Abstract
Objective
ACAT2 is a major cholesterol esterification enzyme specifically expressed in hepatocytes and may control the amount of hepatic free (unesterified) cholesterol available for secretion into bile or into HDL. This study aims to further elucidate physiologic roles of ACAT2 in human hepatic cholesterol metabolism.
Methods and Results
Liver biopsies from 40 normolipidemic, non-obese gallstone patients including some gallstone-free patients (female/male, 18/22) were collected and analyzed for microsomal ACAT2 activity, protein and mRNA expression. Plasma HDL-cholesterol (HDL-C) was significantly higher in females than in males, while triglycerides were significantly lower. ACAT2 activity in females was significantly lower than observed in males, regardless of the presence of gallstone disease. Moreover, the activity of ACAT2 correlated negatively with plasma levels of HDL-C (r=−0.57, P<0.05) and with Apo AI (r=−0.49, P<0.05).
Conclusion
This is the first description of a gender-related difference in hepatic ACAT2 activity in normolipidemic non-obese Chinese patients suggesting a possible role for ACAT2 in the regulation of cholesterol metabolism in humans. The negative correlation between ACAT2 activity and HDL-C or Apo AI may reflect this regulation. Since ACAT2 activity generally has been found to be pro-atherogenic in animal models, the observed sex-related difference may contribute to female protection from complications of coronary heart disease (CHD).
Keywords: ACAT2, Cholesteryl ester, HDL, liver, gender
Introduction
Deposit of cholesteryl esters in the arterial wall is critical for the formation of atherosclerosis. Intracellular free cholesterol is converted into cholesteryl ester by acyl-Coenzyme A: cholesterol acyltransferase (ACAT), also known as steroyl O-acyltransferase (SOAT). Two different ACAT enzymes have been identified in vertebrates: ACAT1 and ACAT2 1. Whereas ACAT1 is located in most tissues of the body, the expression of the ACAT2 protein is limited to only two cell types, enterocytes in the small intestine and hepatocytes 1. Immunofluorescence analysis of human liver has shown that ACAT2 is expressed in the endoplasmic reticulum of the hepatocytes, whereas ACAT1 was only detected within Kupffer cells 2. In newly secreted ApoB-containing lipoproteins, ACAT2 determines the amount of cholesteryl esters 3, of which the most commonly generated products are cholesteryl oleate and cholesteryl palmitate. The function of ACAT2 has been studied in mice 3–5 and in non-human primate models 6–8. Acat2 deficiency in mice can prevent atherosclerosis mainly due to decreased VLDL and LDL-cholesteryl oleate formation which leads to a shift in lipoprotein particle cholesteryl ester composition 9, 10. Further, ACAT2 antisense oligonucleotide knockdown mice have shown a reduced hepatic cholesterol content, normal to low biliary cholesterol secretion, but fecal neutral sterol excretion equivalent to that seen in wild type mice11. The data suggest that ACAT2 depletion causes an upregulation of the putative pathway for hepatic secretion of cholesterol with direct trafficking to the intestine, as has also been suggested by others12. However, the applicability of these data to humans and the role of ACAT2 in human liver in this aspect of cholesterol metabolism have yet to be studied. Many of the known aspects of regulation of hepatic ACAT2 were presented in a recent report on Swedish gallstone patients 2 but, in humans, indications of hepatic cholesterol physiology relative to ACAT2 are much more difficult to derive.
In order to further elucidate the physiologic role of ACAT2 in human cholesterol metabolism, the microsomal activity and expression of ACAT2 was evaluated in the liver tissue of gallstone and gallstone-free patients. Some of the patient material originated from a cohort previously enrolled in a study evaluating the genesis of gallstone disease in Chinese patients 13. We applied this strategy because this study did not show any correlations between ACAT2 activity and underlying gallstone disease 13. A clear gender-related difference was discovered in hepatic ACAT2 activity and furthermore, when graphed across genders, a significant inverse correlation existed between ACAT2 activity and HDL-cholesterol (HDL-C) as well as between ACAT2 activity and Apo AI levels in plasma.
Methods
Subjects
Eighteen females and 22 males with or without cholesterol gallstones were either selected from a cohort previously investigated for putative molecular defects underlying gallstone formation13 or were donors of liver transplantation (n=9). None of the patients or the liver donors had any medical history of disorders affecting the hepatic, gastrointestinal, renal and endocrine functions. Ongoing lipid lowering treatments or hormone replacement therapies represented criteria for exclusion. The patients were all normolipidemic non-obese Chinese. Informed consent was obtained from each participant and the study protocol was approved by the Ethical Committees at Ruijin Hospital, Shanghai Jiaotong University School of Medicine and the Karolinska University Hospital at Huddinge. Except for the transplantation liver donors, patients were fasted overnight prior to surgery, which was performed between 9 and 10 AM. During surgery, a wedge biopsy of about 0.5g was taken from the liver and immediately snap-frozen in liquid nitrogen. All samples were stored at −80°C.
Analysis of plasma lipids
Plasma total and HDL-cholesterol, triglycerides, Apo AI, and Apo B were analyzed on automated bioanalyzers (Roche Hitachi Modular P800, Japan) according to standard procedures. LDL-cholesterol (LDL-C) in plasma was calculated according to the Friedewald’s equation. The conversion factor for cholesterol from mmol/L to mg/dL is 38.67 and for triglycerides is 88.57.
Analysis of hepatic cholesterol
Crude liver homogenates were prepared as described previously 14. In brief, to 20 μl of homogenized liver suspension, 2H7-cholesterol and chloroform-methanol (2:1, v/v) were added. The chloroform phase was collected and then evaporated. The residue was either hydrolyzed with 0.5M KOH, extracted with hexane, and converted into trimethylsilyl ether derivatives, or directly converted into trimethysilyl ether derivatives before analysis by gas-liquid chromatography-mass spectrometry15. Esterified cholesterol was calculated by difference. Protein content of the homogenate was determined by the Lowry method 16.
Assay of microsomal ACAT1 and ACAT2 activity
Total ACAT enzymatic activity was determined in hepatic microsomes as previously reported 17, except that the pre-incubation step with cholesterol-saturated solution of β-hydroxypropyl cyclodextrin was prolonged to 30 minutes before the addition of 14C-oleoyl Co-A. In order to separately identify ACAT1 and ACAT2 activities, pyripyropene A, a specific ACAT2 inhibitor18, was added to the pre-incubation and reaction mixture at a concentration of 5 μM as described previously 2.
Relative RNA expression level measurements
Hepatic total RNA was extracted with Trizol (In vitrogen, Carlsbad, USA). One microgram of total RNA was transcribed into cDNA using the Omniscript kit (Qiagen Inc, Valencia, USA). Real-time quantitative PCR assay was carried out with cDNA samples in triplicate using SYBR Green I (MedProbe, Oslo, Norway), using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, USA). The primers, bridging exon-exon boundaries, were designed with Primer Express 2.0 (Applied Biosystem; primer sequences are available on request). The data are expressed in arbitrary units that were normalized to the signal obtained when the same cDNA was analyzed for cyclophilin A mRNA.
Western Blot analysis
Western blot analysis of ACAT2 was performed as described 2, 8 using antibodies directed against the N-terminal sequence of monkey ACAT2 2, 8. Prior to loading onto gels, the microsomal protein samples were suspended in Laemli buffer and incubated at 37°C for 30 minutes with dithiothreitol (final concentration of 100mmol/L). Detection was accomplished by chemiluminescence and quantified using a Fuji BAS 1800 analyzer (Fuji Photo Film Co.) with the Image Gauge software (Science Lab, 98, version 3.12, Fuji Photo Film Co.), and standardized for the intensity of the reference sample (R) loaded on each blot.
Stastistics
Data are presented as means ± SEM. The significance of differences between groups was tested by 2-Way ANOVA (factor 1 = sex; factor 2 = gallstone disease) followed, when appropriate, by post-hoc analysis according to LSD-test (Statistica software, Stat Soft, Tulsa, OK). Variables were correlated by linear Least Squares Regression. In order to meet the criteria of homoscedasticity between variables, ACAT2 activity was log-transformed, prior regression analysis. Statistical significance was set at P < 0.05.
Results
Plasma lipid analysis
All the male and female patients were matched by age and BMI [age: 44.9±2.1 years (range: 30–50) v.s. 39.4±2.7 years (range 23–61); BMI: 22.2±0.81 v.s. 23.7 ± 0.61 in females and males respectively]. No significant differences were seen in total cholesterol or in LDL-C, while HDL-C was significantly higher in females (Table 1, P < 0.01). Conversely, the plasma triglycerides were significantly lower in females (Table IP < 0.05).
Table 1.
Clinical characteristics and plasma lipids between sexes
| Female (n=18) | Male (n=13) | |
|---|---|---|
| Cholesterol mmol/L | 4.16 ± 0.36 | 3.99 ± 0.28 |
| Triglycerides mmol/L | 1.38 ± 0.19* | 2.11 ± 0.22 |
| HDL-cholesterol mmol/L | 1.21 ± 0.10** | 0.84 ± 0.05 |
| LDL-cholesterol mmol/L | 2.39 ± 0.24 | 2.19 ± 0.23 |
| Apo AI g/L | 1.21 ± 0.08** | 1.01 ± 0.06 |
| Apo B g/L | 0.75 ± 0.05 | 0.72 ± 0.05 |
P < 0.05 and
P <0.01 compared with males. Plasma sample were not available from liver donor of transplantation. Conversion factor for cholesterol from mmol/L to mg/dL is 38.67 and for triglycerides is 88.57.
Lower hepatic microsomal ACAT2 activity in females
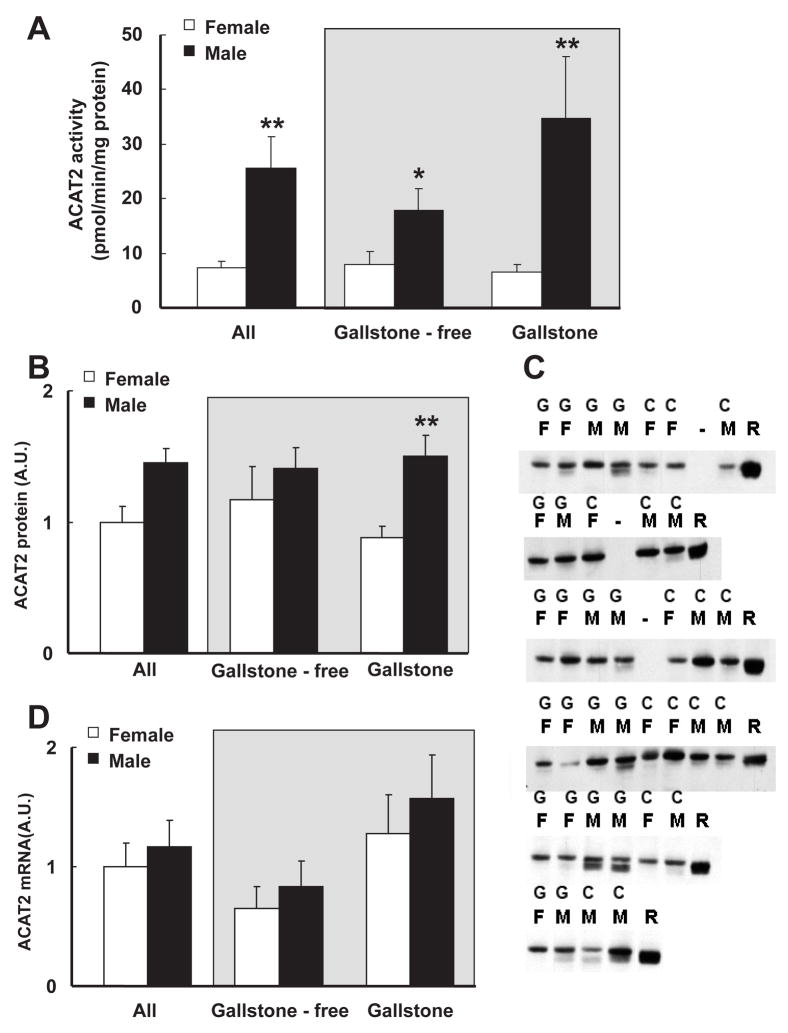
In the total material (n=40), the liver microsomal activity of ACAT2 was 72% lower in females than in males (7.24 ± 1.22 pmol/min/mg protein in females vs. 25.52 ± 5.75 pmol/min/mg protein in males, P < 0.01, Figure 1A). The sex-related difference could still be distinguished when the data on the liver microsomal activity of ACAT2 was stratified by gallstone disease (Figure 1A, gray inset). Due to shortage of liver material Western blot analysis was only possible in 37 patients. The gender-related pattern was only preserved for the ACAT2 protein content in gallstone patients, as the protein expression of ACAT2 in liver microsomes was lower in females (Figure 1B). No significant differences in mRNA expression of ACAT2 were observed between females and males, regardless of the presence of gallstone disease (Figure 1D).
Figure 1. Activity, protein and mRNA expression levels of ACAT2 in liver microsomes between females and males.
A ACAT2 activities between females (open bar, n=18) and males (close bar, n=22). ACAT2 activity was 71% lower in females than in males, P < 0.01, regardless of the presence of gallstone disease (grey inset).
B. Protein levels of ACAT2 between females (open bar, n=17) and males (close bar, n=20). ACAT2 protein level was decreased by 41% in female gallstone patients compared with male gallstone patients, P < 0.05.
C. ACAT2 protein level determined by Western Blot. Equal amounts of microsomal protein were loaded, separated by SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking, blots were incubated with rabbit anti-ACAT2 antibodies overnight. Washing with PBST, anti-rabbit IgG secondary antibody was added. Detection was preceded with chemiluminescent reagents and exposed on films. Bands at around 48 kDa representing ACAT2 protein were present in all the samples. ‘F’ represents female, ‘M’ represents male and ‘R’ represents a reference sample in each blot.
D. No difference of ACAT2 mRNA expression level was observed between females (open bar, n=18) and males (close bar, n=22).
A negative correlation exists between the activity of ACAT2 and plasma HDL and Apo A1 levels
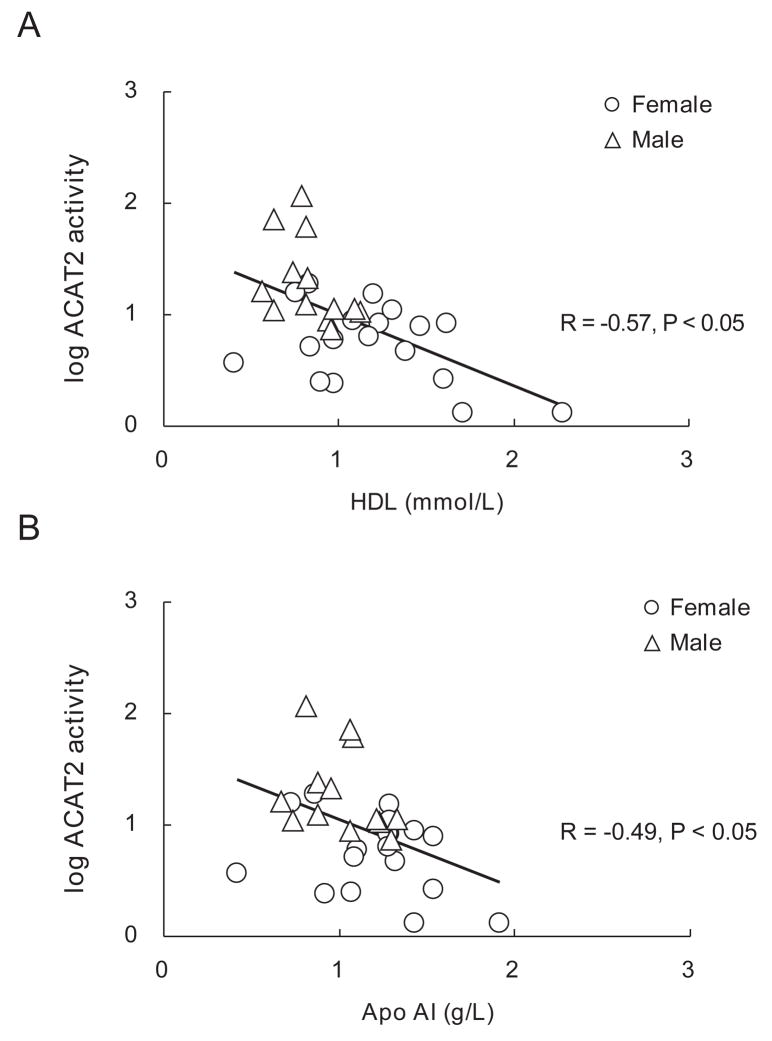
As ACAT2 catalyzes the formation of cholesteryl esters available for secretion into nascent VLDL, lower activity may lead to an increase in the flux of free cholesterol into HDL cholesterol secretion via hepatic ABCA1, although our data in mice do not typically support this. Interestingly, when plotted across genders, a significant negative correlation was observed between the liver ACAT2 activity and plasma HDL-C (r = −0.57, P < 0.05, Figure 2A), and a similar negative correlation was discovered between the liver ACAT2 activity and the plasma levels of Apo AI (r = −0.49, P < 0.05, Figure 2B). However, no correlation was detected between the hepatic ACAT2 mRNA and the plasma levels of HDL-C or Apo A1. Neither was any correlation observed between the plasma levels of triglycerides and HDL-C.
Figure 2. Correlation between ACAT2 activities with plasma lipids.
A. Negative correlation between ACAT2 activity with plasma HDL-C level (n=31), R = −0.57, P<0.05. Plasma sample were not available from liver donor of transplantation.
B. Negative correlation between ACAT2 activity with plasma Apo AI level (n=31), R = −0.49, P<0.05. Plasma sample were not available from liver donor of transplantation.
The gender-related difference in HDL-C is not related to differences in hepatic cholesterol, or expression of SRBI, ABCA1, or Apo A1
In liver homogenates, free cholesterol and cholesteryl ester concentrations were similar in female and male participants (Supplementary figure 1).
No gender-related differences were observed for the protein and mRNA expression of scavenger receptor B type I (SRBI), the mRNA expression of ATP binding cassette (ABC) A1 or the mRNA expression of Apo AI (Supplementary figure 2), with one exception: Apo AI mRNA levels were significantly higher in female gallstone-free patients (Supplementary figure 2). Neither did the expression levels of these genes show any correlations with plasma levels of HDL-C, or the microsomal activity of ACAT2.
mRNA expression of other genes involving hepatic cholesterol metabolism
The mRNA levels for eight other genes regulating hepatic cholesterol and lipoprotein metabolism (e.g. cholesterol synthesis, secretion and uptake) were also measured (Supplementary figure 2). None of these genes significantly differed between the sexes except for the LDL receptor. In female patients with gallstone disease, the LDL receptor mRNA levels were lower by approximately 70% compared to the levels in males.
Discussion
This study carried out on age and weight matched Chinese patients with and without gallstones showed for the first time: an important sex-related difference in hepatic ACAT2 activity, and an interesting negative correlation between the hepatic activity of ACAT2 and the plasma levels of HDL-C and Apo A1. However, no significant differences were observed for HDL-C or ACAT2 activity in the liver when patients with and without gallstones were compared, confirming our previous results13.
Hepatic ACAT2 plays a key role in the synthesis and composition of Apo B-containing lipoproteins, as documented in ACAT2 deficient mice, where the hepatic CE secretion into Apo B-containing lipoproteins is reduced 3, 9. In several mouse models, ACAT2 has been shown to be “pro-atherogenic” and its depletion by targeted disruption leads to a significant reduction of atherosclerotic lesions 1, 19. This reduction occurs despite elevations in plasma Apo B 9, and the composition of these lipoprotein particles contain more triglycerides than ACAT2-derived cholesteryl esters. In humans, several publications describe that higher proportions of cholesteryl linoleate - the enzymatic product of LCAT - in plasma cholesteryl esters are associated with a reduced incidence of complications from coronary heart disease (CHD) (for review see reference1). In the Atherosclerosis Risk in Communities (ARIC) study, Ma et al.20 found that the average carotid intima-media thickness was positively associated with the proportion of ACAT2-derived CE in plasma (cholesteryl palmitate and cholesteryl oleate), and the association was significant for both men and women. This association was independent of age, cigarette smoking, LDL-C, HDL-C, body mass index, diabetes, and hypertension. The ULSAM studies of Warensjo, et al 21 provide even stronger evidence for the importance of ACAT2 derived cholesteryl esters in CHD because, in over 2000 men with 461 cases of death from cardiovascular disease, a statistically significant positive association was found between the cholesteryl oleate and cholesteryl palmitate content of plasma lipoproteins and cardiovascular disease death. Interestingly, the percentage of cholesteryl linoleate was inversely associated with death from cardiovascular disease.
In this light, the observation that a lower microsomal activity of ACAT2 in the liver of female Chinese patients has even greater potential significance. It is well-known that in females atherosclerosis development starts later than in males, a phenomenon also observed in the Chinese population. In the MONICA study, it has been shown that the number of CHD events rate in Chinese males is 79/100000 persons/year and 37/100000 persons/year in Chinese females 22. Similarly, in the InterAsia survey, it has been reported that the incidence of CVD in China is increasing and is 36–60% higher in males than in females 23. This difference is irrespective of the area in which the population lives: urban vs. rural areas and north vs. south parts of the country. Moreover, in another study on the Chinese population, it has been shown that gender is a risk factor related to acute myocardial infarction24. Thus, the gender-related difference in ACAT2 activity may contribute to the difference in relative female protection against CVD incidence. Since almost all the females included in this study were pre-menopausal, further studies are needed to evaluate whether hepatic microsomal ACAT2 activity differs between fertile and post-menopausal females.
It has been recognized that ABCA1 is required to facilitate efflux of cholesterol and phospholipids to newly secreted, lipid-poor Apo AI in order to form nascent HDL particles - for review see 25. This is supported by results showing that in mice genetically deficient for the hepatic ABCA1, plasma HDL cholesterol was reduced by 80% 26. Increased availability of free cholesterol for delivery to HDL cannot be excluded by the observation of similar free hepatic cholesterol levels in men and women. On the contrary, this observation suggests that the free cholesterol might be effluxed and not stored in the hepatocytes. The lack of correlation between the molar percentage of biliary cholesterol and the liver microsomal ACAT2 activity 13 argues against an increased elimination of free cholesterol via the bile. It is also less probable that a larger quantity of free cholesterol is converted into bile acids since females have lower bile acid synthetic rate 27, 28. Thus the greater availability of free cholesterol, which should result from a lower activity of ACAT2 in the liver, may be secreted into HDL by ABCA1 or may be secreted into plasma for transport to the intestine for direct excretion 11. We were unable to determine ABCA1 protein expression in liver membranes from our patients. Thus, we cannot demonstrate if the elevated HDL-cholesterol levels are secondary to an increased expression of the ABCA1 protein. Nevertheless, treatment of 0.2% cholesterol fed C57Bl/6 mice with an ACAT2-antisense oligonucleotide targeted to disrupt the hepatic expression (with an associated decrease in ACAT2 activity in the liver) resulted in a >2-fold increase in ABCA1 protein expression and in the formation of larger HDL particles (Parini, personal observation).
Most correlations observed in our study between protein levels, activity, and the mRNA expression of ACAT2 did not reach significance, suggesting that post-transcriptional regulation of the enzyme may exist. Previous studies performed with rat liver microsomes suggested that total ACAT activity could be modulated by phosphorylation/de-phosphorylation 29, 30. Unfortunately, those studies were performed prior to the identification of the two ACAT isoforms (ACAT1 and ACAT2). Thus, it has not been demonstrated whether or not ACAT2 is phosphorylated to regulate activity.
In ACAT2 knockout mice 3, 9 and in mice treated with ACAT2-anti-sense oligonucleotides 31, the decrease in hepatic ACAT2 activity was accompanied by a corresponding increase in triglycerides in the VLDL particles. Magkos et al 32 recently reported that in women, the liver turns out fewer VLDL particles which are enriched in their triglyceride content, a finding which would be consistent with the sex-related difference we have documented for hepatic ACAT2 activity. Unfortunately, due to insufficient supply of sample material, we were not able to characterize the lipid and apolipoprotein content of each lipoprotein class in our patients.
In conclusion, we have described a gender-related difference in hepatic ACAT2 activity in normolipidemic non-obese Chinese patients, with females having significantly lower activity than males. The data are consistent with a possibility that ACAT2 may have a role in HDL metabolism, although the candidate mechanisms are still to be identified. The inverse correlation between hepatic ACAT2 activity and plasma HDL cholesterol levels might not necessarily be evident in populations of different ethnicity, because different dietary habits could influence the results. Nonetheless, our patient cohort originates from the Shanghai urban area, in which during the last decades the percentage of energy from dietary fat has risen to levels similar to what is described for the Malmö area in Sweden 33, 34.
Supplementary Material
Supplementary figure 1. Hepatic free cholesterol and cholesteryl ester between females and males.
Hepatic free cholesterol and cholesteryl ester concentrations (A. mg/g protein; B. mg/g weight of liver) were similar in females (n=14) and males (n=20). Data expressed as means ± SEM.
Supplementary figure 2. Expression of genes involving hepatic lipids metabolism
A. mRNA and protein expression levels of SRBI and mRNA expression levels of ABCA1 and APOA1 between females (open bar, n=18) and males (close bar, n=22). Data expressed as means ± SEM.
B. mRNA expression of other genes involving hepatic lipids metabolism between females (open bar, n=18) and males (close bar, n=22). Data expressed as means ± SEM.
Acknowledgments
We would like to thank Lisbet Benthin, Lillian Larsson, Xing-Xing Cai and Zhi-Hong Jiang for the valuable technical help. Dr H. Tomoda and Dr S. Ōmura for kindly donating PPPA.
This work was supported by the Swedish Research Council, by an NIH grant (HL-49373), by the Swedish Medical Association, and by the Swedish Heart-Lung and the Throne Holst Foundations, and by the Ruth and Richard Julin Foundation, and by the Karolinska Institute, and by the National Natural Science Foundation of China (No30672042 and No30700310).
Paolo Parini is recipient of a grant from AstraZeneca AB, Sweden.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudel LL, Lee RG, Parini P. ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1112–1118. doi: 10.1161/01.ATV.0000166548.65753.1e. [DOI] [PubMed] [Google Scholar]
- 2.Parini P, Davis M, Lada AT, Erickson SK, Wright TL, Gustafsson U, Sahlin S, Einarsson C, Eriksson M, Angelin B, Tomoda H, Omura S, Willingham MC, Rudel LL. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 2004;110:2017–2023. doi: 10.1161/01.CIR.0000143163.76212.0B. [DOI] [PubMed] [Google Scholar]
- 3.Lee RG, Shah R, Sawyer JK, Hamilton RL, Parks JS, Rudel LL. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J Lipid Res. 2005;46:1205–1212. doi: 10.1194/jlr.M500018-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Lee RG, Kelley KL, Sawyer JK, Farese RV, Jr, Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circulation research. 2004;95:998–1004. doi: 10.1161/01.RES.0000147558.15554.67. [DOI] [PubMed] [Google Scholar]
- 5.Repa JJ, Buhman KK, Farese RV, Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- 6.Rudel LL, Davis M, Sawyer J, Shah R, Wallace J. Primates highly responsive to dietary cholesterol up-regulate hepatic ACAT2, and less responsive primates do not. J Biol Chem. 2002;277:31401–31406. doi: 10.1074/jbc.M204106200. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 8.Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res. 2000;41:1991–2001. [PubMed] [Google Scholar]
- 9.Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV., Jr Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1262–1267. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nature medicine. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 11.Brown JM, Bell TA, 3rd, Alger HM, Sawyer JK, Smith TL, Kelley K, Shah R, Wilson MD, Davis MA, Lee RG, Graham MJ, Crooke RM, Rudel LL. Targeted depletion of hepatic ACAT2-driven cholesterol esterification reveals a non-biliary route for fecal neutral sterol loss. J Biol Chem. 2008;283:10522–10534. doi: 10.1074/jbc.M707659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plosch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 13.Jiang ZY, Parini P, Eggertsen G, Davis MA, Hu H, Suo GJ, Zhang SD, Rudel LL, Han TQ, Einarsson C. Increased expression of LXR alpha, ABCG5, ABCG8, and SR-BI in the liver from normolipidemic, nonobese Chinese gallstone patients. J Lipid Res. 2008;49:464–472. doi: 10.1194/jlr.M700295-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Reihner E, Angelin B, Bjorkhem I, Einarsson K. Hepatic cholesterol metabolism in cholesterol gallstone disease. J Lipid Res. 1991;32:469–475. [PubMed] [Google Scholar]
- 15.Schaffer R, Sniegoski LT, Welch MJ, White VE, Cohen A, Hertz HS, Mandel J, Paule RC, Svensson L, Bjorkhem I, Blomstrand R. Comparison of two isotope dilution/mass spectrometric methods for determination of total serum cholesterol. Clinical chemistry. 1982;28:5–8. [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Carr TP, Parks JS, Rudel LL. Hepatic ACAT activity in African green monkeys is highly correlated to plasma LDL cholesteryl ester enrichment and coronary artery atherosclerosis. Arterioscler Thromb. 1992;12:1274–1283. doi: 10.1161/01.atv.12.11.1274. [DOI] [PubMed] [Google Scholar]
- 18.Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res. 2004;45:378–386. doi: 10.1194/jlr.D300037-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Bell TA, 3rd, Kelley K, Wilson MD, Sawyer JK, Rudel LL. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in ApoB100 only, LDLr−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1396–1402. doi: 10.1161/ATVBAHA.107.142802. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Folsom AR, Lewis L, Eckfeldt JH. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. The American journal of clinical nutrition. 1997;65:551–559. doi: 10.1093/ajcn/65.2.551. [DOI] [PubMed] [Google Scholar]
- 21.Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. The American journal of clinical nutrition. 2008;88:203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 22.Chambless L, Keil U, Dobson A, Mahonen M, Kuulasmaa K, Rajakangas AM, Lowel H, Tunstall-Pedoe H. Population versus clinical view of case fatality from acute coronary heart disease: results from the WHO MONICA Project 1985–1990. Multinational MONItoring of Trends and Determinants in CArdiovascular Disease. Circulation. 1997;96:3849–3859. doi: 10.1161/01.cir.96.11.3849. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y. Current status of major cardiovascular risk factors in Chinese populations and their trends in the past two decades. The Collaborative Study Group on Trends of Cardiovascular Disease in China and Preventive Strategy. Zhong Hua Xin Xue Guan Bing Za Zhi. 2001;29:74–79. [Google Scholar]
- 24.Zhang M, Li J, Cai YM, Ma H, Xiao JM, Liu J, Zhao L, Guo T, Han MH. A risk-predictive score for cardiogenic shock after acute myocardial infarction in Chinese patients. Clinical cardiology. 2007;30:171–176. doi: 10.1002/clc.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oram JF, Vaughan AM. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Current opinion in lipidology. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA–I. The Journal of clinical investigation. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Einarsson K, Hellstrom K, Kallner M. Bile acid kinetics in relation to sex, serum lipids, body weights, and gallbladder disease in patients with various types of hyperlipoproteinemia. The Journal of clinical investigation. 1974;54:1301–1311. doi: 10.1172/JCI107876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gälman C, Angelin B, Thorsson B, Bröijersen A, Rudling M. Bile acid synthesis in man relates to plasma triglycerides, gender and presence of gallbladder but not to aging. Falk Symposium. 2008;165:A21. [Google Scholar]
- 29.Scallen TJ, Sanghvi A. Regulation of three key enzymes in cholesterol metabolism by phosphorylation/dephosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:2477–2480. doi: 10.1073/pnas.80.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavey KL, Trujillo DL, Scallen TJ. Evidence for phosphorylation/dephosphorylation of rat liver acyl-CoA:cholesterol acyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:2171–2174. doi: 10.1073/pnas.80.8.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell TA, 3rd, Brown JM, Graham MJ, Lemonidis KM, Crooke RM, Rudel LL. Liver-specific inhibition of acyl-coenzyme a:cholesterol acyltransferase 2 with antisense oligonucleotides limits atherosclerosis development in apolipoprotein B100-only low-density lipoprotein receptor−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1814–1820. doi: 10.1161/01.ATV.0000225289.30767.06. [DOI] [PubMed] [Google Scholar]
- 32.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. The Journal of clinical endocrinology and metabolism. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 33.Du S, Lu B, Zhai F, Popkin BM. A new stage of the nutrition transition in China. Public health nutrition. 2002;5:169–174. doi: 10.1079/PHN2001290. [DOI] [PubMed] [Google Scholar]
- 34.Mattisson I, Wirfalt E, Andren C, Gullberg B, Berglund G. Dietary fat intake--food sources and dietary correlates in the Malmo Diet and Cancer cohort. Public health nutrition. 2003;6:559–569. doi: 10.1079/phn2003474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Hepatic free cholesterol and cholesteryl ester between females and males.
Hepatic free cholesterol and cholesteryl ester concentrations (A. mg/g protein; B. mg/g weight of liver) were similar in females (n=14) and males (n=20). Data expressed as means ± SEM.
Supplementary figure 2. Expression of genes involving hepatic lipids metabolism
A. mRNA and protein expression levels of SRBI and mRNA expression levels of ABCA1 and APOA1 between females (open bar, n=18) and males (close bar, n=22). Data expressed as means ± SEM.
B. mRNA expression of other genes involving hepatic lipids metabolism between females (open bar, n=18) and males (close bar, n=22). Data expressed as means ± SEM.