Abstract
Cyclooxygenase (COX)-derived prostaglandin E2 (PGE2) plays a role in the development and progression of several tumor types including head and neck squamous cell carcinoma (HNSCC). Measurements of urinary PGE metabolite (PGE-M) can be used as an index of systemic PGE2 production. In ever smokers, increased levels of urinary PGE-M reflect increased COX-2 activity. In this study, we determined whether baseline levels of urinary PGE-M were prognostic for ever smoker HNSCC patients. A retrospective chart review of ever smoker HNSCC patients treated with curative intent was performed. Fifteen of 31 evaluable patients developed progressive disease (recurrence or a second primary tumor) after a median follow-up of 38 months. There were no statistically significant differences between patients with (n=15) or without disease progression (n=16) with regard to stage, site, treatment received, smoking status and aspirin use during follow-up. Median urinary PGE-M levels were significantly higher in HNSCC patients with disease progression (21.7 ng/mg creatinine) compared with patients without (13.35 ng/mg creatinine), P=0.03. Importantly, patients with high baseline levels of urinary PGE-M had a significantly greater risk of disease progression (HR=4.76, 95% CI= (1.31, 17.30), P<0.01) and death (HR=9.54, 95% CI= (1.17, 77.7), P=0.01) than patients with low baseline levels of urinary PGE-M. These differences were most evident among patients with early stage disease. Taken together, our findings suggest that high baseline levels of urinary PGE-M indicate a poor prognosis in HNSCC patients. Possibly, HNSCC patients with high COX-2 activity manifested by elevated urinary PGE-M will benefit from treatment with a COX-2 inhibitor.
Keywords: Smoking, biomarker, HNSCC, prognosis, prostaglandin
Introduction
Cyclooxygenases (COXs) catalyze the first step in the synthesis of prostaglandin E2 (PGE2)from arachidonic acid. There are two isoforms of COX, designated COX-1 and COX-2. COX-1 is constitutively expressed in most tissues and mediates various physiological functions (1). In contrast, COX-2 is not detected in most normal tissues but is rapidly induced by a variety of mitogenic and inflammatory stimuli (2,3) resulting in elevated levels of PGE2 in neoplastic and inflamed tissues (4–7). Multiple lines of evidence suggest that COX-2 and PGE2 play a significant role in carcinogenesis. Levels of COX-2 and PGE2 are increased in a variety of malignancies including head and neck squamous cell carcinoma (HNSCC) (3–6,8). Tumor formation and growth are reduced in animals that are engineered to be COX-2-deficient (9–11). Treatment with selective inhibitors of COX-2, prototypic inhibitors of PGE2 synthesis, or an anti- PGE2 monoclonal antibody inhibited the growth of transplantable tumors including HNSCC (12–15). In humans, selective COX-2inhibitors have proven chemopreventive efficacy in the management of colorectal polyps and may be beneficial in the treatment of non-small cell lung cancer (16–18).
Several mechanisms have been identified that can explain the link between COX-2, PGE2, and malignancy. PGE2 can stimulate cell proliferation, motility and angiogenesis while inhibiting apoptosis and immune surveillance (3, 19–23). COX-2-derived PGE2 may also promote metastasis by stimulating epithelial-mesenchymal transition and cell invasion (24,25). In HNSCC, high levels of intratumor COX-2 and PGE2 have been associated with poor prognosis (26). Levels of COX-2 are also increased in the oral mucosa of seemingly healthy smokers (27).
Since it is rapidly catabolized in the lungs, PGE2 in plasma does not accurately reflect endogenous production of PG (28). 15-hydroxyprostaglandin dehydrogenase initiates the catabolism of PGE2, leading to a stable end metabolite (PGE-M), or 11-α -hydroxy-9, 15-dioxo-2,3,4,5-tetranor-prostane-1, 20-dioicacid, which is excreted in the urine (29,30). The value of urinary PGE-M as an index of systemic PGE2 production has been shown in previous studies (31,32). Urinary levels of PGE-M were found to be increased in non-small cell lung cancer (NSCLC) and colon cancer patients and in ever smokers without cancer or its history (33–35). HNSCC patients also were found to have a small, nearly significant increase in levels of urinary PGE-M (36). The suggestion that increased levels of urinary PGE-M reflect enhanced COX-2 activity has come from previous studies (33–35).
In this study, we determined whether baseline levels of urinary PGE-M were a prognostic factor for ever smoker HNSCC patients. Importantly, patients with high baseline levels of urinary PGE-M had a significantly greater risk of cancer progression and death than did patients with low baseline levels of urinary PGE-M. We speculate that HNSCC patients with elevated levels of urinary PGE-M may benefit from treatment with a COX-2 inhibitor as an adjunct to curative primary therapy and/or as adjuvant therapy to prevent second primary or recurrent cancer.
Materials and Methods
Study Population
The HNSCC patients included in the current study have been described previously (36). Briefly, patients with histologically confirmed HNSCC (newly diagnosed or recurrent) were enrolled. Patients with any surgery, chemotherapy (including corticosteroids), hormonal and/or radiation therapy within 6 weeks of enrollment, known unrelated malignancy, chronic inflammatory disease, renal disease or active infectious process and patients on nonsteroidal anti-inflammatory drugs (NSAIDs) within one week of enrollment to study were excluded. Participant exposure to known HNSCC risk factors, including tobacco and alcohol, were documented. Former smokers were defined as subjects who quit at least 12 months before presentation. Daily 81 mg aspirin use, defined as routine intake including within 48 h of urine collection, was documented. Information regarding site and stage of disease was then extracted from the medical record. All tumors were staged according to the American Joint Committee on Cancer staging system. When available, pathologic staging was preferred over clinical staging. Previous cancer history and any applied therapeutic interventions were identified and recorded as applicable. Prior to initiation of cancer treatment, single void urine specimens were collected from each participant, aliquoted into 2 mL cryovials and stored at −80°C. An informed consent was obtained from each participant. The Institutional Review Board of Memorial Sloan-Kettering Cancer Center approved this study.
Forty of the 58 HNSCC patients in the original study were ever smokers, and 18 were never smokers (36). In this study, the 18 never smokers were excluded because the etiology and prognosis of HNSCC arising in never smokers are very different from those of smoking-related HNSCC and it was not possible to meaningfully analyze such a small sample size (only 3 of the 18 progressed) in this study (37,38). A retrospective chart review of 40 ever smokers was performed by a head and neck surgeon who independently confirmed the status of each case. Patients who received treatment with curative intent, had documentation of disease-free status by clinical or radiological examination following completion of treatment, and had a minimum of one year follow-up after completion of treatment were included in the analysis, for a total of 31 patients. 15 of the 31 patients developed progressive disease (14 clinically classified recurrences, 1 second primary tumor); the remaining 16 patients were disease free. Data concerning smoking status during treatment, smoking status during follow-up, aspirin or NSAID use, local failure, regional failure, distal failure and death due to disease were obtained from the medical records. Details of surgery, radiotherapy and chemoradiation were also obtained.
Urinary PGE-M
Analyses of urine specimens were contemporaneous and blinded. As described previously, we measured urinary PGE-M via mass spectrometry using stable isotope dilution methodology with chemically synthesized(2H6)PGE-M in order to quantify PGE2 production (36). We converted endogenous urinary PGE-M to an unlabeled O-methyloxime derivative and then extracted it. Mass spectrometry was performed using a Thermo Scientific Quantum Ultra instrument fitted with an electrospray source and was operated in the negative ion mode employing multiple reaction monitoring. The transition of the precursor ions for endogenous (m/z 385) and (2H6)-labeled (m/z 391) O-methyloxime PGE-M were collisionally activated at 21eV and product ions m/z 336 and m/z 339 were monitored. We then calculated the specimen levels of endogenous PGE-M from the ratio of the mass chromatogram peak areas of the m/z 385 →336 and m/z 391 →339 transitions. The urinary creatinine concentration was used to normalize the results.
Statistical Analysis
The primary objective of this study was to evaluate the prognostic role of urinary PGE-M. Outcomes of interest include disease-free and overall survival. Variables describing patient demographics, smoking, aspirin use, disease and treatment characteristics are summarized for cases with and without progression separately, in terms of mean ± standard deviation (sd) and median (range) for continuous variables and count (proportion) for categorical variables. Differences in the means between the two groups were compared using a parametric t-test, with log transformation of the data applied when the distribution of data deviated from the normality assumption. Differences in the medians and proportions between the two groups were compared using the non-parametric Wilcoxon rank-sum test and Fisher’s exact test, respectively. Time-to-event data for subjects with high (above median) and low (below median) PGE-M levels are summarized using Kaplan-Meier curves. Two year disease-free survival probability and three year overall survival probability and their respective 95% confidence intervals (CIs) were determined. Log-rank test was used to examine the association between each of the independent variables and a time-to-event outcome univariately. The Cox proportional hazard model was used for multivariable analysis of the association between urinary PGE-M and the outcome adjusting for other covariates which were identified using a significance level of 0.20 based on results from the univariate analyses. The hazard ratios (HR) with 95% CI and P values are reported. All tests were two-sided, and P-values less than 0.05 were considered statistically significant.
Results
Patient and tumor characteristics
The characteristics of the 31 HNSCC patients are shown in Table 1. Patients were grouped as those with (n=15) and without (n=16) disease progression (recurrence or a second primary tumor). Patients who developed local, regional or distant disease following treatment with curative intent were grouped as having progressive disease. The median ages at initial presentation for patients with and without tumor progression were 66.6 and 64.7 years, respectively (P=0.64). A greater proportion of patients were male and former smokers. However, there were no statistically significant differences in gender and smoking status between the two groups. Smoking status during treatment and follow-up and long-term aspirin or NSAID use during follow-up was recorded. Two patients (13.3%) in the progression group continued smoking through last follow-up. Four patients (26.7%) in the progression group and three patients (18.8%) whose tumors failed to progress used 81 mg of aspirin before urine collection; two patients in each group used aspirin during follow-up. The differences between the two groups were not statistically significant.
Table 1.
Patient Characteristics
| Variable | HNSCC cases with progression (n=15) | HNSCC cases without progression (n=16) | P |
|---|---|---|---|
| Age, y | |||
| Median (range) | 66.6 (53.9, 81.0) | 64.7 (45.1, 79.1) | 0.64 (Wilcoxon) |
| Mean ± SD | 66.2 ± 8.9 | 64.2 ±10.9 | 0.57 (t-test) |
| Gender, n (%) | 0.70 (Fisher’s exact test) | ||
| Male | 11 (73.3) | 10 (62.5) | |
| Female | 4 (26.7) | 6 (37.5) | |
| Tobacco use at time of sample collection, n (%) | 0.70 | ||
| Current | 4 (26.7) | 6 (37.5) | |
| Former | 11(73.3) | 10 (62.5) | |
| Pack year exposure* | |||
| Median (range) | 28.1 (2, 100.5) | 25.0 (2.5, 80.0) | 0.88 |
| Mean ± SD | 30.2 ± 28.6 | 28.2 ± 22.7 | 0.84 |
| Tobacco use during follow-up, n (%) | 0.23 | ||
| Yes | 2 (13.3) | 0 (0) | |
| No | 13 (86.7) | 16 (100) | |
| Aspirin use before urine collection, n (%) | 0.69 | ||
| Yes | 4 (26.7) | 3(18.8) | |
| No | 11 (73.3) | 13 (81.2) | |
| Aspirin use during follow-up, n (%) | 1.00 | ||
| Yes | 2 (13.3) | 2 (12.5) | |
| No | 13 (86.7) | 14 (87.5) | |
Excludes 1 pipe smoker in both groups
Medical records were reviewed to obtain information regarding the known risk factors of progression in HNSCC. Summary statistics of variables that can affect the disease outcome, including stage, site, size of the tumor and the treatment received, are listed in the Table 2. Most patients with newly diagnosed disease in both groups were in advanced stages, with stage III and IV accounting for 66.7% and 56.3% in the progression and progression-free groups, respectively. Seven patients with recurrent disease who were offered treatment with curative intent were included in the analysis. Three of these patients eventually developed progressive disease and four patients remained free of disease. The difference between the two groups was not statistically significant. Median tumor size was 2.9 cm with a range of 0–4.5 cm for the progression group and 1.7 cm with a range of 0–6.0 cm in the group that failed to progress. The difference between the two groups was not significant statistically. Tumors sites were well matched in the two groups; however, a higher percentage of cases of laryngeal cancer were found in the group that progressed vs. remained progression free. All patients received current standard of care curative intent treatment, which include surgery alone, radiation alone, chemoradiation or a combination of these three modalities. The difference in the type of treatment received between the two groups was not statistically significant.
Table 2.
Tumor Characteristics
| Variable | HNSCC cases with progression (n=15) | HNSCC cases without progression (n=16) | P |
|---|---|---|---|
| Stage, n (%) | 1.00 | ||
| I/II | 2 (13.3) | 3 (18.7) | |
| III/IV | 10 (66.7) | 9 (56.3) | |
| Recurrent | 3 (20.0) | 4 (25.0) | |
| Tumor size (cm) | |||
| Median (range) | 2.9 (0, 4.5) | 1.7 (0, 6.0) | 0.12 |
| Mean ± SD | 2.4 ± 1.3 | 1.9 ± 1.5 | 0.25 |
| Tumor site, n (%) | 0.69 | ||
| Oral cavity | 2 (13.4) | 4 (25.0) | |
| Oropharynx | 3 (20.0) | 5 (31.1) | |
| Hypopharynx | 1 (6.8) | 1 (6.3) | |
| Larynx | 8 (53.0) | 4 (25.0) | |
| Unknown | 1 (6.8) | 1 (6.3) | |
| Others | 0 (0) | 1 (6.3) | |
| Treatment, n (%) | 0.55 | ||
| Surgery | 3 (20) | 7 (43.8) | |
| Radiation | 2 (13.3) | 2 (12.5) | |
| Chemoradiation | 7 (46.7) | 5 (31.2) | |
| Surgery+RT/CRT | 3 (20) | 2 (12.5) | |
| PGE-M | |||
| Median (range) | 21.7 (2.4, 69.7) | 13.4 (4.9, 38.2) | 0.03 |
| Mean ± SD | 25.1 ± 17.4 | 14.5 ± 8.8 | 0.04 |
Pretreatment Urinary PGE-M levels
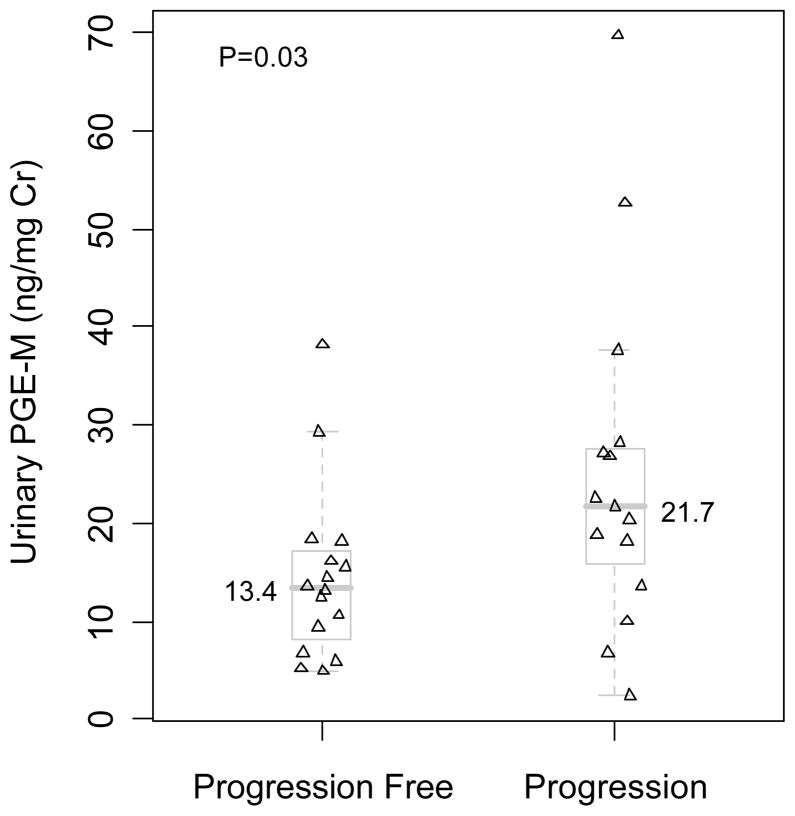
Baseline levels of urinary PGE-M were compared in the two groups (progression vs. progression free) of HNSCC patients. A significantly higher median baseline urinary PGE-M level was found in HNSCC patients who developed progressive disease compared to HNSCC patients whose disease did not progress (Table 2, Fig. 1). Notably, there was considerable variability in levels of urinary PGE-M within each of the two groups of patients. Several individuals with low baseline urinary PGE-M levels developed progressive disease. Two patients whose disease did not recur had high urinary PGE-M levels.
Fig. 1.
Significantly higher baseline urinary PGE-M levels [median (range)] were observed for patients developing disease progression [21.7 (2.4, 69.7)] compared to those without progression [13.4 (4.9, 38.2)], P=0.03 (Wilcoxon rank-sum test). Distribution of urinary PGE-M levels in patients with or without disease progression is illustrated with scatter diagram and the overlapping box plots. Levels of urinary PGE-M are expressed as ng/mg Cr.
Relationship between pretreatment urinary PGE-M and progression free and overall survival
Univariable analysis
The log-rank test was used to examine the association between urinary PGE-M and disease-free survival. The result suggests that higher urinary PGE-M levels were associated with significantly increased risk of progression (HR=1.03, 95% CI=(1.00, 1.07), P=0.04). Dichotomizing PGE-M at the median (16.1 ng/mg creatinine), patients with high urinary PGE-M (above median) had significantly increased risk of progression compared to patients with low urinary PGE-M (HR=4.76, 95% CI= (1.31, 17.30), P<0.01). The two year disease-free survival probability for patients with high urinary PGE-M was estimated to be 46.7% (95% CI = (27.2%, 80.2%)) and 87.1% (95% CI= (71.8%, 100%)) for those with low urinary PGE-M (Fig. 2A). Similarly, the log-rank test was used to examine the association between urinary PGE-M levels and overall patient survival. The result suggests that higher urinary PGE-M was significantly associated with increased risk of death (HR=1.05, 95% CI= (1.01, 1.09), P=0.01). Patients with high urinary PGE-M (above median) had significantly increased risk of death compared to patients with low urinary PGE-M (HR=9.54, 95% CI= (1.17, 77.7), P=0.01). Specifically, the three-year survival probability for patients with high urinary PGE-M and low urinary PGE-M was estimated to be 53.3% (95% CI = (33.2%, 85.6%)) and 91.7% (95% CI= (77.3%, 100%)), respectively (Fig. 2B).
Fig. 2.
Baseline urinary PGE-M levels predict disease-free and overall survival in patients with HNSCC. Kaplan Meier survival curves illustrate A. Disease-free survival was significantly lower for patients with high baseline urinary PGE-M values (P<0.01). The two-year disease-free-survival probabilities were estimated to be 46.7% (95% CI = (27.2%, 80.2%)) for patients with high urinary PGE-M, and 87.1% (95% CI= (71.8%, 100%)) for those with low urinary PGE-M. B. Overall survival was significantly lower for patients having high baseline urinary PGE-M values (P=0.01). The three-year probability of survival for patients with high and low baseline urinary PGE-M values was estimated to be 53.3% (95% CI = (33.2%, 85.6%)) and 91.7% (95% CI= (77.3%, 100%)), respectively. The baseline urinary PGE-M values were dichotomized as high and low categories by using the median (16.1 ng/mg creatinine) as the cutoff. This definition of high and low PGE-M categories is used throughout this manuscript.
Multivariable Analysis
For disease-free survival, only tumor size and treatment showed significance at the level of 0.20. In multivariable analysis using the Cox proportional hazard model, elevated baseline urinary PGE-M appeared to be associated with increased risk of progression (HR=1.03, 95% CI=(1.00, 1.06), P=0.07) after adjusting for tumor size and treatment. Examining the association between disease-free survival and dichotomized PGE-M adjusting for these two variables suggested that patients with high PGE-M had increased risk of progression compared to patients with low PGE-M (HR=3.93, 95% CI = (1.03, 15.06), P=0.05). For overall survival, only tumor size showed association at significance level of 0.20. Multivariable analysis with the Cox proportional hazard model suggested that high urinary PGE-M remained significantly associated with increased risk of death (HR=1.06, 95% CI= (1.02, 1.12), P=0.007) after adjusting for tumor size. Examining the association between overall survival and dichotomized PGE-M adjusting for tumor size suggested that patients with high urinary PGE-M had increased risk of death compared to patients with low urinary PGE-M (HR=7.49, 95% CI = (0.87, 64.23), P=0.07).
Relationship between pretreatment urinary PGE-M and stage specific-progression free and overall survival
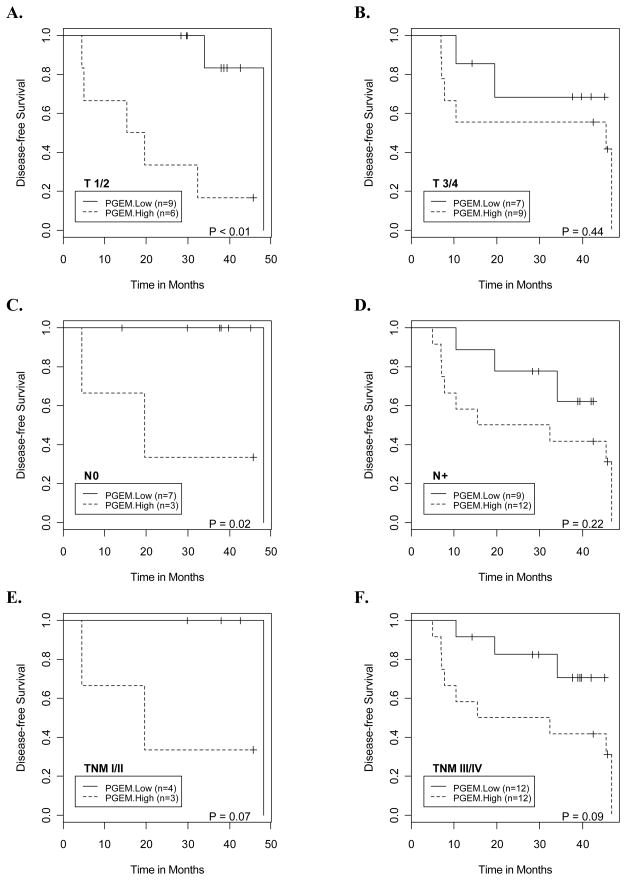
A prognostic biomarker, which indicates adverse prognosis for patients with early stage disease, would be of significant clinical importance. It may help to stimulate appropriate therapies and screening for prevention and/or early detection of the recurrence. Therefore, we examined the association between pretreatment urinary PGE-M and stage-specific disease-free and overall survival. Because of the small sample size, the results of this analysis should be considered to be hypothesis-generating. Elevated levels of urinary PGE-M were associated with increased risk of progression for patients with early stage disease. For patient with primary T-stage T1/T2, node negative and TNM stage I/II disease, high baseline urinary PGE-M was associated with increased risk of disease progression, and the association was significant or close to significance (P values were <0.01, 0.02 and 0.07, respectively; Figs. 3A, 3C & 3E). Consistent with increased risk for disease progression, patients with early stage disease and high baseline urinary PGE-M had lower overall survival (P values were <0.01, 0.03 and 0.07 respectively, Figs. 4A, 4C & 4E). For patients with higher T-stage, nodal metastasis and advanced stage III/IV disease, a trend towards higher risk of progression (Figs. 3B, 3D & 3F) and poorer overall survival (Figs. 4B, 4D & 4F) was suggested for those with higher baseline urinary PGE-M levels. Given the limited sample size of the study, this did not reach the level of statistical significance.
Fig. 3.
Kaplan Meier curves illustrate a consistent pattern of lower disease-free survival probabilities for patients with high baseline urinary PGE-M levels compared to those with low baseline urinary PGE-M levels at the following disease stage categories: A. T1/T2 primary tumors, B. T3/T4 primary tumors, C. N0 neck, D. N+ neck, E. Stage I/II and F. Stage III/IV.
Fig. 4.
Kaplan Meier curves illustrate a consistent pattern of lower overall survival probabilities for patients with high baseline urinary PGE-M levels compared to those with low baseline urinary PGE-M levels at the following disease stage categories: A. T1/T2 primary tumors, B. T3/T4 primary tumors, C. N0 neck, D. N+ neck, E. Stage I/II and F. Stage III/IV.
Discussion
In the current study, high levels of urinary PGE-M marked a poor prognosis in HNSCC patients. This finding raises numerous issues. To begin with, the source of increased PGE2 synthesis that resulted in high levels of urinary PGE-M in a subset of patients should be considered. One strong possibility is the tumor itself. Increased levels of urinary PGE-M have been found in both NSCLC and colorectal cancer patients (33,34). We previously detected a nearly statistically significant increase (P=0.07) in levels of urinary PGE-M in HNSCC patients (36). Many of these HNSCC patients were smokers, an independent cause of elevated urinary PGE-M (35,36). Our previous study was underpowered to detect a small increase in urinary PGE-M due to HNSCC. By contrast increased levels of urinary PGE-M were readily detected in NSCLC patients, another smoking-related cancer. Taken together, this suggests that any increase in urinary PGE-M due to HNSCC would be smaller than the increase due to NSCLC. In fact, the mean levels of urinary PGE-M were 27.2 ng/mg Cr and 17.9 ng/mg Cr in NSCLC and HNSCC patients, respectively (36,39). Hence, it seems likely that HNSCC contributes to increased urinary PGE-M levels in some patients. This is highly relevant since prior studies have shown that both elevated COX-2 and PGE2 at the invasive front of the tumor predict for increased risk of lymph node metastases, local recurrence, and worse disease-free and overall survival in HNSCC patients (26,40). In all likelihood, high levels of intratumor COX-2 cause elevated PGE2 production in HNSCC patients, leading, in turn, to increased urinary PGE-M levels that mark a poor prognosis. Overexpression of microsomal prostaglandin E synthase-1 (mPGES-1), the enzyme that converts COX-derived PGH2 to PGE2, may also contribute to increased levels of PGE2 in HNSCC (41). In addition to the tumor being a likely source of increased urinary PGE-M, smoking is an independent cause of increased COX-2 activity resulting in elevated urinary PGE-M (35). Although the location of the smoking-related increase in COX-2 activity has not been confirmed, the upper aerodigestive tract has been suggested to be a likely source (27,35). Smoking-mediated increases in procarcinogenic PGE2 levels might also have a negative impact on tumor progression in HNSCC patients. Additional studies will be required to determine the relative importance of intratumor PGE2 vs. smoking-related increases in mucosal PGE2 as determinants of urinary PGE-M and disease progression. Perhaps the major overriding issue raised by our results is their implications for therapy and prevention in the setting of curatively treated HNSCC patients (discussed later).
As mentioned in the introduction, PGE2 has numerous effects that can potentially explain the link between elevated urinary PGE-M levels and a poor prognosis. PGE2 exerts its biological actions by binding to any one of four E-series of PG (EP) receptors in tumor or stromal cells resulting in increased cell proliferation, enhanced angiogenesis, reduced apoptosis and inhibition of immune surveillance (3,19–23). For example, stimulation of either EP2 or EP4 activates T-cell transcription factor/β-catenin-mediated transcription that leads, in turn, to enhanced expression of a variety of genes, e.g., cyclin D1 and c-myc, which have been implicated in carcinogenesis (42). Cross talk between EP receptors and the epidermal growth factor receptor may also contribute to increased cell growth (43). Recently, PGE2 was found to activate Wnt signaling and thereby impact stem cell function, which could also be important for tumor progression (44). Additional known effects of PGE2 that may contribute to tumor progression and metastasis include induction of VEGF and matrix metalloproteinase-9, and stimulation of epithelial-mesenchymal transition (3,24,45). PGE2 also exhibits potent immunosuppressive effects by modulating dendritic cell function and causing an imbalance between type 1 and type 2 cytokines (46). In addition to explaining how increased levels of PGE2 can contribute to disease progression, these effects of PGE2 may also create resistance to chemotherapy and radiation therapy (47).
Numerous preclinical studies indicate that inhibiting COX-derived PG synthesis is useful for preventing or treating a variety of tumor types including HNSCC (9–15). Moreover, selective COX-2 inhibitors such as celecoxib have proven efficacy in the treatment of colorectal adenomas in humans (16,17). The results of a small clinical trial suggested that indomethacin, a dual inhibitor of COX-1 and COX-2, reduced the growth of HNSCC (48). Prolonged use of selective COX-2 inhibitors led to a small increase in cardiovascular complications, which is a significant barrier for routine use in chemoprevention (49). Clearly, the risk vs. benefit calculation is different in cancer patients. Nonetheless, it would be a major advance if a biomarker could be used to identify the subset of HNSCC patients who are most likely to benefit from treatment with a COX-2 inhibitor. In a Phase II trial of celecoxib and docetaxel in NSCLC patients, patients who experienced the greatest proportional decline in urinary PGE-M following treatment with celecoxib were at significantly reduced risk of death relative to patients with no change or an increase in PGE-M levels (39). This result was also suggested in a second study of patients with unresectable NSCLC (50). Another recent trial involving NSCLC patients suggested that treatment with a selective COX-2 inhibitor was clinically beneficial in the subset of patients with the highest intratumor COX-2 levels (18). Encouraging responses were seen in a small trial of celecoxib plus gefitinib for the treatment of incurable HNSCC (51). If one can extrapolate from NSCLC to HNSCC, our results suggest that HNSCC patients with high baseline urinary PGE-M levels will be most likely to benefit from treatment with a COX-2 inhibitor. Given the findings in NSCLC, it will be worthwhile to determine whether the magnitude of decline in urinary PGE-M following treatment with a COX-2 inhibitor also predicts for clinical benefit in HNSCC patients.
It is important to acknowledge the potential limitations of our study. Although the important clinical, demographic and tumor characteristics were well matched in patients with and without disease progression, our study had a small sample size. Moreover, determining whether levels of urinary PGE-M had prognostic significance was not a prespecified endpoint of the initial study, and our results are based on a retrospective chart review. A larger prospective study is warranted to validate and extend our findings. For example, a larger study is needed to determine the utility of urinary PGE-M as a prognostic biomarker for different tumor sites within the head and neck and stages of HNSCC. The current results suggest that urinary PGE-M may be a better prognostic biomarker for early- than late-stage disease. However, it is uncertain whether this will be true for all tumor sites within the head and neck. Due to the small size of the current study, the results should be viewed as hypothesis generating. If a larger study confirms that high levels of urinary PGE-M mark a poor prognosis in patients with early-stage disease, this biomarker could potentially be used to inform clinical decision-making.
In conclusion, our finding that elevated levels of urinary PGE-M indicate a poor prognosis in HNSCC patients is consistent with previous data on the effects of PGE2 on tumor progression and metastasis (52). Measurements of urinary PGE-M may provide insights that will enable the identification of subsets of patients who are most likely to benefit from primary or adjuvant/preventive treatment with a COX-2 inhibitor.
Acknowledgments
Grant Support: Flight Attendant Medical Research Institute; Memorial Sloan-Kettering Cancer Center Prevention Control and Population Research Program Pilot Project Award; American Society of Clinical Oncology Young Investigator Award; NIH grants P01 CA77839, P01 CA106451, T32 CA09685, DK48831, GM15431, ES13125 and CTSC UL1-RR024996.
Footnotes
Note: J.D. Morrow is deceased. His passing is a great loss to all of us.
References
- 1.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 2.DuBois RN, Awad J, Morrow J, Roberts LJ, Bishop PR. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-α and phorbol ester. J Clin Invest. 1994;93:493–8. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–6. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 4.Lupulescu A. Prostaglandins, their inhibitors and cancer. Prostaglandins Leukot Essent Fatty Acids. 1996;54:83–94. doi: 10.1016/s0952-3278(96)90064-2. [DOI] [PubMed] [Google Scholar]
- 5.Jung TT, Berlinger NT, Juhn SK. Prostaglandins in squamous cell carcinoma of the head and neck: a preliminary study. Laryngoscope. 1985;95:307–312. doi: 10.1288/00005537-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–23. [PubMed] [Google Scholar]
- 7.Sharon P, Ligumsky M, Rachmilewitz D, Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978;75:638–640. [PubMed] [Google Scholar]
- 8.Chan G, Boyle JO, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–4. [PubMed] [Google Scholar]
- 9.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in ApcΔ 716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 10.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–401. [PubMed] [Google Scholar]
- 11.Howe LR, Chang SH, Tolle KC, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–9. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 12.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–12. [PubMed] [Google Scholar]
- 13.Sheng H, Shao J, Kirkland SC, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–9. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase-2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 15.Zweifel BS, Davis TW, Ornberg RL, Masferrer JL. Direct evidence for a role of cyclooxygenase-2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res. 2002;62:6706–6711. [PubMed] [Google Scholar]
- 16.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 17.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 18.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy-Cancer and Leukemia Group B Trial 3023. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 19.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–81. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–7. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 21.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–6. [PubMed] [Google Scholar]
- 22.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 23.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. [PubMed] [Google Scholar]
- 24.Dohadwala M, Yang SC, Luo J, et al. Cyclooxygenase-2 dependent regulation of E- cadherin: prostaglandin E2 induces transcriptional repressors ZEB1 and Snail in non small cell lung cancer. Cancer Res. 2006;66:5338–5345. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol. 2002;33:708–714. doi: 10.1053/hupa.2002.125376. [DOI] [PubMed] [Google Scholar]
- 27.Moraitis D, Du B, De Lorenzo MS, et al. Levels of cyclooxygense-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor and its ligands. Cancer Res. 2005;65:664–70. [PubMed] [Google Scholar]
- 28.Piper PJ, Vane JR, Wyllie JH. Inactivation of prostaglandins by the lungs. Nature. 1970;225:600–4. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- 29.Hamberg M, Samuelsson B. The structure of the major urinary metabolite of prostaglandin E2 in man. J Am Chem Soc. 1969;91:2177–8. doi: 10.1021/ja01036a092. [DOI] [PubMed] [Google Scholar]
- 30.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E1 and E2 in man. J Biol Chem. 1971;246:6713–21. [PubMed] [Google Scholar]
- 31.Ferretti A, Flanagan VP, Roman JM. Quantitative analysis of 11 α-hydroxy-9,15-dioxo-2,3,4,5,20-pentanor-19-carboxyprostanoic acid, the major urinary metabolite of E prostaglandins in man. Anal Biochem. 1983;128:351–8. doi: 10.1016/0003-2697(83)90385-8. [DOI] [PubMed] [Google Scholar]
- 32.Seyberth HW, Sweetman BJ, Frolich JC, Oates JA. Quantifications of the major urinary metabolite of the E prostaglandins by mass spectrometry: evaluation of the method’s application to clinical studies. Prostaglandins. 1976;11:381–97. doi: 10.1016/0090-6980(76)90160-x. [DOI] [PubMed] [Google Scholar]
- 33.Murphey LJ, Williams MK, Sanchez SC, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JD, Schmidt CR, Shrubsole MJ, et al. Urine PGE-M: a metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006;4:1358–1365. doi: 10.1016/j.cgh.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Duffield-Lillico AJ, Boyle JO, Zhou K, et al. Levels of prostaglandin E metabolite and leukotriene E4 are increased in the urine of smokers. Evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res. 2009;2:322–9. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross ND, Boyle JO, Morrow JD, et al. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin Cancer Res. 2005;11:6087–93. doi: 10.1158/1078-0432.CCR-05-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 38.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 39.Csiki I, Morrow JD, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–40. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 40.Gallo O, Franchi A, Magnelli L, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen EG, Almahmeed T, Du B, et al. Microsomal prostaglandin E synthase-1 is overexpressed in head and neck squamous cell carcinoma. Clin Cancer Res. 2003;9:3425–30. [PubMed] [Google Scholar]
- 42.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–19. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 43.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nature Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 44.Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlovic S, Du B, Sakamoto K, et al. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]
- 46.Sharma S, Stolina M, Yang SC, et al. Tumor cyclooxygenase-2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–68. [PubMed] [Google Scholar]
- 47.Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance? J Natl Cancer Inst. 2003;95:1440–52. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- 48.Panje WR. Regression of head and neck carcinoma with a prostaglandin-synthesis inhibitor. Arch Otolaryngol. 1981;107:658–63. doi: 10.1001/archotol.1981.00790470006003. [DOI] [PubMed] [Google Scholar]
- 49.Solomon SC, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials. Circulation. 2008;117:2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutter R, Lu B, Carbone DP, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009;15:2158–65. doi: 10.1158/1078-0432.CCR-08-0629. [DOI] [PubMed] [Google Scholar]
- 51.Wirth LJ, Haddad RI, Lindeman NI, et al. Phase I study of gefitinib plus celecoxib in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:6976–6981. doi: 10.1200/JCO.2005.02.4182. [DOI] [PubMed] [Google Scholar]
- 52.Klapan I, Katic V, Culo F, Cuk V. Prognostic significance of plasma prostaglandin E concentration in patients with head and neck cancer. J Cancer Res Clin Oncol. 1992;118:308–13. doi: 10.1007/BF01208621. [DOI] [PubMed] [Google Scholar]