Abstract
Background
To determine the predictive value of cardiac T2* magnetic resonance (MR) for heart failure and arrhythmia in thalassemia major.
Methods and results
We analyzed cardiac and liver T2* MR, and serum ferritin on 652 thalassemia major patients from 21 UK centers, with 1,442 MR scans. The relative risk (95% CI) for heart failure with cardiac T2* values <10ms (compared with >10ms) was 160 (39, 653). Heart failure occurred in 47% of patients within one year of a cardiac T2* <6ms with relative risk 270 (64, 1129). The area under the ROC curve for predicting heart failure was significantly greater for cardiac T2* (0.948) than for liver T2* (0.589, P<0.001) or serum ferritin (0.629, P<0.001). Cardiac T2* was <10ms in 98% of scans in patients who developed heart failure. The relative risk for arrhythmia with cardiac T2* values <20ms (compared with >20ms), was 4.6 (2.66, 7.95). Arrhythmia occurred in 14% of patients within one year of a cardiac T2* of <6ms. The area under the ROC curve for predicting arrhythmia was significantly greater for cardiac T2* (0.747) than for liver T2* (0.514, P<0.001) or serum ferritin (0.518, P<0.001). The cardiac T2* was <20ms in 83% of scans in patients who developed arrhythmia.
Conclusions
Cardiac T2* MR identifies patients at high risk of heart failure and arrhythmia from myocardial siderosis in thalassemia major, and is superior to serum ferritin and liver iron. Using cardiac T2* for the early identification and treatment of patients at risk is a logical means towards reducing the high burden of cardiac mortality in myocardial siderosis.
Keywords: Thalassaemia, Cardiac siderosis, T2 star, Magnetic resonance, Heart, Iron overload
INTRODUCTION
Thalassaemia is the commonest single gene disorder worldwide, with approximately 94 million heterozygotes for beta thalassaemia and 60,000 homozygotes born each year.1 Although survival is improving in cohorts of patients in whom deferoxamine was introduced at a younger age,2 myocardial siderosis resulting in heart failure remains the major cause of death (50–70%) in thalassaemia major patients.2,3 This occurs at a strikingly young age with between 15–50% dying by the age of 35 years.2,3
Severe myocardial siderosis causes a toxic dilated cardiomyopathy which can be reversed if aggressive chelation is commenced early,4 but clinical diagnosis is often delayed due to the typically late onset of symptoms. Catastrophic deterioration in cardiac function resulting in death may occur rapidly once clinically obvious heart failure is present. Methods for predicting heart failure have been developed based on established measures of iron loading, most importantly time averaged serum ferritin >2500µg/L,2,5 and liver iron concentration >15mg/kg dry weight,6 but the persistently high mortality rate from heart failure indicates that high risk patients are not being identified in time for effective intervention to be made. Measurement of ventricular function, such as alteration over time in ejection fraction, has also been proposed in thalassemia but it identifies patients at a relatively late stage,7 and furthermore, dysfunction may be masked because of supranormal left ventricular function in thalassemia patients in the absence of myocardial iron loading.8 Most recently, direct assessment of myocardial iron using magnetic resonance (MR) relaxation has been used, because iron deposits shorten T1, T2 and T2*.
Of these, the measurement of T2* has become the widest used in the heart because it is easily combined with cardiac gating, is fast and robust, and the most sensitive to iron deposition.9 The cardiac T2* technique is transferrable with good interscanner agreement,10 and has now been implemented in at least 50 centers worldwide on 1.5T scanners from the three largest scanner manufacturers. Direct calibration of the cardiac T2* value to the myocardial iron concentration is reported in animals,11 and in humans.12 However, whilst this work is important for a complete scientific understanding, the relationship between cardiac T2* and prediction of cardiac events can be made independently of this data, and in terms of patient care, is the more important. We now report the value of cardiac T2* MR to predict cardiac events from a large prospective database of thalassaemia major patients, in comparison with the established predictors of outcome, liver iron and serum ferritin.
METHODS
Patient population
A total of 652 patients (1442 scans) with beta-thalassaemia major were included in this study. Their clinical care was undertaken at 21 hematology centers throughout the United Kingdom, and they were scanned at the Cardiovascular MR Unit of Royal Brompton Hospital, London between 1999 and 2006. This is a substantial majority of the UK thalassemia major patients (approximately 800 patients), and therefore represents a multicentre national sample. Patients were referred for clinical evaluation according to local practice at each caring centre, and were unselected. All patients were included in the database, but 17 were excluded from the predictive analysis reported in this study because of clinical heart failure (N=11) or arrhythmia (N=6) at the time of first MR scan. Of these patients, 319 were male and 333 female with a mean age at time of first scan of 27.1 ±9.6 years, and detailed patient demographics are shown in table 1. The prospective database of all patients was maintained from local patient records, and follow-up was completed by local case note review by a single researcher using a standard case record form. At the time of analysis, the follow up was complete (100%) for a total of 1285 patient years. At the time of the first scan, 22 patients were receiving no chelation, having had bone marrow transplantation. Of those patients who were treated with chelation, 433 patients were receiving deferoxamine, 72 patients deferiprone, 105 patients deferoxamine combined with deferiprone, 19 patients deferasirox only and 1 patient deferasirox combined with deferoxamine. Ferritin levels were recorded within 22.5 ±22.4 days of all MR scans. The mean number of blood units transfused per year per patient was 32.6 ±11.5. The prediction of heart failure and arrhythmia in the year after each scan was pre-defined as the primary endpoint of this study. The holding, follow-up and reporting of the results of this database was fully approved by Trent NHS Research Ethics Committee.
Table 1. Demographics.
Patient demographics at first MR scan. CI- confidence interval.
| Total number of patients | 652 | |
|---|---|---|
| Age (years) | 27.1 ±9.6 | |
| Sex | ||
| Male | 319 | |
| Female | 333 | |
| Race/ethnicity | ||
| White | 296 | |
| South Asian | 283 | |
| Chinese | 23 | |
| Arabic | 31 | |
| African | 19 | |
| Blood measures: | ||
| Transfusional red blood cell input (mL/kg/year) | 113.9 ±49.7 | |
| Pre-transfusion hemoglobin (g/dL) | 9.5 ±1.5 | |
| Hepatitis C positive | ||
| Yes | 38 | |
| No | 614 | |
| Biochemistry | ||
| Serum ferritin (µg/L) [M: 5–104, F: 4–254] | 2231 ±1801 | |
| CMR measures: | ||
| Cardiac T2* (ms) geometric mean (95% CI) | 19.0 (18.4 – 19.7) | |
| Liver T2* (ms) geometric mean (95% CI) | 3.6 (3.5–3.8) | |
| LV end diastolic volume index (mL/m2) | 109 ±112 | |
| LV end systolic volume index (mL/m2) | 43.2 ±21.8 | |
| LV ejection fraction (%) | 66.1 ±8.5 | |
| Chelation (median + Q1,Q3) | ||
| Deferoxamine only (N=433,66.4%) | 202 mg/kg/week (164,270); 5 days/week (5, 5) | |
| Deferiprone only (N=72, 11.0%) | 70 mg/kg/day (57,82) | |
| Deferasirox only (N=19, 2.9%) | 10 mg/kg/day (7.5,15) | |
| Deferoxamine & deferiprone (N=105, 16.1%) |
|
|
| Deferasirox with Deferoxamine (N=1, 0.2%) |
|
|
| No Chelation (N=22, 3.4%) | ||
Diagnostic criteria
A diagnosis of heart failure was made only if the patient complained of worsening dyspnea at rest or during exercise, and objective left ventricular dysfunction was present with an ejection fraction of less than 56%,9,13 and the caring clinician made the clinical diagnosis of heart failure. A diagnosis of arrhythmia was made only if the patient complained of palpitations, and arrhythmia was objectively demonstrated by electrocardiography using 24 hour monitoring or a standard 12 lead recording. Arrhythmias were categorized according to AHA/ACC guidelines,14 and included atrial fibrillation (AF) defined as a cardiac arrhythmia arising from the atrium with an atrial rate >300 bpm and an irregularly irregular ventricular response in the presence of conduction (>10 minutes sustained arrhythmia); supraventricular tachycardia (SVT) defined as a tachycardia that emanates from or requires participation of supraventricular tissue, other than atrial fibrillation/flutter (>10 minutes sustained arrhythmia); ventricular tachycardia (VT) defined as 3 or more consecutive complexes in duration emanating from the ventricles at a rate >100/min; and ventricular fibrillation (VF) defined as rapid, usually more than 300/min, grossly irregular ventricular rhythm with marked variability in QRS cycle length, morphology, and amplitude. Cardiac T2* values >20ms were considered normal.9 A liver T2* value of <0.96ms is calibrated to a dry weight equivalent of >15mg/g dry weight.9
Magnetic Resonance
Patients were scanned with a 1.5T scanner (Sonata, Siemens Medical Solutions, Erlangen, Germany) using previously reported techniques.15 In brief, cardiovascular MR was performed using a cardiac gated, single breath-hold, 8-echo sequence (2.6 – 16.7ms, increasing in 2.02ms increments) of a single mid-ventricular short axis slice. A single breath-hold, 20 echo sequence (1.07–0.21ms) of a transaxial slice of the liver was also acquired. Long axis cines and a contiguous stack of short axis cines were also acquired to assess left ventricular dimensions and function using standard techniques.16 Data analysis was performed using CMRtools and its plug-in ThalassemiaTools (Cardiovascular Imaging Solutions, London UK) for the liver T2* (a large region of interest excluding vascular structures) and heart T2* (a large region of the interventricular septum excluding regions in proximity to the coronary veins), as well as left ventricular ejection fraction using semi-automated planimetry of endocardial borders.16 All scans were reported at the time of acquisition by multiple operators, and a clinical report generated for the referring physician.
Statistics
All statistical analysis was performed by the Medical Statistics Department at our institution (MR). Many patients in this study had multiple scans and therefore we used a mixed-model Poisson regression with nested values (N=1442 scans) to produce univariate and multivariate analysis of cardiac T2*, liver T2* and serum ferritin for cardiac outcomes, and presented as relative risk (95% confidence intervals). Nesting incorporates all data from all scans in the predictive model for cardiac outcomes, and all repeated scans from any patient are modeled as non-independent. The unit of analysis therefore is each individual scan with the 1 year of follow up the follows it. Scans from the same patient are then nested within that patient. Each scan is assessed with regards to the outcomes following that individual scan, with the patient entered as a random effect to account for within-patient correlation. For purposes of comparison only, the relative risks were also calculated for per-scan (non-nested analysis where all scans are considered to be independent; N=1442 scans) and for patient first scans only (non-nested analysis considering each patient’s first scan only, ignoring any repeated scans; N=652 scans). These data are shown in supplementary tables as they show similar results to the main analysis. Patients were censored from further analysis after a first outcome. All patient demographics are presented according to the 1st scan for each patient. The distribution of liver T2*, cardiac T2*, ferritin and ejection fraction were not significantly deviated from normal by Kolmorogov-Smirnov testing and are therefore presented using mean and standard deviation (SD), and presented as per scan. Receiver operating characteristic (ROC) graphs were produced for cardiac T2*, liver T2* and serum ferritin to compare predictive accuracy for cardiac outcomes across the full range of measured values using the area under the curve. Kaplan Meier curves for time to heart failure and arrhythmia were generated. Data with a normal distribution are presented as mean ±SD, and those with non-normal distributions are presented as median with the inter-quartile range. Statistical significance was set at p<0.05, and all p-values quoted are 2-tailed. All analysis was performed using STATA 10.2 (StataCorp, Texas).
RESULTS
Summary of primary endpoint events
At 1 year of follow-up, there were 80 episodes of heart failure and 98 episodes of arrhythmia. There were 4 deaths, with 3 patients dying from sepsis following bone marrow transplant and 1 patient dying following an episode of ventricular tachycardia. There were 32 instances where a patient was recorded as having both a heart failure event and an arrhythmia event within 1 year of a scan.
Heart Failure
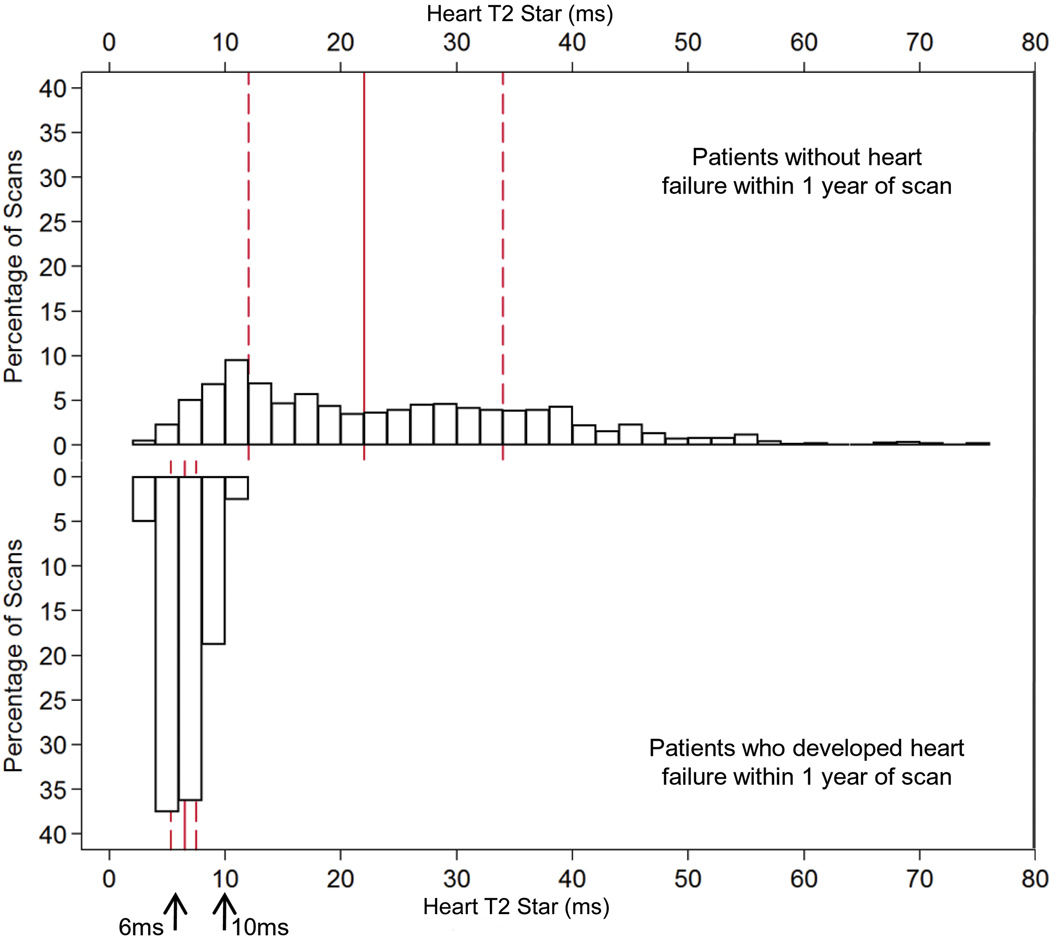
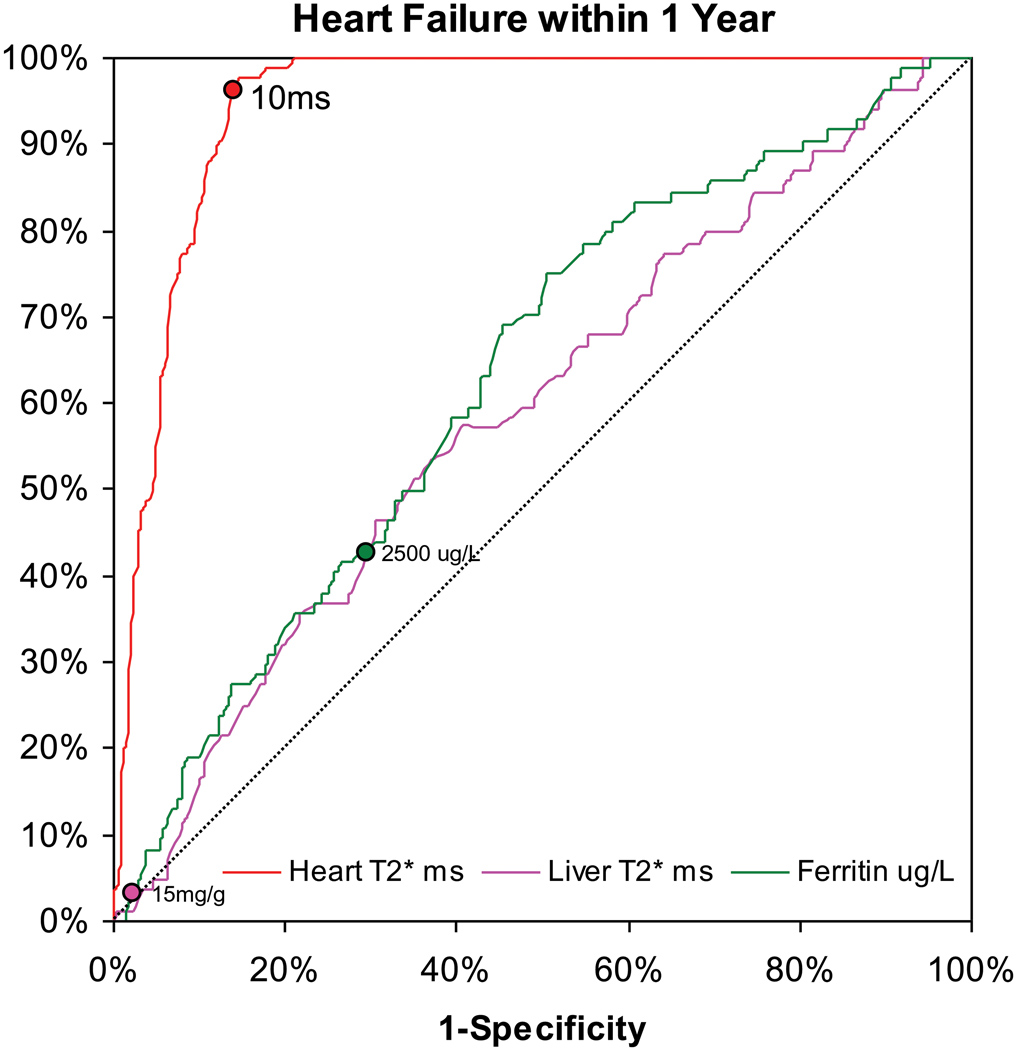
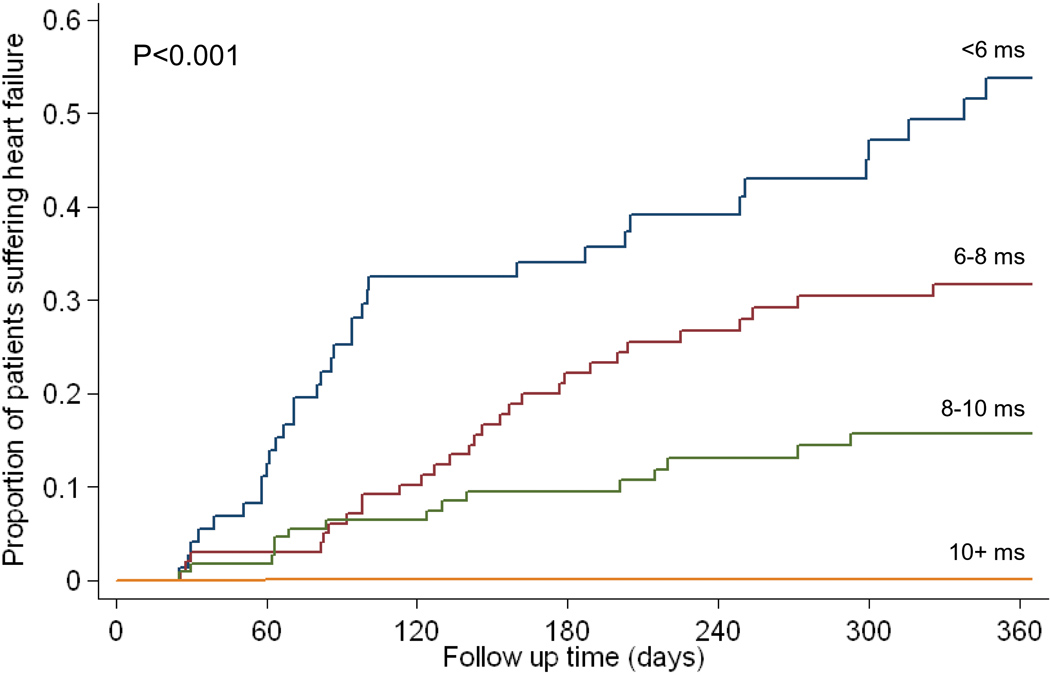
For the 80 heart failure episodes, 60 presented in New York Heart Association (NYHA) class two, 16 were NYHA class three, and 4 were NYHA class four. The mean ejection fraction of these 80 patients at the time of diagnosis of heart failure was 43.1 ±7.2%. The median (Q1, Q3) time of onset from time of T2* MR scan to an episode of heart failure was 158 (52, 342) days. In these heart failure patients, the preceding cardiac T2* was 6.7 ±1.8ms, the liver T2* 3.9 ±3.7ms and ferritin 2,713 ±1,686µg/L. The distribution of cardiac T2* values in those patients who went on to develop heart failure in comparison with the T2* values of those patients who remained free of heart failure is shown in figure 1a. The relative risks associated with cardiac T2*, liver T2* and ferritin are given in table 2. The cardiac T2* was <10ms in 98% of patients who developed heart failure. The one year incidence of heart failure in patients with the lowest cardiac T2* of <6ms was 47%. In comparison with cardiac T2* values >10ms, there was a significantly increased risk of heart failure associated with cardiac T2* values <10ms, with a relative risk of 160 (39, 653) in the nested analysis. The relative risk for cardiac T2* <6ms was 270 (64, 1129). Serum ferritin using the conventional threshold (>2500µg/L) was a significant but weaker predictor of heart failure with relative risk 0.56 (0.34, 0.91) vs ferritin <2500 µg/L, table 2). Liver T2*<0.96 ms (equivalent to the conventional threshold of >15 mg/g/dw iron) was not a significant predictor of heart failure with relative risk 0.81 (0.23, 2.80) vs liver T2* >0.96ms.. ROC curves for the prediction of heart failure by cardiac T2*, liver T2* and serum ferritin are shown in figure 1b. The area under the curve for serum ferritin (0.629) and liver T2* (0.589) were similar (P=0.21), but the area under the curve for cardiac T2* (0.948) was substantially and significantly greater (P<0.001 vs liver T2*, and P<0.001 vs serum ferritin). The relative risks for the first scan only (non-nested) and per scan (non-nested) groups give similar conclusions (supplemental table 1). The Kaplan Meier curves for occurrence of heart failure stratified into 4 levels of cardiac T2* are shown in figure 1c, and show a significant increase in risk with increasing cardiac iron loading (p<0.001). Overall, the T2* threshold of 10ms predicted heart failure with a sensitivity of 97.5% (95% CI: 91.3, 99.7) and specificity of 85.3% (95% CI: 83.3, 87.2). The data was also analyzed for prediction of asymptomatic left ventricular dysfunction at the time of the scan, and showed significant incremental relative risk (RR) for each level of cardiac T2* <10ms (T2* 8–10ms RR 2.97; T2* 6–8ms 3.48; T2* <6ms 4.51; all p<0.001).
Figure 1.
A) The frequency distribution of cardiac T2* values in the 80 patients who developed heart failure within 1 year of scan (lower panel) in comparison with the other 572 patients (upper panel). Note the segregation of cardiac T2* in the patients who went on to develop heart failure into the lowest values, such that 98% of patients who developed heart failure had a cardiac T2* of <10ms. The solid vertical red line is the median and the dashed red lines are the upper and lower quartiles.
B) Receiver operating characteristic curve for prediction of heart failure within one year of MR scanning. The diagonal line shows the performance of a non-diagnostic test. Whilst the liver T2* and serum ferritin are weakly predictive, the cardiac T2* is greatly superior to both these conventional measures (P<0.001). The points marked on each line indicate a threshold of 10ms for cardiac T2*, 0.96ms for liver T2* (equivalent to 15mg/kg dry weight) and 2500ug/L for serum ferritin.
C) Kaplan Meier curves showing occurrence of heart failure over 1 year according to baseline cardiac T2* values of >10ms, 8–10ms, 6-<8ms, and <6ms (p<0.001).
Table 2. Heart failure.
A) Heart failure relative risks (RR) for increments in cardiac T2* from <6 to 10ms in comparison with the reference value of T2* >10ms.
B) Heart failure relative risks for increments in liver T2* from <0.96ms in comparison with the reference value of >6.3ms;
C) Heart failure relative risks for serum ferritin above and below 2500 ug/L.
| Cardiac T2* (ms) |
No. in group |
No. with heart failure |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| <6 | 72 | 34 | 270 | 64 | 1129 | <0.001 |
| 6 to <8 | 98 | 29 | 171 | 41 | 718 | <0.001 |
| 8 to <10 | 108 | 15 | 81 | 19 | 357 | <0.001 |
| 10+ | 1164 | 2 | 1 | Reference | ||
| Liver T2* (ms) |
No. in group |
No. with heart failure |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| <0.96 | 63 | 3 | 1.25 | 0.33 | 4.76 | 0.74 |
| 0.96 to <1.4 | 136 | 14 | 2.59 | 1.16 | 5.79 | 0.021 |
| 1.4 to <2.7 | 382 | 26 | 1.68 | 0.85 | 3.31 | 0.13 |
| 2.7 to <6.3 | 484 | 22 | 1.22 | 0.61 | 2.45 | 0.57 |
| 6.3+ | 377 | 15 | 1 | Reference | ||
| Ferritin µg/L |
No. in group |
No. with heart failure |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| 2500+ | 450 | 35 | 0.56 | 0.34 | 0.91 | 0.02 |
| <2500 | 992 | 45 | 1 | Reference | ||
Arrhythmia
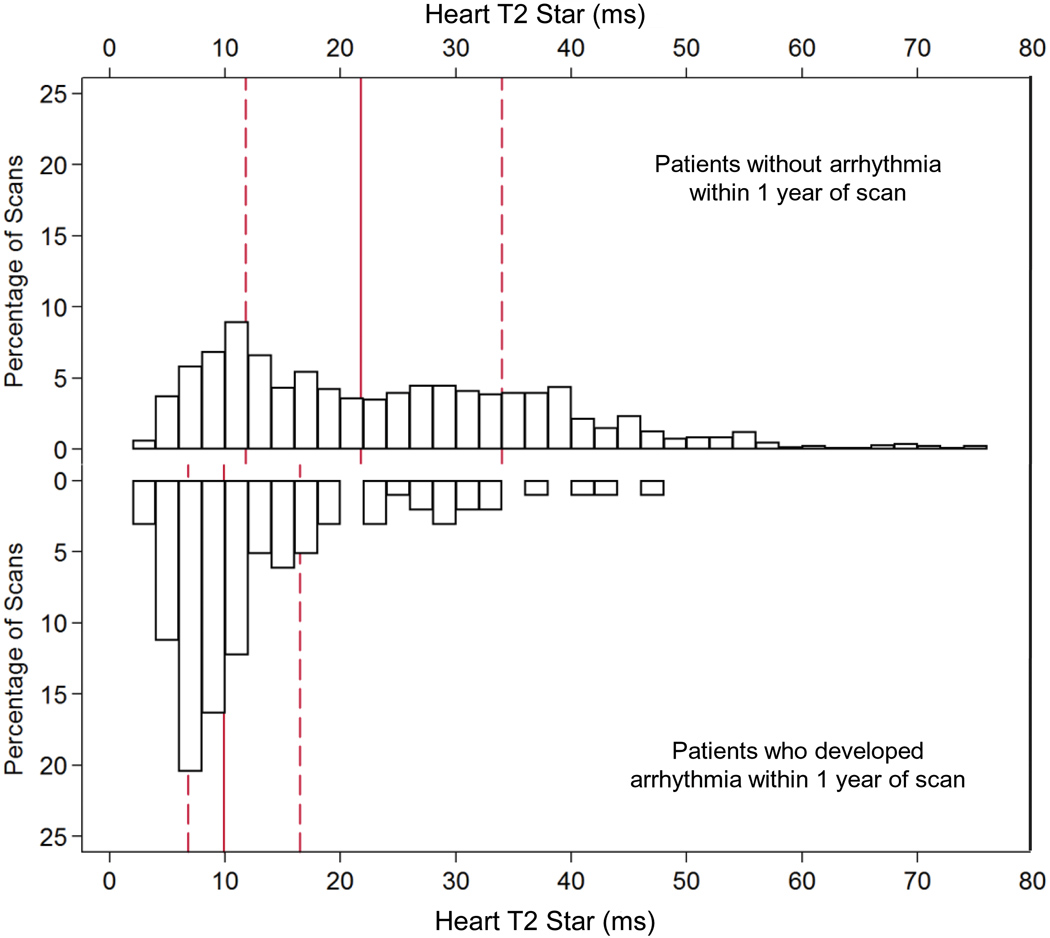
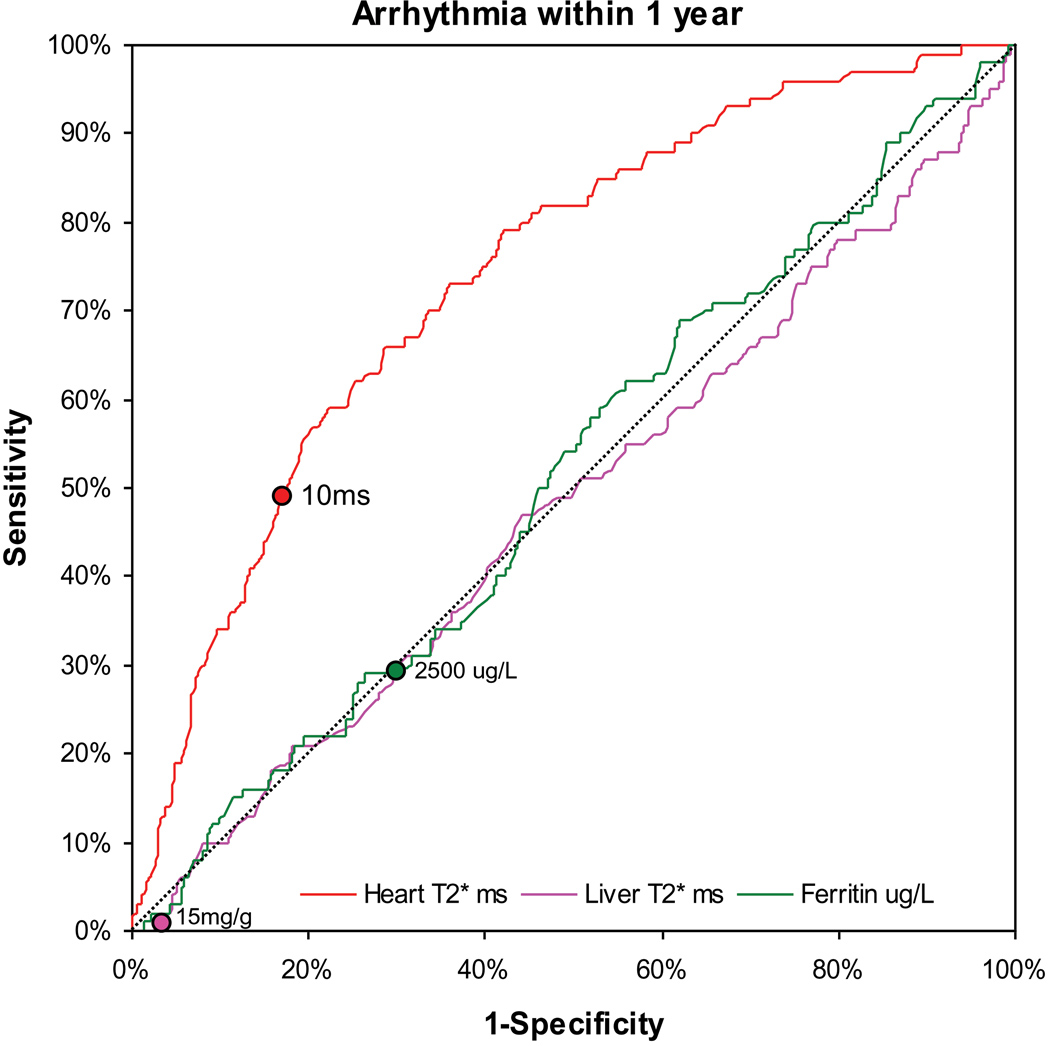
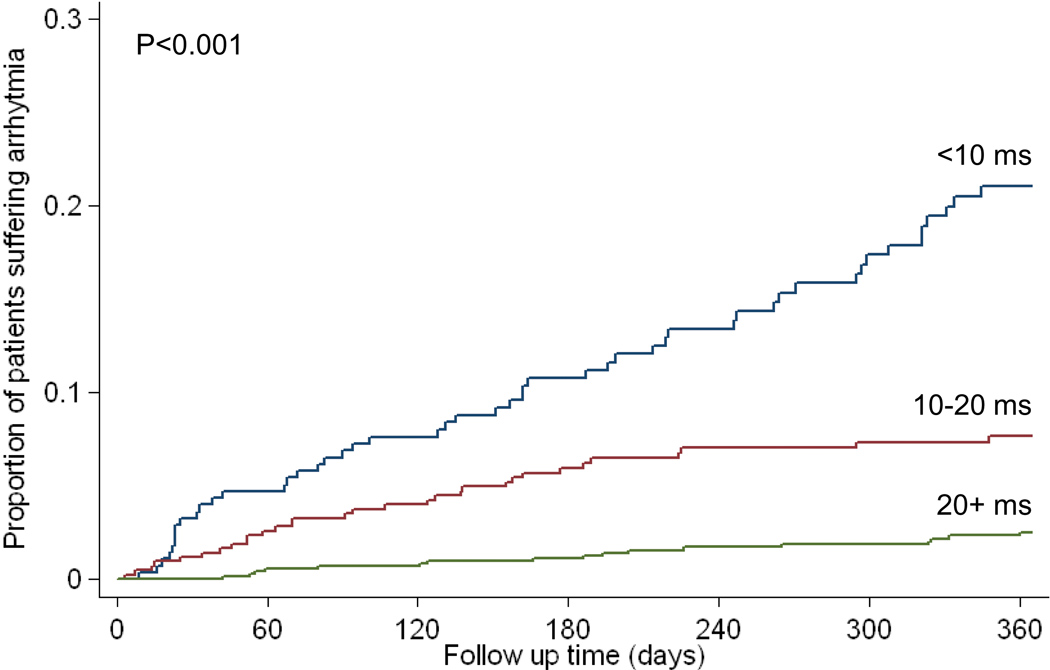
For the 98 patients who developed arrhythmias within 1 year of MR scanning, 78 patients had AF, 14 patients had SVT, 5 patients had VT, and 1 patient had VF. In these patients, at the time of first MR scan, the mean cardiac T2* was 13.5 ±9ms, mean liver T2* 6.0 ±6.4ms, mean serum ferritin 2140 ±1540µg/L, and the mean ejection fraction was 60.7 ±9.3%. The median time to developing arrhythmia was 133 days. The distribution of cardiac T2* values in the patients who went on to develop arrhythmia in comparison with those who remained arrhythmia free is shown in figure 2a. The relative risks associated with cardiac T2*, liver T2* and ferritin are given in table 3. A cardiac T2* of <20ms was present in 83% of patients who developed arrhythmia. The incidence of arrhythmia at one year in patients with the lowest cardiac T2* of <6ms was 14%. The mean cardiac T2* in the arrhythmia patients was: AF 13.6 ±9.9ms, SVT 11.9 ±7.1ms, VT 16.5 ±9.3ms, and VF 9.0ms. There was no significant difference between the cardiac T2* values for atrial and ventricular arrhythmias (P=0.28). In comparison with cardiac T2* values >20ms, there was a significantly increased risk of arrhythmia associated with cardiac T2* values <20ms, with a relative risk of 4.60 (2.66, 7.95). The relative risk for a cardiac T2* <6ms was 8.79 (4.03, 19.2). There was no significant predictive value using the conventional thresholds of ferritin (>2500µg/L) with relative risk 0.90 (0.55, 1.45) vs ferritin <2500µg/L) or liver T2* (<0.96ms) with relative risk 0.81 (0.24, 2.74) vs T2* >0.96ms). ROC curves for the prediction of arrhythmia by cardiac T2*, liver T2* and serum ferritin are shown in figure 2b. The area under the curve for serum ferritin (0.518) and liver T2* (0.514) were similar (P=0.99), but the area under the curve for cardiac T2* (0.747) was substantially and significantly greater (P<0.001 vs liver T2*, and P<0.001 vs serum ferritin). The relative risks for the first scan only (non-nested) and per scan (non-nested) groups give similar conclusions, and are shown in supplemental table 2. The Kaplan Meier curves for occurrence of arrhythmia stratified into 3 levels of cardiac T2* are shown in figure 2c, and show a significant increase in risk with increasing cardiac iron loading (p<0.001). Overall, the T2* threshold of 20ms predicted arrhythmia with a sensitivity of 82.7% (95% CI: 73.7, 89.6) and specificity of 53.5% (95% CI: 50.8 56.2).
Figure 2.
A) The frequency distribution of cardiac T2* values in the 98 patients who developed arrhythmia within 1 year of scan (lower panel) in comparison with the other 554 patients (upper panel). Note the segregation of cardiac T2* in the patients who went on to develop arrhythmia into the lowest values, such that 83% of patients who developed arrhythmia had a cardiac T2* of <20ms, and also that the cardiac T2* values are higher and wider spread than for patients developing heart failure (figure 1). The solid vertical red line is the median and the dashed red lines are the upper and lower quartiles.
B) Receiver operating characteristic curve for prediction of arrhythmia within one year of MR scanning. The diagonal line shows the performance of a non-diagnostic test. The liver T2* and serum ferritin are not predictive. The cardiac T2* is significantly superior to both these conventional measures (P<0.001). The points marked on each line indicate a threshold of 10ms for cardiac T2*, 0.96ms for liver T2* (equivalent to 15mg/kg dry weight) and 2500ug/L for serum ferritin.
C) Kaplan Meier curves showing occurrence of arrhythmia over 1 year according to cardiac T2* values of >20ms, 10–20ms, and <10ms (p<0.001).
Table 3. Arrhythmia.
A) Arrhythmia relative risks (RR) for increments in cardiac T2* from <6 to 10ms in comparison with the reference value of T2* >10ms;
B) Arrhythmia relative risks for increments in liver T2* from <0.96ms in comparison with the reference value of >6.3ms;
C) Arrhythmia relative risks for serum ferritin above and below 2500 ug/L.
| Cardiac T2* (ms) |
No. in group |
No. with Arrhythmia |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| <6 | 72 | 14 | 8.79 | 4.03 | 19.2 | <0.001 |
| 6 to <8 | 98 | 20 | 7.5 | 3.71 | 15.2 | <0.001 |
| 8 to <10 | 108 | 16 | 6.82 | 3.28 | 14.2 | <0.001 |
| 10 to <15 | 263 | 21 | 3.23 | 1.65 | 6.3 | 0.001 |
| 15 to <20 | 165 | 10 | 2.21 | 0.97 | 5.02 | 0.058 |
| 20+ | 736 | 17 | 1 | Reference | ||
| Liver T2* (ms) |
No. in group |
No. with Arrhythmia |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| <0.96 | 63 | 3 | 0.82 | 0.23 | 2.96 | 0.77 |
| 0.96 to <1.4 | 136 | 10 | 1.21 | 0.54 | 2.69 | 0.64 |
| 1.4 to <2.7 | 382 | 28 | 1.13 | 0.64 | 2 | 0.68 |
| 2.7 to <6.3 | 484 | 27 | 0.83 | 0.48 | 1.46 | 0.52 |
| 6.3+ | 377 | 30 | 1 | Reference | ||
| Ferritin µg/L |
No. in group |
No. with Arrhythmia |
Nested Results | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | p | ||||
| 2500+ | 450 | 30 | 0.9 | 0.55 | 1.45 | 0.65 |
| <2500 | 992 | 68 | 1 | Reference | ||
Effect of MR result on risk
The reporting of the cardiac T2* value may have caused alterations in treatment which could have affected the outcome events. Therefore we analyzed the relative risks for an increase or decrease in each chelator (relative to not changing dose), and included these in an adjusted analysis looking at heart T2* for prediction of heart failure or arrhythmia. There was no statistically significant effect of changing dose, except that an increased dose of deferoxamine was associated with a small increased relative risk of arrhythmia, and the adjusted relative risk relating heart T2* to outcomes remained very similar to the unadjusted ones. Therefore, there is little statistical evidence from the database that short-term changes in chelation dose after scans altered the ability of T2* to predict outcomes over the period of collection of data.
Effect of prior history of heart failure or arrhythmia on outcomes
All patients reported in this study did not have heart failure or arrhythmia at the time of first MR scan, but 9 patients had a prior (and resolved) history of heart failure and 1 patient had a prior (and resolved) history of arrhythmia. Therefore we re-analyzed the predictive power of cardiac T2* using prior history of heart failure or arrhythmia as covariates, and including in the analysis those patients who developed endpoints within 1 year of follow-up who had additional MR scans. The relative risk of prior heart failure predicting future heart failure was 1.93 (1.19, 3.13), and the relative risk of prior heart failure or arrhythmia predicting future heart failure was 1.60 (0.92, 2.81) and 1.59 (0.84, 3.02) respectively. The relative risk of prior arrhythmia predicting future arrhythmia was 2.67 (1.41, 5.09), and the relative risk of prior arrhythmia or heart failure predicting future arrhythmia was 1.84 (0.86, 3.94) and 2.08 (1.07, 4.02) respectively. There was no significant interaction between heart T2* and prior heart failure when looking at heart failure, and no significant interaction between heart T2* and prior arrhythmia when looking at arrhythmia. Overall therefore, these results show that there were significant associations between prior disease and new disease, but these were independent of the predictive ability of T2*, and T2* was still a much more powerful predictor.
Regression between iron parameters
There were significant relationships between all three iron variables (cardiac T2* vs liver T2* R2=0.003, p=0.040; cardiac T2* vs ferritin R2=0.003, p=0.041; liver T2* vs ferritin R2=0.37, p<0.001). These results should be interpreted in the context of the large sample size (1442) which highly powered to find significant results for regression analysis, and on data inspection only the relationship between liver T2* and ferritin is meaningful.
Effect of age, gender and baseline medication on heart failure
Neither age nor gender were significantly associated with either of the study outcomes (p>0.3 in all cases). There was no association between baseline treatment and heart failure events either when assessed as a single variable (p>0.2 in all cases) or in conjunction with T2* (p>0.4 in all cases).
DISCUSSION
Failure to control serum ferritin and liver iron over sustained periods has been linked to increased risk of heart disease,2,5,6,7 and other complications of iron overload.17 However low values of liver iron or serum ferritin do not necessarily signify low risk of iron induced cardiomyopathy.9 This may arise because iron chelation therapy can remove iron more rapidly from the liver than from the heart, which may normalize liver iron while myocardial iron remains high.9,18,19 Other mechanisms may also be involved, including potential genetic variations in function of cardiac iron transport channels, such as the L-type calcium channel and the divalent metal transporter 1.20,21 Therefore there is often a need to identify those patients most at risk of cardiomyopathy even when serum ferritin and liver iron values are currently well controlled. While sequential quantification of heart function identifies a patient group at increased risk of cardiac mortality,7 it would be preferable to identify high risk patients early before cardiomyopathy develops, and use targeted treatment.22,23 The estimation of heart iron by MR offers this possibility. This has been done using the MR relaxation parameter T2*, which is sensitive to the presence of storage iron microaggregates which disturb the homogeneity of the magnetic microenvironment. Evidence has been accumulating that low values of cardiac T2*, which reflect high cardiac iron levels, are associated with heart failure. In a cross-sectional study of 28 patients presenting with heart failure, 89% of cases had a cardiac T2* <10ms.24 Limited other data has supported this experience.25 In the current study therefore, we analyzed our prospective database on all thalassemia major patients living in the United Kingdom having a cardiac T2* scan since 1999. The data unequivocally demonstrate that cardiac T2* is a powerful predictor of the subsequent development of heart failure. The relative risk was substantial at 270 for the highest risk group with a cardiac T2* <6ms in comparison with a cardiac T2* of >10ms. Analysis of the incidence of heart failure following the finding of a cardiac T2* of <6ms was impressive with 47% of such patients developing this ominous complication within 12 months. The ROC analysis showed that the predictive value of cardiac T2* for heart failure was significantly and substantially greater than for conventional iron measures for prediction of cardiac complications (liver iron, serum ferritin).
The results for prediction of arrhythmia by cardiac T2* are also significant but of lower magnitude than for prediction of heart failure. In addition the distribution of cardiac T2* in patients presenting with arrhythmia had a greater spread with higher cardiac T2* values. However, 83% of cases occurred in patients with cardiac T2* values <20ms, indicating myocardial siderosis as the primary cause. The majority of the arrhythmias were atrial in origin and these were not dependent on failing ventricular function, as they were observed at higher (though still abnormal) T2* levels, than those seen with heart failure. The atrial myocardium might be more sensitive to iron deposition than the ventricle in the causation of arrhythmias, and this is somewhat supported by historical data prior to the use of iron chelators, which suggested that iron deposition is greater in the ventricles than the atria.26 Unfortunately T2* measurements of the thin atrial myocardium are limited by partial volume effects and are unlikely to be robust, and therefore this issue is not easy to address with current non-invasive MR techniques. Thalassemia patients are also susceptible to non-iron dependent causation of atrial arrhythmia because of chronically raised cardiac output and atrial dilation.
There are some limitations to our study. We have not formally validated our results in a different cohort. However, we have not attempted to build a multivariate model from a number of available factors, but looked at a small number of pre-specified iron measures and prospectively shown their relation to the outcomes of interest. However, it would still be of interest to reproduce these results in another cohort. We used the widely accepted normal cardiovascular MR threshold for the lower limit of normality of left ventricular ejection fraction of 56% to categorize ventricular dysfunction, although data from a single center, which has not been reproduced, suggests that slightly higher threshold values may pertain to thalassemia major patients in the absence of cardiac siderosis.27
In conclusion, this study shows that cardiac T2* is highly predictive over 1 year for the development of heart failure and arrhythmia, and is significantly more predictive than contemporaneous measures of liver iron and serum ferritin. This indicates that the inclusion of cardiac T2* assessment in concert with conventional long-term assessments of tissue iron loading is mandatory for the comprehensive evaluation of iron loading. A widespread program using cardiac T2* in beta-thalassemia major has considerable potential to reduce mortality from heart failure by the early identification and treatment of patients at risk.28 These data will also be of direct value in management of different iron loading conditions such as hemochromatosis and other transfusion dependent anemias.
Supplementary Material
Acknowledgements
We thank the participating centers and the doctors and staff who assisted in contributing patient data to this study: Birmingham Children’s Hospital, Birmingham; City Hospital, Birmingham; Bristol Royal Hospital for Children, Bristol; Bradford Royal Infirmary, Bradford; Leicester Hospital, Leicester; St Bartholomew’s Hospital, London; The Homerton Hospital, London; The Royal London Hospital, London; St George’s Hospital, London; Hammersmith Hospital, London; Kings College Hospital, London; Queen Mary’s Hospital, London; St Mary’s Hospital, London; Central Middlesex Hospital, London; North Middlesex Hospital, London; University College Hospital, London; The Whittington Hospital, London; Central Manchester and Children’s University Hospitals Manchester; Trafford General Hospital, Manchester; John Radcliffe Hospital, Oxford; Peterborough District Hospital, Peterborough.
Funding sources: This study was supported by the UK National Institutes of Health Research Biomedical Research Unit, the NIH [Grant Award 5 R01 DK066084-02], and CORDA-The Heart Charity.
Footnotes
www.clinicaltrials.gov Identifier: NCT00520559
Subject codes: [110] Congestive; [11] Other heart failure; [30] CT and MRI
Conflict of interest disclosures: Kirk P: None; Roughton M: None; Porter JB: Research funding, speaker’s bureau, advisory board Novartis; Walker JM: None; Tanner MA: None ; Patel J: None; Wu D: None; Taylor J: None; Westwood MA: None; Anderson LJ: None; Pennell DJ: Consultancy, advisory board, speaker’s bureau, research funding Novartis. Consultancy, advisory board, speaker’s honoraria ApoPharma. Director and stockholder Cardiovascular Imaging Solutions.
References
- 1.Weatherall DJ. Oxford Textbook of Medicine. Oxford University Press; 1996. Anaemia as a World Health Problem; pp. 3463–3482. [Google Scholar]
- 2.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 3.Modell B, Khan M, Darlison M. Survival in beta thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355:2051–2052. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 4.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Pibiri M, Nair SV, Walker JM, Pennell DJ. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson. 2008;10:12. doi: 10.1186/1532-429X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 6.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farell DE, Harris JW. Efficacy of desferrioxamine in preventing complications of iron overload in patients with thalassaemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 7.Davis BA, O'Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 8.Westwood MA, Anderson LJ, Maceira AM, Shah FT, Prescott E, Porter JB, Wonke B, Walker JM, Pennell DJ. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J Magn Reson Imaging. 2007;25:1147–1151. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LJ, Holden S, Davies B, Prescott E, Charrier C, Bunce NH, Firmin DN, Porter JB, Wonke B, Walker JM, Pennell DJ. Cardiovascular T2* (T2 star) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 10.Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, Vasili B, Pennell DJ. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21:531–538. doi: 10.1007/s10554-005-0651-2. [DOI] [PubMed] [Google Scholar]
- 11.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, Pollack H, Moats R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD, Coates TD, Wood JC. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–686. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo controlled, double blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 14.American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology) Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology) Circulation. 2006;114:2534–2570. doi: 10.1161/CIRCULATIONAHA.106.180199. [DOI] [PubMed] [Google Scholar]
- 15.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 16.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 17.Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, Ripalti M, Sodani P, Tomassoni S, Visani G, Lucarelli G. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17–21. doi: 10.1182/blood.v100.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 19.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oudit GY, Trivieri MG, Khaper N, Liu PP, Backx PH. Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J Mol Med. 2006;84:349–364. doi: 10.1007/s00109-005-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwiczek S, Theurl I, Muckenthaler MU, Jakab M, Mair SM, Theurl M, Kiss J, Paulmichl M, Hentze MW, Ritter M, Weiss G. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007;13:448–454. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- 22.Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, Gotsis ED, Tanner MA, Smith GC, Westwood MA, Wonke B, Galanello R. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107:3738–3744. doi: 10.1182/blood-2005-07-2948. [DOI] [PubMed] [Google Scholar]
- 23.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 24.Tanner MA, Porter JB, Westwood MA, Nair SV, Anderson LJ, Walker JM, Pennell DJ. Myocardial T2* in patients with cardiac failure secondary to iron overload. Blood. 2005;2005:406. Abstract 3838. [Google Scholar]
- 25.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 26.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 27.Westwood MA, Anderson LJ, Maceira AM, Shah FT, Prescott E, Porter JB, Wonke B, Walker JM, Pennell DJ. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J Magn Reson Imaging. 2007;25:1147–1151. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]
- 28.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.