Abstract
Through undefined mechanisms, dominant mutations in (Cu/Zn) superoxide dismutase-1 (mSOD1) cause the non-cell-autonomous death of motoneurons in inherited amyotrophic lateral sclerosis (ALS). Microgliosis at sites of motoneuron injury is a neuropathological hallmark of ALS. Extracellular mSOD1 causes motoneuron injury and triggers microgliosis in spinal cord cultures, but it is unclear whether the injury results from extracellular mSOD1 directly interacting with motoneurons or is mediated through mSOD1-activated microglia. To dissociate these potential mSOD1-mediated neurotoxic mechanisms, the effects of extracellular human mSOD1G93A or mSOD1G85R were assayed using primary cultures of motoneurons and microglia. The data demonstrate that exogenous mSOD1G93A did not cause detectable direct killing of motoneurons. In contrast, mSOD1G93A or mSOD1G85R did induce the morphological and functional activation of microglia, increasing their release of pro-inflammatory cytokines and free radicals. Furthermore, only when microglia were co-cultured with motoneurons did extracellular mSOD1G93A injure motoneurons. The microglial activation mediated by mSOD1G93A was attenuated using toll-like receptors (TLR) 2, TLR4 and CD14 blocking antibodies, or when microglia lacked CD14 expression. These data suggest that extracellular mSOD1G93A is not directly toxic to motoneurons but requires microglial activation for toxicity, utilizing CD14 and TLR pathways. This link between mSOD1 and innate immunity may offer novel therapeutic targets in ALS.
Keywords: mutant SOD1, CD14, Toll-like receptors, microglia, motoneurons
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating and chronic neurodegenerative disease, characterized by selective loss of lower and upper motoneurons (Lomen-Hoerth, 2008). Although the cause of sporadic ALS (sALS) is not known, approximately 10% of ALS cases are familial (fALS) with 20-25% of these cases resulting from various mutations in the SOD1 (Gurney et al., 1994). Several human mSOD1 genes have been over-expressed in mice and cause a disease that replicates many of the clinical and pathological features seen in ALS patients (Gurney et al., 1994; Wong et al., 1995). In addition, a recent study demonstrated an altered SOD1 species within the spinal cords of sALS patients that must have originated from the misfolded wild-type SOD1 (SOD1WT), possibly linking a shared pathophysiologic pathway between sALS and fALS (Gruzman et al., 2007).
The toxicity of mSOD1 in ALS is not due to decreased enzymatic activity because mice lacking SOD1 expression did not develop a motoneuron disease (Reaume et al., 1996). Furthermore, motoneuron disease develops with different SOD1 mutations regardless of their enzymatic activities (Cleveland and Rothstein, 2001; Wang et al., 2002). In addition, motoneuron specific expression of mSOD1 either does not initiate disease (Pramatarova et al., 2001; Lino et al., 2002) or, when expressed homozygously, causes a late onset and slowly progressing disease (Jaarsma et al., 2008). Thus, the aggressive development of disease in mSOD1 transgenic mice may be non-cell-autonomous (Lobsiger and Cleveland, 2007; Yamanaka et al., 2008a).
Current evidence suggests that glial over-expression of mSOD1 may contribute to disease progression in this model of inherited ALS. Several studies support the importance of both microglia and astroglia in mediating motoneuron injury (Zhao et al., 2004; Beers et al., 2006; Boillee et al., 2006; Nagai et al., 2007; Yamanaka et al., 2008b). Over-expression of mSOD1 in microglia enhances toxicity and accelerates disease progression compared with WT microglia (Beers et al., 2006; Xiao et al., 2007). Insights into the role of mSOD1 and microglia-mediated motoneuron injury are derived from the demonstration that mSOD1 can directly augment microglial NADPH oxidase-dependent superoxide production; mSOD1 stimulated microglial NADPH oxidase activity leading to increased production of toxic superoxide (Harraz et al., 2008). A key question not addressed by these studies is whether extracellular mSOD1 also interacts with and activates microglia which subsequently injures motoneurons.
A previous study suggests that the neurosecretory proteins, chromogranin A and B, interact with and mediate the secretion of mSOD1 protein from neurons and astrocytes (Urushitani et al., 2006). Unlike SOD1WT protein, extracellular mSOD1 protein triggered microgliosis and neuronal death in whole spinal cord mixed cultures. Furthermore, recent evidence suggests that oxidation of SOD1WT results in misfolded protein that may acquire the binding and toxic properties of mSOD1, suggesting a possible shared pathway between sporadic and inherited ALS cases (Ezzi et al., 2007; Gruzman et al., 2007; Kabashi et al., 2007). These studies, taken together with our data that mSOD1G93A expressing microglia are more neurotoxic than WT microglia (Beers et al., 2006; Xiao et al., 2007), indicate that mSOD1 is not only harmful within the cell, but also gains toxic functions when outside the cell. However, no study has investigated whether extracellular mSOD1 has direct effects on microglia and/or motoneurons, and by what mechanisms.
Materials and Methods
Materials
Culture media, sera and antibiotics were purchased from Gibco BRL (Rockville, MD), and all other reagents were from Sigma (St. Louis, MO) unless otherwise noted.
Recombinant protein purification
Recombinant human mSOD1G93A, mSOD1G85R and SOD1WT proteins were purified from E. coli, metallated with copper and zinc (Urushitani et al., 2004). Briefly, E. coli were transformed with the expression plasmid, pGEX6p-1 carrying human mSOD1G93A or SOD1WT gene. GST-fused hSOD1protein was induced by 1mM IPTG (Isopropyl β-D-1-thiogalactopyranoside) and absorbed with glutathione sepharose beads. After washing three times in PBS, the beads were incubated with a protease (Precision, Amersham-Pharmacia) to release hSOD1 from the GST tag. The hSOD1 proteins were then dialyzed against a buffer containing 50mM Tris-HCl pH7.5 and 100mM NaCl. Metallation was performed by incubation in two equivalent parts of zinc chloride for 24 hrs, followed by further incubation with two-equimolar copper chloride for 24 hrs. Metallated hSOD1 was dialyzed against the same buffer. The purity of the recombinant protein was verified by Western blotting and the activity of metallated recombinant SOD1 was confirmed using a SOD1 activity assay kit (Dojindo, Kumamoto, Japan), in which dismutase activity against the superoxide anion, generated from the reaction of xanthine with xanthine oxidase, was quantified (data not shown). Purified hSOD1 proteins were stored at −80°C.
Mice
Animal protocols were approved by the Methodist Research Institute's Institutional Animal Care and Research Advisory Committee in compliance with National Institutes of Health guidelines. mSOD1G93A mice [C57B6.Cg-Tg(SOD1*G93A)1Gur/J] and CD14−/− mice were originally purchased from Jackson Laboratories (Bar Harbor, ME). All transgenic animals were bred and maintained in our animal facility. CD4−/− mice were initially bred with mSOD1G93A mice for at least 8 generations. The mSOD1 and CD14 mice were identified by PCR (Eppendorf Mastercycler). mSOD1 copy number was determined by quantitative PCR.
Cell cultures
Primary motoneuron cultures were prepared from spinal cords of embryonic day 13-14 C57BL/6 or CD14−/− mice by HistoDenz gradient centrifugation (Arce et al., 1999; Zhao et al., 2006). Briefly, the ventral cords digested for 15 min with 0.025% trypsin at 37°C. After treatment with DNase, the cell suspension was centrifuged through a 6.8% HistoDenz cushion at 800g for 15 min, the sharp band on top of the HistoDenz cushion was collected and centrifuged through a 4% BSA cushion at 450g for 10 min. The cells were resuspended in the neurobasal medium supplemented with glutamate (25μM), glutamine (200mM), B27 (50×, 2ml), penicillin (100IU/ml), streptomycin (100μg/ml), and horse serum (2%). Cells were plated at a density of 15,000 cells/well in 24-well-plates containing coverslips coated with poly-L-ornithine (2μg/ml) and laminin (3μg/ml).
Microglia cultures were prepared from 7-8-day-old C57BL/6 or CD14−/− mice as previously described (Zhao et al., 2004). Greater than 95% of the floating cells were microglia as determined by OX42 immunocytochemical staining. Co-cultures of microglia and motoneurons were carried out by plating microglia (20,000/well) on motoneurons after motoneurons were incubated for 1 day. mSOD1G93A, mSOD1G85R or SOD1WT was added to culture medium 4 hours after microglia plating. The addition of blocking antibodies or inhibitors was made 2 hrs prior to mSOD1G93A or SOD1WT.
Immunocytochemistry
For motoneuron staining, cultures were fixed for 25 min with 4% paraformaldehyde at room temperature. After washing, cultures were stained by SMI-32 antibody (1:1000, Covance) and M.O.M kit (Vector Laboratories) following the manufacturer's instructions. SMI-32, non-phosphorylated neurofilament H, is a commonly used specific marker for motoneuron staining (Carriedo, et al., 1996). Motoneuron survival was assessed by direct counting of large SMI-32 positive cells (cell bodies >25 μm) displaying intact neurites longer than three cell diameters. Microglial cultures were stained by OX42 antibody (1:100, Chemicon) and biotinylated goat anti-rat IgG (1:200, Serotec). Images of microglia were obtained using a Zeiss Imager-Z1m microscope equipped with a Zeiss AxioCam MRc5 color camera and Zeiss digital image analysis system (Karl Zeiss, Oberkochen, Germany).
ELISA
ELISA Duoset kit (R & D Systems) was used to determine the concentration of TNF-α, IL-1β, and free IGF-1 protein in cell culture supernatant according to manufacture's instructions.
Superoxide assay
The release of superoxide was determined by WST-1 assay (Tan and Berridge, 2000). Briefly, microglia cultures grown in 96-well plates were treated with mSOD1G93A and SOD1WT with or without CD14-Ab for 4 days. After washing, WST-1 (250μM) and catalase (10U/ml) in HBSS were added to each well. The reaction was started by the addition of 800nM phorbol myristate acetate (PMA) (final volume of 200μl/well). The OD values at 450nm were measured at 0 min (served as background) and 5 hrs in a microplate reader. The increase of absorbance was used to determine O2·− generation.
Immunoprecipitation and western blot
mSOD1G93A or SOD1WT (12μg) was incubated with recombinant murine soluble CD14 (rCD14, Cell Sciences, 5.4 μg) at 4°C overnight. Anti-CD14 rabbit antiserum (Cell sciences, 2ul) was added and incubated for 30 min. Immunoprecipitation was performed by incubation with protein G-sepharose. After centrifugation, pellets were run through 15% gel (Bio-Rad Laboratories) and electroblotted onto PVDF membranes (Millipore). After blocking, blots were incubated with anti-SOD1 antibody (1:1000; Calbiochem) and peroxidase-conjugated anti-sheep IgG (1:2000, Vector Laboratories). The ECL plus detection system (Amersham Pharmacia Biotech) was used for protein detection. Negative control conditions include incubation of mSOD1G93A only or rCD14 only with anti-CD14 Ab. iNOS and β-actin were detected by anti- iNOS antibody (1:400; Chemicon) and anti- β-actin antibody (1:10,000; Sigma), respectively.
Quantitative RT-PCR
Cells were lysed in TRIzol Reagent (Invitrogen) and total RNA was prepared according to the manufacturer's instructions. RNA was further purified by RNeasy kit (Qiagen). Real-time RT-PCR was performed using one-step RT-PCR kit with SYBR Green (Bio-Rad Laboratories or Qiagen) and the iQ5 Multicolor Real-time PCR detection System (Bio-Rad Laboratories) according to the manufacturer's recommendations. The RT-PCR conditions were as follows: NOX2 primers: 5′-TGA ATG CCA GAG TCG GGA TTT-3′ and 5′-CCC CCT TCA GGG TTC TTG ATT T-3′, Tm=60°C; iNOS primers: 5′-CAG CAC AGG AAA TGT TTC AGC-3′ and 5′-TAG CCA GCG TAC CGG ATG A-3′, Tm=55°C; β-actin primers: 5′-TTG CTG ACA GGA TGC AGA AG-3′ and 5′-CAG TGA GGC CAG GAT AGA GC-3′, Tm=69.1°C or 58.8°C. Primer efficiency was assessed by analyzing a series dilution of RNA. The relative expression levels of each mRNA were calculated using the ΔΔCt method normalizing to β-actin and relative to the control samples. The presence of one product of the correct size was verified by both 2% agarose gel electrophoresis and melt curve analyses containing a single melt curve peak.
Statistics
Comparisons were performed by a one-way analysis of variance (ANOVA) when WT microglia were studied. Differences among WT and CD14−/− groups were analyzed using a two-way ANOVA. Onset and survival time differences determined using Kaplan–Meier survival statistics (log-rank-sum test; Number Cruncher Statistical Systems, Kaysville, UT). Data are expressed as mean±SE and p values less than 0.05 were considered statistically significant.
Results
Extracellular mSOD1G93A is not directly toxic to isolated motoneurons
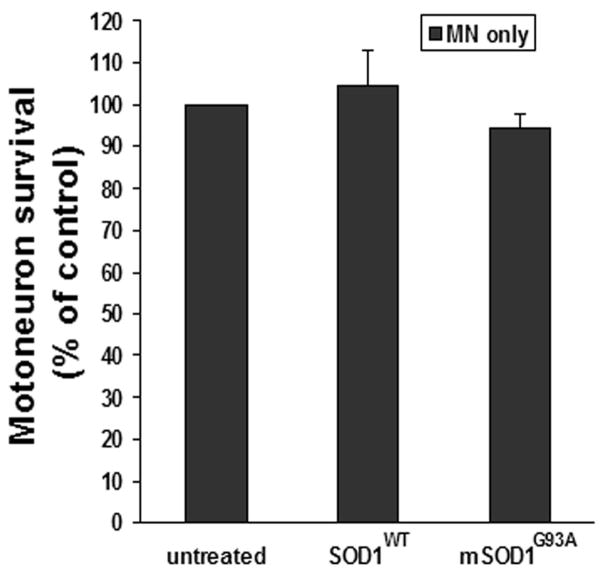
Extracellular mSOD1G93A has been documented to cause neuronal death in spinal cord mixed cell cultures (Urushitani et al., 2006). In the present study, 5 μg/ml metallated mSOD1G93A was incubated with primary motoneuron cultures (>90% purity) to examine the direct effect of extracellularly applied mSOD1G93A on motoneuron survival. After 7 days, motoneuron survival (94.7±3.2%) was not compromised compared with untreated-motoneuron (100%, representing 248±88 counted cells/coverslip; p=0.18) or SOD1WT-treated cultures (104±9.0%; p=0.37; Fig. 1). Thus, after 7 days in vitro, mSOD1G93A has no direct toxic effects on motoneurons.
Fig. 1.
Extracellular mSOD1G93A was not directly toxic to motoneuron cultures. Primary motoneuron cultures were treated with 5 μg/ml metallated (Cu/Zn) SOD1WT or mSOD1G93A for 7 days. Motoneuron survival was not decreased in mSOD1G93A-treated motoneuron cultures compared with untreated or SOD1WT-treated cultures. Motoneuron survival was expressed as a percentage of untreated cultures (100% represents 248±88 counted cells/coverslip). Data shown as mean±SE of three independent experiments with duplicate or triplicate wells. MN = motoneurons; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A.
Extracellular mSOD1 morphologically and functionally activates microglia
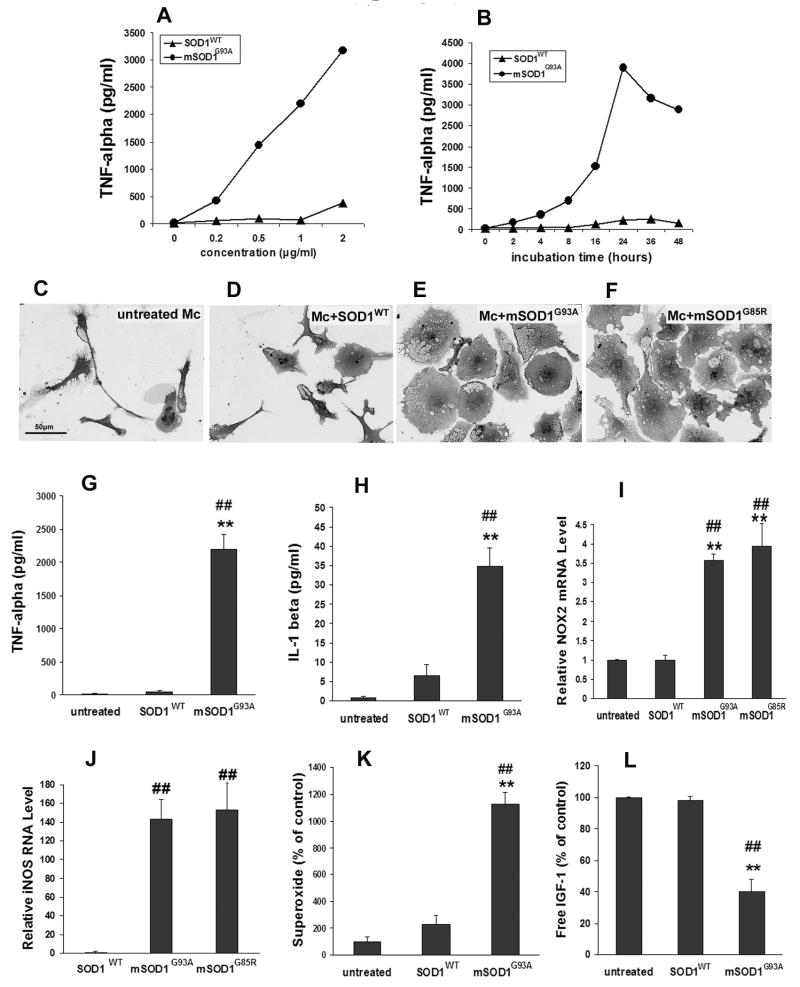
Initial experiments revealed that mSOD1G93A increased microglial production of TNF-α in both a dose- and time-dependent manner (Fig. 2A,B). To determine the direct effects of mSOD1G93A on microglia, 1 μg/ml mSOD1G93A was added to primary microglial cultures and incubated for 48hrs. Incubation of microglia with mSOD1G93A protein induced morphological changes of microglia with cell shape changing from a ramified configuration (Fig. 2C) into enlarged amoeboid cell bodies with vacuoles (Fig. 2E), suggestive of an activated microglial morphology. In contrast, most SOD1WT-treated microglia maintained a ramified morphology; only a small percentage of SOD1WT-treated microglia had an amoeboid shape (Fig. 2D). As an index of “functional” microglial activation, the levels of TNF-α and IL-1β were measured in the culture supernatants (Fig. 2G,H). TNF-α and IL-1β protein production increased 39-fold and 5.4-fold, respectively, in mSOD1G93A-treated microglia compared with SOD1WT-treated microglia (p=8E-5 and p=0.006, respectively). No changes in TNF-α and IL-1β production were observed between untreated and SOD1WT-treated microglia. NOX2, a subunit of NADPH oxidase predominantly expressed in phagocytic cells, is the major source of microglial superoxide. Following treatment with mSOD1G93A, microglial NOX2 mRNA was upregulated compared with SOD1WT-treated microglia (p=0.0003; Fig. 2I). Superoxide released from microglia was also evaluated after treatment with mSOD1G93A protein. While no difference was observed after 2 days, microglia incubated with mSOD1G93A for 4 days produce more superoxide than SOD1WT-treated microglia (4.8-fold increase, p=0.001; Fig. 2K); untreated and SOD1WT-treated microglia released similar levels of superoxide. Since inducible nitric oxide synthase (iNOS) is upregulated after microglial activation (Zhao et al., 2004; Xiao et al., 2007), iNOS mRNA expression in microglia was examined using quantitative RT-PCR after a 2-day-treatment with mSOD1G93A (Fig. 2J). Although it was undetectable in untreated microglia, iNOS mRNA increased 140-fold in mSOD1G93A-treated microglia compared with SOD1WT-treated microglia (p=0.001). Furthermore, after incubation for 2 days, mSOD1G93A reduced microglial insulin-like growth factor-1 (IGF-1) production compared with SOD1WT (p=0.0003; Fig. 2L). To determine whether the microglial response to mSOD1G93A protein was a unique response to the specific mutation, microglia were treated with a different mutant SOD1 protein, mSOD1G85R. The extracellular mSOD1G85R morphologically activated microglia (Fig. 2F) and increased NOX2 and iNOS mRNA expression in microglia to a similar extent as mSOD1G93A (Fig. 2I,J). The two mSOD1 proteins, which differ with respect to SOD1 enzymatic activity (Borchelt et al., 1994), had similar activating effects on microglia.
Fig. 2.
Effects of mSOD1G93A and mSOD1G85R on microglia cultures. A-B: Microglia were incubated with different concentrations (0-2 μg/ml) of SOD1WT or mSOD1G93A for 2 days (A) or with 1 μg/ml SOD1WT or mSOD1G93A for 0-48 hours (B). TNF-α levels were measured as the index of microglial activation. C-E: Morphology of microglia without treatment (C), treated with 1μg/ml SOD1WT (D), 1 μg/ml mSOD1G93A (E) or 1 μg/ml mSOD1G85R (F) for 2 days. G-L: After a 2-day-treatment with 1 μg/ml SOD1WT, mSOD1G93A, or mSOD1G85R, the production of TNF-α (G), IL-1β (H), NOX2 mRNA (I), iNOS mRNA expression (J) increased in mSOD1G93A- or mSOD1G85R-treated microglial cultures compared with untreated or SOD1WT-treated microglia cultures. K: mSOD1G93A-treated microglia produced more superoxide than untreated or SOD1WT-treated microglia at 4 days. L: Free IGF-1 was decreased in mSOD1G93A-treated microglial cultures compared with untreated or SOD1WT-treated cultures. There was no difference between untreated and SOD1WT-treated microglial cultures. Data shown as mean±SE of at least three independent experiments with duplicate or triplicate wells. Mc = microglia; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A, mSOD1G85R = mutant SOD1G85R. **p<0.01 vs untreated microglia; ##p<0.01 vs SOD1WT-treated microglia. Scale bar = 50 μm.
Since mSOD1G93A has been reported to induce microglial proliferation (Urushitani et al., 2006), microglial numbers were counted to evaluate the influence of cell number on the increased markers of “functional” microglial activation. Microglial numbers in the mSOD1G93A-treated cultures (1541±261 counted cells/coverslip) were only 2-fold greater than the numbers of microglia in untreated (720±65 counted cells/coverslip) or SOD1WT-treated cultures (792±91 counted cells/coverslip). Therefore, the fact that the 4.8-140 fold increase in markers for microglia activation following treatment with mSOD1G93A was greater than the two-fold microglia proliferative response induced by mSOD1G93A suggests that mSOD1G93A directly activates microglia and enhances the release of these toxic molecules from each microglial cell.
mSOD1G93A-activated microglia are neurotoxic
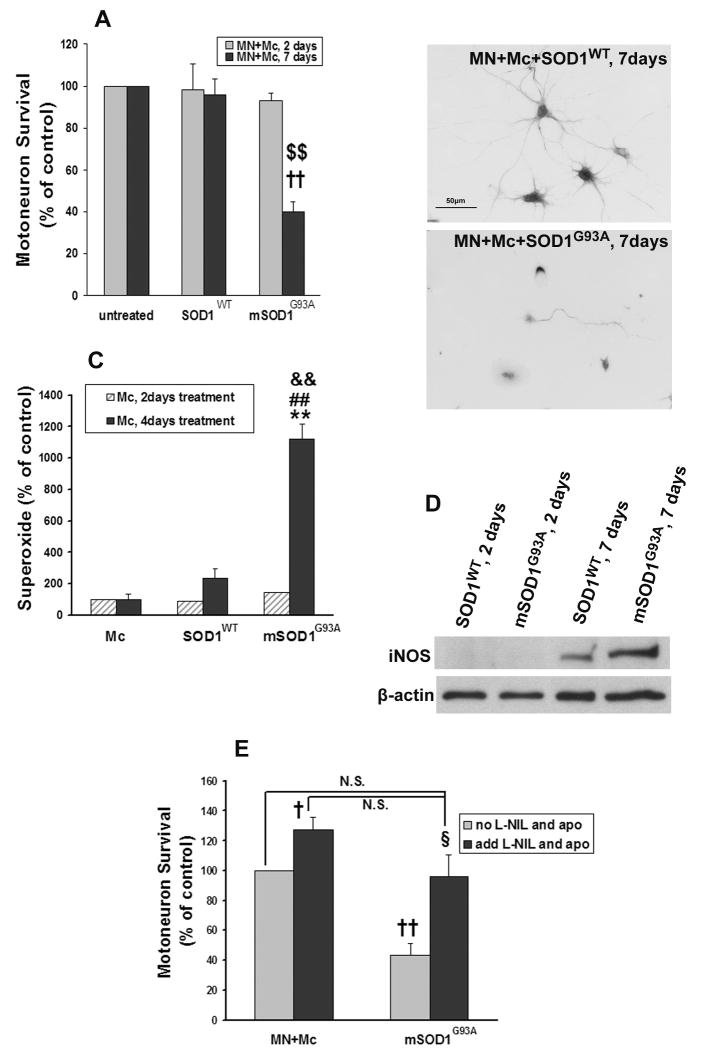
To ascertain whether mSOD1G93A-activated microglia are injurious to motoneurons, primary motoneurons were co-cultured with microglia in the presence of 1 μg/ml mSOD1G93A or SOD1WT, the identical concentration used to activate microglia. Incubating primary motoneurons and microglia with mSOD1G93A for 2 days did not induce motoneuron injury when compared with untreated or SOD1WT-treated co-cultures. However, prolonging the incubation time to 7 days and increasing the dose of mSOD1G93A to 5 μg/ml, a dose shown above not to be directly toxic to motoneurons, resulted in motoneuron loss in mSOD1G93A-treated co-cultures compared with SOD1WT-treated co-cultures (p=0.007); motoneuron survival in SOD1WT-treated co-cultures was similar to untreated co-cultures (p=0.61; Fig. 3A). Motoneuron morphology in the co-cultures was observed by immunostaining with anti-SMI-32 (Carriedo, et al., 1996). Surviving motoneurons were smaller and had fewer neurites in the mSOD1G93A-treated microglia/motoneuron co-cultures. Motoneurons in SOD1WT-treated co-cultures were uninjured and maintained large somas with extensive neuritic arborizations (Fig. 3B); motoneuron morphology was similar in appearance to the motoneurons in untreated co-cultures or when motoneurons were cultured in the absence of microglia. These data, in combination with the data presented in Fig. 1, suggest that the toxic effects of mSOD1G93A are mediated predominantly through microglia; in the absence of microglia, 5 μg/ml mSOD1G93A was not detectably toxic to motoneurons after 7 days.
Fig. 3.
mSOD1G93A induces motoneuron injury in the presence of microglia. A: mSOD1G93A reduced motoneuron survival in microglial/motoneuron co-cultures after a 7-day-treatment with 5 μg/ml mSOD1G93A. MN survival was expressed as a percentage of untreated co-cultures. B: Motoneuron morphology after a 7-day-treatment with 5 μg/ml SOD1WT or mSOD1G93A, stained by SMI-32. C: Microglia treated with mSOD1G93A for 2 days did not produce more superoxide than untreated or SOD1WT-treated microglia. After treatment for 4 days, superoxide levels in mSOD1G93A-treated microglial cultures increased compared with untreated or SOD1WT-treated microglia. D: iNOS was not detectible from microglia at 2 days with either SOD1WT or mSOD1G93A. At 7 days, mSOD1G93A-treated microglia express more iNOS than SOD1WT-treated microglia. E: The addition of apocynin and L-NIL rescued motoneuron death induced by mSOD1G93A-treated microglia. Motoneuron survival was expressed as a percentage of untreated co-cultures. MN = motoneurons; Mc = microglia; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A; Ab = antibody; apo = apocynin. †p<0.05, ††p<0.01 vs untreated microglial/motoneuron co-cultures; $$p<0.01 vs SOD1WT-treated microglial/motoneuron co-cultures; **p<0.01 vs untreated microglia; ##p<0.01 vs SOD1WT-treated microglia; &&p<0.01 vs 2-day-treatment in each group; §p<0.05 vs SOD1G93A-treated, “no apo and L-NIL” group; N.S. = no significant difference compared with “untreated groups with or without ‘apo and L-NIL’ ”. Data shown as mean±SE of three independent experiments with duplicate or triplicate wells.
Nitric oxide and superoxide mediates mSOD1G93A-induced microglia toxicity
To explore why microglia treated with 1 μg/ml mSOD1G93A for 2 days could not induce detectable motoneuron injury, the levels of superoxide and iNOS were assayed after different treatment periods. At 2 days, 1 μg/ml mSOD1G93A did not increase the release of superoxide from microglia (p=0.2), while after 4 days, mSOD1G93A treated microglia produced higher levels of superoxide than either untreated or SOD1WT-treated co-cultures (p=0.001 and p=0.002, respectively; Fig. 3C). In addition, although iNOS protein was not detectable by western analyses from microglia after 2 days of incubation with 1 μg/ml SOD1WT or mSOD1G93A, at 7 days, 5 μg/ml mSOD1G93A-treated microglia expressed more iNOS protein than SOD1WT-treated microglia (p=0.03; Fig. 3D). As additional evidence for the important roles that superoxide and nitric oxide play in mSOD1G93A-mediated neurotoxicity, apocynin, a NADPH oxidase inhibitor, and L-NIL, an iNOS inhibitor, were added to the microglial/motoneuron co-cultures. The combined application of apocynin and L-NIL to the mSOD1G93A-treated microglial/motoneuron co-cultures restored motoneuron survival compared with mSOD1G93A-treated co-cultures in the absence of apocynin and L-NIL (p=0.03; Fig. 3E).
Extracellular mSOD1G93A activates microglia through CD14 and Toll-like receptors
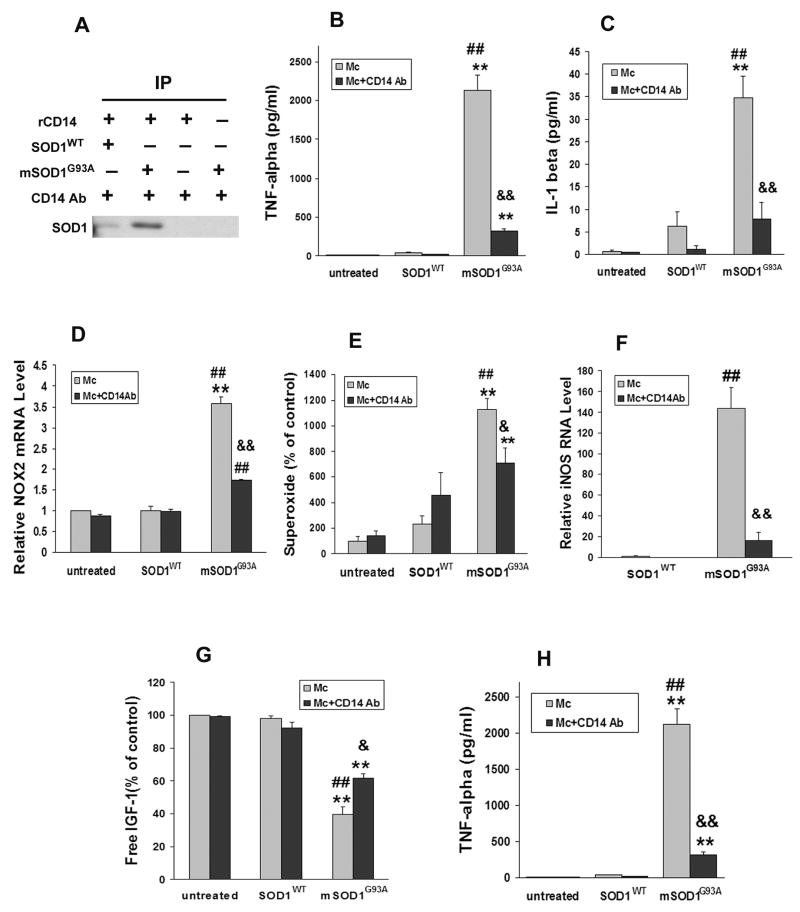
Fassbender et al. (2004) previously demonstrated that β-amyloid interacts with CD14, a lipopolysaccharide (LPS) receptor known to be involved with cellular activation. Using the immunoprecipitation technique used by Fassbender et al. (2004), mSOD1G93A was co-incubated with recombinant soluble murine CD14 (rCD14) protein. The complexes were subsequently immunoprecipitated with anti-CD14 antibody, and western analyses were performed to detect SOD1. The results showed a clear SOD1 band, demonstrating binding of mSOD1G93A to CD14 (Fig. 4A). Although anti-CD14 did immunoprecipitate SOD1WT, the signal intensity was less compared with CD14 and mSOD1G93A, suggestive of a stronger interaction between CD14 and mSOD1G93A than CD14 and SOD1WT proteins, possibly due to the misfolded confirmation of mSOD1. This weak interaction between CD14 and SOD1WT may help explain the modest, though non-significant, increase in IL-1β and superoxide release from SOD1WT stimulated microglia. The omission of rCD14 protein prevented the precipitation of mSOD1G93A.
Fig. 4.
mSOD1G93A activates microglia through CD14. A: Immunoprecipitation with anti-CD14 antibody and subsequent western blot with anti-SOD1 antibody showed binding of mSOD1G93A to rCD14 was greater than SOD1WT. Negative control included incubation of mSOD1G93A only or rCD14 only with anti-CD14 antibody. B-G: The blocking CD14 antibody (1 μg/ml) attenuated mSOD1G93A-induced microglial activation by suppressing the release of TNF-α (B), IL-1β (C), NOX2 mRNA (D), superoxide (E), iNOS expression (F), and increasing the production of free IGF-1 (G). H: WT or CD14−/− microglia were treated with 1 μg/ml SOD1WT or mSOD1G93A for 2 days. mSOD1G93A induced CD14−/− microglia to produce less TNF-α than WT microglia. Data shown as mean±SE of at least three independent experiments with duplicate or triplicate wells. Mc = microglia; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A; Ab = antibody. *p<0.05; **p<0.01 vs untreated microglia; ##p<0.01 vs SOD1WT-treated microglia; &p<0.05; &&p<0.01 vs mSOD1G93A-treated microglia.
To determine whether CD14 mediates microglial activation induced by mSOD1G93A, an antibody to CD14 was added to microglial cultures 2hrs prior to the addition of SOD1WT or mSOD1G93A. After incubating for 2 days, the CD14 blocking antibody attenuated TNF-α production by 86% and IL-1β release by 77% from mSOD1G93A-treated microglia (p=0.0001 and p=0.0044, respectively; Fig. 4B,C). Increases in NOX2 mRNA levels after 2 days and superoxide release after 4 days were also inhibited by the CD14 antibody in mSOD1G93A-treated microglial cultures (p=0.0009 and p=0.04, respectively; Fig. 4D); the change in microglial NOX2 mRNA expression closely mirrored the change in microglial superoxide production following treatment with either mSOD1G93A or the combination of mSOD1G93A and CD14 antibodies. In addition, increased iNOS expression by extracellular mSOD1G93A to microglia cultures was suppressed 88% with CD14 blocking antibodies (p<0.01; Fig. 4E). CD14 blocking antibodies also inhibited the decrease in IGF-1 levels in mSOD1G93A-treated microglia (p=0.016; Fig. 4F).
To further substantiate the role of CD14 and its involvement in mSOD1G93A-mediated microglial activation, microglia were isolated and cultured from CD14−/− mice. Microglia lacking CD14 produced 96% less TNF-α following extracellular treatment with mSOD1G93A than similarly cultured WT (CD14+/+) microglia (p=0.0006; Fig. 4F). Thus, in vitro, the activating effects of mSOD1G93A on microglia are possibly transduced through a CD14 dependent pathway.
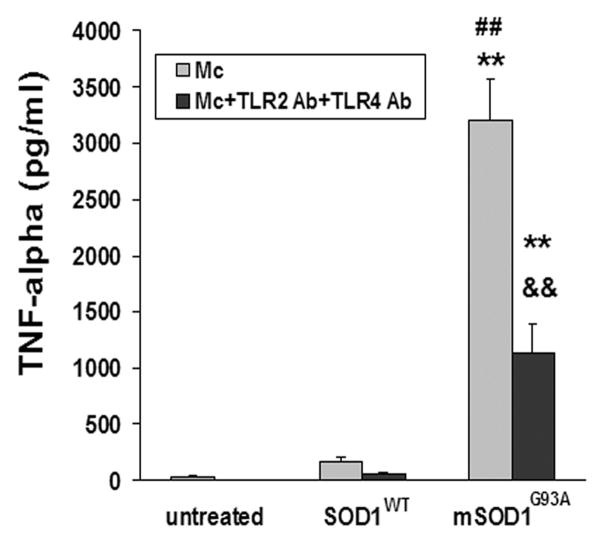
Because CD14 mediates signal transduction in conjunction with TLR2 and TLR4 (Leung et al., 2005), the inhibitory effects of extracellular mSOD1G93A-induced microglia activation were assayed using blocking antibodies specific for TLR2 and/or TLR4. TNF-α production in mSOD1G93A-treated microglia was reduced following the addition of both TLR2 and TLR4 blocking antibodies (p=0.008; Fig. 5). Motoneurons express CD14, TLR2 and TLR4 mRNAs (Tang et al., 2007); therefore, the lack of direct toxic effects of mSOD1G93A on motoneurons was not due to absence of these receptors.
Fig. 5.
TLR2 and TLR4 participate in mSOD1G93A-induced microglial activation. Combination of TLR2 and TLR4 blocking antibodies (1 μg/ml and 10 μg/ml, respectively) suppressed microglial production of TNF-α induced by mSOD1G93A. Data shown as mean±SE of at least three independent experiments with duplicate or triplicate wells. Mc = microglia; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A; Ab = antibody. **p<0.01 vs untreated microglia; ##p<0.01 vs SOD1WT-treated microglia; &&p<0.01 vs mSOD1G93A-treated microglia.
CD14 is involved in mSOD1G93A-induced neurotoxicity in vitro
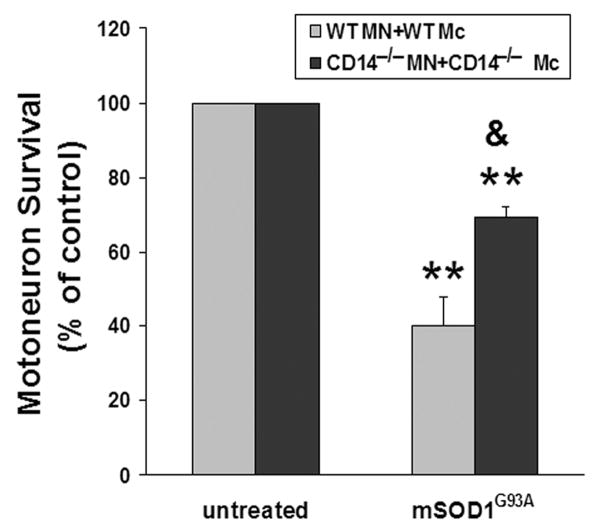
To determine whether the neurotoxicity of mSOD1G93A-activated microglia is also dependent on CD14, both microglia and motoneurons were isolated from CD14−/− mice. When CD14−/− motoneurons were co-cultured with CD14−/− microglia, motoneuron survival was partially increased compared with WT microglial/motoneuron co-cultures after incubation with 5 μg/ml mSOD1G93A for 7 days (p<0.02; Fig. 6). These data indicate that CD14 is involved in the extracellular mSOD1G93A-induced microglial neurotoxicity, although CD14 may not be the only pathway that mediates these neurotoxic effects.
Fig. 6.
CD14 is involved in mSOD1G93A-induced neurotoxicity in vitro. WT microglial/motoneuron co-cultures or CD14−/− microglial/CD14−/− motoneuron co-cultures were incubated with 5 μg/ml mSOD1G93A for 7 days. Motoneuron survival was enhanced in mSOD1G93A-treated CD14−/− co-cultures than mSOD1G93A-treated WT co-cultures. Motoneuron survival was expressed as a percentage of untreated co-cultures in each genotype group. Data shown as mean±SE of three independent experiments with duplicate or triplicate wells. MN = motoneurons; Mc = microglia; SOD1WT = wild-type SOD1; mSOD1G93A = mutant SOD1G93A. **p<0.01 vs untreated co-cultures; &p<0.05 vs mSOD1G93A-treated WT co-cultures.
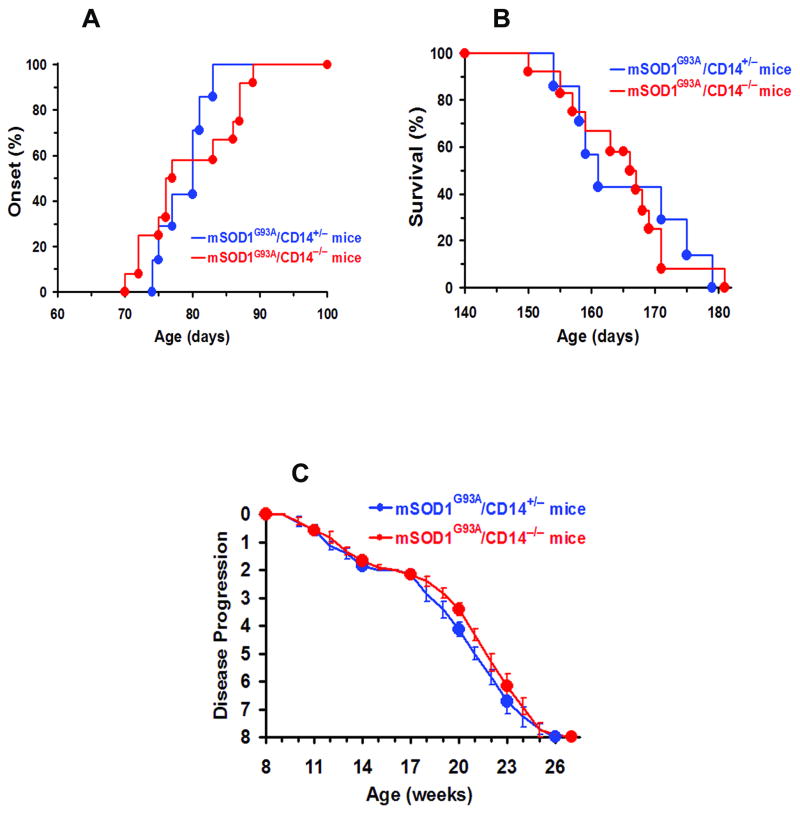
Disease course is unaltered in mSOD1G93A/CD14−/− mice
To examine whether the lack of CD14 expression alters the in vivo course of disease, mSOD1G93A mice were bred with CD14−/− mice to generate both mSOD1G93A/CD14+/− and mSOD1G93A/CD14−/− mice. However, there were no significant differences in either symptom onset or lifespan between mSOD1G93A/CD14+/− and mSOD1G93A/CD14−/− mice using previously defined criteria (Beers et al., 2008; Beers et al., 2006; Fig. 7A,B). Furthermore, the disease progression profiles were similar between mSOD1G93A/CD14+/− and mSOD1G93A/CD14−/− mice (Fig. 7C).
Fig. 7.
Disease course is unchanged in mSOD1G93A/CD14−/− mice. The disease courses were compared between mSOD1G93A/CD14−/− mice (n=12) and mSOD1G93A/CD14+/− mice (n=7). The onset (A), survival (B), and disease progression (C) of these two groups were not different.
Discussion
Previous studies have reported that mSOD1G93A or oxidized SOD1WT injure motoneurons and trigger microgliosis in spinal cord cultures (Urushitani et al., 2006; Ezzi et al., 2007). In those studies, the direct neurotoxic properties of mSOD1G93A were not differentiated from the possible indirect toxic effects mediated through microglia, a component of the innate immune system. The present study demonstrates that exogenous mSOD1G93A has minimal direct toxic effects on motoneurons and only when microglia were incubated with mSOD1G93A was mSOD1G93A toxic to motoneurons. Furthermore, mSOD1G93A was demonstrated to bind CD14 and initiate microglia activation in concert with TLR2 and TLR4. Thus, in addition to the toxic intracellular effects of mSOD1 (Beers et al., 2006; Xiao et al., 2007), these data suggest an additional mechanism whereby mSOD1 gains an extracellular toxic function.
To test the hypothesis that microglia are involved in mSOD1G93A-mediated neurotoxicity, motoneurons were co-cultured with microglia. Using the same conditions that were used in the motoneuron only cultures, co-cultures treated with the same dose of mSOD1G93A over an identical time period, the presence of microglia caused motoneuron death. The ratio between microglia and motoneurons in these co-cultures was 4:3, a substantially lower number of microglia to motoneurons than that is found in lumbar spinal cords of mSOD1 mice. This reduces the possibility that excess microglia may lead to spurious neurotoxicity. Additionally, because both components of these co-cultures were greater than 95% pure, it is unlikely that other cells contributed to the toxicity. Therefore, the present study establishes that the neurotoxic properties of exogenous mSOD1G93A were indirect and mediated through microglia; since the SOD1WT protein was purified identically to mSOD1, the observed effects were due to mutations in the SOD1 protein and not due to the protein preparation.
Several lines of evidence document the importance of microglia in mSOD1-induced motoneuron death. Microglia isolated from mice over-expressing mSOD1G93A induced more motoneuron injury than WT microglia (Xiao et al., 2007). In vivo studies have shown that replacing all mSOD1 expressing microglia with WT microglia or reducing expression of mSOD1 in microglia/macrophages, slowed motoneuron loss and prolonged disease duration and survival (Beers et al., 2006; Boillee et al., 2006). In addition, a recent study demonstrated the presence of microgliosis in mice homozygously expressing high levels of mSOD1G93A specifically in neurons (Jaarsma et al., 2008). These data indicate that other cells such as microglia may actively contribute to the disease of ALS.
Treatment for 2 days with 1 μg/ml of mSOD1G93A morphologically and functionally activated microglia. However, motoneuron death was only observed after 7 days of treatment with 5 μg/ml mSOD1G93A. A comparison of superoxide and iNOS protein between microglia from 2-day-treatment and 4- or 7-day-treatment with mSOD1G93A demonstrated that the levels of free radicals increased only after 4 days. The ability of apocynin and L-NIL to enhance motoneuron survival after 7 days also supports roles of superoxide and nitric oxide in the mSOD1G93A neurotoxicity. The fact that the loss of NOX2 expression or NADPH oxidase inhibition by apocynin slowed disease progression and prolonged survival of mSOD1G93A mice (Marden et al., 2007; Harraz et al., 2008) emphasizes the importance of oxidative stress in ALS. Thus, the neurotoxic properties of mSOD1G93A are temporal and dependent upon sufficient production of free radicals.
These data are in accord with our previous report documenting that motoneuron injury resulted from the reaction of superoxide and nitric oxide, which form the highly unstable and toxic compound peroxynitrite. Peroxynitrite initiates motoneuron injury by increasing the susceptibility of the motoneuron AMPA/kainate receptor to the toxic effects of glutamate; CNQX treatment prevented motoneuron injury (Zhao et al., 2004). Although other studies have reported that microglia-induced cortical neural injury is signaled through an NMDA receptor (Takeuchi et al., 2005), the NMDA receptor antagonist APV did not protect motoneurons from microglia-mediated injury (Zhao et al., 2004). Therefore, enhancing the vulnerability of motoneurons to glutamate by altering the AMPA/kainate receptor may be an underlying mechanism of extracellular mSOD1-induced neurotoxicity.
Because of the technical complexity, it is very difficult to determine the precise levels of extracellular mSOD1 in spinal cords of mSOD1 transgenic mice - lysed neurons within the spinal cord would contribute to the extracellular pool of mSOD1G93A. However, the estimated mSOD1 concentration in the CNS of mSOD1G93A mice is 4000 μg/ml (Gurney et al., 1994). Furthermore, extracellularly released mSOD1, mediated by chromogranin (Urushitani et al., 2006) or lysed motoneurons, may have a much higher effective local concentration. Thus, compared with the CNS concentration of 4000 μg/ml, the dose of mSOD1 used in the present study (1 or 5 μg/ml) is far below that observed in the CNS of mSOD1 mouse.
Since CD14 is a pattern recognition receptor and mutations in, or oxidation of, SOD1 leads to misfolded protein (Furukawa and O'Halloran 2005; Furukawa et al., 2006; Kabashi et al., 2007), mSOD1G93A may be recognized by CD14 and in turn activate microglia. This study demonstrated that mSOD1G93A binds to CD14 and that CD14 blocking antibody attenuated the production of several pro-inflammatory cytokines, free radicals and increase IGF-1 release from microglia treated with mSOD1G93A. CD14−/− microglia treated with mSOD1G93A produced less TNF-α and when co-cultured with CD14−/− motoneurons, motoneuron survival was enhanced compared with WT microglia/motoneuron co-cultures. When mSOD1G93A treated CD14−/− microglia were co-cultured with WT motoneurons (data not shown), the increased motoneuron survival was modest, most likely due to the release of soluble CD14 from the motoneurons. Therefore, these data support the important role of CD14 in the activation and toxicity of mSOD1G93A-treated microglia.
Two important CD14 co-receptors are TLR2 and TLR4. Using antibodies that block TLR2/TLR4 signal induction inhibited microglial activation caused by extracellular mSOD1G93A, suggesting that TLRs are involved in the signal cascade of microglial activation. However, individually each inhibitory antibody resulted in only a slight inhibitory effect. Only with the combination of TLR2 and TLR4 inhibitory antibodies was the microglial suppression significant; therefore, the toxic signals induced by extracellular mSOD1G93A are possibly transduced through both TLR2 and TLR4 pathways. Other lines of evidence indeed suggest that CD14/TLRs may contribute to the inflammatory responses initiated by microglia (Olson and Miller, 2004; Jack et al., 2005). Expression of CD14 is increased in spinal cords of both ALS patients and mSOD1 mice (Henkel et al., 2004; Henkel et al., 2006). Upregulation of CD14 and TLR2 in phagocytes are common in transgenic models of Alzheimer's disease, Parkinson's disease, and ALS (Letiembre et al., 2007). Aβ fibrils bind to CD14 and activate microglia (Fassbender et al., 2004), and anti-CD14 strategies reduced the Aβ-stimulated microglia neurotoxicity (Bate et al., 2004). Additionally, chronic stimulation of CD14/TLR pathway by LPS was found to exacerbate disease in ALS mice (Nguyen et al., 2004).
An important component of the CD14/TLR signaling cascade is MyD88, which is utilized by all TLR except TLR3 and part of TLR4 (Watters et al., 2007). In agreement with our present data, Kang and Rivest (2007) demonstrated that mSOD1 injected into the central nervous system of mice activated microglia and increased TLR2 expression via a MyD88 dependent pathway. While the lack of MyD88 expression in mSOD1G37R mice did not influence the disease course, MyD88-deficient bone marrow cells accelerated disease onset and reduced survival in mSOD1G37R mice, suggesting that peripheral cells expressing MyD88 are beneficial; the absence of an altered disease course in MyD88 deficient mSOD1G37R mice may be that any attributable protective effects of peripheral MyD88 cells are counteracted by the toxic effects of CNS MyD88 cells. In our study, the lack of CD14 expression in mSOD1 mice also did not influence disease onset or survival. Since CD14 and MyD88 share similar downstream pathways, a similar hypotheses could be applicable to CD14, namely that the peripheral and CNS CD14 cells may have opposite functions. Therefore, to further examine the effects of CD14 or the TLR in mSOD1 mice, these receptors may need to be specifically depleted in the CNS. Secondly, these in vivo results suggest that in the absence of CD14, other receptors may substitute for CD14 and mediate mSOD1-induced microglial neurotoxicity. Echchannaoui et al. (2005) reported that although knocking-out CD14 did not impair systemic host defenses, CD14 deficiency caused excessive meningeal inflammation and early death of CD14−/− mice after S. pneumoniae infection. In our in vitro systems, the lack of CD14 expression only partially reversed motoneuron injury and TLR2/TLR4 were also involved in mSOD1G93A-induced microglial activation. Unlike CD14, TLR can bind to ligands and directly transduce cellular signals. In addition, Aβ interacts with microglia to induce neurotoxicity through numerous microglial receptors including scavenger receptors (El Khoury et al., 2003, 1996), CD40 (Townsend et al., 2005), and complement receptors (Eikelenboom and Veerhuis, 1996). Collectively, these data support the notion of putative compensatory mechanisms due to the developmental absence of CD14 and that other microglial receptors also mediate extracellular mSOD1 neurotoxicity.
Microgliosis is also a prominent neuropathological feature observed in the spinal cords of sALS patients (McGeer and McGeer, 2002). Because altered SOD1 species are found within the spinal cords of sALS patients and since oxidized SOD1WT can acquire toxic properties similar to mSOD1 triggering microgliosis (Gruzman et al. 2007; Ezzi et al., 2007), possibly linking a shared pathophysiologic pathway between sALS and fALS, the present data may be relevant to sALS. Thus, this study demonstrates a link between extracellular mSOD1, microglial activation, and neurodegeneration and adds to the cumulative evidence supporting the non-cell-autonomous nature of motoneuron toxicity in ALS.
Acknowledgments
We thank Jinghong Wang, Meiyue Chen, Yvonne K Henry, Derek Cridebring, Shixiang Wen and Xiaoli Wang for their technical assistance. This study was supported by grants from the NIH (NS048950), the Muscular Dystrophy Association. The authors have no conflicting financial interests.
Abbreviations list
- ALS
amyotrophic lateral sclerosis
- fALS
familiar ALS
- sALS
sporadic ALS
- IGF-1
Insulin-like Growth Factor-1
- LPS
lipopolysaccharide
- SOD1
Cu2+/Zn2+ superoxide dismutase 1
- mSOD1
mutant SOD1
- mSOD1G93A
G93A mutant form of SOD1
- SOD1WT
wild-type SOD1
- WT
wild-type
- TLR
Toll-like receptor
References
- Arce V, Garces A, de Bovis B, Filippi P, Henderson C, Pettmann B, deLapeyrière O. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55:119–126. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bate C, Veerhuis R, Eikelenboom P, Williams A. Microglia kill amyloid-beta1-42 damaged neurons by a CD14-dependent process. Neuroreport. 2004;15:1427–1430. doi: 10.1097/01.wnr.0000132203.76836.16. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T-Cells Support Glial Neuroprotection, Slow Disease Progression, and Modify Glial Morphology in an Animal Model of Inherited ALS. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-Type Microglia Extend Survival in PU.1 Knockout Mice with Familial Amyotrophic Lateral Sclerosis. Proc Natl Acad Sci USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci USA. 1994;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Echchannaoui H, Frei K, Letiembre M, Strieter RM, Adachi Y, Landmann R. CD14 deficiency leads to increased MIP-2 production, CXCR2 expression, neutrophil transmigration, and early death in pneumococcal infection. J Leukoc Biol. 2005;78:705–715. doi: 10.1189/jlb.0205063. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1996;17:673–680. doi: 10.1016/0197-4580(96)00108-x. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kühl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, O'Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo- and reduced form of SOD1, leading to unfolding and oxidative aggregation. J Biol Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Fu R, Deng HX, Siddique T, O'Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci USA. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R, Wood TD, Cleveland DW, Lingappa VR, Liu J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Rouleau GA. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann Neurol. 2007;62:553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- Kang J, Rivest S. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J Cell Biol. 2007;179:1219–1230. doi: 10.1083/jcb.200705046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Leung TF, Tang NLS, Wong GWK, Fok TF. CD14 and toll-like receptors: potential contribution of genetic factors and mechanisms to inflammation and allergy. Curr Drug Targets Inflamm Allergy. 2005;4:169–175. doi: 10.2174/1568010053586336. [DOI] [PubMed] [Google Scholar]
- Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomen-Hoerth C. Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol. 2008;28:205–211. doi: 10.1055/s-2008-1062265. [DOI] [PubMed] [Google Scholar]
- Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, D'Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KP, Town T, Mori T, Lue LF, Shytle D, Sanberg PR, Morgan D, Fernandez F, Flavell RA, Tan J. CD40 signaling regulates innate and adaptive activation of microglia in response to amyloid beta-peptide. Eur J Immunol. 2005;35:901–910. doi: 10.1002/eji.200425585. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Kurisu J, Tateno M, Hatakeyama S, Nakayama K, Kato S, Takahashi R. CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J Neurochem. 2004;90:231–244. doi: 10.1111/j.1471-4159.2004.02486.x. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR. Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol Dis. 2002;10:128–138. doi: 10.1006/nbdi.2002.0498. [DOI] [PubMed] [Google Scholar]
- Watters TM, Kenny EF, O'Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH. Mutant SOD1G93A microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci USA. 2008a;105:7594–7599. doi: 10.1073/pnas.0802556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008b;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J Neuropathol Exp Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]