) and anti-β-actin antibody (A and B, middle panels) for normalization. Coomassie-stained gels are also presented (A and B, lower panels) to further demonstrate equal protein loading. The relative ratio of the 3-NT level detected between the APAP-exposed samples and the corresponding control, which was set at 1, is shown in the top.
To provide direct evidence that nitrated proteins were actually ubiquitinated, nitrated proteins in wild-type mice treated with 400 mg/kg APAP for 4 h were immunoprecipitated with monoclonal antibody against 3-NT. The immunoprecipitated proteins were then subjected to immunoblot analyses using specific antibody against ubiquitin (Fig. 5, arrows). The total level of ubiquitin-conjugated proteins in the immunoprecipitated nitrated proteins from APAP-exposed mice was markedly increased by ~2.6-fold compared with their control (Fig. 5). These data suggest that nitrated proteins following APAP treatment are more susceptible to ubiquitination and degradation than the native proteins, as demonstrated for other proteins [37].
3.4. Increased nitration, ubiquitination, degradation and decreased activity of SOD1 in wild-type mice treated with APAP
Earlier reports showed opposing results about the role of SOD1 in APAP hepatic toxicity [38,39]. However, nitration of SOD1 and subsequent loss of activity through its degradation are well-established [37,40]. Because of the maximum levels of nitration and ubiquitination following treatment with 400 mg/kg APAP for 24 h in wild-type mice (Figs. 2-5), we selected this dose and time point for evaluating the levels of nitration and ubiquitination of a protein as an example. Because of the well-established nitration and degradation [40], we chose to study the levels of SOD1 protein and activity in both wild-type and Cyp2e1-null mice. The SOD1 protein levels were slightly decreased in APAP-exposed Cyp2e1-null mice compared to control (Fig. 6A). In contrast, SOD1 protein level was markedly decreased by ~62% in APAP-treated wild-type mice compared with their control (Fig. 6A), suggesting the possibility of SOD1 protein degradation. We next evaluated whether SOD1 was nitrated and/or ubiquitinated in APAP-exposed wild-type mice by examining the immunoreactivity of the immunoprecipitated SOD1 with the specific antibody against 3-NT or ubiquitin (Fig. 6B). Consistent with the aforementioned results, SOD1 in Cyp2e1-null mice (Fig. 6B, top and middle panels) was slightly nitrated and ubiquitinated (increased by ~ 1.2- and 1.6-fold, respectively, compared with their control after APAP treatment. In contrast, the levels of SOD1 nitration and ubiquitination were profoundly increased (~4.4- and 13.1-fold, respectively) in APAP-exposed wild-type mice, compared with their corresponding controls (Fig. 6B). The levels of immunoprecipitated SOD1 protein were similar in Cyp2e1-null mice regardless of APAP exposure (Fig. 6B, bottom panel). However, the SOD1 level in APAP-exposed wild-type mice was much lower than saline-treated control. We further studied whether nitration and ubiquitination would actually decrease the SOD1 activity (Figs. 6C and D). For this experiment, we chose to analyze the liver extracts from mice treated with 200 and 400 mg/kg APAP to investigate the dose- and time-dependent effects on nitration and/or degradation of SOD1 protein (Figs. 6C and D). We could not detect any inhibition of SOD1 activity in response to either dose of APAP in Cyp2e1-null mice (Figs. 6C and D). However, SOD1 activity was clearly decreased in a time- and dose-dependent manner in APAP-exposed wild-type mice (Figs. 6C and D). In wild-type mice treated with 200 mg/kg for 4 h, SOD1 activity was inhibited by ~16%, while its activity was suppressed by ~79% at 24 h after APAP treatment. The SOD1 activity was decreased by ~54% and 90% following treatment with 400 mg/kg APAP for 4 and 24 h, respectively, compared with their corresponding controls (Figs. 6C and D). Collectively, these data demonstrate that APAP can promote nitration and/or ubiquitination of SOD1 prior to its degradation, resulting in clear reduction of its catalytic activity.
3.5. Increased lipid peroxidation in wild-type and Cyp2e1-null mice treated with APAP
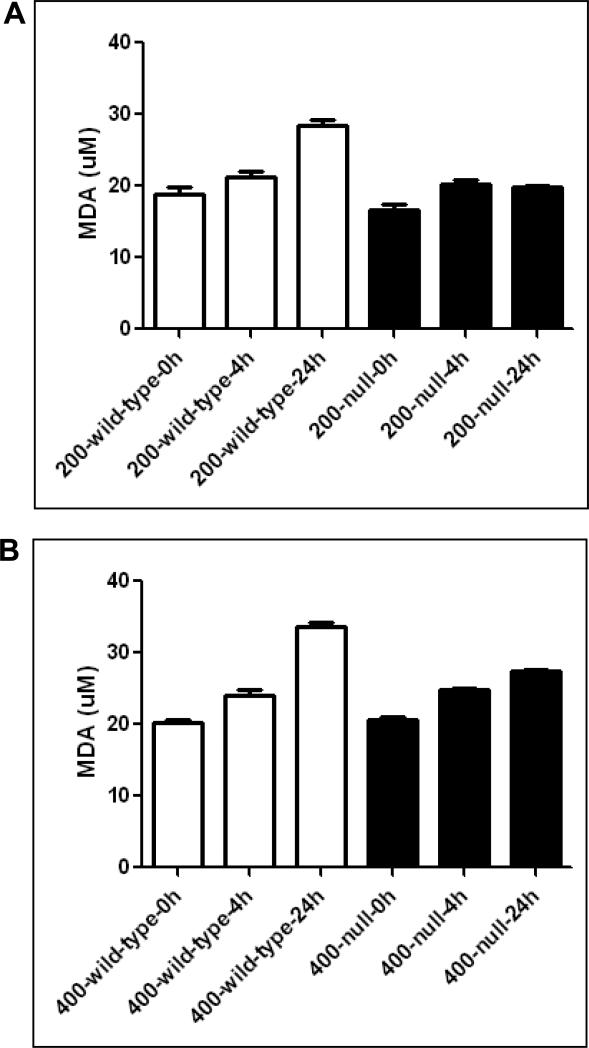
By using iNos-null mice and wild-type mice, Michael et al [27] suggested an important role of lipid peroxidation in APAP-mediated liver toxicity. We, therefore, evaluated the levels of lipid peroxidation, using MDA as a marker, in both wild-type and Cyp2e1-null mice exposed to APAP (Fig. 7A). Both wild-type and Cyp2e1-null mice treated with APAP (200 and 400 mg/kg) exhibited a similar trend of increased lipid peroxidation; however, the increase was more prominent in wild-type mice treated with 200 or 400 mg/kg APAP, which exhibited the maximum increase of lipid peroxidation following 24 h treatment (Figs. 7A and B). These data indicate that lipid peroxidation may not be the major cause in promoting APAP-related liver toxicity, mainly because there was little liver damage observed in Cyp2e1-null mice [18,22,23] and in our current study.
Fig. 7. Levels of hepatic MDA in wild-type and Cyp2e1-null mice subjected to treatment with APAP.
Equal amounts of liver cytosolic proteins (0.5 mg/sample) were used to measure the levels of MDA according to the manufacturer's protocols.
4. Discussion
GSH depletion and the formation of NAPQI adducts are very well-established as hallmarks of hepatoxicity in response to toxic doses of acetaminophen [6,41,42]. Recently, peroxynitrite and 3-NT formation have received much attention as critical mediators of APAP as suggested by several reports which also showed that 3-NT formation was predominant in the centrilobular regions where necrosis takes place [11,13,27,43,44]. One of the best evidence of a role for peroxynitrite in APAP-mediated hepatoxicity was provided by Knight et al. [15], who showed that the delayed treatment with GSH following APAP led to increased levels of hepatic GSH and decreased peroxynitrite levels, resulting in the attenuation of APAP-induced liver toxicity despite the fact that elevated mitochondrial oxidative stress persists following GSH induction. These investigators suggested that peroxynitrite per se is a critical mediator of hepatoxicity in response to APAP and that elevated peroxynitrite may have a limited role in increasing the mitochondrial oxidative stress that was initiated in response to NAPQI [15]. Thus, the exact mechanism(s) of peroxynitrite-mediated liver toxicity still elusive and require further investigation.
Since CYP2E1 is widely recognized as a major enzyme in the biotransformation of APAP to NAPQI, it was conceivable to hypothesize that Cyp2e1-null mice should exhibit less hepatotoxic response to APAP and consequently less 3-NT formation. Therefore, a major aim of this study was to determine the role of CYP2E1 in protein nitration and the mechanism of protein degradation in APAP-related hepatotoxicity. In support of the possible role for CYP2E1 in mediating liver damage through oxidative/nitrative stress, we recently showed that at higher doses of APAP, both wild-type and Cyp2e1-null mice exhibited a similar pattern of APAP metabolism despite the huge differences in liver toxicity with markedly increased serum ALT and AST levels in wild-type mice but not in Cyp2e1-null mice [22,23]. These results suggest a limited role for CYP2E1 and the possible involvement of other P450s such as CYP3A and CY1A2 in NAPQI production with the dosages used in these studies. These results also indicate the possibility that another mechanism such as oxidative/nitrative stress may be involved in mediating the differences in the hepatotoxicity observed in both mouse strains.
Formation of 3-NT, which is considered as a footprint of peroxynitrite, is mediated by reactive nitrogen species such as peroxynitrite anion (ONOO.−) and nitrogen dioxide (NO2.−), formed as a secondary product of NO.− interaction with oxidants such as superoxide radicals (O2.−), H2O2, and transition metal centers [45]. Peroxynitrite can cause oxidative damage to all kinds of cellular macromolecules [12] and may promote inactivation of a variety of target proteins [46]. The inducible form of NOS (iNOS) was suggested to be the main source of NO following APAP treatment since there was marked reduction of 3-NT staining in mice lacking iNOS following APAP treatment [27,47] and also in mice pretreated with the inhibitors of NOS [44]. In contrast, the current results show that NOS was not induced following treatment with APAP, and was actually inhibited despite the evidence of 3-NT immunostaining, similar to the earlier results [11]. Further, 3-NT adducts were formed as early as 30 min following APAP treatment despite the absence of iNOS induction [11,48]. Thus, the role of iNOS in the formation of 3-NT is not very clear and it may not be the only possible source of NO in APAP-mediated hepatotoxicity. In fact, Gow et al. [36] reported that many other metalloproteins such as P450 enzymes, myeloperoxidase, eosinoperoxidase, and myoglobin are also responsible for 3-NT formation. The results shown in this study are in agreement with this view.
The source of superoxide and underlying mechanism(s) of its increase following APAP is still under debate. Potential sources of superoxides could be CYP2E1, which produces reactive oxygen species [21,49] even in the absence of its substrates [50], damaged mitochondria [51], and/or NADPH oxidase in Kupffer cells [52]. It was reported that gadolinium chloride, an inhibitor of superoxide formation in Kupffer cells, inhibited APAP-induced liver injury [24]. However, the role of Kupffer cells in the formation of peroxynitrite is questionable since the independence of NADPH oxidase expressed in Kupffer cells on APAP hepatoxicity has been reported [53]. Further, Kupffer cells have been proposed to be actually protective against APAP-mediated liver toxicity [54]. Taken together, the role of Kupffer cells in APAP still needs further investigation.
Our results (Figs. 2 and 3) suggest that in our system, even low, basal levels of NO may be sufficient enough to produce peroxynitrite in the presence of extra amounts of additional superoxides, resulting in increased levels of 3-NT. These data also suggest that at the APAP doses used in this study, CYP2E1 seems essential for the formation of 3-NT, possibly through superoxide formation because CYP2E1 possesses high NADPH oxidase activity [20,21], and may actually be more important than iNOS whose role seems controversial [27,43,44,47,55]. This view is also in agreement with our previous study where H2O2 was only increased in wild-type mice, but not in Cyp2e1-null mice despite APAP exposure, further implying a possible role for oxidative stress through CYP2E1-mediated events [22]. Although we can not totally exclude the formation of 3-NT in Cyp2e1-null mice, especially with the higher doses of APAP, the substantial differences between wild-type and Cyp2e1-null mice were very clear. It is noteworthy to mention that GSH was rapidly restored in Cyp2e1-null mice following treatment with 400 mg/kg APAP (4 h) while its recovery was delayed in wild-type mice (16 h) [23]. This result (differential rates of GSH recovery) may be critically important since GSH is a potent peroxynitrite scavenger and may confer a small window of opportunity to prevent hepatotoxicity in Cyp2e1-null mice following APAP treatment, as previously shown [15]. Consequently, the rapidly restored levels of GSH may be another possible reason of the decreased formation of 3-NT in Cyp2e1-null mice treated with APAP. These data validate our original hypothesis that CYP2E1 may be important in promoting its hepatotoxic effects through increasing oxidative/nitrative stress rather than promoting the formation of NAPQI since its presence seems necessary for peroxynitrite formation under our experimental conditions. We however can not totally exclude the possible role of NAPQI in GSH depletion which indirectly may lead to the formation of reactive oxygen or nitrogen species (ROS/RNS).
Protein ubiquitination is an important pathway by which many proteins, especially those that are improperly folded and damaged, are degraded [34,35]. Protein ubiquitination and degradation play an essential role in many vital processes such as cell division and differentiation, apoptosis, DNA repair, signal transduction, membrane transport, oncogenesis and degrading abnormal proteins [34,35]. Ubiquitination is a multicatalytic process that tags target proteins with a chain of multiple ubiquitin moieties. This is followed by their degradation by the cytosolic 26S protease complex (the 26S proteasomes) [26]. Proteasomal degradation of nitrated proteins, resulting in shorter half-lives than the native protein counterparts, has been well-established [25,26,37,56]. Proteasomal degradation has been suggested to be part of the cellular defense against protein aggregation during oxidative stress which would cause more cellular damage. In agreement with these earlier reports, protein ubiquitination only increased in wild-type mice following treatment with 200 and 400 mg/kg APAP and slightly increased in Cyp2e1-null mice following 400 mg/kg APAP treatment. We also provided direct evidence that nitrated proteins were actually ubiquitinated (Figs. 5 and 6) following APAP exposure. Given the massive liver necrosis we observed in APAP-exposed wild-type mice, cell ability to replace the degraded proteins would be highly questionable. Consequently, the inefficient or reduced rates of replacement of the damaged proteins (with less native proteins) may also contribute to cellular damage. Furthermore, it is possible that cell damage can occur since nitration of tyrosine residues in many proteins may interfere with the normal cell signaling, as discussed [36,57].
The role of SOD1 in APAP hepatic toxicity is still controversial. The protective role of SOD1 against APAP may be implicated in preventing 3-NT formation as over-expression of SOD1 in mice abolished the increased nitration levels of tyrosine hydroxylase [58] and APAP toxicity [38]. In contrast, studies with Sod1-null mice suggested that SOD1 is actually essential for 3-NT formation in mice treated with APAP [39]. Regardless of the role of SOD1 in APAP toxicity, its nitration and subsequent loss of activity and/or degradation are well-established [37,40]. We thus selected SOD1 as an example to demonstrate protein nitration and/or degradation during APAP toxicity. Indeed, our data (Fig. 6) with decreased levels of SOD1 further support the earlier findings of rapid degradation of nitrated proteins [37,38]. Identification of nitrated proteins in response to APAP will thus provide a new insight in understanding the underlying mechanism of APAP-mediated liver necrosis. In addition, the role of the ubiquitin/proteasome system in APAP-induced liver toxicity will require further investigation.
The role of lipid peroxidation in mediating APAP toxicity is also not clear and it was suggested that despite its occurrence, lipid peroxidation may not be a critical event in the mechanism of APAP hepatoxicity; rather multifactorial events were suggested to mediate the hepatotxic effect [4]. The level of lipid peroxidation was not increased in APAP-exposed wild-type mice while its level was increased in iNos-null mice (27) despite liver necrosis in both mouse groups. These investigators suggested that the elevated levels of lipid peroxidation instead of peroxynitrite production are critically important in causing liver damage in the iNos-null mice following APAP treatment. Unlike this study, our current data (Fig. 7B) showed increased levels of lipid peroxides in Cyp2e1-null mice exposed to APAP (400 mg/kg) for 4 and 24 h despite very little histological damage and biochemical indicators of hepatotoxicity (such as elevated serum ALT and AST levels). Therefore our current data suggest that peroxynitrite may be more important than lipid peroxidation in promoting liver toxicity and/or an additional factor may be needed for lipid peroxidation to mediate liver toxicity, as suggested [4]. Alternatively, lipid peroxidation may not be sufficient to produce liver damage due to the quick restoration of GSH in Cyp2e1-null mice [22,23]. Nonetheless, oxidative stress seems to also take place in Cyp2e1-null mice following higher doses of APAP, but its increase seems to be under control possibly through rapid restoration of GSH [23].
In summary, we showed that CYP2E1 appears to be necessary for the formation of 3-NT adducts, which likely represent oxidative protein modification (i.e., nitration), contributing to decreased levels and/or loss of function of many nitrated proteins through ubiquitin-dependent degradation. Collectively, this chain event would contribute to liver necrosis and eventually liver failure. Identification of nitrated proteins and investigating the role of ubiquitin/proteasome system in APAP-liver toxicity, by using the already existing mouse models such as Cyp2e1-null mice, would provide a new insight in understanding the possible mechanism(s) and down-stream effects through which peroxynitrite mediates its hepatotoxic effects.
Acknowledgement
This research was supported by the Intramural Research Program of National Institute on Alcohol Abuse and Alcoholism. We are thankful to Dr. Klaus Gawrisch for supporting this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors do not have conflict of interest.
E-mail addresses of other co-authors are: Mohamed A. Abdelmegeed: abdelmegeedm@mail.nih.gov; Kwan-Hoon Moon: kwanhoonm@mail.nih.gov; Chi Chen: chichen@umn.edu; Frank J. Gonzalez: gonzalef@mail.nih.gov.
References
- 1.Prescott LF. Hepatotoxicity of mild analgesics. Br J Clin Pharmacol. 1980;10(Suppl 2):373S–9S. doi: 10.1111/j.1365-2125.1980.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas SH. Paracetamol (acetaminophen) poisoning. Pharmacol Ther. 1993;60:91–120. doi: 10.1016/0163-7258(93)90023-7. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 5.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, et al. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Hart SG, Cartun RW, Wyand DS, Khairallah EA, Cohen SD. Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: correspondence of covalent binding with toxicity. Fundam Appl Toxicol. 1995;24:260–74. doi: 10.1006/faat.1995.1029. [DOI] [PubMed] [Google Scholar]
- 8.Pumford NR, Halmes NC, Hinson JA. Covalent binding of xenobiotics to specific proteins in the liver. Drug Metab Rev. 1997;29:39–57. doi: 10.3109/03602539709037572. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SD, Khairallah EA. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab Rev. 1997;29:59–77. doi: 10.3109/03602539709037573. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–53. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 11.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–20. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 12.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 13.Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604–7. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 15.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 16.Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–8. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 17.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 18.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 19.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:449–57. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 20.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984;259:6812–7. [PubMed] [Google Scholar]
- 21.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Krausz KW, Idle JR, Gonzalez FJ. Identification of novel toxicity-associated metabolites by metabolomics and mass isotopomer analysis of acetaminophen metabolism in wild-type and Cyp2e1-null mice. J Biol Chem. 2008;283:4543–59. doi: 10.1074/jbc.M706299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Krausz KW, Shah YM, Idle JR, Gonzalez FJ. Serum metabolomics reveals irreversible inhibition of fatty acid beta-oxidation through the suppression of PPARalpha activation as a contributing mechanism of acetaminophen-induced hepatotoxicity. Chem Res Toxicol. 2009;22:699–707. doi: 10.1021/tx800464q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–95. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 25.Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63–6. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 26.Poppek D, Grune T. Proteasomal defense of oxidative protein modifications. Antioxid Redox Signal. 2006;8:173–84. doi: 10.1089/ars.2006.8.173. [DOI] [PubMed] [Google Scholar]
- 27.Michael SL, Mayeux PR, Bucci TJ, Warbritton AR, Irwin LK, Pumford NR, et al. Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric Oxide. 2001;5:432–41. doi: 10.1006/niox.2001.0385. [DOI] [PubMed] [Google Scholar]
- 28.Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, et al. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250–60. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–12. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 30.Moon KH, Upreti VV, Yu LR, Lee IJ, Ye X, Eddington ND, Veenstra TD, Song BJ. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8:3906–18. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyagi M, Sakaguchi H, Darrow RM, Yan L, West KA, Aulak KS, et al. Evidence that light modulates protein nitration in rat retina. Mol Cell Proteomics. 2002;1:293–303. doi: 10.1074/mcp.m100034-mcp200. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–65. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 33.Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, et al. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–30. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 34.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 35.Ciechanover A, Schwartz AL. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc Natl Acad Sci U S A. 1998;95:2727–30. doi: 10.1073/pnas.95.6.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–8. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 37.Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M, et al. Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys. 2000;380:360–6. doi: 10.1006/abbi.2000.1940. [DOI] [PubMed] [Google Scholar]
- 38.Mirochnitchenko O, Weisbrot-Lefkowitz M, Reuhl K, Chen L, Yang C, Inouye M. Acetaminophen toxicity. Opposite effects of two forms of glutathione peroxidase. J Biol Chem. 1999;274:10349–55. doi: 10.1074/jbc.274.15.10349. [DOI] [PubMed] [Google Scholar]
- 39.Jian-Hong Z, Zhang X, Roneker CA, McClung JP, Zhang S, Thannhauser TW, et al. Role of copper,zinc-superoxide dismutase in catalyzing nitrotyrosine formation in murine liver. Free Radic Biol Med. 2008;45:611–8. doi: 10.1016/j.freeradbiomed.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, et al. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–22. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 42.Corcoran GB, Mitchell JR, Vaishnav YN, Horning EC. Evidence that acetaminophen and N-hydroxyacetaminophen form a common arylating intermediate, N-acetyl-p-benzoquinoneimine. Mol Pharmacol. 1980;18:536–42. [PubMed] [Google Scholar]
- 43.Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL, et al. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27:748–54. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]
- 44.Hinson JA, Bucci TJ, Irwin LK, Michael SL, Mayeux PR. Effect of inhibitors of nitric oxide synthase on acetaminophen-induced hepatotoxicity in mice. Nitric Oxide. 2002;6:160–7. doi: 10.1006/niox.2001.0404. [DOI] [PubMed] [Google Scholar]
- 45.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–8. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, et al. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- 48.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 49.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–30. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 50.Bell LC, Guengerich FP. Oxidation kinetics of ethanol by human cytochrome P450 2E1. Rate-limiting product release accounts for effects of isotopic hydrogen substitution and cytochrome b5 on steady-state kinetics. J Biol Chem. 1997;272:29643–51. doi: 10.1074/jbc.272.47.29643. [DOI] [PubMed] [Google Scholar]
- 51.Jaeschke H, Mitchell JR. Mitochondria and xanthine oxidase both generate reactive oxygen species in isolated perfused rat liver after hypoxic injury. Biochem Biophys Res Commun. 1989;160:140–7. doi: 10.1016/0006-291x(89)91632-x. [DOI] [PubMed] [Google Scholar]
- 52.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–50. [PubMed] [Google Scholar]
- 53.James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–97. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- 54.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–13. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 55.Kamanaka Y, Kawabata A, Matsuya H, Taga C, Sekiguchi F, Kawao N. Effect of a potent iNOS inhibitor (ONO-1714) on acetaminophen-induced hepatotoxicity in the rat. Life Sci. 2003;74:793–802. doi: 10.1016/j.lfs.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Grune T, Blasig IE, Sitte N, Roloff B, Haseloff R, Davies KJ. Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteasome. J Biol Chem. 1998;273:10857–62. doi: 10.1074/jbc.273.18.10857. [DOI] [PubMed] [Google Scholar]
- 57.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, et al. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci U S A. 1998;95:7659–63. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]