Abstract
Gap junctional intercellular communication (GJIC) plays a critical role in the regulation of tissue homeostasis and carcinogenesis and is modulated by the levels, subcellular localization and posttranslational modification of gap junction proteins, the connexins (Cx). Here, using oval cell-like rat liver epithelial cells, we demonstrate that the RNA-binding protein HuR promotes GJIC through two mechanisms. First, HuR silencing lowered the levels of Cx43 protein, Cx43 mRNA and Cx43 mRNA half-life. This regulation was likely due to the direct stabilization of Cx43 mRNA by HuR, since HuR associated directly with Cx43 mRNA, a transcript that bears signature AU- and U-rich sequences in its 3′UTR. Second, HuR silencing reduced both half-life and the levels of β-catenin mRNA, also a target of HuR; accordingly, HuR silencing lowered the levels of whole-cell and membrane-associated β-catenin. Co-immunoprecipitation experiments showed a direct interaction between β-catenin and Cx43. SiRNA-mediated depletion of β-catenin recapitulated the effects of decreasing HuR levels: it attenuated GJIC, decreased Cx43 levels and redistributed Cx43 to the cytoplasm, suggesting that depletion of β-catenin in HuR-silenced cells contributed to lowering Cx43 levels at the membrane. Finally, HuR was demonstrated to support GJIC under conditions of exposure to a genotoxic agent, doxorubicin, or an inducer of differentiation processes, retinoic acid, thus pointing to a crucial role of HuR in the cellular response to stress and in physiological processes modulated by GJIC.
Conclusion
HuR promotes gap junctional intercellular communication in rat liver epithelial cells through two related regulatory processes, by enhancing the expression of Cx43 and by increasing the expression of β-catenin which, in turn, interacts with Cx43 and is required for proper positioning of Cx43 at the plasma membrane.
Keywords: Connexin-43, Gap junctions, β-catenin, mRNA stability, oval cells, retinoic acid, doxorubicin
Gap junctional intercellular communication (GJIC) plays a critical role in the regulation of cellular proliferation, differentiation and during carcinogenesis 1. Gap junctions are clusters of intercellular channels connecting the cytoplasms of two adjacent cells. The channels, composed of two connexin-hexamer hemi-channels provided by each of the neighboringcells, allow for a controlled diffusion of compounds of low molecular mass (< 1 kDa) between cells, including nutrients, signaling molecules and ions 2,3. In the liver, connexin (Cx) 32 and Cx26 are the major connexins expressed in hepatocytes, while Cx43 is expressed at high levels in bile duct epithelial cells, hepatic stellate cells and oval cells 4,5, the two latter cell types being capable of further differentiating into hepatocytes or epithelial cells 6–8. GJIC is controlled at different levels, including connexin gene expression, posttranslational modification and subcellular distribution of connexin molecules as well as the stabilization or anchoring of gap junctional channels in the cell membrane.
Cx phosphorylation may serve as a means of modulating GJIC in immediate response to extracellular stimuli, such as growth factors 9,10 or stressful agents 11–13. In contrast, changes in connexin expression may serve long-term control of GJIC. In addition to reports on transcriptional regulation 14, there is evidence for posttranscriptional control of connexin expression that was found with murine Cx43 mRNA 15. However, no RNA-binding protein mediating such effects has been identified so far. Similar to Cx43, the expression of membrane-bound adhesion proteins interacting with Cx43 and stabilizing gap junctional clusters in the membrane, such as the adherens junction-associated protein β-catenin, was hypothesized to be controlled by RNA-binding proteins: in colon carcinoma cells, β-catenin expression was described to be controlled by HuR 16, an mRNA stabilizing protein related to the Drosophila ELAV (embryonic lethal abnormal vision) family of proteins 17 known to be modulated by mitogenic and stress-causing agents 18,19.
The present study examines whether Cx43-based GJIC is regulated by HuR both directly, e.g. by controlling Cx43 levels, or indirectly, e.g. by controlling gap junctional channel integrity. As model system, an oval cell-like rat liver epithelial cell line (WB-F344) was employed, which expresses high levels of Cx43 and is capable of differentiating into hepatocytes 6,20. Oval cells are liver progenitor cells activated during liver regeneration stimulated by liver injury induced by drugs, viruses, or toxins 21. We identify HuR as an RNA-binding protein that controls GJIC at least in part by enhancing Cx43 levels. Interestingly, modulation of Cx43 function by HuR is also indirect, via β-catenin, suggesting that GJIC is controlled by interaction of Cx43 with adherens junction proteins and at the posttranscriptional level. We further demonstrate that HuR promotes GJIC in cells exposed to retinoic acid or to a genotoxic agent, doxorubicin. Our data establish novel links between HuR, Cx43, and β-catenin and may offer an explanation for changes of GJIC and Cx43 levels in differentiating cells and during carcinogenesis.
Materials and Methods
Cell Culture and transfections
WB-F344 rat liver epithelial cells 22 with stem cell-like properties 6 were a kind gift of Dr. James E. Trosko, Michigan State University, East Lansing, MI, USA. Cells were maintained as described previously 10. For siRNA transfections, cells were transferred to 3 cm dishes one day before transfection. Cells were transfected using Oligofectamine reagent (Invitrogen) and siRNAs (Table 1) using standard procedures.
Table 1.
Sequences of primer pairs and siRNAs employed in this study
| Target | Sequences 5′ → 3′ | Genbank entry |
|---|---|---|
|
PCR Primers | ||
| 18S rRNA | Gccgctagaggtgaaattcttg cattcttggcaaatgctttcg |
X01117 |
| β-actin | gtcgtaccactggcattgtg ctctcagctgtggtggtgaa |
NM_031144 |
| β-catenin #1 | ggccagtggtggaccccaagcct aggccccagtgcctgcatccca |
NM_053357 |
| β-catenin #2 | acgctgcataatctcctgct gagcttgctttcctgattgc |
NM_053357 |
| Cx43 #1 | ggcgtgaggaaagtaccaaa acagcgaaaggcagactgtt |
X06656, NM_012567 |
| Cx43 #2 | aacagtctgcctttcgctgt aaagcgagagacaccaagga |
X06656, NM_012567 |
| Cx43 #3 | agcctgaactctcatttttcctt ccatgtctgggcacctct |
X06656, NM_012567 |
| GAPDH | gatcgtggaagggctaatga ggatgcagggatgatgttct |
NM_017008 |
| HuR | ttcgggataaagttgcagga tttgcagtaacatagttcacaaagc |
XM_344063 |
| p21 | agcaaagtatgccgtcgtct ggcgcttggagtgatagaaa |
NM_080782 |
| SDHA | cgagatccgtgaaggaagag gcccatgttgtaatgcacag |
NM_130428 |
| siRNAs (supplier) | ||
| Ctrl #1 (Qiagen) | uucuccgaacgugucacguuu | |
| Ctrl #2 (Dharmacon) | ugguuuacaugucgacuaa | |
| HuR #1 (Qiagen) | aagaggcaauuaccaguuuca | XM_344063 |
| HuR #2 (Dharmacon) | caacaagucccacaaauaauu | XM_344063 |
| Cx43 (Smart Pool, Dharmacon) | caacaaccuggcugcgaaa, ugauugaaaugucgaguua, cgugaagggaagaagcgau, uuacugagauucugcgaua | NM_012567 |
| β-catenin (Smart Pool, Dharmacon) | gaucuuagcuuacggcaauuu, gggagaagcccuuggauuu, Aagcugaccucauggaguuuu, guagaaacagcccguuguauu | NM_053357 |
Determination of Gap Junctional Intercellular Communication
GJIC was determined as described earlier 10 by microinjecting the fluorescent dye Lucifer Yellow CH (Sigma; 10 % (w/v) in 0.33 M LiCl) into selected cells. One minute after injection, fluorescent cells surrounding the cells loaded with the dye were counted and taken as a measure of GJIC. Ten individual cells were loaded with dye per dish and means of the numbers of fluorescent neighboring cells were calculated 23.
Determination of RNA stability, RT-PCR
The stability of Cx43 mRNA in cells treated with HuR siRNA or control siRNA was assessed by blocking transcription by addition of actinomycin D (ActD; final concentration: 2–5 μg/ml) and following the decay of Cx43 mRNA levels over time. RNA was isolated at various times following addition of ActD. Reverse transcription (Omniscript, Qiagen, Hilden, Germany) was followed by amplification of specific cDNAs using classical PCR procedures or Real-Time PCR with primer pairs listed in Table 1.
Western blotting, immunoprecipitation, immunocytochemistry
All immunochemical assays were described earlier 24. For Western blotting, cells were lysed in 0.5% (w/v) sodium dodecyl sulfate and protein concentrations determined in a bicinchoninic acid (BCA)-based protein assay (Pierce/Thermo Scientific, Bonn, Germany). Samples were applied to SDS-polyacrylamide gels of 10% (w/v) acrylamide, followed by electrophoresis, blotting and immunodetections using the following antibodies: rabbit polyclonal anti-Cx43(Sigma), mouse monoclonal anti-HuR (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti β-catenin (Abcam, Cambridge, UK), mouse monoclonal anti-GAPDH (Chemicon, Temecula, CA, USA) and horseradish peroxidase-coupled goat anti-mouse and goat anti-rabbit as secondary antibodies (Amersham Pharmacia Biotec).
For immunoprecipitations, cells were grown to 80–90% confluence on 10 cm dishes. Lysates prepared on ice in [10 mM Tris-HCl (pH 7.4), 5 mM EDTA, 2 mM DTT, 1 mM PMSF, 2 μg/ml leupeptin, 2μg/ml pepstatin, 140 mM NaCl, 1% (v/v) Triton X-100] were briefly centrifuged and supernatants taken for further analysis. Anti-Cx43 or β-catenin antibodies or non-specific rabbit IgG (BD Biosciences) were added to lysates (75 μg of total protein) and incubated at 4°C overnight. Immunocomplexes were collected using protein A or G-agarose (Roche); agarose beads were washed 5 times with 0.1% SDS/1% Triton-X in PBS. Precipitated proteins were then solubilised in SDS-PAGE buffer and analysed by SDS-PAGE and Western blotting.
Immunoprecipitation of RNA-protein complexes and analysis of coprecipitated RNA were performed as previously described 25,26.
Immunocytochemistry was performed as described 24 using the above-mentioned antibodies and Alexa 546- or Alexa 488-coupled secondary antibodies. Cells were embedded with ProLong Gold/DAPI mounting medium (Invitrogen), followed by fluorescence microscopic analysis with an AXIOVERT 200 M microscope (Zeiss, Oberkochen, Germany) or a confocal laser scanning microscope (LSM510 META, Zeiss).
Results
HuR binds to Cx43 mRNA and controls gap junctional communication
Analysis of the mRNA sequence of rat Cx43 (Genbank entry X06656) for the presence of AU-rich elements (ARE) revealed an AU-rich region in the 3′-untranslated region (3′-UTR). The presence of this sequence in Cx43 mRNA of WB-F344 cells was verified by RT-PCR, cloning and sequencing of a region of approx. 300 bp (boxed region in Figure 1A; data not shown). This AU-rich part of Cx43 mRNA contains several AREs (AU-rich elements), such as the AUUUA pentamer sequences and UUAUUUA(U/A)(U/A) nonamer regions, which generally confer altered stability 27,28. Increases in the half-lives of mRNAs carrying such AREs may be achieved by interaction with stabilising RNA-binding proteins such as HuR. To test for an interaction of Cx43 mRNA with HuR, HuR was immunoprecipitated from WB-F344 cell lysates, followed by extraction of coprecipitated RNA and analysis by RT-PCR. Primers specific for Cx43 yielded a positive signal, suggesting that Cx43 mRNA was bound to precipitated HuR (Figure 1B). Detection of p21waf1 mRNA served as a positive control of HuR/target mRNA interaction 18. In contrast, neither Cx43 mRNA nor p21 mRNA were detected in precipitates collected with an unspecific antibody (IgG lanes). Another control was the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA, an abundant housekeeping transcript which was amplified comparably in both the IgG and HuR samples (although slightly higher in HuR IP samples); the detection of GAPDH mRNA is expected in ribonucleoprotein/RNA coprecipitation assays, and it serves as a measure of nonspecific binding of any cellular RNA to beads or antibodies and further serves to monitor the evenness in sample input (Figure 1B).
Figure 1.
Modulation of GJIC by HuR. (A) Schematic representation of rat Cx43 mRNA and localization of AU-rich elements. (B) Binding of HuR to Cx43 mRNA. Immunoprecipitates from lysates of WB-F344 cells were prepared employing an anti-HuR antibody or a control IgG. Coprecipitated mRNAs were identified by RT-PCR. Data are representative of at least three independent experiments. (C) Analysis of GJIC by dye transfer analysis in WB-F344 cells 48 h after transfection of HuR-specific or control siRNA. Data are means ± SEM (n=3). Pictures of cultures with Lucifer Yellow microinjected into the central cell were taken after diffusion of Lucifer Yellow to neighboring cells had occurred. (D) WB-F344 cells were transfected with control or Cx43-specific siRNA, followed by determination of GJIC after 48 h. Data are means ± SD (n=3).
If HuR stabilized Cx43 mRNA, depletion of HuR would likely result in lower cellular levels of Cx43 and a loss in GJIC. In fact, cells depleted of HuR using an siRNA approach were signifiscantly less capable of GJIC, as intercellular spreading of microinjected fluorescent Lucifer Yellow was lowered by approximately 60% (Figure 1C). This loss of GJIC is attributed almost entirely to changes in activity of Cx43 rather than any other connexin: depletion of Cx43 by siRNA diminished GJIC to 7% of control (Figure 1D).
HuR depletion lowers Cx43 and Cx43 mRNA and reduces Cx43 mRNA stability
Depletion of HuR was reflected in a reduction in Cx43 protein levels, as seen in Western blots detecting at least three distinct bands of Cx43 that are known to correspond to nonphosphorylated Cx43 and to two different phosphorylation stages of Cx43. Real-time, quantitative (q)PCR analysis revealed a 50% decrease in Cx43 mRNA steady-state levels for cells depleted of HuR (Figures 2A, 2B). The half-life of Cx43 mRNA was also affected by depletion of HuR, changing from > 6 h in the Ctrl group to ~5 h in the HuR siRNA group (Figure 2C). The stability of a housekeeping transcript (GAPDH mRNA) was comparable between both Ctrl and HuR siRNA groups (Figure 2C). Thus, while GAPDH mRNA stability was unaltered by depletion of HuR, Cx43 mRNA stability was drastically lowered in the absence of HuR, as verified by Real-time qRT-PCR of mRNA levels remaining after addition of actinomycin D to cell cultures (Figure 2D). In summary, HuR stabilizes Cx43 mRNA: depletion of HuR lowered Cx43 mRNA steady-state levels and stability, diminished Cx43 protein levels, and reduced GJIC.
Figure 2.
Depletion of HuR decreases Cx43 levels and Cx43 mRNA stability. (A) WB-F344 cells were treated with HuR-specific or control siRNA for 48 h, followed by Real-time RT-PCR analysis of HuR and Cx43 mRNA levels. Data were normalized against 18S rRNA levels and control treatments were set to 1. Data are means of two independent experiments. (B) Western blot analysis of Cx43 levels in WB-F344 cells treated with control or HuR-specific siRNA. Depletion of HuR and loading of gels were controlled for by Western analysis of HuR and GAPDH levels. Data are means ± SEM (n=3). (C) WB-F344 cells were transfected with control or HuR-specific siRNA for 30 h, followed by exposure to actinomycin D for the indicated periods of time, lysis of cells and analysis of Cx43 mRNA levels by RT-PCR. Changes in mRNA levels were assessed by densitometric analysis of ethidium bromide-stained PCR product bands in agarose gels and normalized against GAPDH mRNA. Normalized Cx43 mRNA levels prior to addition of actinomycin D were set to 100%. Data are means of two to three independent experiments ± SEM. (D) Cells were treated with actinomycin D as in (C), followed by Real-time quantitative RT-PCR analysis of Cx43 and GAPDH mRNA levels in cells treated with control or HuR-specific siRNA. Data were normalized to 18S rRNA levels and are means of two independent experiments.
HuR depletion affects subcellular distribution of Cx43
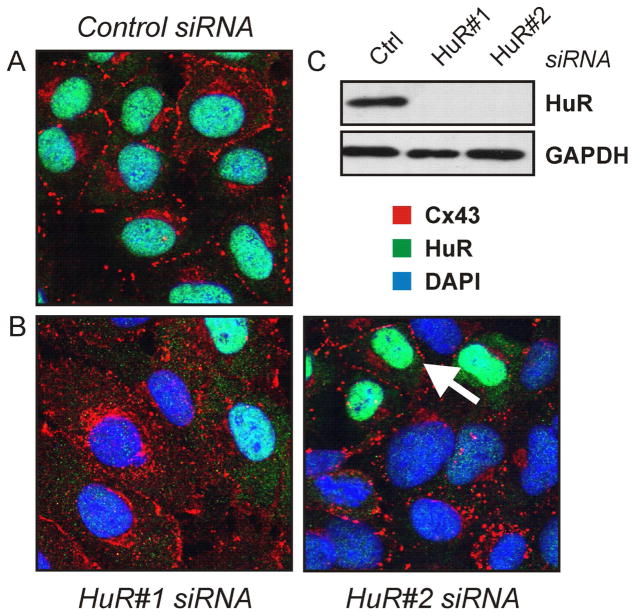
Immunocytochemical analyses revealed that, under control conditions, most of the cellular Cx43 (red) was detected as spots lined up at the plasma membrane (Figure 3A). On the contrary, HuR (green) was mostly nucleoplasmic, with a minor fraction detected in the cytoplasm, as reported previously 29. In cell cultures with silenced HuR (Figure 3B, 3C) cells with insufficient depletion were detected in the culture dishes; such regions were selected for display in Figure 3B, as the effect of HuR depletion on Cx43 subcellular distribution is most obvious in these areas. Depletion of HuR caused an extensive redistribution of Cx43 from the cell membrane to the cytoplasm, with aggregates found in the perinuclear region (Figure 3B). Two different siRNAs targeting different regions of the HuR mRNA (HuR#1 and HuR#2) were employed, resulting in a similar phenotype. In support of the hypothesis that depletion of HuR causes subcellular redistribution of Cx43, Cx43 is found in the plasma membrane in cells insufficiently deprived of HuR in cultures treated with HuR-specific siRNA (i.e., cells with green nuclei; see arrow in Figure 3B). We set out to study the molecular basis for Cx43 redistribution in HuR-silenced cells.
Figure 3.
Subcellular localization of connexin-43 in cells depleted of HuR. (A, B) WB-F344 cells were treated with control or one of two different HuR-specific siRNAs for 48 h, followed by immunocytochemical analysis by confocal microscopy of Cx43 (red) and HuR (green) localization. Nuclei were stained with DAPI (blue). The arrow indicates a cell with insufficiently depleted HuR displaying Cx43 localization similar to control cell conditions. (C) Western analysis of HuR levels in cells treated with control or HuR-specific siRNAs. GAPDH levels were analysed after treatment with control and HuR siRNA. Images are representative of at least three independent experiments
Depletion of HuR causes loss of β-catenin
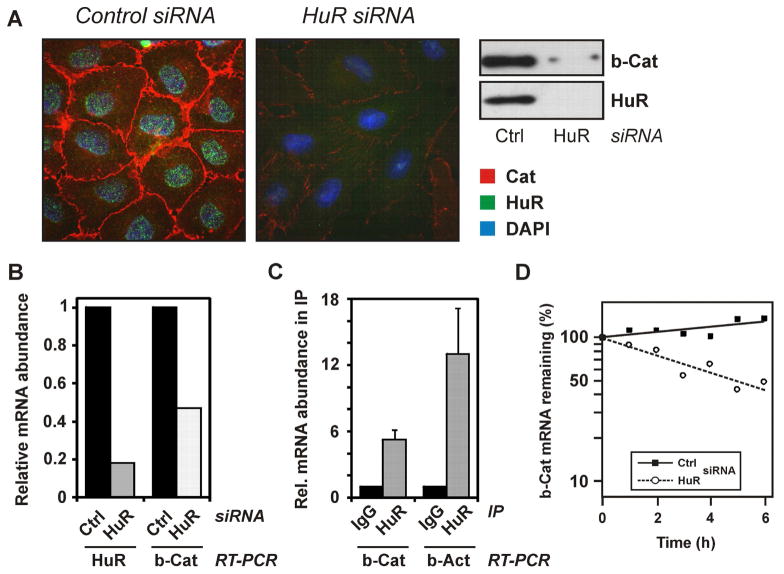
Cx43 is known to interact with adherens junction proteins, including β-catenin 30. In line with previous reports on HuR interacting with β-catenin mRNA and regulating its expression 16, β-catenin was found to be significantly lowered in cells treated with HuR siRNA (Figure 4A). Similarly, β-catenin mRNA levels were decreased in these cells (Figure 4B). Moreover, HuR was found to interact with β-catenin mRNA, as the transcript was detected in HuR immunoprecipitation samples, but not in immunoprecipitates with an unspecific IgG (Figure 4C). The Interaction of HuR with β-actin mRNA, a known HuR target, was tested as a positive control 31. Furthermore, the half-life of β-catenin mRNA was drastically lowered in rat liver epithelial cells depleted of HuR (Figure 4D).
Figure 4.
Loss of β-catenin after HuR depletion and binding of HuR to β-catenin mRNA. (A) WB-F344 cells were treated with control or HuR-specific siRNA for 48 h, followed by immunocytochemical and Western analysis of subcellular localization and levels of β-catenin (red) and HuR (green). Nuclei were stained with DAPI (blue). Data are representative of three independent experiments. (B) Real-Time RT-PCR analysis of HuR and β-catenin mRNA levels in cells treated with control or HuR-specific siRNA. Data were normalized against 18S rRNA levels and control treatments were set to 1. Data are means of two independent experiments. (C) Binding of HuR to β-catenin mRNA. Immunoprecipitates from lysates of WB-F344 cells were prepared employing an anti-HuR antibody or control IgG. Coprecipitated mRNAs were identified by real-time RT-PCR. RNA levels were normalized against succinate dehydrogenase mRNA. As a positive control, levels of coprecipitated β-actin mRNA were analysed. (D) Cells were transfected and exposed to actinomycin D as described in the legend to Figure 2C, followed by Real-time quantitative RT-PCR analysis of β-catenin mRNA levels in cells treated with control or HuR-specific siRNA. Data were normalized to 18S rRNA levels and are means of two independent experiments.
Lowering β-catenin levels recapitulates HuR depletion in reducing Cx43 levels and attenuating GJIC
We then tested whether a reduction in the interaction of β-catenin and Cx43, such as might occur after HuR silencing, could explain the redistribution of Cx43 and the loss of GJIC. In cells depleted of β-catenin, GJIC decreased by 59% (Figure 5A). Moreover, depletion of β-catenin coincided with a loss of Cx43 (Figure 5B) of approximately 54% (48h), similar to that observed after HuR depletion (Figure 1B). This loss was still detectable, albeit less pronounced, at later time points following siRNA treatment.
Figure 5.
Depletion of β-catenin decreases GJIC and expression of Cx43 in WB-F344 cells. (A) Cells were transfected with control or β-catenin-specific siRNA for 48 or 72 h, followed by determination of GJIC. Data are means ± SEM of three (48 h) or means of two (72 h) independent experiments. (B) Western analysis of β-catenin and connexin-43 levels in WB-F344 cells treated with β-catenin-specific siRNA for 48 or 72 h. Cx43 protein levels as determined by densitometric analyses were related to GAPDH levels. Cx43/GAPDH ratios are given as means ± SEM (n=3), with the 48 h control set to 100. (C) Physical interaction of β-catenin and Cx43 as demonstrated by co-immunprecipitation. Western blotting analysis of immunoprecipitates prepared from WB-F344 cell lysates (75 μg of total protein) employing anti-β-catenin or anti-Cx43 antibodies or a nonspecific rabbit IgG as negative control. Cell lysate (20 μg of protein) was analysed as input control.
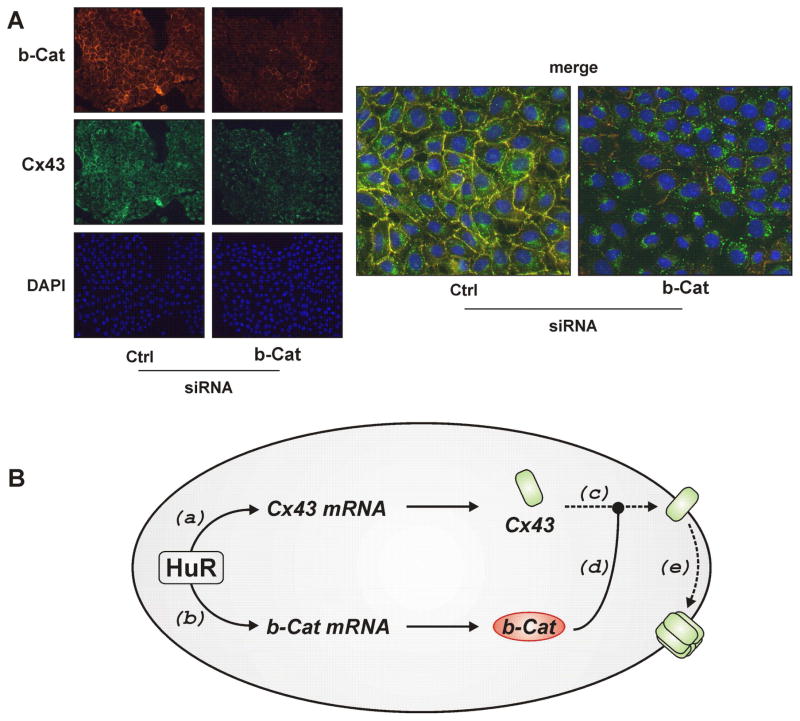
In coimmunoprecipitation experiments, a physical interaction of β-catenin and Cx43 was demonstrated to occur: β-catenin was detected in Cx43 immunoprecipitates and, vice versa, Cx43 detected in β-catenin immunoprecipitates, whereas no signals were detected in immunoprecipitates employing an unspecific antibody (Figure 5C). In support of an interaction between Cx43 and β-catenin, extensive colocalisation of these proteins was detected by immunocytochemistry (see merged pictures in Figure 6A).
Figure 6.
Depletion of β-catenin alters the subcellular distribution of Cx43. (A) WB-F344 cells were treated with control or β-catenin-specific siRNA for 48 h. Subcellular localization of β-catenin (red) and Cx43 (green) were analyzed by immunostaining and fluorescent microscopy. Nuclei were stained with DAPI (blue). Images are representative of results from three independent experiments. (B) Schematic summary of the suggested role of HuR in control of GJIC: HuR, by stabilizing Cx43 (a) and β-catenin mRNA (b) supports Cx43 and β-catenin protein accumulation. Cx43 transport to the cell membrane (c) is supported by β-catenin (d). Once in the membrane, Cx43 may contribute to formation of functional connexin hexamer hemichannels (e). See text for further details.
The subcellular redistribution and cytoplasmic accumulation of Cx43 that was induced by depletion of HuR (Figure 3) was also seen in cells depleted of β-catenin (Figure 6A). Both the extent of β-catenin/Cx43 colocalisation (merged pictures in Figure 6A) and the levels of both β-catenin and Cx43 (left panel in Figure 6A) were significantly lowered by depletion of β-catenin. In summary, the effects induced by HuR depletion, i.e. loss of GJIC, redistribution of Cx43 and downregulation of Cx43 levels, were mimicked by depletion of β-catenin, the expression of which is controlled by HuR and which interacts with Cx43 (see Figure 6B).
Role of HuR in the modulation of GJIC during exposure to doxorubicin or retinoic acid
As the abundance and activity of HuR are affected both by genotoxic agents and during differentiation 19,32, we set out to test for the consequences of HuR depletion for the modulation of GJIC by doxorubicin, a DNA intercalator and topoisomerase inhibitor 33, and by a stimulator of cellular differentiation processes, retinoic acid.
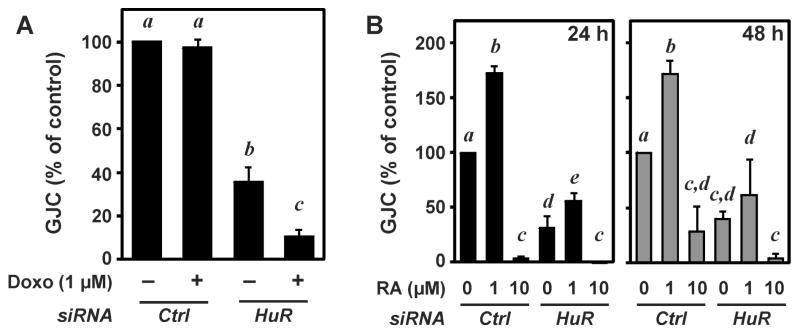
High concentrations of doxorubicin (> 25 μM) cause a loss of GJIC in WB-F344 cells 12. In line with these observations, lower doses of doxorubicin (1 μM) did not affect GJIC, even after 24 h of exposure (Figure 7A). These same conditions of doxorubicin treatment, however, caused a highly significant loss in GJIC in cells depleted of HuR in addition to the loss elicited by HuR depletion per se (Figure 7A). These findings imply that HuR stabilizes Cx43-dependent GJIC and protects against doxorubicin-induced loss of GJIC.
Figure 7.
Doxorubicin and retinoic acid modulate GJIC: role of HuR. WB-F344 cells were transfected with control or HuR-specific siRNA, followed by exposure to doxorubicin (A; 1 μM for 24 h) or all-trans-retinoic acid (B; RA, 1 or 10 μM for 24 and 48 h) and determination of GJIC. Data are given as means ± SD (n=3). ANOVA with Student-Newman-Keuls post-test was used for the determination of statistical significance between treatment groups. P < 0.05 was selected as the level of significance. Treatment groups are significantly different from each other if no labeling letter (a to e) is shared between groups.
GJIC was previously described to be modulated by exposure of cells to all-trans retinoic acid (RA) 34,35. Here, a biphasic response to RA was observed in WB-F344 cells, with lower concentrations (1 μM) enhancing and higher concentrations (10 μM) strongly attenuating GJIC after exposure for 24 or 48 h (Figure 7B). In the absence of HuR, RA (1 μM)-induced elevation of GJIC is less pronounced and no longer significant after 48 h of incubation with RA. Again, HuR appears to support GJIC by promoting low dose-RA-induced elevation of GJIC.
Discussion
HuR is a novel modulator of GJIC
HuR was reported to stabilize mRNAs encoding crucial regulators of cellular proliferation, differentiation and stress response, such as p21waf, p53, cyclins, and SIRT1 18,19. A strong correlation between the abundance of HuR and cancer has been established and the posttranscriptional modulatory effects of HuR on the expression of a variety of target genes were suggested to affect carcinogenesis 36.
The present study documents a role for HuR in controlling GJIC. GJIC has been recognized as a modulator of carcinogenesis. Trosko et al. 1 indicated that this may occur at different levels: GJIC is low or even absent in many tumor cells, and deficiency in certain connexins renders cells prone to carcinogenic changes. For example, various genotoxic agents such as ultraviolet radiation and oxidative stress 11,37 or tumor promoters and carcinogens decrease GJIC, either by suppressing connexin expression, by impairing intracellular connexin trafficking, or by inducing posttranslational modifications such as phosphorylation, causing a decreased gap junction channel conductance (for review, see 38).
Although Cx43 is the major connexin in WB-F344 cells, these cells also express Cx26 and, depending on culture conditions and degree of differentiation, Cx32 39,40. The changes in GJIC observed in the present study were almost entirely changes in Cx43-dependent GJIC, as siRNA-based knockdown of Cx43 lowered GJIC in WB-F344 cells to approx. 7% of control (Figure 1D).
Interestingly, it is HuR depletion, rather than overexpression, that causes a loss in GJIC (Figure 1C). While this may seem contradictory in view of the aforementioned hypothesized procarcinogenic roles of abundant HuR and of an impaired GJIC, it should be noted that the general statement that HuR levels are enhanced in tumor versus normal tissue does not apply to all tissues analyzed so far; for example, cytoplasmic HuR levels in human hepatocellular carcinoma samples tended to be lower than in non-tumor (cirrhotic) samples 16. Moreover, the effects observed after depletion of HuR by siRNA not necessarily require the absence of HuR protein but may also be due to the absence of HuR activity. Modulation of HuR activity may be achieved by posttranslational modification, most prominently phosphorylation. While phosphorylation of HuR by the cell cycle regulating kinase Cdk1 41 results in a predominantly nuclear localization, phosphorylation by PKC isoforms coincided with its translocation to the cytoplasm 32. Furthermore, checkpoint kinase Chk2-dependent phosphorylation in the RNA binding regions of HuR causes a loss of interaction between HuR and SIRT1 mRNA 26. Interestingly, while Chk2 is generally regarded a tumor suppressor, the latter mechanism would provide a potential link between an initiation event, i.e. DNA damage, and a loss of GJIC: DNA damage-induced stimulation of Chk2-dependent HuR phosphorylation reduces interaction with target mRNA, resulting in a decreased GJIC. Such a response is indeed observed in the present study, provided HuR levels are well below basal levels: in cells largely depleted of HuR by siRNA, doxorubicin causes a loss of GJIC (Figure 7A). A reason for this effect being observed only under conditions of HuR deficiency could be that it is a route of minor importance that would normally be overruled by abundant HuR. Whether or not this effect indeed relies on residual HuR surviving siRNA-based depletion of HuR (see Figure 3B) and on DNA damage-induced modulation of HuR activity remains to be established.
Modulation of GJIC by HuR via Cx43/β-catenin interaction
In addition to HuR directly controlling Cx43 levels by stabilizing Cx43 mRNA, Cx43 levels also appear to be controlled by HuR in an indirect fashion, via β-catenin. β-Catenin contributes to cell-cell adhesion by interacting with cadherins and by establishing physical interaction of adhesion proteins with the actin cytoskeleton. Intact cell adhesion complexes appear to be required for proper assembly of connexins to form functional gap junctional intercellular channels 42,43. The physical interaction of β-catenin and Cx43 was demonstrated in this work (Figure 5C) and elsewhere 44. Hence, depletion of β-catenin is likely to result in a loss of GJIC. In addition to β-catenin controlling Cx43 expression levels 44 and proper positioning of Cx43 hemichannels in the plasma membrane, β-catenin was recently demonstrated to also govern transition of Cx43 from the microtubular transport machinery to the plasma membrane 30: hence, accumulation of Cx43 in the cytoplasm under conditions of depletion of HuR (Figure 3) or β-catenin (Figure 6) most likely reflects an insufficient transport of Cx43 to the plasma membrane rather than internalization of improperly positioned Cx43.
Connexins and β-catenin in oval cells
Oval cells are liver progenitor cells activated during liver regeneration stimulated by liver injury 21. β-Catenin expression is enhanced following stimulation of oval cell differentiation 45. Similarly, connexin levels are altered during differentiation to hepatocytes: while Cx43 is the major connexin in hepatic stellate or oval cells, hepatocytes express Cx32 at high levels and carry almost no Cx43 5. It is presently unknown if HuR levels and/or function is affected by oval cell activation and whether HuR affects the differentiation of oval cells. However, our present study suggests that changes in GJIC that are known to occur during differentiation and to affect its progress are affected by HuR levels: retinoic acid-induced changes in GJIC were modulated by HuR depletion (Figure 7B).
Conclusions
It is demonstrated here for the first time that the RNA-binding protein HuR controls GJIC by two molecular mechanisms. First, HuR interacts with Cx43 mRNA, enhances its stability and elevates Cx43 protein levels. Second, HuR interacts with and stabilizes β-catenin mRNA, enhancing β-catenin abundance; β-catenin, in turn, helps to maintain Cx43 levels to localize Cx43 properly on the plasma membrane, thereby ensuring the integrity of GJIC. These data are summarized in Figure 6B. Our results provide a novel link between HuR, Cx43, β-catenin as well as GJIC and may set a basis for further understanding of changes in GJIC in differentiating liver cells or during hepatocarcinogenesis.
Acknowledgments
We thank Elisabeth Sauerbier for excellent technical assistance and Professors Andre Menke and Klaudia Giehl, University of Ulm, Germany, for helpful discussions.
Financial support
This study was supported by Deutsche Forschungsgemeinschaft (DFG, Bonn, Germany), SFB 575/B4. S.G, K.A, and M.G. were supported by the NIA-IRP, NIH. H.S. is a Fellow of the National Foundation for Cancer Research, Bethesda, MD, USA.
List of abbreviations
- GJIC
gap junctional intercellular communication
- Cx
connexin
- RT-PCR
reverse transcription polymerase chain reaction
- ActD
actinomycin D
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- SDHA
succinate dehydrogenase, subunit A
- ARE
AU-rich element
- IP
immunoprecipitation
- RBP
RNA-binding protein
References
- 1.Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci. 1998;3:d208–d236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 2.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 3.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, et al. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36:631–640. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 5.Fischer R, Reinehr R, Lu TP, Schonicke A, Warskulat U, Dienes HP, et al. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology. 2005;128:433–448. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 6.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, et al. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 7.Kordes C, Sawitza I, Müller-Marbach A, Ale-Agha N, Keitel V, Klonowski-Stumpe H, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelmohsen K, Sauerbier E, Ale-Agha N, Beier J, Walter P, Galban S, et al. Epidermal growth factor- and stress-induced loss of gap junctional communication is mediated by ERK-1/ERK-2 but not ERK-5 in rat liver epithelial cells. Biochem Biophys Res Commun. 2007;364:313–317. doi: 10.1016/j.bbrc.2007.09.132. [DOI] [PubMed] [Google Scholar]
- 11.Upham BL, Kang KS, Cho HY, Trosko JE. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis. 1997;18:37–42. doi: 10.1093/carcin/18.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Abdelmohsen K, von Montfort C, Stuhlmann D, Gerber PA, Decking UK, Sies H, et al. Doxorubicin induces EGF receptor-dependent downregulation of gap junctional intercellular communication in rat liver epithelial cells. Biol Chem. 2005;386:217–223. doi: 10.1515/BC.2005.027. [DOI] [PubMed] [Google Scholar]
- 13.Melchheier I, von Montfort C, Stuhlmann D, Sies H, Klotz LO. Quinone-induced Cdc25A inhibition causes ERK-dependent connexin phosphorylation. Biochem Biophys Res Commun. 2005;327:1016–1023. doi: 10.1016/j.bbrc.2004.12.107. [DOI] [PubMed] [Google Scholar]
- 14.Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005;1719:6–23. doi: 10.1016/j.bbamem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Clairmont A, Sies H. Evidence for a posttranscriptional effect of retinoic acid on connexin43 gene expression via the 3′-untranslated region. FEBS Lett. 1997;419:268–270. doi: 10.1016/s0014-5793(97)01468-3. [DOI] [PubMed] [Google Scholar]
- 16.Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, et al. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22:7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 17.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, et al. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol. 2000;20:760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem. 2008;389:243–255. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993;142:1373–1382. [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen BE. Hepatic “stem” cells: coming full circle. Blood Cells Mol Dis. 2001;27:590–600. doi: 10.1006/bcmd.2001.0422. [DOI] [PubMed] [Google Scholar]
- 22.Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 23.Klotz LO, Patak P, Ale-Agha N, Buchczyk DP, Abdelmohsen K, Gerber PA, et al. 2-Methyl-1,4-naphthoquinone, vitamin K(3), decreases gap-junctional intercellular communication via activation of the epidermal growth factor receptor/extracellular signal-regulated kinase cascade. Cancer Res. 2002;62:4922–4928. [PubMed] [Google Scholar]
- 24.Abdelmohsen K, Patak P, von Montfort C, Melchheier I, Sies H, Klotz LO. Signaling effects of menadione: from tyrosine phosphatase inactivation to connexin phosphorylation. Methods Enzymol. 2004;378:258–272. doi: 10.1016/S0076-6879(04)78020-9. [DOI] [PubMed] [Google Scholar]
- 25.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagnado CA, Brown CY, Goodall GJ. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A) Mol Cell Biol. 1994;14:7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubiaga AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol Cell Biol. 1995;15:2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dormoy-Raclet V, Menard I, Clair E, Kurban G, Mazroui R, Di MS, et al. The RNA-binding protein HuR promotes cell migration and cell invasion by stabilizing the beta-actin mRNA in a U-rich-element-dependent manner. Mol Cell Biol. 2007;27:5365–5380. doi: 10.1128/MCB.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 34.Rivedal E, Sanner T. Regulation of gap junctional communication in Syrian hamster embryo cells by retinoic acid and 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1992;13:199–203. doi: 10.1093/carcin/13.2.199. [DOI] [PubMed] [Google Scholar]
- 35.Ara C, Massimi M, Devirgiliis CL. Retinoic acid modulates gap junctional intercellular communication in hepatocytes and hepatoma cells. Cell Mol Life Sci. 2002;59:1758–1765. doi: 10.1007/PL00012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. 2005;2:11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- 37.Upham BL, Trosko JE. Oxidative-Dependent Integration of Signal Transduction with Intercellular Gap Junctional Communication in the Control of Gene Expression. Antioxid Redox Signal. 2009;11:297–307. doi: 10.1089/ars.2008.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 39.Matesic DF, Rupp HL, Bonney WJ, Ruch RJ, Trosko JE. Changes in gap-junction permeability, phosphorylation, and number mediated by phorbol ester and non-phorbol-ester tumor promoters in rat liver epithelial cells. Mol Carcinog. 1994;10:226–236. doi: 10.1002/mc.2940100407. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg E, Faris RA, Spray DC, Monfils B, Abreu S, Danishefsky I, et al. Correlation of expression of connexin mRNA isoforms with degree of cellular differentiation. Cell Adhes Commun. 1996;4:223–235. doi: 10.3109/15419069609010768. [DOI] [PubMed] [Google Scholar]
- 41.Kim HH, Abdelmohsen K, Lal A, Pullmann R, Jr, Yang X, Galban S, et al. Nuclear HuR accumulation through phosphorylation by Cdk1. Genes Dev. 2008;22:1804–1815. doi: 10.1101/gad.1645808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jongen WM, Fitzgerald DJ, Asamoto M, Piccoli C, Slaga TJ, Gros D, et al. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J Cell Biol. 1991;114:545–555. doi: 10.1083/jcb.114.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 44.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]