Abstract
Porous Si is a nanostructured material that is of interest for molecular and cell-based biosensing, drug delivery, and tissue engineering applications. Surface chemistry is an important factor determining the stability of porous Si in aqueous media, its affinity for various biomolecular species, and its compatibility with tissues. In this study, the attachment and viability of a primary cell type to porous Si samples containing various surface chemistries is reported, and the ability of the porous Si films to retain their optical reflectivity properties relevant to molecular biosensing is assessed. Four chemical species grafted to the porous Si surface are studied: silicon oxide (via ozone oxidation), dodecyl (via hydrosilylation with dodecene), undecanoic acid (via hydrosilylation with undecylenic acid), and oligo(ethylene) glycol (via hydrosilylation with undecylenic acid followed by an oligo(ethylene) glycol coupling reaction). Fourier Transform Infrared (FTIR) spectroscopy and contact angle measurements are used to characterize the surface. Adhesion and short-term viability of primary rat hepatocytes on these surfaces, with and without pre-adsorption of collagen type I, are assessed using vital dyes (calcein-AM and ethidium homodimer I). Cell viability on undecanoic acid-terminated porous Si, oxide-terminated porous Si, and oxide-terminated flat (non-porous) Si are monitored by quantification of albumin production over the course of 8 days. The stability of porous Si thin films after 8 days in cell culture is probed by measuring the optical interferometric reflectance spectra. Results show that hepatocytes adhere better to surfaces coated with collagen, and that chemical modification does not exert a deleterious effect on primary rat hepatocytes. The hydrosilylation chemistry greatly improves the stability of porous Si in contact with cultured primary cells while allowing cell coverage levels comparable to standard culture preparations on tissue culture polystyrene.
1. Introduction
Silicon, in both bulk crystalline and nanostructured forms, has emerged as an interesting platform for tissue engineering [1-3], cell culture [4], and for interfacing cells with electronic devices [5, 6]. The porous form of Si shows significantly improved mammalian cell adhesion and viability [7, 8], and improved implant stability in whole organisms [9] in comparison to flat crystalline Si. The ability to tune both nanostructure and surface chemistry of electrochemically prepared porous Si provides a means to adjust these parameters for successful integration with cells in culture or within the body. Indeed much research is underway to take advantage of the tunable porous nature of the material for controlled drug release [10-13], and the material is being assessed in clinical studies [14].
When porous Si is exposed to physiological conditions or cell growth media [15, 16] the native Si hydride surface rapidly oxidizes and subsequently degrades to the aqueous forms of silicic acid. Silicic acid is the soluble, bioavailable form of Si that is essential for normal bone development [17, 18], however silicic acid can be toxic at high doses [19]. In our previous investigation of cell compatibility with porous Si [20], we used primary rat hepatocytes as a probe for cytotoxicity of the material due to their importance in pharmacological and toxicological studies and as an example of a primary cell type sensitive to culture conditions [21]. We showed that despite surface degradation of ozone-oxidized porous Si, hepatocytes maintained similar viability and function compared to hepatocytes cultured on tissue culture polystyrene (TCPS) [20].
Studies of cell compatibility with porous Si are relevant for in vivo and in vitro applications. Specifically, the ability of this material to detect chemicals [22, 23], biomolecules [24, 25], enzymatic activity [26], and cells [27-29] presents the possibility that porous Si may play a role in in vitro sensing or in vivo diagnostic devices in which the material is in direct contact with live cells. Recently it was demonstrated that porous Si can be used to report loss of viability of hepatocytes in advance of traditional biochemical assays [28]. In this particular set of experiments, the surface of porous Si was protected from degradation by sealing the pores with polystyrene. However, many potential biomedical applications require the pore voids to be accessible and stable. In such cases the inner walls of the porous matrix must be protected from degradation in the aqueous cell culture environment without eliciting any undesirable effects on the cells.
In a prior study on porous Si biocompatibility, immortalized cell lines were cultured on porous Si samples that had been amine-terminated by silanization with 3-aminopropyl trimethoxysilane [30]. This modification provided significantly improved stability and greater cell adhesion in comparison to oxidized porous Si. However, surface silicon species formed during silanization remain susceptible to nucleophilic and hydrolytic attack in aqueous environments due to the electron withdrawing power of the pendant oxygen atoms. In contrast, alkylation of the silicon surface via Si-C bonds results in a kinetically stable bond that has greatly reduced rates of degradation in aqueous environments [31, 32] and can withstand boiling in chloroform, water, acid, base, and fluoride solutions [33].
In this work, thermal hydrosilylation is used to graft chemical species via surface Si-C bonds to generate a stable substrate for culturing primary rat hepatocytes. We attach three chemical species for cell adhesion and viability studies: dodecene, undecylenic acid, and oligo(ethylene) glycol. For comparison, cell adhesion and differentiated function was also assessed on ozone-oxidized porous Si, flat Si, and standard tissue culture polystyrene. Viability was assessed using vital dyes, and albumin production was monitored over the course of a week as a sensitive measure of liver-specific function over time. Cell adhesion and short-term viability were studied with and without a collagen layer adsorbed to the surface, because collagen I has been shown to play an important role in hepatocyte adhesion [34] and is a major structural component in many tissues. The goal of this work is to identify classes of chemical modifications to porous Si that can be used for in vitro and in vivo studies in which long-term surface stability is achieved and cell viability is maintained.
2. Materials and Methods
Porous Silicon Formation.
Porous Si samples used in cell culture were prepared from p-type silicon (boron doped, 7 Ω-cm resistivity, <100> orientation) by electrochemical etch in a Teflon etch cell employing a 2-electrode con figuration. A Pt mesh electrode functioned as the counter electrode. Current density of 15 mA/cm2 was applied for 5 min in an electrolyte consisting of 1:1 v/v solution of 100% ethanol (Pharmco-AAPER, Brookfield, CT) and aqueous hydrofluoric acid (48%, EMD Chemicals Inc., Gibbstown, NJ).
Chemical Modification of Porous Silicon
1-dodecene (Sigma-Aldrich, St. Louis, MO) and undecylenic acid (Sigma-Aldrich, St. Louis, MO) modified surfaces were prepared by thermal hydrosilylation of freshly-prepared porous Si. Employing standard Schlenk and syringe inert atmosphere handling methods [35], samples were submerged in neat alkene and degassed with 3 freeze-pump-thaw cycles prior to heating at 120 °C for 2 hours in a N2(g) environment. Samples were then rinsed with ethanol, dried, and stored under vacuum until use. Samples modified with amino-dPEG4-t-butyl ester (Quanta Biodesign, Ltd., Powell, OH) were prepared using a technique similar to a previously reported procedure [36]. Briefly, the surface was first modified with undecylenic acid (Sigma-Aldrich, St. Louis, MO), then coupled to amino-dPEG4-t-butyl ester (25 μL, Quanta BioDesign) in 10mM N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDAC, Sigma-Aldrich, St. Louis, MO) in ethanol for two hours at room temperature. Oxidized porous Si samples were prepared by exposing freshly etched porous Si to ozone from a Trio3 Ozone Systems T-12 ozone generator for 15 min.
Characterization by FTIR and Contact Angle Measurements
Chemically modified porous Si samples were characterized by Fourier-transform infrared (FTIR) spectroscopy using a Nicolet-Magna 550 spectrometer in transmission mode. Sessile drop contact angle measurements were collected on a minimum of two samples per surface chemistry using a sample stage, commercial digital camera, and Adobe Photoshop for analysis. Contact angles from three separate deionized water droplets with a volume of 5 μL were measured on both sides of the droplet and averaged for each chip. Contact angle measurements were also performed on freshly etched porous Si samples for comparison.
Hepatocyte Isolation and Culture
Hepatocytes were isolated from 2-3 month old adult female Lewis rats (Charles River Laboratories) by collagenase perfusion as previously described [37]. Less than one hour after isolation, 1.5x106 cells were seeded per Petri dish in media. Cells were cultured in Dulbecco’s modified eagle medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 0.5 U/mL insulin, 7 ng/mL glucogon, 20 ng/mL epidermal growth factor, 7.5 μg/mL hydrocortisone, 200 U/mL penicillin, 200 μg/mL streptomycin. During the experiments cells were incubated at 37 °C in air containing 5% CO2.
Cell Culture on Porous Si and Polystyrene Petri Dishes
Modified porous Si samples were sterilized with 70% ethanol for 1 hour and rinsed twice with sterile water prior to collagen adsorption and cell seeding. Type I collagen, isolated from rat tails [37], was adsorbed on samples of modified porous Si or the tissue culture polystyrene (TCPS) control by incubation in 2 mL of 0.1 mg/mL type I collagen for 1 hr. at 37 °C, followed by rinsing with sterile water and cell media. Primary rat hepatocytes were seeded on the sample surfaces in fresh media and incubated at 37 °C, in air containing 5% CO2 with shaking every 15 min. After 90 min, samples were rinsed with media to remove any unattached cells and then placed in new cell culture dishes containing 2 mL of fresh media with serum for overnight incubation.
Cell Attachment and Viability Assays
Cell attachment and viability on the chemically modified porous Si surfaces were studied using a different hepatocyte isolate on three separate days. Cells were seeded on porous Si or TCPS control with and without collagen pre-adsorbed to the surface. Hepatocyte attachment and viability were assessed after 24 h using the vital dyes calcein acetoxymethyl (calcein-AM) and ethidium homodimer-1 (EthD-1), both from Molecular Probes, Inc. The dyes were reconstituted as 1 mg/mL solutions in anhydrous DMSO. Samples with adhered cells were incubated with calcein-AM and EthD-1 for 30 min at a final concentration of 2.5 μg/mL. The samples were then washed with fresh DMEM, inverted onto coverslips and examined by fluorescence microscopy. Cells were observed and recorded with an inverted epifluorescence microscope (Nikon TE200) and attached camera (CoolSnap HQ). Images were analyzed using the Metamorph Image Analysis System (Universal Imaging, Westchester, PA) and data were normalized to the cell coverage on a tissue culture polystyrene standard (collagen pre-adsorbed), that was seeded with the same hepatocyte isolate. Normalized data obtained on 3 separate days were averaged. Coverage was taken to be total cells adhered to the chip after 24 hrs.
Functional Analysis of Hepatocytes on Flat and Chemically Modified Porous Si Substrates
5 or 6 samples each of undecanoic acid-terminated porous Si, ozone-oxidized porous Si, and hydrophilic non-porous Si were sterilized, adsorbed with collagen, and seeded with primary rat hepatocytes. After 24 h the media was removed, a collagen gel overlay was applied to maintain cell function [37], and cells were re-incubated in media. The collagen gel overlay consisted of a 1mg/mL collagen solution (in DMEM) that was applied for 1 h at 37°C for gelation to occur. Cell media was collected and changed daily for 7 days beginning 48 h after initial cell seeding. Media samples collected for albumin content analysis were stored at −80 °C. Albumin concentrations were measured using enzyme linked immunosorbent assays (ELISA) as previously described [38]. Antibodies were purchased from ICN/Cappel Laboratories (Cockranville, PA, USA). Albumin analyses were performed in triplicate from each sample on each day. The results were averaged and a cumulative sample average was obtained for each surface type studied. Total cells on each surface were calculated from the coverage and viability averages measured at 24 hours. Separate coverage experiments and calculations were performed for crystalline Si samples. The data were then normalized to 106 cells to give units of μg/mL/day/106 cells.
Porous Si Surface Stability
Optical reflectance spectra were obtained on chemically modified porous Si and porous Si samples that had been used for the long-term hepatocyte culture viability studies. After termination of the long-term experiment, the samples were carefully rinsed with cell media to remove the collagen gel layer and hepatocytes and were further rinsed with water and air dried. A bifurcated optical cable was used to direct white light from a tungsten lamp source to the porous Si chip at normal incidence [28]. Reflected light was collected and transmitted through the other arm of the bifurcated fiber optic cable to a CCD spectrometer (Ocean Optics).
Statistics and Data Analysis
Coverage experiments and short-term viability experiments were performed on 3 separate days with a different cell isolate. Long term viability experiments were performed on 5 or 6 samples for the 3 surface chemistries tested. Error bars represent the standard error of the mean. Statistical significance was determined using one-way ANOVA (analysis of variance) on Microsoft EXCEL and Tukey’s post-test analysis with p < 0.05.
3. Results and Discussion
Porous Si etching and characterization
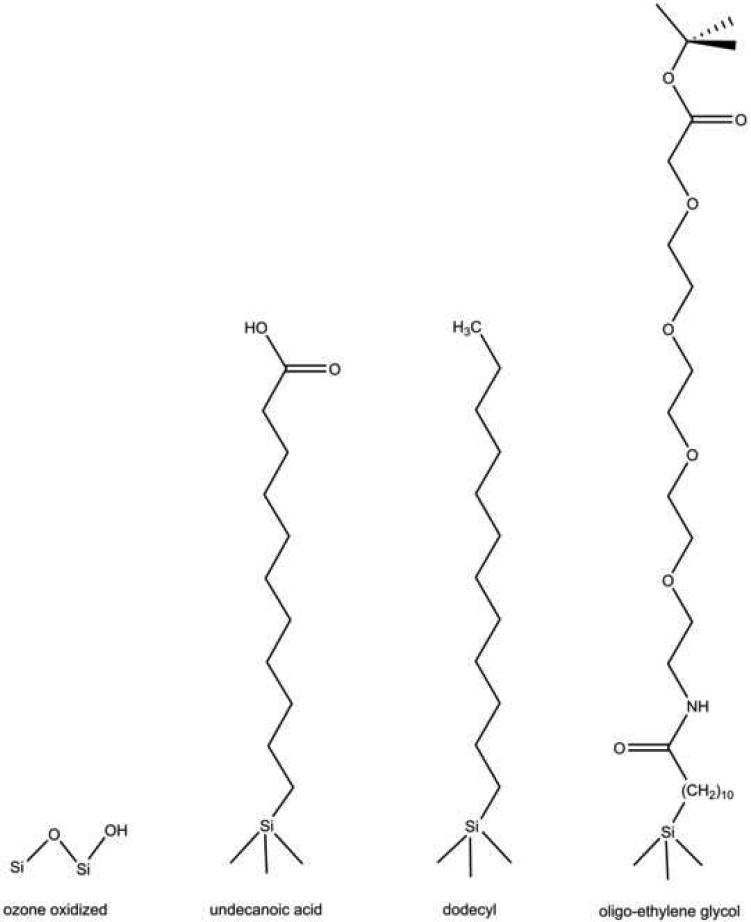
The four types of chemistries studied in this work are shown in Fig. 1. The first type shown is generated by room-temperature ozone oxidation. Ozone oxidation transforms the hydrophobic porous Si surface to a hydrophilic surface, possessing a combination of Si-OH and Si-O-Si surface bonds. Thermal hydrosilylation grafts organic species to the surface; the surface affinity is then determined by the specific functional groups on the organic molecule. This technique allows a comparison between surfaces containing a saturated hydrocarbon and carboxylic acid functionality. In addition, it has been shown that the terminal carboxylic acid group of a grafted undecanoic acid species can be used to further modify the porous Si surface [36]. Here we used a standard coupling reaction to incorporate a tert-butyl ester-oligo(ethylene) glycol unit onto the porous Si surface. The 4-subunit oligo(ethylene) glycol (OEG)-modified surface provides a versatile, non-adsorbing surface that is also relevant for cell patterning studies. Furthermore, the terminal tert-butyl ester group on the OEG unit can be used for additional covalent attachment of a molecule of interest, such as an antibody. The four products of the chemical reactions were characterized by FTIR (Fig. 2) and sessile contact angle measurements (Table 1).
Figure 1.
Chemical structures of modified porous silicon surfaces used in the study of primary rat hepatocyte cell adhesion and viability. Ozone oxidation results in a combination of Si-O-Si bonds and surface Si-OH species. Hydrosilylation with undecylenic acid results in an undecanoic acid-terminated surface. Hydrosilylation with dodecene results in a dodecyl-terminated surface. Hydrosilylation with undecylenic acid, followed by bionconjugate coupling chemistry, results in a four-subunit oligo(ethylene) glycol-terminated porous Si surface.
Figure 2.
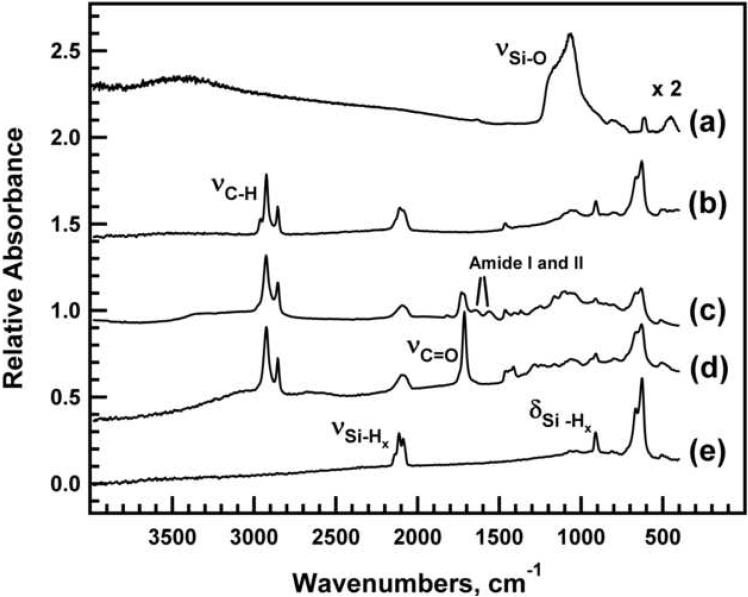
Fourier transform infrared (FTIR) spectra of freshly etched porous Si and the chemically modified porous Si samples used in this study. (a) ozone-oxidized porous Si. (b) dodecyl-terminated porous Si. (c) Oligo(ethylene) glycol-modified porous Si. (d) Undecanoic acid-terminated porous Si. (e) freshly etched porous Si. Spectra are offset along the y-axis for clarity.
Table 1.
Sessile drop contact angles for freshly etched and chemically derivatized porous Si
| Surface preparation | contact angle, degrees |
|---|---|
| Freshly etched, Si-H | 111 ± 1 |
| Dodecene, Si-(CH2)11CH3 | 115 ± 5 |
| Ozone oxidized, Si-O-Si and Si-OH | < 6 |
| Undecylenic acid, Si-(CH2)10COOH | 60 ± 1 |
| Oligoethylene glycol, Si-(CH2)10CONH(CH2CH2O)4-CH2COO-C(CH3)3 | 67 ± 2 |
The FTIR spectrum of a freshly etched porous Si sample (Fig. 2e), indicates the presence of surface hydrides, with bands assigned to Si-Hx stretching modes at 2137 cm-1 (ν ), 2114 cm-1 Si-H3 (ν ), and 2087 cm-1 Si-H2 (νSi-H). Bands characteristic of Si-H deformations are also apparent at 910 cm-1. The dodecyl-terminated porous Si sample (Fig. 2b) displays bands assigned to asymmetric and symmetric νCH2 stretching vibrations at 2924 cm-1 and 2855 cm-1, respectively. An absorption band due to C-H stretching vibrations of the terminal CH group is also apparent at 2959 cm-1 3 . The undecanoic acid-terminated surface displays similar νCH2 stretching bands (Fig. 2d). Additionally, there is a strong band associated with the carboxylic acid νC=O stretching vibration at 1710 cm-1. Attachment of the OEG molecule (Fig. 2c) to the undecanoic acid-terminated surface is verified by bands assigned to νC=O stretching of the ester (1728 cm-1) and the appearance of amide I (1643 cm-1) and amide II (1559 cm-1) absorptions resulting from the peptide bond formed between the EDAC-activated carboxylic acid and the amine-terminated OEG reagent. A shoulder is apparent on the νC=O stretching of the ester indicating unreacted undecanoic acid remains on the surface. Ozone-oxidized porous Si (Fig. 2a) shows a strong vibrational band assigned to asymmetric Si-O-Si stretching in the region 1200-1000 cm-1.
Stretching and deformation bands associated with the Si-H bonds are still present after hydrosilylation, indicating that the procedure does not replace all of the surface hydrides. Incomplete modification is the result of steric hindrance [39]. However, the chemistry is still effective at influencing bulk hydrophobic or hydrophilic character as indicated by the measured contact angles, Table 1.
The order of increasing hydrophobicity of the examined surfaces is ozone-oxidized < undecanoic acid < OEG < dodecyl. Silicon etched in ethanolic hydrofluoric acid is terminated with surface hydrides and results in a hydrophobic surface. Porous Si hydrosilylated with dodecene retains its hydrophobicity, although its stability in aqueous media is significantly improved relative to the freshly etched surface due to the kinetic stability of the Si-C bond. Reacting freshly etched porous Si with ozone produces surface oxides and hydroxides; the surface is correspondingly very hydrophilic. Hydrosilylation with undecylenic acid, an 11 carbon aliphatic containing a terminal carboxylic acid group, generates a moderately hydrophilic surface. The hydrophilic nature is retained if this surface is further modified with a 4-subunit oligo(ethylene) glycol molecule (OEG) containing a terminal tertiary butyl ester group.
The addition of the 4-subunit OEG molecule with the tert-butyl end group results in a slight increase in contact angle, by ~ 7°, compared to the acid-terminated surface. The increase in hydrophobicity is ascribed to the replacement of the terminal carboxylic acid group with a terminal tertiary butyl ester moiety. FTIR results show remaining carboxylic acid groups on the surface, therefore the wettability of the surface is likely influenced by a combination of the undecanoic acid termination and the t-butyl ester OEG termination. The contact angle measured for the 4-subunit OEG surface, 67°, is only slightly larger than values measured for 3-subunit, methoxy-terminated OEG layers formed on flat surfaces, which exhibit contact angles between 57-65° [40-42]. The nanotexture of the porous Si films may contribute to the observed contact angles; surfaces with high aspect ratio features such as those exhibited by porous Si have been found to display superhydrophobic properties [43, 44].
The tert-butyl ester-oligo(ethylene glycol)-modified surface is expected to reduce nonspecific binding of cells by minimizing adsorption of the applied collagen and serum proteins present in the cell media, both of which are important in mediating cell adhesion and viability in vivo and in vitro. Studies have shown that surfaces presenting OEGs with terminal methyl groups are effective at reducing nonspecific binding of proteins [45, 46]. A recent study on OEG-modified porous Si showed that hydrophobic and hydrophilic ethylene glycol termination both display comparable inhibition of protein adsorption [47].
Cell Adhesion
Cell survival, differentiation, and response to their surroundings are mediated by interactions between cell surface integrins and extracellular matrix proteins present in serum. In the absence of these interactions, most non-transformed cells will undergo apoptosis [48]. Collagen type I is an important extracellular matrix protein in the liver, and hepatocyte attachment to collagen is primarily mediated by α1β1 integrins [34]. Therefore, the ability of primary rat hepatocytes to adhere and survive for 24 h on chemically modified porous Si was assessed and compared with a standard laboratory cell culture preparation. The control consisted of cells cultured in media containing serum on tissue culture polystyrene (TCPS) with adsorbed collagen.
Porous Si surfaces were studied in the presence of serum, with and without added collagen I adsorbed to the surface. Cells were allowed to adhere for 90 min before wash and incubation in fresh media. Cell coverage on the porous Si samples was determined by imaging cells stained with calcein acetoxymethyl and ethidium homodimer-1 and normalizing to the TCPS control. Normalized results are presented in Fig. 3 and representative micrographs are presented in Fig. 4.
Figure 3.
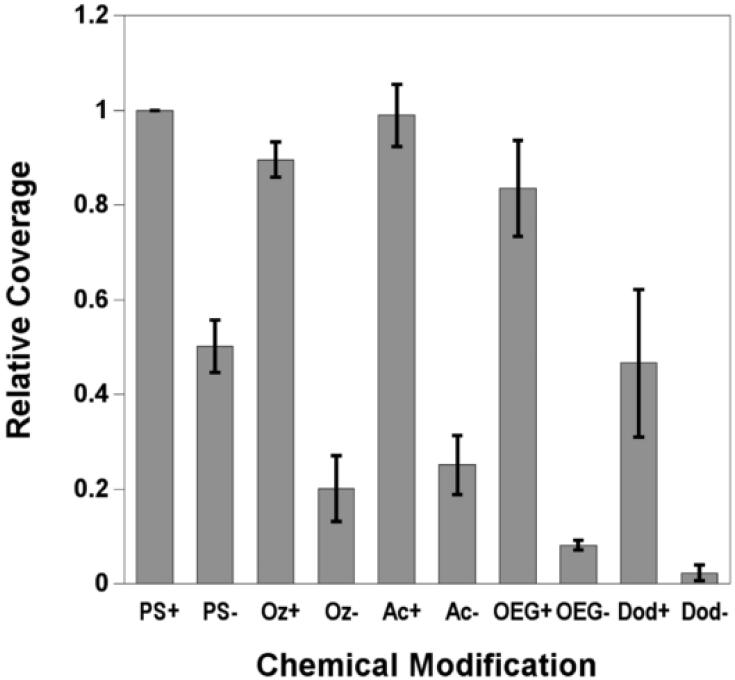
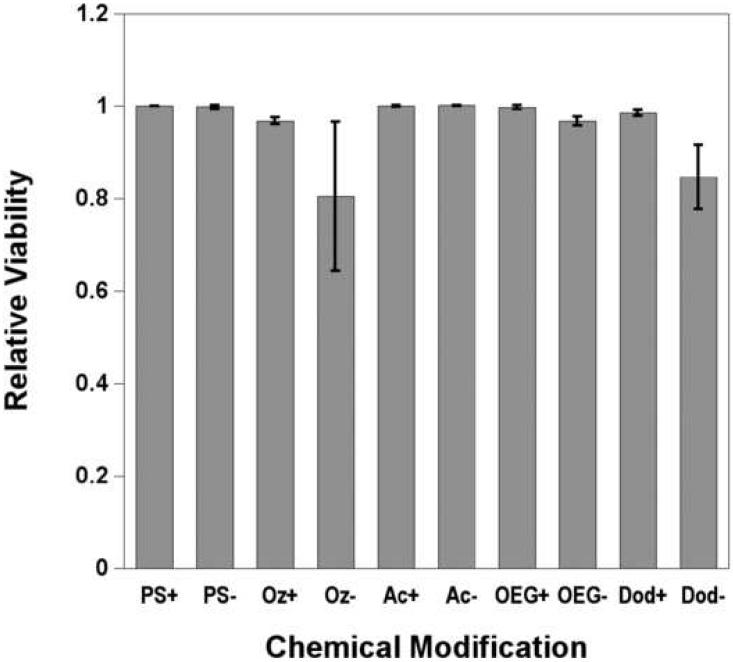
Relative coverage of primary rat hepatocytes at 24 hours determined from viability assays of hepatocytes cultured on tissue culture polystyrene (TCPS) and chemically modified porous Si with (+) and without (−) adsorbed collagen. Coverage on the surfaces is normalized to TCPS with adsorbed collagen. Abbreviations for the control and the modified porous Si samples are indicated on the x-axis: tissue culture polystyrene (PS), ozone-oxidized (Oz), undecanoic acid (Ac), oligo(ethylene) glycol (OEG), and dodecyl (Dod). Cell densities for the surface modifications are listed: (PS+) 870 cells/mm2; (PS−) 400 cells/mm2; (Oz+) 780 cells/mm2; (Oz−) 170 cells/mm2; (Ac+) 850 cells/mm2; (Ac−) 210 cells/mm2; (OEG+) 700 cells/mm2; (OEG−) 70 cells/mm2; (Dod+) 400 cells/mm2; (Dod−) 20 cells/mm2. Cell adhesion studies were performed in cell media containing 10% FBS. Normalized results are means ± standard error of the mean of three separate cell preparations.
Figure 4.
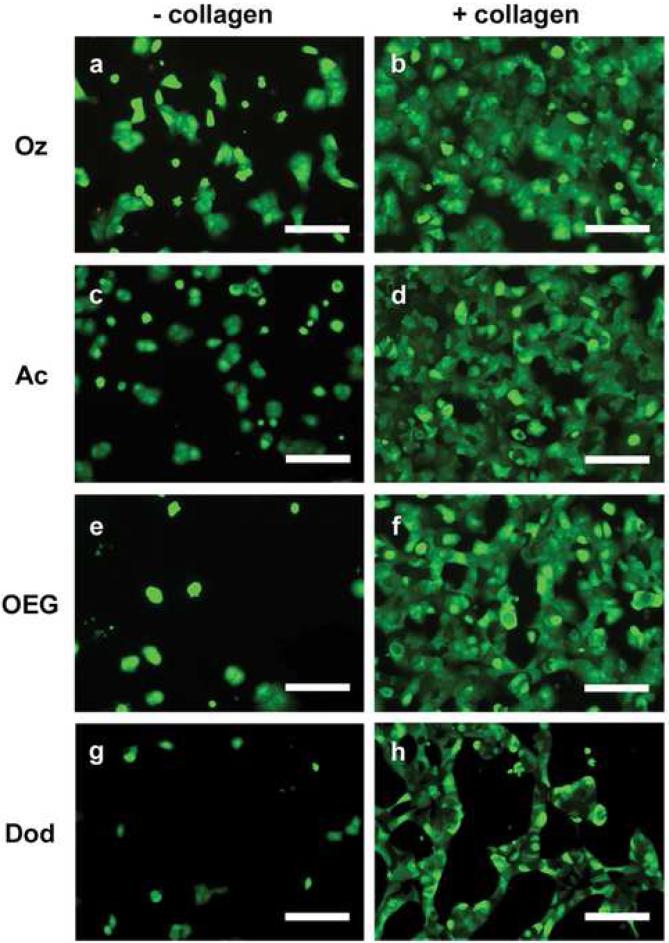
Representative optical micrographs of primary rat hepatocytes seeded on 2 types of chemically modified porous Si samples with (+ collagen) and without (− collagen) adsorbed collagen type I. Abbreviations for the samples are indicated on the left: tissue culture polystyrene (PS), ozone-oxidized (Oz), undecanoic acid (Ac), oligo(ethylene) glycol (OEG), and dodecyl (Dod). Cells are stained with the vital dyes calcein acetoxymethyl and ethidium homodimer I. (a) Hepatocytes on ozone-oxidized porous Si. (b) Hepatocytes on ozone-oxidized porous Si pretreated with collagen. (c) Hepatocytes on undecanoic acid-terminated porous Si. (d) Hepatocytes on undecanoic acid-terminated porous Si pretreated with collagen. (e) Hepatocytes on OEG-modified porous Si. (f) Hepatocytes on OEG-modified porous Si pretreated with collagen. (g) Hepatocytes on dodecyl-terminated porous Si. (h) Hepatocytes on dodecyl-terminated porous Si pretreated with collagen. Scale bar is 100 μm.
In the absence of collagen adsorbed to the sample, significantly fewer cells attach to the modified porous Si surfaces compared with the control and to TCPS without collagen adsorbed. The highly hydrophobic dodecyl surface displays the fewest attached cells (2%) if collagen is not present to promote cell adhesion. The difference in cell coverage between the dodecyl surface and the undecanoic acid surface, which differs only by the terminal carboxylic acid, is apparent in the images of Figs. 4g and 4c, respectively. The observation that the hydrophilic surface leads to superior hepatocyte attachment and spreading in comparison to the hydrophobic surface is in agreement with related studies on hydrophilic glass and polymer supports [49-51].
Hepatocyte coverage increases significantly if collagen is adsorbed on the modified porous Si samples prior to cell seeding. This improvement in cell adhesion when collagen is present, is in agreement with previous results observed on oxidized porous Si [20] and on acrylonitrile copolymers [51]. When the undecanoic acid, ozone-oxidized, and OEG-modified surfaces are exposed to collagen type I prior to cell seeding, cells adhere at levels comparable to the TCPS control (Fig. 3). Although the hydrophobic, dodecyl-terminated surface with pre-adsorbed collagen shows substantial improvement in cell coverage relative to the same surface without collagen (Figs. 3 and 4g-h), this surface displayed significantly less cell attachment compared to the other collagen-coated surfaces. This result is interesting, because it is known that collagen I adsorption is driven by hydrophobic interactions, and more collagen adsorbs on hydrophobic surfaces than hydrophilic ones [52, 53]. While adsorption of collagen and other serum proteins [54] increases on hydrophobic surfaces, the conformation of the adsorbed protein is different and can influence cell adhesion and morphology [54-56]. Total quantities of adsorbed protein were not measured in the present study, but in both experimental cases observed here (serum without collagen and serum with collagen), it is possible that serum proteins and collagen I adsorb to the hydrophobic surface in conformations that do not significantly enhance hepatocyte adhesion. Although hydrosilylation with both undecylenic acid and dodecene have been used to stabilize porous Si in aqueous conditions, our results show that the carboxylic acid-terminated surface is better at maximizing hepatocyte coverage to levels observed using standard lab culture preparations (on TCPS).
Oligo(ethylene) glycol is commonly used to inhibit nonspecific binding of biomolecules to material surfaces and to create nonadhesive domains in patterning cells [57]. We tested hepatocyte adhesion to oligo(ethylene) glycol terminated porous Si in order to determine if the short chain OEG can inhibit cell adhesion to porous Si. Cell coverage on the OEG-terminated surface is low without adsorbed collagen (Fig. 4e) and is likely due to the antifouling properties of the OEG surface and the inhibition of serum protein adhesion necessary to mediate cell binding to the surface. However, the result on the OEG surface is not significantly different from the other hydrophilic surfaces in this study (undecanoic acid and oxidized, Fig. 3). This observation is consistent with previous results comparing hydroxy-terminated OEG to silicon oxide on porous Si [30]. As noted above, exposure of the OEG surface to collagen I results in cell adhesion comparable to the control surface. Presumably, sufficient quantities of collagen are adsorbed to promote adhesion of significant numbers of hepatocytes (Fig 4e-f). This may be due to incomplete conversion of undecanoic acid residues to the OEG functionality (Fig. 2c), thereby forming an inadequate surface density of the OEG. The result is also consistent with observations by Leckband and colleagues [58] that, in the presence of serum proteins, cells adhere to 3-subunit, methoxy-terminated OEG-functionalized surfaces at levels comparable to polystyrene culture dishes. The prior study found that the cells were loosely bound to the 3-subunit OEG surface, and near complete inhibition of cell attachment was observed by increasing the chain length of the OEG polymer. Both chain length and surface density of the OEG molecule influence the antifouling properties of poly(ethylene) glycol (PEG) [45, 59]. In order to achieve the best antifouling properties for a given ethylene glycol chain, the coverage must be increased with decreasing molecular weight [60]. Inhibiting cell adhesion on porous Si may be improved with higher grafting density of the OEG or, alternatively, increasing the chain length.
Cell Viability after 24 hours
Ethidium homodimer-1, a stain that intercalates in the DNA of cells with compromised membranes, was used to identify and count non-viable cells after 24 h in culture. No apparent toxicity towards hepatocytes is observed for any of the porous Si samples tested. Primary rat hepatocytes survive equally well on all substrates studied over this time frame, irrespective of their chemical functionality or the presence of a collagen coating, Fig. 5. Although no significant differences in viability are observed, greater variance was observed on the collagen-free ozone-oxidized and dodecene samples.
Figure 5.
Relative viability of primary rat hepatocytes at 24 hours on tissue culture polystyrene and chemically modified porous silicon with (+) and without (−) collagen adsorbed, quantified by ethidium homodimer-1 stain. Abbreviations for the control and the modified porous Si samples are indicated on the x-axis: polystyrene (PS), undecanoic acid (Ac), ozone-oxidized (Oz), oligo(ethylene) glycol (OEG), and dodecyl (Dod) modified. Cell viability studies were performed in cell media containing 10% FBS. Results are means ± standard error of the mean of three separate cell preparations.
As previously noted, there are significant differences in the extent of cellular attachment between surfaces. Therefore, while the attached cells remain viable for all surfaces, the total number of cells varies drastically. The use of porous Si as a cell culturing platform requires not only that the cells be viable; they must also adhere to the surface.
Long-Term Viability
In order to use the optical properties of porous Si for tissue based biosensing [28], the cells must maintain function for an experimentally relevant time period. In previous work [20], albumin production from hepatocytes cultured on flat Si and ozone-oxidized porous Si were comparable to cells cultured on polystyrene, suggesting there are no cytotoxic effects of nanoporous silicon on primary hepatocytes. Albumin production, an indicator of liver-specific function, was measured for 7 days starting on day 2 after hepatocyte seeding and encapsulation in the collagen sandwich con figuration. Measurements were performed on hepatocytes cultured on collagen-coated undecanoic acid and ozone-modified surfaces, because results from coverage and viability were most similar to the control, collagen coated TCPS. Albumin production was also measured from cells grown on collagen-coated, hydrophilic, nonporous crystalline Si samples to allow comparison with previous work [20] and results are presented in Figure 6.
Figure 6.
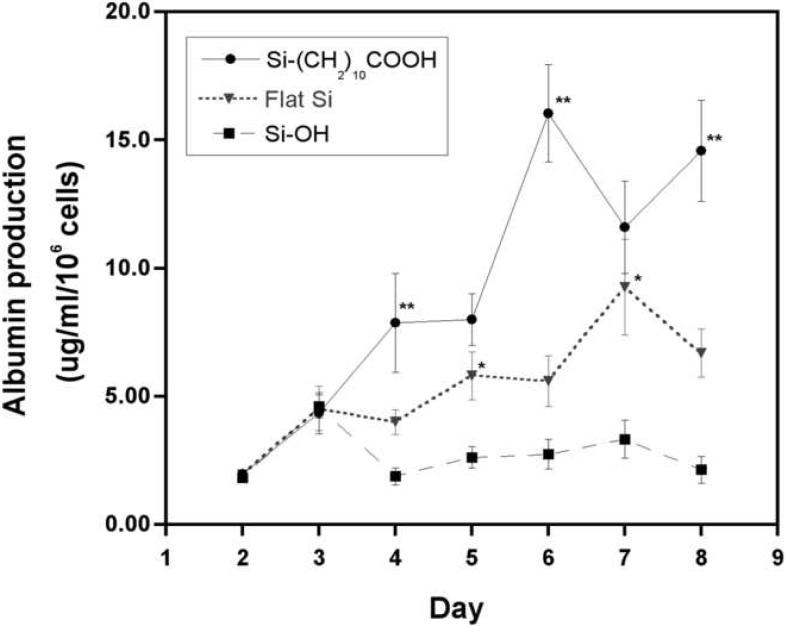
Albumin production by primary rat hepatocytes cultured on undecanoic acid-terminated porous Si (Si—(CH2)10COOH), ozone-oxidized porous Si (Si-OH), and on a crystalline Si wafer (Flat Si) over the course of 8 days. All surfaces contained pre-adsorbed collagen I, and cells were overlayed with a collagen gel for maintenance of cell function. Double asterisks (**) denotes data points with significantly higher albumin production from cells cultured on undecanoic acid-terminated porous Si in comparison to cells on flat Si. Single asterisk (*) denotes data points with significantly higher albumin production from cells on flat Si in comparison to ozone oxidized porous Si. p < 0.05
Our results indicate that albumin production from hepatocytes cultured on crystalline and ozone-oxidized porous Si surfaces is at a level similar to that observed in the previous study. Slightly higher albumin production was observed from hepatocytes cultured on the undecanoic acid surface in comparison to the other hydrophilic Si samples.
It has been shown that the chemical properties of a material surface can influence the adhesion and function of cells in culture [61-66]. Improved adhesion of mammalian cells is typically observed on hydrophilic surfaces as was observed here for hepatocytes on porous Si (Fig. 3). However, the interplay between surface chemistry, adsorbed serum proteins, and hepatocyte protein production (particularly albumin) is not well established [49, 67] and some investigators have noted that hepatocyte protein expression can vary between hydrophilic surfaces [51]. Grant et al. showed that hydrophilic, primary amine-terminated copolymer surfaces have a deleterious effect on the maintenance of primary hepatocyte function in comparison to other hydrophilic polymers. The hydrophilic surfaces studied here appear to have no harmful effect on primary rat hepatocytes, and the ease of surface modification allows flexibility in tailoring the stability and functionality of porous Si while maintaining successful cell culture of primary rat hepatocytes.
Effect of Porous Si Surface Chemistry on Optical Sensing in Culture
Optical biosensors based on porous Si have been used to detect proteins [25], DNA [24], viruses [29], and proteases [68] in well-controlled aqueous matrices. An important consideration for adapting this technology to in vitro or in vivo environments is the chemical and structural stability of porous Si in culture or other biologically relevant media. Silicon and SiO2 are both thermodynamically unstable in aqueous solutions, and the nanostructured forms present in porous Si can degrade and dissolve rather quickly [69]. Certain surface modification chemistries reduce the rate of degradation to a negligible degree; alkylated porous Si in particular has shown considerable promise [31]. The applicability of porous Si as an optical biosensor in cell culture was assessed using optical interferometry.
The porous Si samples used in this study are uniform enough to display thin film Fabry-Perot interference fringes in the white light reflectance spectrum. Representative reflectance spectra of undecanoic acid-modified and ozone-oxidized porous Si samples are presented in Fig. 7. The spectral pattern results from interference of light reflected from the air-porous Si interface and the porous Si-bulk Si interface. The wavelength, λ, of a given interference fringe is related to the refractive index, n, and thickness, L, of the porous Si film by the equation
| (1) |
where m is the spectral order of the particular fringe. Shifting of these fringes to shorter wavelengths can be used to quantify changes in refractive index [70] as the porous Si matrix oxidizes or dissolves.
Figure 7.
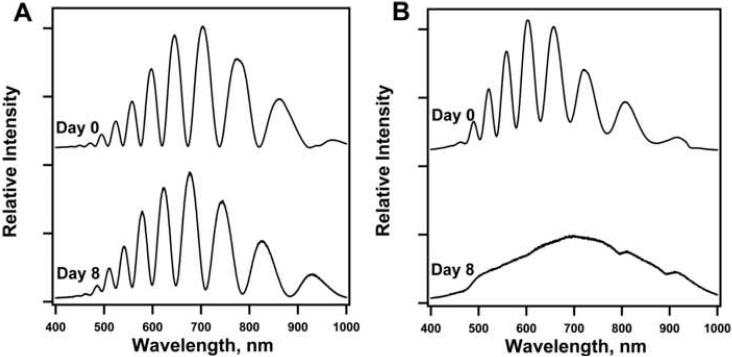
Representative optical reflectance spectra of (a) undecanoic acid-modified porous Si and (b) ozone-oxidized porous Si before and after 8 days in cell culture. Electrochemical anodization of the Si wafer produces a film uniform and thin enough to display Fabry-Perot interference fringes. After 8 days in cell culture, the optical spectrum of the undecanoic acid-modified sample indicates a moderate degree of oxidation and/or dissolution, while the ozone-oxidized sample has degraded to the point that thin film interference can no longer be observed
At the conclusion of the long-term hepatocyte culture experiments, the optical interference spectra of the acid-terminated and ozone-oxidized porous Si samples were probed to assess their degradation while in culture and in direct contact with cells. Whereas the thin film interference spectrum of the undecylenic acid-modified material is still apparent after 8 days in culture (Fig. 7A), the ozone-oxidized sample has degraded to the point that the interference fringes are no longer observable (Fig. 7B). Fourier analysis [71] of the spectra of the undecylenic acid-modified material indicate that the refractive index of the film is significantly reduced after this period. The result indicates that the porous Si matrix has undergone oxidation or dissolution, though the extent of the degradation is not as large as with the ozone-oxidized sample. While adsorbed protein can slow degradation of ozone- oxidized porous Si [30], the structure did not withstand 8 days in cell culture. Of the samples studied here, undecylenic acid-modified porous Si provides the greatest coverage of primary cells while maintaining the integrity of the underlying optical sensor when in contact with live cells. However, the significant drift of the optical signal over the course of 8 days is probably too large to allow accurate biosensing, even with the most stable surface chemistry tested in this study. In order to perform in vitro biosensing, more stable porous Si modification chemistries such as methyl end-capping [72] or thermal carbonization via acetylene decomposition [73] may be necessary.
4. Conclusions
Successful culture of primary rat hepatocytes has been demonstrated on chemically modified porous Si. Primary rat hepatocytes adhere best to hydrophilic surfaces that are coated with collagen prior to cell seeding. The 4-subunit OEG-modified surface is not effective at inhibiting cell adhesion when collagen is physisorbed onto the surface at a concentration of 0.1 mg/mL. The extent of cellular adhesion is low on hydrophobic porous Si regardless of serum protein adsorption. Once seeded on the substrates, viability of hepatocytes is comparable to that observed on tissue culture polystyrene. Long-term viability is maintained on collagen-coated crystalline Si and on collagen-coated ozone-oxidized porous Si, in agreement with previous studies, and albumin protein secretion is maintained on the collagen-coated undecanoic acid-terminated substrate. Of the porous Si samples studied, the surface that has been hydrosilylated with a hydrophilic, carboxylic acid species (collagen-coated undecanoic acid-modified) shows the best stability and compatibility with primary rat hepatocytes.
Acknowledgments
Acknowledgements
This project has been funded in part with Federal funds from the National Science Foundation (Grant DMR-0806859). MJS is a member of the Moores UCSD Cancer Center and the UCSD NanoTUMOR Center under which this research was conducted and partially supported by NIH grant U54 CA 119335. S.D.A. is grateful for fellowships provided by the Department of Education, Graduate Assistance in Areas of National Need (GANN) program (P200A030163), and the San Diego Fellowship, administered by the University of California, San Diego. M.P.S. thanks the Burroughs Welcome Fund for a La Jolla Interfaces in Science postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scholl M, Sprossler C, Denyer M, Krause M, Nakajima K, Maelicke A, et al. Ordered networks of rat hippocampal neurons attached to silicon oxide surfaces. Journal of Neuroscience Methods. 2000;104(1):65–75. doi: 10.1016/s0165-0270(00)00325-3. [DOI] [PubMed] [Google Scholar]
- 2.Lopez CA, Fleischman AJ, Roy S, Desai TA. Evaluation of silicon nanoporous membranes and ECM-based microenvironments on neurosecretory cells. Biomaterials. 2006;27(16):3075–3083. doi: 10.1016/j.biomaterials.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Sun W, Puzas JE, Sheu TJ, Liu X, Fauchet PM. Nano- to Microscale Porous Silicon as a Cell Interface for Bone-Tissue Engineering. Advanced Materials. 2007;19(7):921–924. [Google Scholar]
- 4.Baca HK, Ashley C, Carnes E, Lopez D, Flemming J, Dunphy D, et al. Cell-Directed Assembly of Lipid-Silica Nanostructures Providing Extended Cell Viability. Science. 2006;313(5785):337–341. doi: 10.1126/science.1126590. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Sheldon EL, Wu L, Heller MJ, O′Connell JP. Isolation of Cultured Cervical Carcinoma Cells Mixed with Peripheral Blood Cells on a Bioelectronic Chip. Anal Chem. 1998;70(11):2321–2326. doi: 10.1021/ac971274g. [DOI] [PubMed] [Google Scholar]
- 6.Andersson H, van den Berg A. Microtechnologies and nanotechnologies for single-cell analysis. Current Opinion in Biotechnology. 2004;15(1):44–49. doi: 10.1016/j.copbio.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss S, Buckberry L, Harris P, Rousseau C. Nanostructured semiconductors: compatibility with biomaterials. Thin Solid Films. 1997;297:308–310. [Google Scholar]
- 8.Bayliss SC, Heald R, Fletcher DI, Buckberry LD. The Culture of Mammalian Cells on Nanostructured Silicon. Advanced Materials. 1999;11(4):318–321. [Google Scholar]
- 9.Rosengren A, Wallman L, Danielsen N, Laurell T, Bjursten LM. Tissue reactions evoked by porous and plane surfaces made out of silicon and titanium. Biomedical Engineering, IEEE Transactions on. 2002;49(4):392–399. doi: 10.1109/10.991167. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Coffer JL, Chen Y, Pinizzotto RF, Newey J, Canham LT. Transition Metal Complex-Doped Hydroxyapatite Layers on Porous Silicon. J Am Chem Soc. 1998;120(45):11706–11709. [Google Scholar]
- 11.Foraker Amy B., Walczak Rob J., Cohen Michael H., Boiarski Tony A., Grove Carl F., Swaan PW. Microfabricated Porous Silicon Particles Enhance Paracellular Delivery of Insulin Across Intestinal Caco-2 Cell Monolayers. Pharmaceutical Research. 2003;V20(1):110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 12.Anglin EJ, Schwartz MP, Ng VP, Perelman LA, Sailor MJ. Engineering the Chemistry and Nanostructure of Porous Silicon Fabry-Perot Films for Loading and Release of a Steroid. Langmuir. 2004;20(25):11264–11269. doi: 10.1021/la048105t. [DOI] [PubMed] [Google Scholar]
- 13.Salonen J, Laitinen L, Kaukonen AM, Tuura J, Bjorkqvist M, Heikkila T, et al. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. Journal of Controlled Release. 2005;108(23):362–374. doi: 10.1016/j.jconrel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Loong SLE, Connor S, Yu SWK, Tan S-Y, Ng RTH, et al. Complete Tumor Response Following Intratumoral 32P BioSilicon on Human Hepatocellular and Pancreatic Carcinoma Xenografts in Nude Mice. Clin Cancer Res. 2005;11(20):7532–7537. doi: 10.1158/1078-0432.CCR-05-0400. [DOI] [PubMed] [Google Scholar]
- 15.Canham LT. Bioactive silicon structure fabrication through nanoetching techniques. Advanced Materials. 1995;7(12):1033–1037. [Google Scholar]
- 16.Mayne AH, Bayliss SC, Barr P, Tobin M, Buckberry LD. Biologically Interfaced Porous Silicon Devices. physica status solidi (a) 2000;182(1):505–513. [Google Scholar]
- 17.Schwarz K, Milne DB. Growth-promoting Effects of Silicon in Rats. Nature. 1972;239(5371):333–334. doi: 10.1038/239333a0. [DOI] [PubMed] [Google Scholar]
- 18.Carlisle EM. Silicon: an essential element for the chick. Science. 1972;178(61):619–621. doi: 10.1126/science.178.4061.619. [DOI] [PubMed] [Google Scholar]
- 19.Kawanabe K, Yamamuro T, Kotani S, Nakamura T. Acute nephrotoxicity as an adverse effect after intraperitoneal injection of massive amounts of bioactive ceramic powders in mice and rats. Journal of Biomedical Materials Research. 1992;26(2):209–219. doi: 10.1002/jbm.820260207. [DOI] [PubMed] [Google Scholar]
- 20.Chin V, Collins BE, Sailor MJ, Bhatia SN. Compatibility of Primary Hepatocytes with Oxidized Nanoporous Silicon. Advanced Materials. 2001;13(24):1877–1880. [Google Scholar]
- 21.Leffert HL, Paul D. Studies On Primary Cultures Of Differentiated Fetal Liver Cells. J Cell Biol. 1972;52(3):559–568. doi: 10.1083/jcb.52.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart MP, Buriak JM. Chemical and Biological Applications of Porous Silicon Technology. Advanced Materials. 2000;12(12):859–869. [Google Scholar]
- 23.Létant S, Sailor MJ. Detection of HF Gas with a Porous Silicon Interferometer. Advanced Materials. 2000;12(5):355–359. [Google Scholar]
- 24.Chan S, Fauchet PM, Li Y, Rothberg LJ, Miller BL. Porous Silicon Microcavities for Biosensing Applications. physica status solidi (a) 2000;182(1):541–546. [Google Scholar]
- 25.Janshoff A, Dancil KPS, Steinem C, Greiner DP, Lin VSY, Gurtner C, et al. Macroporous p-Type Silicon Fabry-Perot Layers. Fabrication, Characterization, and Applications in Biosensing. J Am Chem Soc. 1998;120(46):12108–12116. [Google Scholar]
- 26.Létant SE, Hart BR, Kane SR, Hadi MZ, Shields SJ, Reynolds JG. Enzyme Immobilization on Porous Silicon Surfaces. Advanced Materials. 2004;16(8):689–693. [Google Scholar]
- 27.Chan S, Horner SR, Miller BL, Fauchet PM. Identification of gram negative bacteria using nanoscale silicon microcavities. J Am Chem Soc. 2001;123(47):11797–11798. doi: 10.1021/ja016555r. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MP, Derfus AM, Alvarez SD, Bhatia SN, Sailor MJ. The Smart Petri Dish: A Nanostructured Photonic Crystal for Real-Time Monitoring of Living Cells. Langmuir. 2006;22(16):7084–7090. doi: 10.1021/la060420n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez SD, Schwartz MP, Migliori B, Rang CU, Chao L, Sailor MJ. Using a porous silicon photonic crystal for bacterial cell-based biosensing. physica status solidi (a) 2007;204(5):1439–1443. doi: 10.1002/pssa.200674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low SP, Williams KA, Canham LT, Voelcker NH. Evaluation of mammalian cell adhesion on surface-modified porous silicon. Biomaterials. 2006;27(26):4538–4546. doi: 10.1016/j.biomaterials.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Canham LT, Reeves CL, Newey JP, Houlton MR, Cox TI, Buriak JM, et al. Derivatized Mesoporous Silicon with Dramatically Improved Stability in Simulated Human Blood Plasma. Advanced Materials. 1999;11(18):1505–1507. [Google Scholar]
- 32.Canham LT, Stewart MP, Buriak JM, Reeves CL, Anderson M, Squire EK, et al. Derivatized Porous Silicon Mirrors: Implantable Optical Components with Slow Resorbability. physica status solidi (a) 2000;182(1):521–525. [Google Scholar]
- 33.Linford MR, Fenter P, Eisenberger PM, Chidsey CED. Alkyl monolayers on silicon prepared from 1-alkenes and hydrogen-terminated silicon. J Am Chem Soc. 1995;117:3145–3155. [Google Scholar]
- 34.Pinkse GGM, Voorhoeve MP, Noteborn M, Terpstra OT, Bruijn JA, Heer Ed. Hepatocyte survival depends on b1-integrin-mediated attachment of hepatocytes to hepatic extracellular matrix. Liver International. 2004;24:218. doi: 10.1111/j.1478-3231.2004.0914.x. [DOI] [PubMed] [Google Scholar]
- 35.Shriver DF, Drezdzon MA. 2nd ed. John Wiley and Sons, Inc; New York: 1986. The Manipulation of Air-Sensitive Compounds. [Google Scholar]
- 36.Schwartz Michael P., Cunin Frédérique, Cheung Ronnie W., Sailor MJ. Chemical modification of silicon surfaces for biological applications. physica status solidi (a) 2005;202(8):1380–1384. [Google Scholar]
- 37.Dunn JCY, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 39.Boukherroub R, Morin S, Wayner DDM, Bensebaa F, Sproule GI, Baribeau JM, et al. Ideal Passivation of Luminescent Porous Silicon by Thermal, Noncatalytic Reaction with Alkenes and Aldehydes. Chem Mater. 2001;13(6):2002–2011. [Google Scholar]
- 40.Bocking T, Gal M, Gaus K, Gooding JJ. Evidence for Why Tri(ethylene oxide) Functionalized Si-C Linked Monolayers on Si(111) Have Inferior Protein Antifouling Properties Relative to the Equivalent Alkanethiol Monolayers Assembled on Gold. Australian Journal of Chemistry. 2005;58(9):660–663. [Google Scholar]
- 41.Herrwerth S, Eck W, Reinhardt S, Grunze M. Factors that Determine the Protein Resistance of Oligoether Self-Assembled Monolayers - Internal Hydrophilicity, Terminal Hydrophilicity, and Lateral Packing Density. J Am Chem Soc. 2003;125(31):9359–9366. doi: 10.1021/ja034820y. [DOI] [PubMed] [Google Scholar]
- 42.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability To Resist Protein Adsorption. J Phys Chem B. 1998;102(2):426–436. [Google Scholar]
- 43.Khung YL, Cole MA, McInnes SJP, Voelcker NH. BioMEMS and Nanotechnology III. Vol. 2007. SPIE; Canberra, ACT, Australia: 2007. Control over wettability via surface modification of porous gradients; pp. 679909–679912. [Google Scholar]
- 44.Suh KY, Jon S. Control over Wettability of Polyethylene Glycol Surfaces Using Capillary Lithography. Langmuir. 2005;21(15):6836–6841. doi: 10.1021/la050878+. [DOI] [PubMed] [Google Scholar]
- 45.Prime KL, Whitesides GM. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): a model system using self-assembled monolayers. J Am Chem Soc. 1993;115(23):10714–10721. [Google Scholar]
- 46.Clare TL, Clare BH, Nichols BM, Abbott NL, Hamers RJ. Functional Monolayers for Improved Resistance to Protein Adsorption: Oligo(ethylene glycol)-Modified Silicon and Diamond Surfaces. Langmuir. 2005;21(14):6344–6355. doi: 10.1021/la050362q. [DOI] [PubMed] [Google Scholar]
- 47.Kilian KA, Bocking T, Gaus K, Gal M, Gooding JJ. Si-C linked oligo(ethylene glycol) layers in silicon-based photonic crystals: Optimization for implantable optical materials. Biomaterials. 2007;28:3055–3062. doi: 10.1016/j.biomaterials.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Malik RK. Regulation of Apoptosis by Integrin Receptors. Journal of Pediatric Hematology/Oncology. 1997;19(6):541–545. doi: 10.1097/00043426-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Krasteva N, Groth T, Fey-Lamprecht F, Altankov G. The role of surface wettability on hepatocyte adhesive interactions and functions. J Biomater Sci Polymer Edn. 2001;12(6):613–627. doi: 10.1163/156856201316883449. [DOI] [PubMed] [Google Scholar]
- 50.Catapano G, Lorenzo MCD, Volpe CD, Bartolo LD, Migliaresi C. Polymeric membranes for hybrid liver support devices: The effect of membrane surface wettability on hepatocyte viability and functions. Journal of Biomaterials Science Polymer Edition. 1996;7(11):1017–1027. doi: 10.1163/156856296x00417. [DOI] [PubMed] [Google Scholar]
- 51.Grant MH, Morgan C, Henderson C, Malsch G, Seifert B, Albrecht W, et al. The viability and function of primary rat hepatocytes cultured on polymeric membranes developed for hybrid artificial liver devices. Journal of Biomedical Materials Research Part A. 2005;73A(3):367–375. doi: 10.1002/jbm.a.30306. [DOI] [PubMed] [Google Scholar]
- 52.Penners G, Priel Z, Silberberg A. Irreversible Adsorption of Triple-Helical Soluble Collagen Monomers from Solution to Glass and Other Surfaces. Journal of Colloid and Interface Science. 1981;80(2):437–444. [Google Scholar]
- 53.Denis FA, Hanarp P, Sutherland DS, Gold J, Mustin C, Rouxhet PG, et al. Protein Adsorption on Model Surfaces with Controlled Nanotopography and Chemistry. Langmuir. 2002;18(3):819–828. [Google Scholar]
- 54.Grinnell F, Feld MK. Fibronectin Adsorption on Hydrophilic and Hydrophobic Surfaces Detected by Antibody Binding and Analyzed during Cell Adhesion in Serum-containing Medium. The Journal of Biological Chemistry. 1982;257(9):4888–4893. [PubMed] [Google Scholar]
- 55.Elliott JT, Woodward JT, Umarji A, Mei Y, Tona A. The effect of surface chemistry on the formation of thin films of native fibrillar collagen. Biomaterials. 2007;28(4):576–585. doi: 10.1016/j.biomaterials.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Keselowsky BG, Collard DM, García AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. Journal of Biomedical Materials Research Part A. 2003;66A(2):247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 57.Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using Microcontact Printing to Pattern the Attachment of Mammalian Cells to Self-Assembled Monolayers of Alkanethiolates on Transparent Films of Gold and Silver. Experimental Cell Research. 1997;235(2):305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 58.Zhu B, Eurell T, Gunawan R, Leckband D. Chain-length dependence of the protein and cell resistance of oligo(ethylene glycol)-terminated self-assembled monolayers on gold. Journal of Biomedical Materials Research. 2001;56(3):406–416. doi: 10.1002/1097-4636(20010905)56:3<406::aid-jbm1110>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 59.Lasseter TL, Clare BH, Abbott NL, Hamers RJ. Covalently Modified Silicon and Diamond Surfaces: Resistance to Nonspecific Protein Adsorption and Optimization for Biosensing. J Am Chem Soc. 2004;126(33):10220–10221. doi: 10.1021/ja047642x. [DOI] [PubMed] [Google Scholar]
- 60.Sofia SJ, Merrill EW. Protein Adsorption on Poly(ethylene oxide)-Grafted Silicon Surfaces. In: Zalipsky S, Harris JM, editors. Poly(ethylene) glycol: chemistry and biological applications. American Chemical Society; Washington, DC: 1997. pp. 342–360. [Google Scholar]
- 61.Mrksich M, Whitesides GM. Using Self-Assembled Monolayers to Understand the Interactions of Man-made Surfaces with Proteins and Cells. Annual Review of Biophysics and Biomolecular Structure. 1996;25(1):55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 62.Healy KE, Lom B, Hockberger PE. Spatial distribution of mammalian cells dictated by material surface chemistry. Biotechnology and Bioengineering. 1994;43(8):792–800. doi: 10.1002/bit.260430814. [DOI] [PubMed] [Google Scholar]
- 63.Webb K, Hlady V, Tresco PA. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. Journal of Biomedical Materials Research. 1998;41(3):422–430. doi: 10.1002/(sici)1097-4636(19980905)41:3<422::aid-jbm12>3.0.co;2-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altankov G, Grinnell F, Groth T. Studies on the biocompatibility of materials: Fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. Journal of Biomedical Materials Research. 1996;30(3):385–391. doi: 10.1002/(SICI)1097-4636(199603)30:3<385::AID-JBM13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 65.Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27(27):4783–4793. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Liu WF, Chen CS. Engineering biomaterials to control cell function. Materials Today. 2005;8(12):28–35. [Google Scholar]
- 67.Krasteva N, Harms U, Albrecht W, Seifert B, Hopp M, Altankov G, et al. Membranes for biohybrid liver support systems--investigations on hepatocyte attachment, morphology and growth. Biomaterials. 2002;23(12):2467–2478. doi: 10.1016/s0142-9612(01)00381-7. [DOI] [PubMed] [Google Scholar]
- 68.Orosco MM, Pacholski C, Miskelly GM, Sailor MJ. Protein-Coated Porous-Silicon Photonic Crystals for Amplified Optical Detection of Protease Activity. Advanced Materials. 2006;18(11):1393–1396. [Google Scholar]
- 69.Anderson SHC, Elliott H, Wallis DJ, Canham LT, Powell JJ. Dissolution of different forms of partially porous silicon wafers under simulated physiological conditions. physica status solidi (a) 2003;197(2):331–335. [Google Scholar]
- 70.Dancil KPS, Greiner DP, Sailor MJ. A Porous Silicon Optical Biosensor: Detection of Reversible Binding of IgG to a Protein A-Modified Surface. J Am Chem Soc. 1999;121(34):7925–7930. [Google Scholar]
- 71.Segal E, Perelman LA, Cunin F, Di Renzo F, Devoisselle JM, Li YY, et al. Confinement of Thermoresponsive Hydrogels in Nanostructured Porous Silicon Dioxide Templates. Advanced Functional Materials. 2007;17(7):1153–1162. [Google Scholar]
- 72.Lees IN, Lin H, Canaria CA, Gurtner C, Sailor MJ, Miskelly GM. Chemical Stability of Porous Silicon Surfaces Electrochemically Modified with Functional Alkyl Species. Langmuir. 2003;19:9812–9817. [Google Scholar]
- 73.Torres-Costa V, Martin-Palma RJ, Martinez-Duart JM, Salonen J, Lehto VP. Effective passivation of porous silicon optical devices by thermal carbonization. Journal of Applied Physics. 2008;103(8):083124. [Google Scholar]