Abstract
X-ray crystal structures of LacY exhibit a large cytoplasmic cavity containing the residues involved in sugar binding and H+ translocation at the apex and a tightly packed side facing the periplasm. However, biochemical and biophysical evidence provide a strong indication that a hydrophilic pathway opens on the external surface of LacY with closing of the cytoplasmic side upon sugar binding. Thus, an alternating-access mechanism in which sugar- and H+-binding sites at the approximate middle of the molecule are alternatively exposed to either side of the membrane is likely to underlie LacY-catalyzed sugar/H+ symport. To further investigate periplasmic opening, paired residues on the tightly packed periplasmic side of LacY were replaced with Cys, and the effect of cross-linking was studied by testing the accessibility/reactivity of Cys148 with the elongated (~29 Å), impermeant hydrophilic reagent maleimide-PEG2-biotin. When the paired-Cys mutant Ile40→Cys/Asn245→Cys containing native Cys148 is oxidized to form a disulfide-bond, the reactivity of Cys148 is markedly inhibited. Moreover, the reactivity of Cys148 in this mutant increases with the length of the cross-linking agent. In contrast, MPB reactivity of Cys148 is unaffected by oxidation two other paired-Cys mutants at the mouth of the periplasmic cavity. The data indicate that residues Ile40 and Asn245 play a primary role in gating the periplasmic cavity and provide further support for the alternating-access model.
Keywords: membranes, transport, membrane proteins, thiol cross-linking, alkylation, protein dynamics
The lactose permease of Escherichia coli (LacY), a member of the Major Facilitator Superfamily (MFS) of membrane transport proteins, catalyzes the coupled translocation of a galactopyranoside and an H+. Thus, LacY transduces the free energy stored in an H+ electrochemical ion gradient (Δμ̃H +) into a sugar concentration gradient. Conversely, in the absence of Δμ̃ H+, LacY transduces the free energy stored in a sugar concentration gradient into Δμ̃H +, the polarity of which depends upon the direction of the sugar gradient 1; 2. LacY has been solubilized from the membrane, purified to homogeneity in a completely functional state 3; 4, and is structurally and functionally a monomer 2; 5.
X-ray crystal structures of LacY 6; 7; 8 and a wealth of biochemical and biophysical data 2; 9; 10; 11; 12; 13; 14 have led to an alternating access model for LacY. Accordingly, sugar binding induces closing of the inward-facing cavity and the opening of an outward-facing periplasmic cavity, thereby allowing alternating access of the sugar- and H+-binding sites to either face of the membrane. A similar model has been proposed for the glycerol phosphate/phosphate antiporter GlpT, a related MFS protein 15; 16; 17, and the ABC transporters Sav 1866 18 and MalF 19. The alternating access model involves a global conformational change, which is consistent with the highly dynamic nature of LacY 2; 14; 20; 21; 22; 23.
The crystal structures of LacY exhibit no pathway to the sugar-binding site from the tightly packed periplasmic side (Fig. 1) 6; 7; 8. Based on the structures, a molecular dynamics simulation suggests that a constricted region at the periplasmic side of LacY, which may be a ‘gate’, involves Ile40 (N-terminal 6-helix bundle) and Asn245 (C-terminal 6-helix bundle) (Fig. 1) 24. In this study, we used maleimide-PEG2-biotin (MPB) to probe key positions controlling the periplasmic gate of LacY in right-side-out (RSO) membrane vesicles. MPB is an elongated (29 Å), flexible, hydrophilic alkylation reagent (Fig. 2) that is membrane impermeant 25, and reactivity is readily assayed by Western blotting.
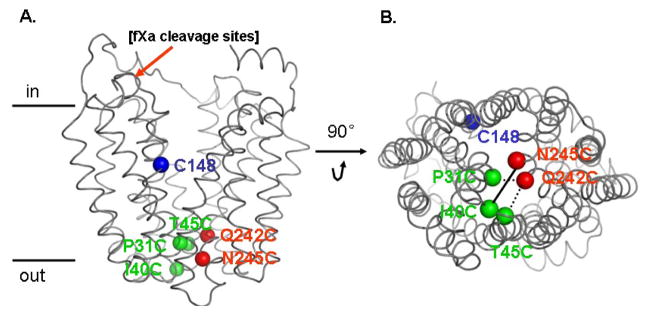
Fig. 1.
Position of Cys148 and paired-Cys replacements on the backbone of the X-ray crystal structure of LacY (PDB ID 2V8N). Left: side view; Cys148 (blue sphere; helix V); pairs are Ile40→Cys (green sphere; loop I/II) with Asn245→Cys (red sphere; helix VII); Pro31→Cys (green sphere; helix I) with Gln242→Cys (red sphere; helix VII) and Thr45→Cys (green sphere; helix II) with Gln242→Cys (red sphere; helix VII). Tandem fXa protease sites inserted between residue 136 and 137 are also shown in black. Right: viewed from the cytoplasm.
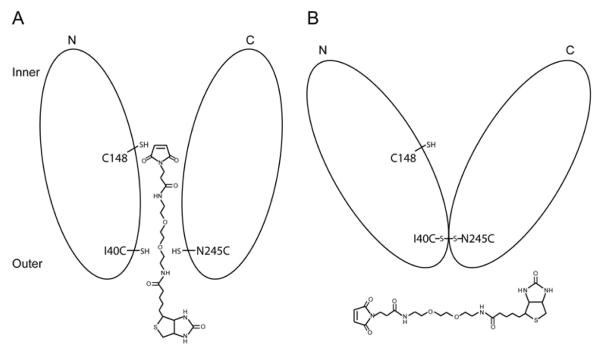
Fig. 2.
Experimental design. A. Reaction of MPB with Cys148 in untreated mutant C148/I40C/N245C; B. Reaction of MPB after disulfide formation with mutant C148/I40C/N245C.
Results
Experimental design and mutant construction
The X-ray crystal structures of LacY 6; 7; 8 show that helices I, II and helix VII are at the interface between the N- and C-terminal 6-helix bundles on the periplasmic side of LacY and appear to play a critical role in sealing the inward-facing cavity from the outside. A number of single-Cys replacement mutants within the tightly packed periplasmic domain of LacY exhibit a marked increase reactivity/accessibility to thiol reagents in the presence of β-D-galactopyranosyl 1-thio-β-D-galactopyranoside (TDG) 9; 12; 13. Moreover, the mutants also react with methanethiosulfonate ethylsulfonate (MTSES), a highly impermeant thiol reagent 9; 26; 27, in the presence of substrate, indicating that a hydrophilic pathway opens on the periplasmic face of LacY during turnover. Further evidence for opening of a periplasmic hydrophilic cavity has been obtained from single molecule Föster resonance energy transfer 10, double electron-electron resonance 11 and site-directed thiol cross-linking 14.
When engineered single-Cys replacements in the periplasmic pathway react with thiol reagents, inactivation correlates for the most part with the size of the modifying reagent; however, sugar binding is apparently unaffected 28. The results suggest that placement of a relatively large moiety in the putative periplasmic cleft of LacY likely prevents closure, an essential step in the transport cycle, without significantly altering access of sugar to the binding site. In order to test this conclusion further, we reasoned that tighter closure of the periplasmic side might be necessary to inhibit access to the sugar-binding site. The experimental approach used is outlined in Fig. 2. Starting with single-Cys 148 LacY, located in the approximate middle of LacY and protected from reactivity with substrate 29; 30; 31, paired Cys residues in the C- and N-terminal 6 helix bundles were engineered across the mouth of the putative periplasmic pathway (Fig. 1). As such, Cys148, as well as the Cys replacements, should react with MBP (Fig. 2A). However, when paired Cys residues across the mouth of the potential outward-facing cavity are oxidized to form a disulfide or cross-linked with short bifunctional reagents in the presence of ligand (which blocks Cys148 and simultaneously causes the Cys replacements in the periplasmic cavity to become more reactive/accessible 9; 12; 13), Cys148 should become inaccessible to MBP (Fig. 2B). Therefore, three paired-Cys mutants--C148/P31C/Q242C, C148/I40C/N245C and C148/T45C/Q242C -- were constructed with tandem factor Xa (fXa) protease sites in the loop between helices IV/V (Fig. 1) so that cross-linking could be tested conveniently without adversely affecting activity 32. Mutants C148/T45C/Q242C or C148/I40C/N245C exhibit about 24% or 19%, respectively of wild-type activity; mutant C148/P31C-Q242C had only 10% of wild-type activity (data not shown).
Effect of disulfide formation on alkylation of Cys148
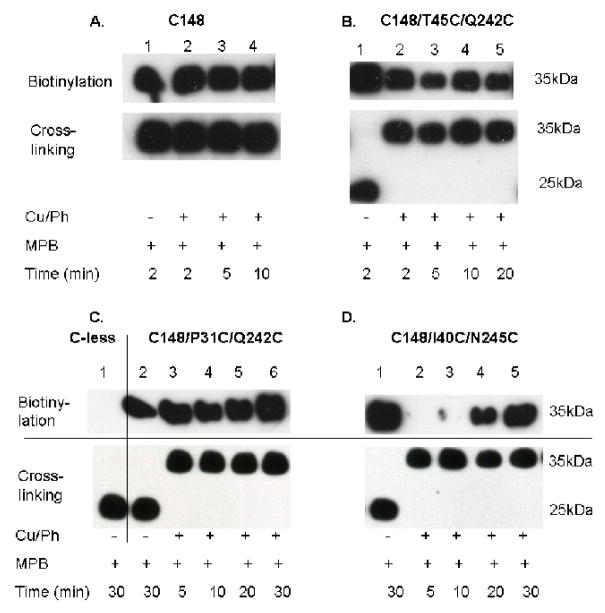
Vesicles containing single-Cys148 LacY devoid of fXa sites incubated with copper(II) o-phenanthroline (Cu/Ph) exhibit complete labeling with MPB at a band corresponding to full-length LacY over the time course tested (Fig. 3A, lanes 1–4, upper and lower panels). Thus, Cys148 is not altered under the oxidizing conditions used. Furthermore, LacY devoid of Cys residues (C-less), but containing tandem fXa protease sites exhibits no reactivity with MPB (Fig. 3C, lane 1, upper panel), but is completely cleaved by the protease (Fig. 3C, lane 1, lower panel). Also, complete cleavage is observed when Cys148 in each mutant is reacted with MPB and then cleaved by fXa protease (Fig. 3B, C & D, lanes 1, upper and lower panels), demonstrating that the MPB adduct with Cys148 has no effect on fXa activity. Oxidation of vesicles containing either mutant C148/T45C/Q242C or C148/P31C/Q242C in the presence of Cu/Ph has no effect on the rapid reaction of Cys148 with MPB, as reflected by the intense biotinylation observed over the time period tested, despite complete disulfide cross-linking of each sample (Figs 3B & C, lanes 2–5 and 3–6, upper and lower panels, respectively). In marked contrast, vesicles containing mutant C148/I40C/N245C, which are completely cross-linked by oxidation prior to incubation with MPB (Fig. 3D, lanes 2–5, lower panel), exhibit a distinct time course of labeling that clearly increases markedly from 5 to 30 min (Fig. 3D, lanes 2–5, upper panel).
Fig. 3.
Disulfide formation and time course of Cys148 labeling with MPB. All experiments were performed as described in Materials and Methods. Lower panels: RSO membrane vesicles with given LacY mutations [400 mg protein in 0.1 M KPi (pH 7.5)] were treated with 0.5 mM Cu/Ph in the presence of 10 mM TDG at room temperature for 30 min. After washing 3 times with excess 0.1M KPi (pH 7.5), the pellet was resuspended in 200 μl KPi (pH 7.5). Samples (2 μg protein) were then digested with 0.2 μg fXa on ice overnight, and cross-linking was determined by western blotting with anti-C-terminal antibody. Upper panels: The remainder of the sample was treated with 475 μM MPB at 0 °C, and the reactions were stopped by addition of 10 mM NEM at given times. Biotinylated proteins were then partially purified by monomeric avidin affinity chromatography separated by SDS-12% PAGE electrophoresis and transferred to polyvinylidene difluoride membranes. Biotinylated bands were detected by western blotting with anti-C-terminal antibody.
Effect of cross-linking agents of different lengths
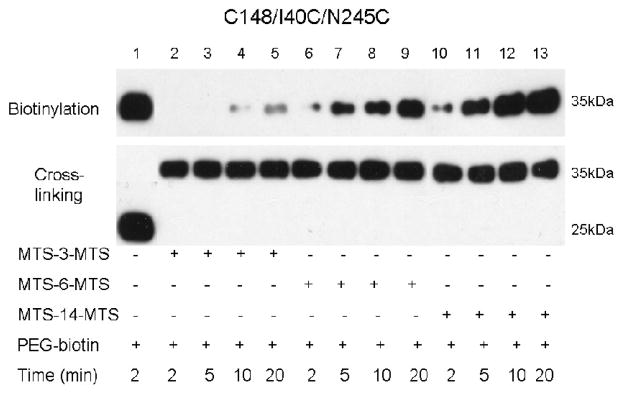
Previous studies 14 demonstrate that all three paired-Cys mutants (I40C/N245C, I32C/N245C or T45C/N245C) lose the ability to catalyze active lactose transport upon cross-linking with homobifunctional methane thiosulfonate reagents less than ~15 Å in length. In order to test the effect of cross-linking agents on accessibility of Cys148 from the periplamic side of the membrane, RSO vesicles containing mutant C148/I40C/N245C were treated with 1,3-propanediyl bis-methanethiosulfonate (MTS-3-MTS; ~5 Å), 1,6-hexanediyl-bis-methane-thio-sulfonate (MTS-6-MTS; ~9 Å) or 3,6,9,12-tetra-oxatetra-decane-1,14-diyl-bis-methanethiosulfonate (MTS-14-O4-MTS; ~17 Å) in the presence of 10 mM TDG. Paired Cys residues at positions 40 and 245 are completely cross-linked after 10 min incubation with each reagent (Fig. 4, lower panel). After cross-linking with MTS-3-MTS, no labeling of Cys148 with MPB is observed after 2 or 5 min, and a very low level of reactivity is observed after 10 or 20 min (Fig. 4, lanes 1–4, upper panel). However, with MTS-6-MTS or MTS-14-MTS, clear biotinylation is observed by 5 min, which increases to maximum intensity by 20 min (Fig. 4, lanes 7–9 and 11–13, upper panel). In other words, the shorter the cross-linking agent, the less accessibility/reactivity of Cys148.
Fig. 4.
Effect of MTS cross-linking reagents on Cys148 labeling by MPB. Experiments were performed as described in Fig. 3 except that RSO vesicles containing mutant C148/I40C/N245C were treated with a given MTS cross-linking reagent at 0.1 mM (final concentration) for 30 min, and the reactions were stopped by adding 10 mM NEM. Treatment with MPB (475 μM) was carried out for given times at 0 °C.
Discussion
In order to catalyze lactose/H+ symport, LacY and many other membrane transport proteins must exist in at least two conformations to allow access of the substrate-binding site to the milieu on both sides of the membrane. However, all X-ray structures of LacY to date 6; 7; 8 exhibit a large hydrophilic cavity on the cytoplasmic side of the molecule with a tightly packed periplasmic side, which blocks access of sugar to the binding site, clearly an inward-facing conformation. If sugar cannot gain access to the binding site from the exterior, transport obviously cannot occur. Notably, five separate lines of evidence—underestimation of cross-linking distances on the cytoplasmic side of LacY relative to the crystal structure 6, site-directed alkylation 9; 13; 33, single molecule Förster resonance energy transfer 10, double electron-electron resonance 11 and thiol cross-linking 14—support the conclusion that sugar binding leads to opening of a relatively large, hydrophilic cavity on the periplasmic face of LacY. Therefore, LacY must exist in a minimum of inward- and outward-facing conformations, which strongly favors the alternating access model for transport 34; 35; 36. Moreover, studies with C154G LacY, a conformationally crippled mutant, indicate that the periplasmic cleft in this mutant is fixed in an open-outward conformation 10; 11; 33. This conformation is not detected in the X-ray structures probably because the crystallization conditions select a lowest free energy conformation of LacY 8.
In this study, we address the role of key positions in the periplasmic gate of LacY. Several pairs of Cys residues were engineered in the region of the putative periplasmic gate in LacY, allowing site-specific cross-linking of positions predicted to be in close proximity. Cys replacements at positions on the periplasmic side of the sugar-binding site become much more reactive with respect to alkylation of N-ethylmaleimide in the presence of substrate (TDG), as well as accessible to methane ethylthiosulfonyl ethylsulfonate 9, a very impermeant, hydrophilic reagent 26; 27; 37. Notably in this regard, the X-ray crystal structures of LacY do not contain re-entrance loops 6; 7; 8. The observations presented here complement conclusions drawn from Cys accessibility/reactivity studies. Thus, Cys148 at the substrate-binding site does not react with the impermeant, elongated and flexible reagent MPB if paired Cys replacements for Ile40 and Asn245 are oxidized to form a disulfide or cross-linked with MTS-3-MTS (~5 Å). Strikingly, Cys148 in two other paired-Cys mutants on the periplasmic side of LacY react readily with MPB even after they are fully oxidized to disulfides or fully cross-linked with MTS-6-MTS (~9 Å) or MTS-14-MTS (~17 Å). The findings clearly suggest that Ile40 and Asn245 play an important role in gating the periplasmic cavity of LacY, which is consistent with the prediction from molecular dynamics simulations 24.
Materials and Methods
Materials
Restriction endonucleases, T4 DNA ligase, and Factor Xa protease were purchased from New England Biolabs (Beverly, MA). The QuickChange II kit was from Stratagene (La Jolla, CA). DNA plasmid purification and DNA fragment gel extraction kits were purchased from QIAGEN (Valencia, CA). MTS-based homobifunctional cross-linking agents were purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). Site-directed rabbit polyclonal antiserum against a dodecapeptide corresponding to the C terminus of LacY was prepared as described 38. Micro BCA protein determination and Supersignal West Pico Chemiluminescent substrate kits were from Pierce Inc. (Rockford, IL). All other materials were of reagent grade and obtained from commercial sources.
Construction of mutants
Plasmid pT7-5/Cys-less/fXa2 encoding Cys-less LacY with tandem factor Xa sites (Ile-Glu-Gly-Arg)2 between Ser136 and Asn137 in cytoplasmic loop IV/V (Fig. 1) was constructed as described 32. Double-Cys mutants were then generated by two-step replacement of a BamH I/Pst I fragment encoding helices I-II and then a BstX I/Hind III fragment encoding helix VII from plasmid pT7-5 containing appropriate single-Cys mutants into plasmid pT7-5/Cys-less/C148/fXa2. DNA sequencing of the entire lacY gene confirmed all constructs, and no other mutations were found.
Expression of LacY
E. coli T184 [lacI+O+Z−Y− (A) rpsL,met−,thr−,recA,hsdM,hsdR/F′,lacIqO+ZD118(Y+A+)]39 transformed with plasmids encoding given double-Cys mutants by electroporation in 0.2 cm cuvettes were grown at 37 °C in Luria-Bertani broth with 100 mg/l ampicillin. Overnight cultures were diluted 10-fold and allowed to grow for 2 h at 37 °C before induction with 1 mM isopropyl 1-thio-β-D-galactopyranoside. After additional growth for 2–3 h at 37 °C, cells were harvested by centrifugation.
Preparation of RSO vesicles
RSO membrane vesicles were prepared by osmotic lysis as described 40; 41, suspended in 100 mM potassium phosphate (KPi, pH 7.5)/10 mM MgSO4 at a protein concentration of about 10–20 mg/ml, frozen in liquid N2, and stored at −80 °C until use.
Cross-linking and alkylation
Unless stated otherwise, cross-linking was carried out with 0.5 mM Cu/Ph for 30 min, 0.1 mM MTS reagents for 20 min at room temperature in presence of 10 mM TDG (final concentrations) 1. Following 10-fold dilution with 100 mM cold KPi (pH 7.5), the vesicles were immediately collected by centrifugation at 4 °C, washed 3 times with 100 mM KPi (pH 7.5) and resuspended in 100 mM KPi (pH 7.5)/10 mM MgSO4 at ~1 mg protein/ml. One portion of the preparation was used for protein determinations and analysis of cross-linking. Cross-linking was tested as follows: aliquots (2 μg protein) were digested with factor Xa protease as described 32, and samples (2 μg protein) were subjected to sodium dodecyl sulfate-12% polycrylamide gel electrophoresis (SDS-12% PAGE) and western blotting with a site-directed polyclonal antibody against the C-terminus of LacY followed by horseradish peroxidase-coupled anti-rabbit antibody (Amersham Biosciences, NA-934) as described 42.
The remaining sample was treated with MPB (475 μM, final concentration) on ice for given times, and the reaction was terminated by addition of 10 mM dithiothreitol (DTT). Following 10-fold dilution with 100 mM cold KPi (pH 7.5), the vesicles were immediately collected by centrifugation at 4 °C and washed 3 times with 100 mM KPi (pH 7.5). The pellet was resuspended in 1 ml of 150 mM NaCl/10 mM Tris-HCl/1% Triton X-100/1% sodium deoxycholate/0.1% SDS (pH 7.4) containing 12% Pefabloc SC at 4 °C for 1 h and centrifuged at 14,000 rpm for 30 min to remove insoluble material. Avidin-agarose beads (10 μl; Pierce) were added to the supernatant, which was incubated overnight at 4 °C with constant mixing. The beads were then resuspended and applied sample to a column. After washing the column 3 times with 3 ml of washing buffer [50 mM NaPi (pH 7.4)/0.1 M NaCl/0.02% n-dodecyl-β-D-maltopyranoside], 50 μl of 5 mM d-Biotin in washing buffer was added to elute protein from the beads. The eluate was electrophoresed by SDS-12% PAGE, transferred to polyvinylidene difluoride membranes and immunoreactivity was detected as described 42.
Acknowledgments
This work was supported by NIH Grants DK51131, DK069463, GM074929, and NSF Grant 0450970 to H.R.K.
Abbreviations
- LacY
the lactose permease of Escherichia coli
- Δμ̃H +
the H+ electrochemical H+ gradient across the membrane
- MFS
Major Facilitator Superfamily
- MPB
maleimide-PEG2-biotin
- RSO
right-side-out
- TDG
β-D-galactopyranosyl 1-thio-β-D-galactopyranoside
- KPi
potassium phosphate
- MTS
methane-thiosulfonate
- MTS-3-MTS
1,3-propanediyl bis-methanethiosulfonate
- MTS-6-MTS
1,6-hexanediyl-bis-methane-thio-sulfonate
- MTS-14-O4-MTS
3,6,9,12-tetraoxatetradecane-1,14-diyl bis-methanethiosulfonate
- PEG
polyethylene glycol
- Cu/Ph
copper(II) o-phenanthroline
- DTT
dithiothreitol
- fXa
factor Xa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guan L, Weinglass AB, Kaback HR. Helix Packing in the Lactose Permease of Escherichia coli: Localization of Helix VI. J Mol Biol. 2001;312:69–77. doi: 10.1006/jmbi.2001.4933. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from Lactose Permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viitanen P, Garcia ML, Kaback HR. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci USA. 1984;81:1629–33. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–52. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 5.Sahin-Tóth M, Lawrence MC, Kaback HR. Properties of permease dimer, a fusion protein containing two lactose permease molecules from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:5421–5425. doi: 10.1073/pnas.91.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–5. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 7.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. Embo J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007;104:15294–8. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–4. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Ermolova N, Kaback HR. Site-directed Alkylation of LacY: Effect of the Proton Electrochemical Gradient. J Mol Biol. 2007;374:356–64. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie Y, Sabetfard FE, Kaback HR. The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A. 2008;105:3774–8. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–20. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 16.Lemieux MJ, Song J, Kim MJ, Huang Y, Villa A, Auer M, Li XD, Wang DN. Three-dimensional crystallization of the Escherichia coli glycerol-3-phosphate transporter: a member of the major facilitator superfamily. Protein Sci. 2003;12:2748–56. doi: 10.1110/ps.03276603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law CJ, Yang Q, Soudant C, Maloney PC, Wang DN. Kinetic evidence is consistent with the rocker-switch mechanism of membrane transport by GlpT. Biochemistry. 2007;46:12190–7. doi: 10.1021/bi701383g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–5. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 19.Khare D, Oldham ML, Orelle C, Davidson AL, Chen J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol Cell. 2009;33:528–36. doi: 10.1016/j.molcel.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogdanov M, Heacock PN, Dowhan W. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 2002;21:2107–16. doi: 10.1093/emboj/21.9.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.le Coutre J, Narasimhan LR, Patel CK, Kaback HR. The lipid bilayer determines helical tilt angle and function in lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1997;94:10167–71. doi: 10.1073/pnas.94.19.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci U S A. 1998;95:6114–7. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of Ligand-induced Conformational Flexibility in the Lactose Permease of Escherichia coli. J Biol Chem. 2006;281:35779–84. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MO, Yin Y, Tajkhorshid E, Schulten K. Sugar transport across lactose permease probed by steered molecular dynamics. Biophys J. 2007;93:92–102. doi: 10.1529/biophysj.107.103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelis RM, Zhang X, Dangprapai Y, Wright SH. Cysteine accessibility in the hydrophilic cleft of human organic cation transporter 2. J Biol Chem. 2006;281:35272–80. doi: 10.1074/jbc.M606561200. [DOI] [PubMed] [Google Scholar]
- 26.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–45. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 27.Kwaw I, Zen KC, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helices IV and V that contain the major determinants for substrate binding. Biochemistry. 2001;40:10491–9. doi: 10.1021/bi010866x. [DOI] [PubMed] [Google Scholar]
- 28.Nie Y, Zhou Y, Kaback HR. Clogging the periplasmic pathway in LacY. Biochemistry. 2009;48:738–43. doi: 10.1021/bi801976r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan L, Kaback HR. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. Proc Natl Acad Sci U S A. 2004;101:12148–52. doi: 10.1073/pnas.0404936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin-Tóth M, Gunawan P, Lawrence MC, Toyokuni T, Kaback HR. Binding of hydrophobic D-galactopyranosides to the lactose permease of Escherichia coli. Biochemistry. 2002;41:13039–45. doi: 10.1021/bi0203076. [DOI] [PubMed] [Google Scholar]
- 31.Frillingos S, Kaback HR. Cysteine-scanning mutagenesis of helix VI and the flanking hydrophilic domains in the lactose permease of Escherichia coli. Biochemistry. 1996;35:5333–5338. doi: 10.1021/bi953068d. [DOI] [PubMed] [Google Scholar]
- 32.Wolin CD, Kaback HR. Thiol cross-linking of transmembrane domains IV and V in the lactose permease of Escherichia coli. Biochemistry. 2000;39:6130–5. doi: 10.1021/bi0001269. [DOI] [PubMed] [Google Scholar]
- 33.Law CJ, Almqvist J, Bernstein A, Goetz RM, Huang Y, Soudant C, Laaksonen A, Hovmoller S, Wang DN. Salt-bridge dynamics control substrate-induced conformational change in the membrane transporter GlpT. J Mol Biol. 2008;378:826–37. doi: 10.1016/j.jmb.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widdas WF. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952;118:23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell P. Molecule, group and electron transport through natural membranes. Biochem Soc Symp. 1963;22:142–168. [Google Scholar]
- 36.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–70. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 37.Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–10. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- 38.Carrasco N, Viitanen P, Herzlinger D, Kaback HR. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 1. Functional studies. Biochemistry. 1984;23:3681–3687. doi: 10.1021/bi00311a017. [DOI] [PubMed] [Google Scholar]
- 39.Teather RM, Müller-Hill B, Abrutsch U, Aichele G, Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Molec Gen Genet. 1978;159:239–48. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- 40.Kaback HR. Bacterial Membranes. In: Kaplan NP, Jakoby WB, Colowick NP, editors. Methods in Enzymol. XXII. Elsevier; New York: 1971. pp. 99–120. [Google Scholar]
- 41.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 42.Carrasco N, Herzlinger D, Mitchell R, DeChiara S, Danho W, Gabriel TF, Kaback HR. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci USA. 1984;81:4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]