Abstract
Individuals with schizophrenia have higher plasma nicotine levels in comparison to non-psychiatric smokers, even when differences in smoking are equated. This difference may be related to how intensely cigarettes are smoked but this has not been well-studied. Mecamylamine (MEC), a non-competitive nicotinic acetylcholine receptor (nAChR) antagonist, which has been shown to increase ad-lib smoking and to affect smoking topography, was used in the current study as a pharmacological probe to increase our understanding of smoking behavior, smoking topography, and resulting nicotine levels in smokers with schizophrenia. This preliminary study used a within-subject, placebo-controlled design in smokers with schizophrenia (n=6) and healthy control smokers (n=8) to examine the effects of MEC (10mg/day) on ad-lib smoking behavior, topography, nicotine levels, and tobacco craving across two smoking deprivation conditions (no deprivation and 12-hr deprivation). MEC, compared to placebo, increased the number of cigarettes smoked and plasma nicotine levels. MEC increased smoking intensity and resulted in greater plasma nicotine levels in smokers with schizophrenia compared to controls, although these results were not consistent across deprivation conditions. MEC also increased tobacco craving in smokers with schizophrenia but not in control smokers. Our results suggest that antagonism of high-affinity nAChRs in smokers with schizophrenia may prompt compensatory smoking, increasing the intensity of smoking and nicotine exposure without alleviating craving. Further work is needed to assess whether nicotine levels are directly mediated by how intensely the cigarettes are smoked, and to confirm whether this effect is more pronounced in smokers with schizophrenia.
Keywords: mecamylamine, ad-lib smoking, schizophrenia, topography, nicotine, nicotinic acetylcholine receptor, craving
1.INTRODUCTION
Individuals with schizophrenia smoke at rates that are two to three times higher than adults in the general population (45–88% versus 22%; Kalman et al., 2005; Lasser et al., 2000). Nicotine use in patients with schizophrenia may reduce negative symptoms and extrapyramidal side effects from antipsychotic medication, and improve mood, neuropsychological performance, and sensory gating (Dalack et al., 1998; Weinberger, Sacco et al., 2007, George et al., 2002; Sacco et al., 2005). It has also been hypothesized that smoking may remediate dysfunctional mesolimbocortical dopamine system functioning in schizophrenia (Dalack et al., 1998; George et al., 2002; George 2007).
Smokers with schizophrenia have higher plasma nicotine levels and higher urine and plasma cotinine (the proximal metabolite of nicotine) levels compared to healthy control smokers, even when matched for the number of cigarettes per day and indices of nicotine dependence (Olincy et al., 1997; Weinberger et al., 2007; Williams et al., 2005). Differences in metabolism or excretion may be responsible for these findings; however, there is little evidence for either possibility (Olincy et al., 1997; Williams et al., 2005). It is more likely that differences in nicotine levels between smokers with and without schizophrenia are related to the intensity with which each cigarette is smoked (i.e., smoking topography). Clinicians have long observed that patients with schizophrenia take deep puffs off their cigarettes and smoke them down to the butt (Masterson & O’Shea, 1984) and it has been hypothesized that this intensity of smoking may target specific nicotine receptors (e.g., the α7 nicotine receptor) also implicated in schizophrenic illness (Olincy et al., 1997).
There is little research on the differences between smokers with and without schizophrenia on how cigarettes are smoked, which is typically assessed by measuring aspects of smoking topography (e.g., puff count, puff volume, inter-puff interval, depth of inhalation). Tidey et al (2005) compared smoking topography of 20 smokers with schizophrenia or schizoaffective disorder with 20 control smokers and found that smokers with schizophrenia took more total puffs, more puffs per cigarettes, and had shorter inter-puff intervals, larger total cigarette puff volumes, and greater increases in carbon monoxide levels than control smokers. There have been no systematic investigations of the relationship between smoking topography and resulting nicotine levels in patients with schizophrenia.
Mecamylamine (MEC) is a ganglionic blocker that has been used as an anti-hypertensive agent (Inversine®) and is known to be a non-competitive antagonist of several nicotinic acetylcholine receptors (nAChR) including the α4β2 receptor (Sacco et al., 2004). As a pharmacological probe, administration of a single dose of mecamylamine (0mg, 10mg) to healthy smokers has been found to increase ad-lib smoking (Rose et al., 2001), nicotine self-administration (Rose et al., 2003), and cravings for cigarettes (McClernon & Rose, 2005) as well as short-term increases in ad-lib smoking behavior after overnight abstinence (Rose et al., 2001). In addition, a study of smoking topography in 8 healthy male smokers found greater base-to-peak elevations of nicotine levels after administration of 12.5 mg of mecamylamine (as compared to placebo) but no differences in puff volume or total number of puffs (Pomerleau et al., 1987). Marx et al. (2000) investigated a single administration of mecamylamine (0mg, 5mg, 10mg) on ad-lib smoking in patients diagnosed with mania, schizophrenia, or alcohol abuse. Mecamylamine produced an increase in the number of cigarettes smoked, expired carbon monoxide levels, and plasma nicotine, however this was not a true ad-lib design as patients were restricted to one cigarette per hour following hospital regulations.
In contrast to the findings presented above, longer-term treatment of mecamylamine administered with transdermal nicotine patch (TNP) has been shown to decrease smoking behavior and promote abstinence (Rose et al., 1994). Furthermore, pretreatment with mecamylamine does not precipitate nicotine withdrawal (Eissenberg et al., 1996) and some studies of smokers with schizophrenia have found no increase in cravings for cigarettes with mecamylamine with overnight abstinence (Sacco et al., 2005) or after exposure to smoking cues (Fonder et al., 2005). Thus, the effects of antagonizing high-affinity nAChRs on smoking behavior are still not clear. In addition, no study to date has examined the influence of mecamylamine pre-treatment on smoking topography in patients with schizophrenia.
The current preliminary study involved a detailed examination of mecamylamine (0mg vs. 10mg/day) on smoking topography, nicotine plasma levels, and craving responses in smokers with schizophrenia and healthy control smokers with similar baseline levels of cigarette use and nicotine dependence. Mecamylamine versus placebo effects on smoking behavior were assessed during non-deprived and nicotine-deprived conditions as differences between smokers with schizophrenia and healthy control smokers have been observed across these conditions (Weinberger, Sacco et al., 2007; Sacco et al., 2005). Based on prior work, it was predicted that mecamylamine would increase ad-lib smoking behavior and plasma nicotine levels, and alter smoking topography. We also predicted that these effects would be more pronounced in smokers with schizophrenia, when compared to smokers without schizophrenia.
2.MATERIALS AND METHODS
2.1 Participants
Participants were eligible to enroll if they were between 18 and 60 years of age, smoked at least 10 cigarettes per day, and met DSM-IV criteria for nicotine dependence by clinical interview (First et al., 1997). Two groups participated in this study, smokers with and without schizophrenia. Additional eligibility criteria for smokers with schizophrenia included meeting criteria for a current DSM-IV diagnosis of a schizophrenia spectrum disorder (schizophrenia or schizoaffective disorder), being on a stable dose of antipsychotic medication, and being in stable remission from positive symptoms of psychosis (as judged by psychiatric evaluation). Smokers with schizophrenia on a stable dose of anxiolytics (benzodiazepines, buspirone), mood stabilizers (lithium, valproate, gabapentin, carbamazepine), or anti-depressants (SSRIs or TCAs excluding bupropion and MAO inhibitors) were eligible. Control smokers were not eligible to participate if they they were currently taking psychotropic medications or met criteria for current DSM-IV Axis I disorders (except nicotine dependence). Participants were excluded if they met criteria for current abuse or dependence for alcohol or any illicit substance within the past 3 months, if they reported any history of renal insufficiency, glaucoma, pyloric stenosis, current prescription of sulfonamide antibiotics, hypersensitivity to mecamylamine, hypotension or current systolic BP of less than 90, or if they were not capable of giving informed consent for participation in this study. Individuals seeking treatment for smoking cessation, including current use of nicotine replacement therapy, were not eligible. Pregnant women and women without a reliable form of birth control did not participate. Eighteen smokers with schizophrenia were screened; 5 did not meet eligibility criteria, 7 declined to participate, and 6 completed the study. Seventeen healthy control smokers were screened; 4 did not meet eligibility criteria, 5 declined to participate, and 8 completed the study.
2.2 Procedures
2.2.1 Intake Sessions
The study was approved by the Yale Human Investigation Committee and written informed consent was obtained at the start of the intake session. The study was carried out in accordance with the Declaration of Helsinki. During the intake session, baseline assessments were obtained and participants completed a physical exam which included a urine drug screens, pregnancy test, EKG, and routine lab tests. The Structured Clinical Interview for DSM-IV (First et al., 1997) was used to exclude individuals who met diagnostic criteria for substance abuse or dependence (other than nicotine dependence) or other Axis I disorders (other than schizophrenia or schizoaffective disorder in the schizophrenia group). Eligible subjects were then scheduled for laboratory sessions.
2.2.2 Laboratory Sessions
This study was a double-blind repeated measures mixed design, examining within-subject effects of mecamylamine (0 mg/day vs. 10 mg/day) and deprivation (non-nicotine deprived vs. 12-hours of nicotine deprivation) on smoking behavior in smokers with and without schizophrenia, adapted from previous studies from our group (e.g., Sacco et al., 2005).
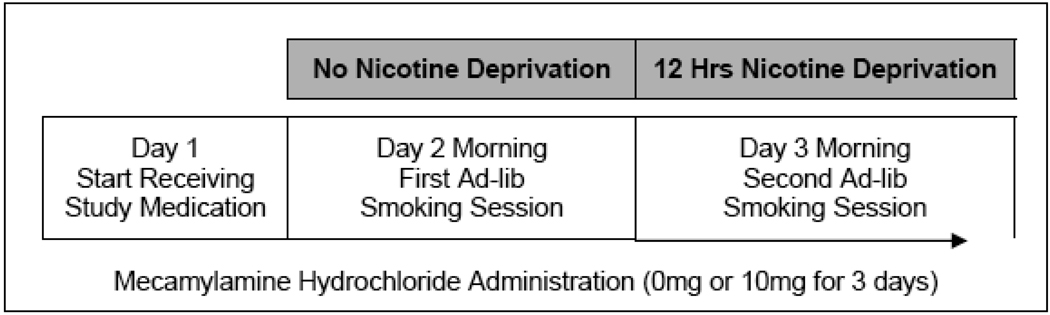
Medication order was randomized and counterbalanced. Test sessions spanned 2 consecutive days per week during 2 consecutive test weeks, thus included a total of 4 laboratory sessions per participant (see Figure 1). All laboratory sessions were conducted at the Yale Center for Clinical Investigations (YCCI) at Yale-New Haven Hospital in New Haven, CT. At the beginning of each session, baseline measurements of breath carbon monoxide (CO) and alcohol levels, vitals, adverse events, and alcohol and cigarette use were assessed. At the beginning of Days 2 and 3, plasma nicotine and cotinine levels, tobacco craving, and nicotine withdrawal were also assessed. Positive and negative symptoms were assessed in participants with schizophrenia at the start of each session.
Figure 1. Single subject study timeline for smokers with schizophrenia and control smokers. Testing for each medication condition occurred on consecutive weeks with medication order counterbalanced.
2.2.2.3 Day 1
After baseline assessments, subjects were familiarized with the smoking topography equipment by smoking a cigarette.
2.2.2.4 Day 2
Subjects arrived at the YCCI at 8:30 am. After baseline assessments, an IV cannula was inserted. Subjects engaged in a 2-hour ad-lib smoking period from 10 am to 12 pm. Nicotine plasma levels were obtained at the following timepoints: 0, +30, +60, +90, and +120 minutes. Tobacco craving was assessed at each timepoint. Subjects were instructed to stop smoking at 10 pm.
2.2.2.5 Day 3
Subjects arrived at the YCCI at 8:30 am. After baseline assessments, an IV cannula was inserted. CO levels less than 50% of intake values, later confirmed with plasma nicotine levels, were used to confirm overnight (12 hours) abstinence. One smoker with schizophrenia was unable to maintain abstinence and was rescheduled. Under placebo, one smoker with schizophrenia did not complete Day 3. Procedures for Day 3 for the 2-hr ad-lib smoking period were identical to Day 2. After completing the two sessions in one medication condition, subjects returned one week later to repeat the sessions under the other medication condition.
2.3 Medication Dosing
The mecamylamine dose was 5 mg po b.i.d (or 10mg/day) or 0 mg po b.i.d. Mecamylamine hydrochloride (Targacept, Inc., Winston-Salem, NC) was obtained from a local supplier. Matching placebo tablets were created by the Yale Investigational Drug Service, New Haven, CT. Medication administration occurred at 9 am on Days 1 to 3 at the YCCI under staff supervision. Subjects were instructed to administer the second dose at 5 pm on Days 1 and 2.
2.4 Smoking Topography Equipment
A Clinical Research Support System (CreSS from Plowshare Technologies, Richmond, VA) was used to assess smoking topography during the ad-lib period (e.g., latency, puff frequency, puff volume, puff duration, inter-puff interval, depth of inhalation, inter-cigarette interval).
2.5 Assessments
2.5.1 Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987)
The PANSS is a 30-item, 7-point rating questionnaire that was used to determine levels of psychotic symptoms (positive, negative, and general symptoms subscale scores, and a total symptoms score on PANSS) during the intake and laboratory sessions. Greater scores indicate more severe symptoms (range: positive & negative scales, 7–49; general scales, 16–112; total (sum), 30–210). Instructions were modified to encompass the timeframe being assessed (Sacco et al., 2005).
2.5.2 Adverse Events Checklist
This 30-item self-report questionnaire (George et al., 2002) assessed the presence and severity of common medication side effects associated with mecamylamine using a Likert scale of 0–4 (not present, minimal, mild, moderate, severe).
2.5.3 Beck Depression Inventory
This self-report depression rating scale consists of 21 items rated on a scale of 0–3, with a score range of 0–63 (Beck et al., 1988). On this scale, ratings of 0–9 are considered to be in the normal range, ratings of 10–19 are consistent with mild depression, and ratings of 20–30 are consistent with moderate depressive symptoms.
2.5.4 Smoking History
Self-reports were obtained about personal history of cigarette use and/or use of smokeless tobacco products.
2.5.5 Fagerström Test of Nicotine Dependence
(FTND; Heatherton et al., 1991). The FTND, a modification of the Fagerström Tolerance Questionnaire (Fagerström, 1978), was used to assess nicotine dependence. The FTND contains six items and is closely rated to biochemical indices of smoking. The score range is 0–10, with scores of 5 or more indicating moderate nicotine dependence.
2.5.6 Minnesota Nicotine Withdrawal Scale
This 8-item scale was used to assess current symptoms of withdrawal and is known to be sensitive to nicotine abstinence (Hughes & Hatsukami, 1986). The score range is 0–32, with higher scores indicating greater nicotine withdrawal.
2.5.7 Tiffany Questionnaire of Smoking Urges (QSU-brief)
The QSU-brief is a 10 item questionnaire which evaluates the structure and function of smoking urges (Cox et al., 2001). The Tiffany scale characterizes urges to smoke in response to two separate affective systems: negative affect related to relief of withdrawal and positive affect related to expectancy of reinforcement.
2.5.8 Expired Breath Carbon Monoxide (CO) Levels
Carbon monoxide levels were measured using Vitalograph Breath CO, from Vitalograph, Inc., which is a precision instrument for detecting carbon monoxide in exhaled breath. Carbon monoxide is known to have a half-life of 4 hours. This instrument measures CO in the range of 0–5000 ppm and has no cross-sensitivity to hydrogen or other positrons.
2.5.9 Nicotine and Cotinine levels
Plasma nicotine and cotinine were measured by reversed-phase high-performance liquid chromatography with UV detection modified from the literature (Hariharan et al. 1988) to include a micro acid back extraction cleanup step, which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4 ng/ml and cotinine was set to 25 ng/ml.
2.6 Data Analyses
Smokers with and without schizophrenia were compared using chi-squares or t-tests for differences on baseline demographic and smoking variables. Repeated measures analyses of variance (ANOVA) were used to examine medication, diagnosis, and nicotine deprivation effects on cigarettes smoked, smoking topography, and nicotine withdrawal measures in all smokers, and medication and nicotine deprivation effects were examined for positive and negative symptoms in smokers with schizophrenia. Mixed model regression analyses, using a compound symmetry covariance structure, were used to examine the main and interactive effects of time (5 timepoints across the 2-hour smoking session), medication, diagnosis, and nicotine deprivation on nicotine levels and cigarette craving. Fixed effects were reported from these analyses. In all analyses, diagnosis was a between-subject effect and medication and nicotine deprivation were within-subject factors.
Smoking topography data were cleaned with a utility program (PuffCleanUp, Plowshare Technologies), which combines or deletes data (i.e., false puffs), which can arise from movement artifacts. We used the recommended parameters for data cleaning. Smoking topography measures (number of puffs, puff duration, puff volume, peak puff volume, and inter-puff interval) were calculated as average scores across all cigarettes smoked during a lab session.
3.RESULTS
3.1 Baseline Characteristics
Smokers with and without schizophrenia were compared on baseline demographics, psychiatric symptoms, and smoking habits, as shown in Table 1.
Table 1.
Demographic and clinical characteristics of schizophrenic and healthy control smokers*
| Variable |
Smokers with Schizophrenia (n = 6) |
Control Smokers (n = 8) |
P value |
|
|---|---|---|---|---|
| Age, years | 41.3 (5.0) | 40.8 (15.3) | 0.93 | |
| Sex^ | 0.09 | |||
| Male | 5 | 3 | ||
| Female | 1 | 5 | ||
| Race^ | 0.05 | |||
| White | 2 | 7 | ||
| African American | 3 | 0 | ||
| Other | 1 | 1 | ||
| Education^ | 0.05 | |||
| Some college | 0 | 5 | ||
| High School | 4 | 2 | ||
| Less than HS | 2 | 1 | ||
| CPD | 21.2 (12.0) | 18.0 (5.5) | 0.57 | |
| CO level | 34.3 (14.3) | 28.5 (9.8) | 0.41 | |
| FTND score | 5.0 (2.1) | 4.3 (1.9) | 0.51 | |
| BDI score | 11.7 (14.6) | 4.0 (3.3) | 0.17 | |
| PANSS score | ||||
| Positive | 12.4 (1.5) | NA | - | |
| Negative | 12.8 (4.0) | NA | - | |
| General | 30.4 (3.8) | NA | - | |
| Total | 55.6 (7.9) | NA | - | |
Abbreviations: CPD, cigarettes per day; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence; BDI, Beck Depression Inventory; PANSS, Positive and Negative Syndrome Scale; NA, not applicable.
Values are expressed as mean (SD) unless otherwise indicated.
Values are expressed as number of subjects.
3.2 Psychotic Symptoms
For smokers with schizophrenia, no main effects of medication or session or medication by nicotine deprivation interactions were significant for any of the PANSS subscores.
3.3 Adverse Events
There were no differences in rates of treatment emergent side effects in smokers with and without schizophrenia following 3 days of 10mg/day mecamylamine. All adverse events were rated as minimal or mild, and the most common adverse events included sedation (35%), blurred vision when reading (29%), and constipation (14%).
3.4 Nicotine Withdrawal
An interaction of diagnosis and nicotine deprivation was observed. Smokers with schizophrenia exhibited a similar magnitude of nicotine withdrawal under both conditions of nicotine deprivation (non-deprived mean=6.67, SD=6.74 vs. deprived mean=6.67, SD=5.68), while control smokers reported greater nicotine withdrawal following 12-hr nicotine deprivation (mean=6.06, SD=2.80), in comparison to no deprivation (mean=2.56, SD=0.62), F=6.43,p=.03. No medication main effect or interactions were observed, ps > .05.
3.5 Ad-lib Smoking
Participants smoked more cigarettes under mecamylamine (mean=4.2, SD=0.9) than under placebo (mean=3.5, SD=0.9), F =13.30,p<.01. No effects of diagnosis or deprivation were demonstrated.
3.6 Smoking Topography
Following 12 hours of nicotine deprivation, mecamylamine increased average puff volume (mecamylamine mean = 52.2 ml, placebo mean = 41.2 ml), puff duration (1.6 seconds, 1.3 seconds), and peak puff volume (55.3 ml, 49.7 ml) in smokers with schizophrenia, compared to placebo but mecamylamine did not affect the average puff volume (mecamylamine mean = 40.7 ml, placebo mean = 42.4 ml), puff duration (1.4 seconds, 1.4 seconds) or peak puff volume (42.3 ml, 43.8 ml) of control smokers, ps<.03. No differences in smoking topography measures were observed on the non-nicotine deprived session. No significant findings were observed with other measures of smoking topography, ps > .05.
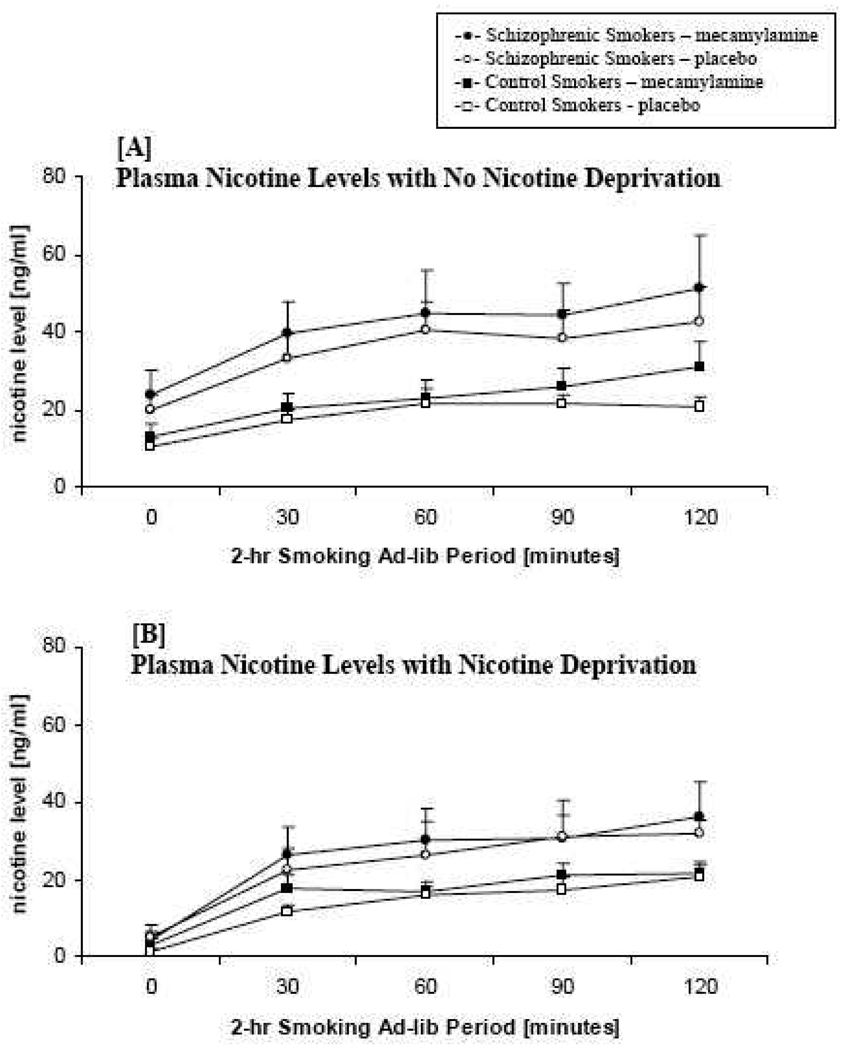
3.7 Plasma Nicotine and Cotinine Levels
A medication main effect was observed on plasma nicotine levels. Across time, nicotine levels were higher under mecamylamine than under placebo, F=3.87, p =.05. An interaction of nicotine deprivation and diagnosis was observed in plasma nicotine levels. Nicotine levels were higher overall at the non-nicotine deprived session than at the 12-hour nicotine deprivation session, and this difference was greater in the smokers with schizophrenia in comparison to the control smokers, F =5.46, p =.02 (see Figure 2).
Figure 2. Mean nicotine plasma levels across the 2-hour ad-lib smoking period no nicotine deprivation [A] and nicotine deprivation [B] by diagnosis (schizophrenic smokers vs. control smokers) and medication (mecamylamine vs. placebo).
Cotinine levels were assessed at the beginning of each session and demonstrated no differences by diagnosis, medication, or deprivation, ps>.12.
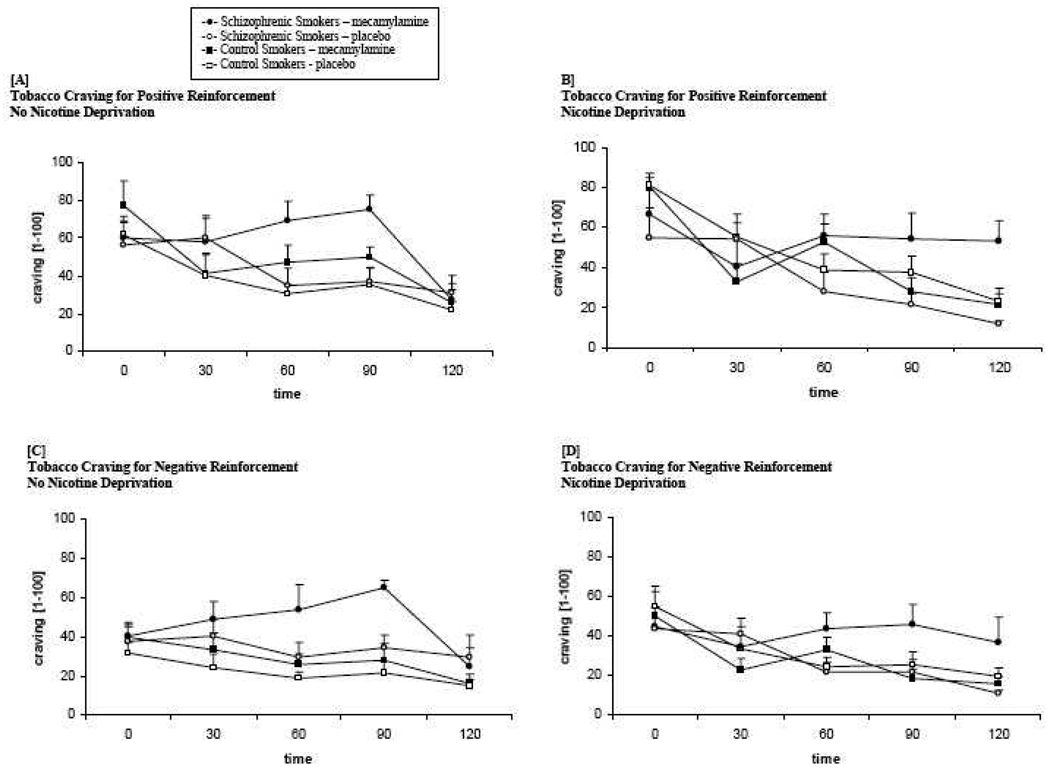
3.8 Cigarette Craving
A significant interaction of diagnosis and medication was observed. Smokers with schizophrenia reported greater craving over time for positive reinforcement (F =4.24, p=.04) and negative reinforcement (F = 4.97, p =.03) under mecamylamine, in comparison with placebo across both deprivation conditions. These ratings did not differ by medication for smokers without schizophrenia (see Figure 3).
Figure 3. Mean cigarette craving for positive reinforcement no nicotine deprivation [A] and 12 hours nicotine deprivation [B] and for withdrawal relief no nicotine deprivation [C] and nicotine deprivation [D] across the 2-hr ad-lib smoking period by diagnosis (schizophrenic smokers vs. control smokers) and medication (mecamylamine vs. placebo).
4.DISCUSSION
Individuals with schizophrenia tend to have higher plasma nicotine levels in comparison to healthy smokers, even when differences in smoking are equated. This difference may be related to how intensely cigarettes are smoked but this has not been well studied. Past research has found that administration of low doses of mecamylamine, a non-competitive nicotinic antagonist, increased ad-lib smoking behavior (e.g., Marx et al., 2000; Pomerleau et al., 1987, Rose et al., 2001), making it a useful probe with which to compare various groups on aspects of smoking behavior. Results from our preliminary investigation suggest a number of differences in smoking behavior in response to mecamylamine between smokers with and without schizophrenia including increased intensity of smoking, increased intake of nicotine, and increased cigarette cravings.
The mecamylamine-induced increases in smoking consumption and nicotine levels found in this study are consistent with past research (Marx et al., 2000; Pomerleau et al., 1987; Rose et al., 1989, 2001) and mecamylamine-induced increases in nicotine levels were greater for smokers with schizophrenia than control smokers. There have been few studies of smoking topography in smokers with schizophrenia and this was the first study to examine both smoking topography and nicotine levels for this group of smokers. In this study, mecamylamine was associated with increased smoking intensity in smokers with schizophrenia following 12-hour smoking abstinence, however, greater increases in nicotine levels in smokers with schizophrenia were observed during the non-deprived condition. This observed disconnect between smoking topography and nicotine levels is consistent with prior findings. Pomerleau et al. (1987) found increased nicotine levels but no differences in smoking intensity (puff number or puff volume) for nonpsychiatric smokers (n=8) receiving mecamylamine. Replication of these findings in larger samples could determine whether nicotine levels are directly mediated by how intensely the cigarettes are smoked, and whether this mediation varies by diagnostic status.
In this study, mecamylamine increased cravings for cigarettes but only for smokers with schizophrenia. Thus, while smokers with schizophrenia engaged in more intense smoking with mecamylamine administration, this increased smoking did not relieve cravings either in response to positive or negative reinforcement. Prior work examining the effect of MEC on craving relief in smokers with and without schizophrenia has yielded mixed findings. Mecamylamine was found to decrease craving in response to visual smoking cues in smokers with schizophrenia but not in healthy controls (Fonder et al., 2005) while Sacco et al. (2005) found no effect of mecamylamine on craving in either group of smokers during overnight smoking abstinence and reinstatement.
Rose and colleagues (2001) have suggested that mecamylamine may attenuate the reinforcing effects of smoking, resulting in smokers engaging in increased smoking to attempt to offset the reduced reinforcement. In smokers with schizophrenia, mecamylamine increased smoking behavior but failed to attenuate craving responses across deprived and non-deprived conditions. This effect was not demonstrated in smokers without schizophrenia suggesting that there was a differential effect of mecamylamine on craving relief in this population. The present findings are consistent overall with the observation that MEC appears to have differential effects on neuropsychological performance (Sacco et al., 2005), prepulse inhibition (George et al., 2006), cue-reactivity (Fonder et al., 2005) and smoking reinstatement (Weinberger et al., 2007) in smokers with schizophrenia versus control smokers.
It should be noted that generalizability of these results are limited by the small sample size of this preliminary investigation. However, despite the small sample size, a number of significant differences were found between the two smoking groups suggesting the need for further study of the effects of mecamylamine on smoking behavior in larger samples. In addition, it should be noted that the primary purpose of the current study was to utilize mecamylamine as a mechanistic probe due to its ability to increase smoking behavior. This study was not a test of mecamylamine as a treatment for nicotine dependence. Due to its properties as a nAChR antagonist, researchers have examined the utility of mecamylamine for smoking cessation treatment with mixed results. While Rose et al. (1994) reported rates of smoking abstinence that were higher with a combination of TNP and mecamylamine than TNP alone, Glover and colleagues (2007) found no statistically significant difference in abstinence rates for adult smokers receiving the TNP/mecamylamine combination versus TNP alone. Given the small sample size of this investigation, adjustments were not made for antipsychotic medications, which might have altered the findings.
The results of this preliminary study found that mecamylamine had a greater impact on smoking and smoking-related behavior of patients with schizophrenia than non-psychiatric smokers. These results suggest that using agents that antagonize high-affinity nAChRs may be an important tool to parse reasons why smokers with schizophrenia smoke at higher rates and have a more difficult time quitting than non-psychiatric control smokers. Knowledge gained from using nAChR antagonists can be used to test the efficacy of treatment for smoking cessation, used to enhance current treatments, or suggest novel pharmacological agents that may show efficacy for treating smokers with schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. American Journal of Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Griffiths RR, Stitzer ML. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology. 1996;127:328–336. doi: 10.1007/s002130050094. [DOI] [PubMed] [Google Scholar]
- Fagerström K-0. Measuring degree of physical dependency to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Washington, DC: American Psychiatric Press; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Fonder MA, Sacco KA, Termine A, Boland BS, Seya AA, Dudas MM, Vessicchio JC, George TP. Smoking cue reactivity in schizophrenia: Effects of a nicotinic receptor antagonist. Biological Psychiatry. 2005;57:802–808. doi: 10.1016/j.biopsych.2004.12.027. [DOI] [PubMed] [Google Scholar]
- George TP. Neurobiological links between nicotine addiction and schizophrenia. J. Dual Diagnosis. 2007;3:27–42. [Google Scholar]
- George TP, Seyal AA, Dolan SL, Dudas MM, Termine A, Vessicchio JC. Nicotine addiction and schizophrenia: A clinical approach. Primary Psychiatry. 2002;9:48–53. [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, Duncan EJ. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: Involvement of nicotinic receptor mechanisms. Schizophrenia Research. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- George TP, Verrico CD, Picciotto MR, Roth RH. Nicotine modulation of mesoprefrontal dopamine neurons: Pharmacological and neuroanatomic characterization. The Journal of Pharmacology and Experimental Therapeutics. 2000;295:58–66. [PubMed] [Google Scholar]
- Glover ED, Laflin MT, Schuh KJ, Schuh LM, Nides M, Christen AG, Glover PN, Strand JV. A randomized, controlled trial to assess the efficacy and safety of a Transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102:795–802. doi: 10.1111/j.1360-0443.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Hariharan M, Van Noord T, Greden JK. A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LK, Frecker RC, Fagerström K-O. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal on Addictions. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler L. The positive and negative syndrome scale for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Masterson E, O’Shea B. Smoking and malignancy in schizophrenia. Brit J Psychiatry. 1984;145:429–432. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- Marx CE, McIntosh E, Wilson WH, McEvoy JP. Mecamylamine increases cigarette smoking psychiatric patients. Journal of Clinical Psychopharmacology. 2000;20:706–707. doi: 10.1097/00004714-200012000-00023. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Rose JE. Mecamylamine moderates cue-induced emotional responses in smokers. Addictive Behaviors. 2005;30:741–753. doi: 10.1016/j.addbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biological Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology. 1987;91:391–393. doi: 10.1007/BF00518198. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacology, Biochemistry, and Behavior. 2001;68:187–197. doi: 10.1016/s0091-3057(00)00465-2. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE. Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacology, Biochemistry, and Behavior. 2003;76:307–313. doi: 10.1016/j.pbb.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clinical Pharmacology & Therapeutics. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Rose JE, Sampson A, Levin ED, Henningfeld JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacology, Biochemistry, and Behavior. 1989;32:933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. Journal of Psychopharmacology. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine AT, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia. Archives of General Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug and Alcohol Dependence. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorders is not a metabolic effect. Schizophrenia Research. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Creeden CL, Sacco KA, George TP. Neurocognitive effects of nicotine and tobacco in individuals with schizophrenia. Journal of Dual Diagnosis. 2007;3:61–78. [Google Scholar]
- Weinberger AH, Sacco KA, Creeden CC, Vessicchio JC, Jatlow PI, George TP. Effects of acute abstinence, reinstatement, and mecamylamine on biochemical and behavioral indices of cigarette smoking in schizophrenia. Schizophrenia Research. 2007;91:217–225. doi: 10.1016/j.schres.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]