Summary
The recently discovered Neuropeptide S (NPS) and its cognate receptor represent a highly interesting system of neuromodulation with unique physiological effects. On one hand, NPS increases wakefulness and arousal. On the other, NPS produces anxiolytic-like effects by acutely reducing fear responses as well as modulating long-term aspects of fear memory, such as attenuation of contextual fear or enhancement of fear extinction. The main sources of NPS in the brain are a few clusters of NPS-producing neurons in the brainstem. NPS binds to a G-protein-coupled receptor that is highly conserved among vertebrates and stimulates mobilization of intracellular Ca2+ as well as activation of protein kinases. In synaptic circuits within the amygdala, which are important for processing of acute fear as well as formation and expression of fear memories, NPS causes increased release of the excitatory transmitter glutamate, especially in synaptic contacts to a subset of GABAergic interneurons. Polymorphisms in the human NPS receptor gene have been associated with altered sleep behavior and panic disorder. In conclusion, the NPS system displays a unique physiological profile with respect to the specificity and time course of its actions. These functions could provide interesting opportunities for both basic research and clinical applications.
Keywords: Neuropeptide S, G-Protein-coupled receptor, Amygdala, Fear Behavior, Anxiety Disorder
Introduction
About 10–15 % of adults are experiencing clinically relevant symptoms of anxiety disorders or post-traumatic stress disorders. Chronic anxiety disorders significantly reduce the quality of life for the affected patients. Research investigating the neurobiological basis of anxiety disorders and stress is therefore not only an academic challenge but could also have significant clinical and public health-related impact. Most importantly, better understanding of intra- and intercellular signal pathways in the critically involved synaptic networks of the brain are expected to yield novel insights for the development of focused drug-based therapies. Besides the “classical” transmitter systems, neuropeptides have received increasing attention since they appear to play a central role in modulation of information processing particularly in those brain areas that are involved in expression of fear or stress. The recently discovered neuropeptide S (NPS) and its cognate receptor (NPSR) display a spectrum of effects that are highly interesting to basic research on anxiety disorders. NPS produces anxiolytic-like effects in rodents while increasing wakefulness at the same time (Xu et al., 2004; Vitale et al., 2008; Rizzi et al., 2008). The anxiolytic-like effect of NPS has been confirmed in a number of preclinical mouse and rat models predictive of anxiolytic action, including the four-plate test, elevated zero maze, stress-induced hyperthermia, and defensive burying (Leonard et al., 2008; Vitale et al., 2008). While NPS is thus considered a potent anxiolytic compound, the studies to date have not revealed it to alter depression-related behavior (Leonard et al., 2008). In any case, and of particular interest here, are genetic studies indicating that a variant of human NPSR with reduced agonist efficacy might be associated with panic disorder (Reinscheid et al., 2005; Okamura et al., 2007). This paper presents recent findings to explain NPS-induced effects at the molecular, cellular and behavioral level, with a focus on circuits of the amygdala and related modulation of fear expression and memory.
Neuropeptide S and the NPS Receptor
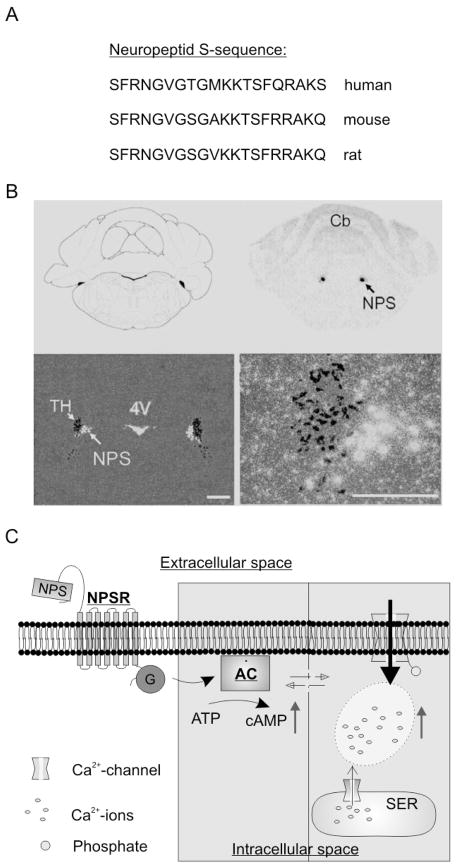
NPS and its receptor NPSR were first described in 2004 and represent a novel transmitter system that is found mainly expressed in the brain (Xu et al., 2004). NPS contains 20 amino acids, is highly conserved among tetrapods, and was termed after its aminoterminal serine residue (S) found in all species examined so far (Reinscheid, 2007) (Fig. 1A). It is processed from a larger precursor protein (89 amino acids in rat). In the rat brain, NPS precursor mRNA expression is detected by in situ hybridization in three nuclei of the brainstem: a previously uncharacterized area between the locus coeruleus and Barrington’s nucleus (Fig. 1B), an area near the lateral parabrachial nucleus and in the principle sensory 5 nucleus of the trigeminal system (Xu et al., 2004; 2007). NPS is co-expressed with excitatory transmitters such as glutamate, acetylcholine or corticotropine releasing factor (CRF). The projection areas of these NPS-producing neurons are currently unknown, but are a focus of current studies. Outside of the central nervous system, the NPS precursor is expressed mainly in endocrine tissues (Xu et al., 2004).
Figure 1. NPS and the NPS-receptor.
A) Neuropeptide S consists of 20 amino acids and displays significant sequence-homology in human, mouse and rat. B) Identification of NPS-producing neurons in the brain-stem of rats through in situ-hybridization against the mRNA of the NPS-precursor peptide (white). NPS-positive neurons are found next to tyrosine hydroxylase-positive neurons (TH) of the locus coeruleus, but do not colocalize. 4V = 4. ventricle, Cb = cerebellum. Scale bars 500 μm (left), 250 μM (right). C) The binding of NPS to the NPS-receptor triggers the synthesis of intracellular cAMP via a G-protein (G) adenylyl cyclase (AC) pathway. Furthermore, the NPS-NPS-receptor interaction leads to a significant increase in intracellular Ca2+-concentration, either by activation of Ca2+-channels in the plasmamembrane and/or the endoplasmatic reticulum. (A–C, modified from Xu et al., 2004).
The NPSR is a typical member of the G-protein-coupled receptor superfamily. It is also known as GPR154, VRR1 or GPRA (Gupte et al., 2004; Laitinen et al., 2004). It is mainly expressed in the brain (Xu et al., 2007) and its mRNA can be detected in brain areas involved in olfactory processing, such as anterior olfactory bulb, prepiriform and endopiriform cortex. NPSR expression was also found in brain areas critical for fear processing (e.g. amygdala and paraventricular nuclei of the hypothalamus) as well as brain regions involved in sleep-wake modulation (e.g. thalamic intralaminar nuclei, preoptic nucleus or tuberomammillary nucleus). Intracellularly, NPSR couples to G-proteins. Based upon the in vitro pharmacology of the NPSR, the Gq and Gs-type of G proteins are likely candidates, although alternative pathways including Ga(olf) or Gβy- signaling cannot be excluded. Agonist binding leads to elevated intracellular Ca2+ concentration and increase of cAMP formation via activation of adenylyl cyclase (Reinscheid et al., 2005). In addition, NPSR activation stimulates mitogen-activated protein kinase (MAPK) phosphorylation (Reinscheid et al., 2005). In essence, NPS binding to its receptor induces a number of intracellular signaling cascades which might produce numerous effects inside the cell. Recently, bicyclic piperazines (SHA 66 and SHA 68; Okamura et al., 2008) and NPS peptide analogues (Camarda et al., 2009; Guerrini et al., 2009) were identified as effective NPSR antagonists. Details of NPS-induced intracellular signal transduction events are currently under investigation.
The human NPSR gene is encoded by at least 9 exons and is located on chromosome 7p14. A number of polymorphisms have been identified in the human NPSR gene and associated with increased risk of asthma (Laitinen et al., 2004) or circadian phenotypes (Gottlieb et al., 2007). One single nucleotide polymorphism leads to anAsn/Ile exchange in position 107 (N107I), which results in an increase in the potency of NPS at NPSR-Ile107 (Reinscheid et al., 2005; Bernier et al., 2006). Structural characterization of NPS and mutagenesis studies showed that the NH(2)-terminal third of NPS are necessary and sufficient for activation of NPSR (Bernier et al., 2006). Furthermore, part of a nascent helix within the peptide might act as a regulatory region that inhibits receptor activation. This inhibition is absent in the Ile107 variant of NPSR, suggesting that residue 107 interacts with the regulatory region of NPS (Bernier et al., 2006). Of particular interest here is a study reporting that the less active isoform NPSR-Asn107 is under-represented in a male cohort of panic disorder patients, while samples from healthy volunteers, patients diagnosed with schizophrenia or attention deficit/hyperactivity disorder displayed no association with any of the two NPSR alleles (Okamura et al., 2007). Therefore, the NPSR gene might be involved in the pathogenetic mechanisms of anxiety or panic disorders in a gender-specific manner.
NPS-mediated Control of Transmitter Release in the Amygdala
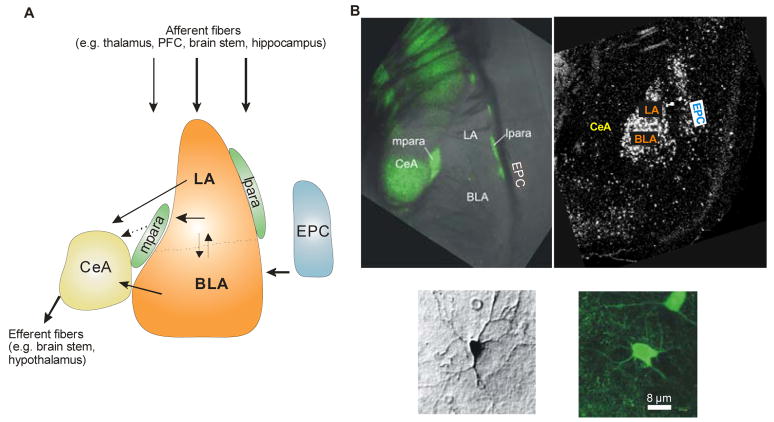
The amygdala – located in the temporal lobe of the brain - is a key structure of the limbic system and is composed of anatomically and functionally distinct nuclei. The amygdala plays a central role in processing and expression of emotions, especially during formation and consolidation of fear-related memory traces (reviewed in Maren and Quirk, 2004). Figure 2 provides an overview about the functional and structural organization of amygdalar nuclei that have been proven relevant for NPS action so far. The lateral (LA), basolateral (BLA) and basomedial amygdala form the basolateral amygdaloid complex. The LA receives afferent inputs from various brain regions (e.g. thalamus, prefrontal cortex (PFC), brainstem, and hippocampus) and is axonally connected to BLA and the central amygdala (CeA), which provides the major output station of signals to the brainstem and hypothalamus. Signal processing within the amygdala involves excitatory projection or principal neurons as well as interneurons (reviewed in Sah et al., 2003) that are characterized by the inhibitory transmitter gamma-amino butyric acid (GABA). There are two main populations of amygdala GABAergic interneurons: a) “local” interneurons that mediate inhibitory activity within particular nuclei and b) so-called “intercalated” or “paracapsular” interneurons that are located between the different subnuclei of the amygdala. The intercalated paracapsular interneurons are subdivided again into two groups: a lateral paracapsular (lpara IN) population located next to the lateral and basolateral amygdala (LA/BLA) thus controlling the main input structure of the amygdala complex, and a second group of medial paracapsular (mpara IN) interneurons located next to the central amygdala (CeA) and thus controlling the main output nucleus (Royer et al., 1999). The anatomical location of these two populations of paracapsular interneurons implies already their distinct functions in controlling either in- or output signals of the amygdala. The amygdala forms connections with an extremely diverse array of structures including cortex, striatum, some thalamic and hypothalamic nuclei, as well as various basal forebrain structures and brainstem nuclei (reviewed in Pitkänen et al., 2000). As a result, the amygdala is in a position to influence a wide variety of processes from autonomic and motor control to memory formation and neuromodulation.
Figure 2. Functional and cellular organization of major nuclei of the mouse amygdala.
A) Functional organization of the lateral amygdala (LA), the basolateral amygdala (BLA), and the central amygdala (CeA).. LA, BLA and the basomedial nucleus form the basolateral complex. Lateral and medial to the basolateral complex, clusters of inhibitory interneurons form the intercalated or paracapsular cell masses (lpara, mpara). The LA receives afferent inputs from various brain regions (e.g. thalamus, prefrontal cortex (PFC), brain stem, and hippocampus) and is axonally connected to BLA and CeA. A sub-population of LA neurons innervates mpara interneurons, which in turn are connected to CeA by inhibitory synapses. The BLA receives information from the LA, the endopiriform cortex (EPC) and other brain regions, and projects to LA and CeA. The CeA represents a main output station of the amygdala to the brain stem and hypothalamus. B) Identification of GABAergic neurons and NPS-receptors in the mouse amygdala. In amygdala slices of transgene GAD67-GFP mice (Tamamaki et al., 2003), GABAergic neurons can be identified in lpara, mpara and CeA by their expression of a GAD67-GFP fusion-protein (upper left panel; GAD67: glutamic acid decarboxylase, 67 kDa; GFP: green fluorescent protein). Higher magnification exemplifies the morphology of a GAD-negative projection neuron (lower panel, left; stained with biocytin) and a GAD-GFP positive GABAergic interneuron (lower panel, right). Detection of NPS-receptor mRNA through in situ hybridization in LA and EPC (upper right panel).
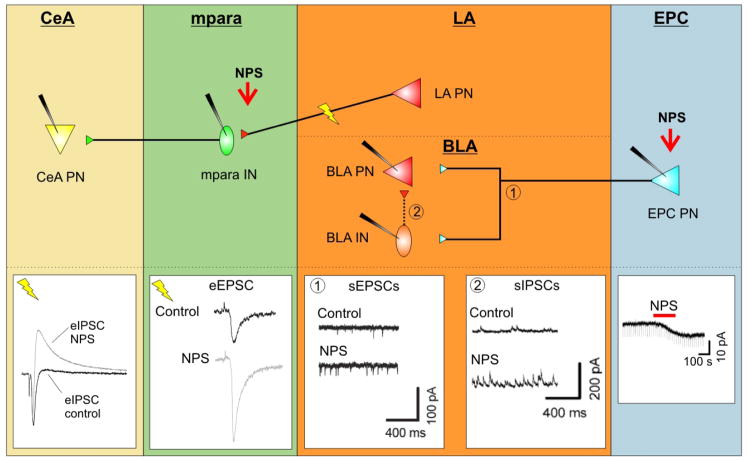
Two recent publications now explain how NPS is modulating this signaling network within the amygdala. The results are summarized in Figure 3. Using electrophysiological recordings of single units in the endopiriform cortex (EPC) in mouse brain slices, Meis et al. (2008) demonstrated that NPS produces a depolarizing membrane current and thus causes an increase in neuronal excitability (Fig. 3 EPC). By releasing the transmitter glutamate, the EPC projection neurons (EPC PN) convey excitatory input onto their targets in the amygdala. Increased discharge of EPC PN has two consequences within the basolateral amygdala complex (Fig. 3 BLA). On one hand it produces increased activation of monosynaptically connected projection neurons as well as local inhibitory interneurons that are measurable as increased frequencies of spontaneous excitatory postsynaptic potentials in both types of neurons (Fig. 3 BLA 1). On the other hand, local interneurons are connecting via GABAergic synapses with projection neurons of the BLA, so that NPS-mediated activation of projection neurons also results in an increase of interneuron inhibitory postsynaptic currents (sIPSCs) via a disynaptic circuit (Fig. 3 BLA 2).
Figure 3. Modulation of synaptic transmission in fear related networks by NPS.
Schematics in upper part illustrate basic synaptic interconnections between projection neurons (PN) and interneurons (IN) in endopiriform cortex (EPC), lateral (LA) and central (CeA) amygdala. Traces in lower part represent synaptic currents recorded at the respective interconnections in slices in vitro, before and during action of NPS (modified from Jüngling et al., 2008; Meis et al., 2008). EPC: Application of NPS into the EPC leads to a depolarizing inward current in projection neurons (EPC PN), resulting in an increase in excitability. LA/BLA: The consequence of the NPS-induced increase in EPC excitability is an increase in spontaneous excitatory postsynaptic currents (sEPSCs) in connected BLA projection and interneurons (1; BLA PN und BLA IN); furthermore, the excitation of local GABAergic interneurons raises the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in connected BLA PN (2). mpara: Stimulation of LA neurons evokes an eEPSC in connected mpara interneurons (mpara IN), which is significantly increased during presence of NPS, most likely through modulation of the presynaptic transmitter release. CeA: The consequence of the NPS-mediated raise in activity of mpara IN is an increase in GABAergic eIPSCs in connected projection neurons in the central nucleus of the amygdala (CeA PN).
In another study from the same year, Jüngling et al. (2008) demonstrated a specific regulatory effect of NPS directly within amygdala networks. An important step was the cell-specific localization of NPSR expression in projection neurons of the amygdala, in particular in LA/BLA principle neurons, through single cell RT-PCR analyses. The GABAergic interneurons of the mouse amygdala lack NPSRs. It is important to note that these studies have been performed in mice, and that evidence demonstrates clear differences in NPSR localization between the rat and mouse amygdala. In any case, a subpopulation of LA PN forms excitatory synapses on medial paracapsular interneurons (mpara IN) that are found in between the LA/BLA complex and the central amygdaloid nucleus (CeA). Electrical stimulation with an extracellular electrode in the LA produces synaptic transmission onto mpara IN and postsynaptic events can thus be recorded (Fig. 3 mpara). NPS-induced activation of NPSR leads to a significant increase in evoked postsynaptic currents in mpara IN. Simultaneously, probability of transmitter release is also increased at the presynaptic endings of LA PN. These results indicate a specific modulation of synaptic transmission between LA PN and mpara IN. The exact mechanism of the NPS-mediated enhancement of transmitter release is however unclear at this point. It is possible that activation of presynaptic protein kinase A (PKA) could contribute to these changes in transmitter release. Such a mechanism has already been demonstrated in presynaptic terminals of cortical inputs to LA/BLA complex (Humeau et al., 2003). The results also imply that increased excitation of mpara IN might lead to an increase in their intrinsic activity. This hypothesis could be verified indirectly by recordings of biphasic postsynaptic currents from projection neurons of the central amygdala (CeA). Biphasic currents are composed of a monosynaptic (direct) excitatory input and a bisynaptic (via interneuron) inhibitory component (IPSC) (Fig. 3 CeA). Recordings of these biphasic currents in CeA PN that were produced by extracelluar stimulation in LA, showed that NPS administration specifically increased the IPSC component of the postsynaptic effect while the excitatory input remained unchanged. Therefore, NPS appears to enhance a feed-forward inhibition of CeA PN that is mediated most likely by mpara IN.
The studies by Meis et al. (2008) and Jüngling et al. (2008) demonstrate for the first time that NPS-induced activation of NPSR is able to modulate both afferent and efferent transmission in the amygdala in a specific manner.
Attenuation of Fear and Modulation of Fear Memories by NPS
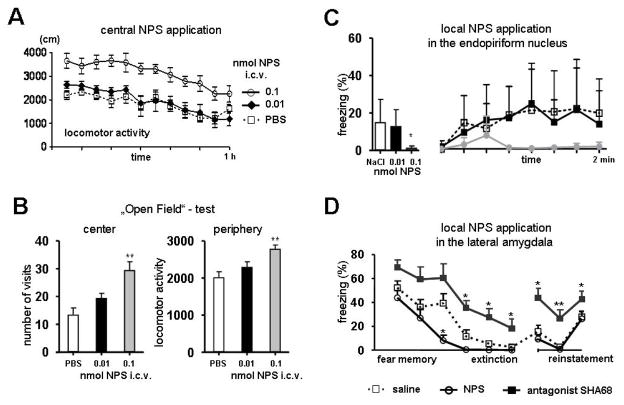
What are the behavioral and physiological consequences of the NPS-mediated specific modulation of synaptic function in the amygdala? In initial experiments, i.c.v. administration of NPS was found to decrease all stages of sleep in rats, accompanied by a dose-dependent increase of locomotor activity (Fig. 4A; Xu et al., 2004). Furthermore, i.c.v. NPS has been shown to be anxiolytic in the marble-burying test and stress-induced hyperthermia test, separating anxiolytic-like effects of NPS from locomotion (Xu et al., 2004; Leonard et al., 2008; Vitale et al., 2008). Other studies investigating potential NPS effects on anxiety-like behaviors and fear memories have used bilateral local injections into the amygdala instead of the i.c.v. route of administration.
Figure 4. Behavioral consequences of central and local NPS application.
A) Intra-cerebroventricular (i.c.v.) infusion of NPS induces hyperlocomotor activity in mice, dependent on NPS concentration. B) In the open-field test, i.c.v. infusion of NPS has an anxiolytic-like effect, as indicated by a higher fraction of center visits, and affects locomotor activity, as indicated by an increase in distance travelled in the „protected“ areas (mean ± SEM; ** p<0.01 compared to saline controls; modified from Xu et al., 2004). C) Local application of NPS into the endopiriform nucleus reduces context-conditioned freezing in a dose-dependent manner. During the time-course of contextual fear memory retrieval less freezing occurs in NPS-injected animals compared to saline-injected controls (mean ± SEM; * p<0.05; modified from Meis et al., 2008). D) Local application of NPS into the lateral amygdala facilitates extinction of conditioned fear. Cue-conditioned (CS) fear-responses (freezing) decline during extinction learning and remain at a low level during extinction recall, but can be re-instated upon CS exposure after unsignaled US presentation in the extinction context (reinstatement). NPS has no effect on the initial responses to the CS, but accelerates extinction learning. During presence of an NPS receptor antagonist (SHA68), extinction learning and recall are significantly impaired (mean ± SEM; * p < 0.05, ** p<0.01; modified from Jüngling et al., 2008).
An assay of general anxiety is, for example, the “open field test”. In this paradigm the animals are placed in an arena consisting of an open field surrounded by walls and can choose to travel close to the “protected and safe” areas close to the walls or explore the “unprotected and potentially dangerous“ center. Acute administration of NPS, either i.c.v. (Xu et al., 2004) or bilateral intra-amygdala (Jüngling et al., 2008), produced a significant decrease of anxiety-related behaviors in mice (Fig. 4B). Increased time spent and increased number of visits in the “unprotected” center of the arena were used as indicators for this anxiolytic-like effect of NPS in the open field test. While i.c.v. administration of NPS also causes increased locomotion in this test (Xu et al., 2004), this effect was absent after local intra-amygdala injections (Jüngling et al., 2008). Additional tests, such as “elevated plus maze” or “light-dark avoidance” confirmed the anxiolytic-like effects of NPS after both i.c.v. (Xu et al., 2004) and local intra-amygdala administration (Jüngling et al., 2008). In order to distinguish between the effects of exogenously administered NPS and endogenously released NPS, the specific NPSR antagonist SHA 68 was injected locally into the amygdala. Under these conditions, the antagonist produced anxiogenic effects, indicating that endogenous NPS release might contribute to an anxiolytic role of the NPS system (Jüngling et al., 2008). The physiological conditions under which NPS release occurs in vivo are, however, currently unknown.
Pavlovian fear conditioning is an established model for emotional memory that can be tested in experimental animals as well as - in a modified version – in humans (LeDoux, 2000). In this paradigm, an aversive stimulus (unconditioned stimulus, US; e.g. short electric foot shock) is associated with a neutral stimulus (conditioned stimulus, CS) if they correlate temporarily. The conditioned stimulus can be linked specifically to either a cue (e.g. tone or light) or a context (e.g. olfactory, visual or tactile environment). After the training (pairing of US-CS), associative fear memory is tested by presentation of CS alone and fearful responses can be recorded reliably by measuring “freezing” behavior Laxmi et al., 2003; Seidenbecher et al., 2003). Test at different intervals after the training allow an assessment of short- or long-term memory formation (Narayanan et al., 2007). Repeated presentation of CS alone without the aversive stimulus leads to a gradual decline in fear responses, which indicates extinction of fear memory. Current theories emphasize that fear extinction requires renewed learning and memory formation, rather than erasing of previous memory traces, implying a competition of newly learned extinction with previously acquired fear memories (Maren and Quirk, 2004). The observation that initially established fear memories can reappear spontaneously or be triggered by cues supports this hypothesis (Quirk and Mueller, 2008). The re-emergence of fear memories is especially relevant in the clinical context of post-traumatic stress disorder where patients often relive previous traumatic experiences.
The effect of NPS on formation of emotional memories was tested in a model of Pavlovian fear conditioning, measuring either cue-induced or context-induced fear conditioning as well as acquisition, consolidation and extinction of these memory traces. After context-induced fear conditioning in mice, local injection of NPS into the endopiriform cortex (EPC) produced a significant decrease of fear behavior over time (Fig. 4C). In contrast, NPS had no effect on acquisition, consolidation or recall of the conditioned fear (Meis et al., 2008; Jüngling et al., 2008). Only when measuring fear extinction, a specific effect of NPS was uncovered. Moreover, after local administration of NPS into the LA/BLA complex of the amygdala, an accelerated reduction of fear responses during repeated presentation of the conditioned stimulus (CS) was detected, indicating accelerated extinction learning (Fig. 4D). Mice in which endogenous NPS signaling was blocked by the specific NPSR antagonist SHA 68 displayed significantly delayed extinction learning as well as attenuated consolidation and recall of extinction 24 h later (Fig. 4D). Neither the acquisition nor the retrieval of fear memory were affected by NPS or the NPSR antagonist. Thus, NPS in the amygdala engages cellular processes that facilitate fear extinction in addition to its effect on fear expression.
How might this dual effect of NPS be mediated? One likely route involves mobilization of intracellular Ca2+ upon activation of NPS receptors (Reinscheid et al., 2005). NPS receptors are located at glutamatergic synapses from LA principal neurons to mpara IN, and receptor activation results in an increase in glutamate release (as described above). Importantly, the mpara IN are essential mediators of fear extinction, as indicated by the finding that pharmacotoxical inactivation prevents extinction of conditioned fear (Likhtik et al., 2008). The NPS-mediated increase in glutamatergic transmission to this population of GABAergic neurons can thus be assumed to facilitate fear extinction. Furthermore, the mpara IN innervate the CeA, and their increased glutamatergic excitation will impose an additional inhibitory influence on the CeA, the major output station of the amygdala for expression of fear behaviour. Importantly, NPS receptors are positively coupled to the cAMP/PKA system (Reinscheid et al., 2005). A result of NPS receptor-activated cAMP/PKA is phosphorylation of MAPK (Reinscheid et al., 2005), potentially giving rise to long term effects involving nuclear regulation of protein synthesis. In fact, fear extinction is sensitive to modulation of kinase activity in the BLA complex, including the MAPK-ERK pathway (Lu et al., 2001; Herry et al., 2006), and requires de novo protein synthesis in the BLA (Yang and Lu, 2005). NPS may thus represent a transmitter system supporting a link to these processes in principal neurons in the LA, thereby enabling a lasting increase in efficacy of synaptic connections to GABAergic mpara IN and a modulation of fear extinction on a long term scale.
Conclusions
The recently discovered Neuropeptide S and its cognate receptor represent a system of neuromodulation with unique physiological and potential clinical effects. The major (albeit not exclusive) source of NPS in the brain is a cluster of NPS-positive neurons in circumscribed regions within the brainstem. The NPS system mediates specific effects on synaptic transmission to and within the amygdala, which are important for processing of acute fear as well as extinction of fear memories. Specifically in mouse models predictive of anxiolytic action, NPS has a dual function to acutely attenuate anxiety-like responses and later facilitate extinction of aversive memories. A polymorphism in the human NPS receptor gene resulting in an alteration in the potency of NPS for NPSR has been found to correlate with panic disorder. Whether or not the specific profile of NPS action in amygdaloid circuits might be therapeutically beneficial during conditions of increased anxiety or posttraumatic stress disorders remains to be delineated.
Acknowledgments
The work of the authors is supported by the Deutsche Forschungsgemeinschaft (SFB-TRR 58; HCP, KJ, TS, JL), the Max-Planck-Society and Humboldt-Foundation (HCP), and the National Institute of Mental Health (RKR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernier V, Stocco R, Bogusky MJ, Joyce JG, Parachoniak C, Grenier K, Arget M, Mathieu MC, O’Neill GP, Slipetz D, Crackower MA, Tan CM, Therien AG. Structure-function relationships in the neuropeptide S receptor: molecular consequences of the asthma-associated mutation N107I. Journal of Biological Chemistry. 2006;281:24704–24712. doi: 10.1074/jbc.M603691200. [DOI] [PubMed] [Google Scholar]
- Camarda V, Rizzi A, Ruzza C, Zucchini S, Marzola G, Marzola E, Guerrini R, Salvadori S, Reinscheid RK, Regoli D, Calò G. In vitro and in vivo pharmacological characterization of the neuropeptide s receptor antagonist [D-Cys(tBu)5]neuropeptide S. Journal of Pharmacology and Experimental Therapeutics. 2009;328:549–555. doi: 10.1124/jpet.108.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, O’Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Medical Genetics. 2007;8(Suppl 1):S9. doi: 10.1186/1471-2350-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Camarda V, Trapella C, Calò G, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, Salvadori S. Synthesis and biological activity of human neuropeptide S analogues modified in position 5: identification of potent and pure neuropeptide S receptor antagonists. Journal of Medical Chemistry. 2009;52:524–529. doi: 10.1021/jm8012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte J, Cutler G, Chen JL, Tian H. Elucidation of signaling properties of vasopressin receptor-related receptor 1 by using the chimeric receptor approach. Proceedings of the National Academy of Science USA. 2004;101:1508–1513. doi: 10.1073/pnas.0308250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Lüthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. European Journal of Neuroscience. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissière S, Lüthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen T, Polvi A, Rydman P, Vendelin J, Pulkkinen V, Salmikangas P, Mäkelä S, Rehn M, Pirskanen A, Rautanen A, Zucchelli M, Gullstén H, Leino M, Alenius H, Petäys T, Haahtela T, Laitinen A, Laprise C, Hudson TJ, Laitinen LA, Kere J. Characterization of a common susceptibility locus for asthma-related traits. Science. 2004;304:300–304. doi: 10.1126/science.1090010. [DOI] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behavioral Brain Research. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews Neuroscience. 2000;23:727–738. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. Journal of Neuroscience. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. Public Library of Science ONE. 2008;3:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta synchronization in amygdalo-hippocampal circuits during various stages of fear memory. European Journal of Neuroscience. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. Journal of Pharmacology and Experimental Therapeutics. 2008;25:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S, Reinscheid RK. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1444–8. doi: 10.1016/j.pnpbp.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Acadamy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide S receptor variants. Journal of Pharmacology and Experimental Therapeutics. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides. 2007;28:830–837. doi: 10.1016/j.peptides.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Britsh Journal of Pharmacology. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. Journal of Neuroscience. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological Reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. Journal of Comparative Neurology. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A, Guerrini R, Calò G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;29:2286–2291. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. Journal of Comparative Neurology. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]