Abstract
A Phase I human vaccine trial of a novel polypeptide vaccine of HIV T helper epitopes (EP-1043) and a DNA vaccine of HIV CTL epitopes was conducted in 84 healthy adult volunteers. The vaccine immunogenicity was assessed by an intracellular cytokine staining assay for IL-2, IL-4, TNFα and IFNγ. Sixty eight percent (32/47) of subjects had a positive CD4+ T response after receiving two vaccinations of the polypeptide vaccine. The responding CD4+ T cells made various combinations of IL-2, IL-4, IFN-γ, and TNF-α. The study demonstrated that the EP-1043 vaccine is safe, well-tolerated, and immunogenic.
Keywords: HIV vaccine, T helper epitopes, phase I clinical trial
INTRODUCTION
An HIV vaccine that is capable of eliciting protective cellular or humoral immune responses is urgently needed. This necessity is underscored by the disappointing results of a large Phase II clinical trial of an HIV vaccine that elicited only CD8+ T cell response [1, 2], and a Phase III clinical trial of an HIV vaccine that stimulated an antibody response [3].
We recently reported a human clinical trial with DNA vaccine EP-1090 which encodes 21 HIV-derived cytotoxic T lymphocyte (CTL) epitopes that are derived from conserved sequences from Clades A, B, C, D, Circulating Recombinant Form (CRF) AE and CRF AG viral isolates, and a pan-DR helper T lymphocyte epitopes sequence (PADRE) which is capable of inducing T helper responses in mice and humans with a diverse range of MHC class II alleles. Results showed that the EP-1090 vaccine was safe and well-tolerated but only weakly immunogenic in healthy volunteers [4].
In the current study, we evaluated a helper T lymphocyte (HTL) vaccine, EP-1043, used either alone or in combination with the EP-1090 vaccine to test whether coordinated delivery of both HTL and CTL epitopes would augment the induction of a CTL response, and whether the EP-1043 vaccine is immunogenic in healthy human volunteers.
EP-1043 is a novel vaccine that contains 18 HTL epitopes derived from conserved sequences among HIV isolates of multiple clades (Walker et al., Vaccine, Submitted). The vaccine is produced in a baculovirus expression system, and formulated with Alhydrogel (aluminum hydroxide). The immunogenicity of the HTL epitopes was first verified in HLA transgenic mice (Walker et al., Vaccine, Submitted), and then characterized using peripheral blood mononuclear cells (PBMCs) from 22 HIV-1-infected patients receiving combination antiretroviral therapy. Half of the patients (13/22) tested had a CD4+ T cell response to one or more of the epitopes, and four of them responded to 10 or more of the epitopes [5]. The immunogenicity of the EP-1043 vaccine has never been tested before in healthy, HIV-uninfected volunteers, and thus will be evaluated in the current human phase I clinical trial.
MATERIALS AND METHODS
Vaccine and clinical trial design
The HVTN-064 study was a multi-site, double-blinded, Phase I human clinical trial of a novel multiepitope polypeptide composed of 18 HTL epitopes (EP-1043) and a DNA vaccine encoding 21 CTL epitopes (EP-1090). EP-1090 is a plasmid vaccine which encodes 21 cytotoxic lymphocyte CTL epitopes of HIV-1 Gag, Pol, Vpr, Nef, Rev, and Env, derived from HIV-1 sequences conserved among Clades A, B, C, D, CRF AE, and CRF AG viral isolates. In a recently completed clinical trial of the EP-1090 vaccine, it was found to be safe, well-tolerated, and weakly immunogenic [4]. EP-1043 is a recombinant protein vaccine consisting of a string of 18 HIV-1 derived HTL epitopes adsorbed to Alhydrogel®. The cGMP-produced product was stable for at least two years when stored at 2–8°C as a suspension of Alydrogel adsorbed protein. These epitopes were selected from HIV-Env (2/18), -Gag (3/18), -Pol (12/18), and -Vpu (1/18) proteins. Each one of the epitopes binds to several HLA-DR antigens (Walker et al., submitted). A vaccine composed of these epitopes would be expected to induce HTL responses in nearly 100% of the worldwide population. Many of the selected epitopes are also present in HIV-1 isolates from non-B clades. Thus, the vaccine may be appropriate for use outside of the US and Europe [5].
The original clinical trial consisted of two parts. Part A was a dose-escalation study for EP-1043 at 50 and 200µg, given as a single injection 4 times at months 0, 1, 3 and 6. A vehicle control group including aluminum hydroxide (Alhydrogel®) was included for each dose. A total of 24 subjects were enrolled into this part of the study. Part B was designed to test the combination of the maximum tolerated doses of the protein vaccine EP-1043 from Part A, along with the DNA vaccine EP-1090. The vaccine schedule was the same as that in the part A. There were three arms: EP-1043 only, EP-1090 only, and both vaccines combined. Vehicle controls were either aluminum hydroxide (Alhydrogel®) for EP-1043 or 3.4% polyvinylpyrrolidone (PVP) (Povidone, USP) in sterile PBS for EP-1090. A total of 96 subjects were to be enrolled in this part of the study (Table 1A). The two vaccines were administered to different and distal sites so that the safety of each component could be monitored. Blood and serum samples were collected at regular intervals for safety monitoring and immunogenicity assays. Signed informed consent was obtained from each of the trial participant. Federal and states rules and regulations regarding human subject research were strictly followed.
Table 1.
| Table 1A. Clinical Trial Design | |||||||
|---|---|---|---|---|---|---|---|
| Study Group |
Planned Enrollment (N) |
Vaccines | Vaccination schedule (months) | ||||
| Proteina(µg) (EP-1043) |
DNAb(mg) (EP-1090) |
V1 (0) | V2 (1) | V3 (3) | V4 (6) | ||
| Part A | |||||||
| 1 | 10 | 50 | - | Protein | Protein | Protein | Protein |
| 2 | - | - | Alhydrogelc | Alhydrogel | Alhydrogel | Alhydrogel | |
| 2 | 10 | 200 | - | Protein | Protein | Protein | Protein |
| 2 | - | - | Alhydrogel | Alhydrogel | Alhydrogel | Alhydrogel | |
| Part B | |||||||
| 3 | 20 | 200 | - | Protein | Protein | Protein | Protein |
| 4 | - | - | Alhydrogel | Alhydrogel | Alhydrogel | Alhydrogel | |
| 4 | 30 | - | 4 | DNA | DNA | DNA | DNA |
| 6 | - | - | PVPd | PVP | PVP | PVP | |
| 5 | 30 | 200 | 4 | Protein+DNA | Protein+DNA | Protein+DNA | Protein+DNA |
| 6 | - | - | Alhydrogen+PVP | Alhydrogen+PVP | Alhydrogen+PVP | Alhydrogen+PVP | |
| Table 1B. Actual Enrollment for Immunogenicity Study | |||||
|---|---|---|---|---|---|
| Study Group |
Actual Enrollment (N) |
Vaccines | Vaccination completed (days) |
||
| Protein (µg) | DNA (mg) | V2 (42) | V4 (182) | ||
| Part A | |||||
| 1 | 10 | 50 | - | 10 | 8 |
| 2 | - | - | 2 | 2 | |
| 1 | 10 | 200 | - | 9 | 7 |
| 2 | - | - | 2 | 1 | |
| Part B | |||||
| 3 | 10 | 200 | - | 10 | 8 |
| 2 | - | - | 1 | 0 | |
| 4 | 20 | - | 4 | 20 | 19 |
| 4 | - | - | 4 | 4 | |
| 5 | 20 | 200 | 4 | 18 | 16 |
| 4 | - | - | 3 | 0 | |
Notes: Protein: EP-1043 vaccine contains multiple HIV epitopes. It is expressed in Baculorvirus as recombinant protein, and adsorbed to alhydrogel.
DNA: EP-1090 plasmid DNA vaccine contains multiple HIV epitopes. It is dissolved in PBS containing 3.4% polyvinylpyrrolidone.
Alhydrogel: Aluminum hydorxide which serves as a diluent for the EP-1043 protein vaccine.
PVP: Phosphate-buffered saline (PBS) containing 3.4% polyvinylpyrrolidone which serves as a diluent for the EP-1090 DNV vaccine.
Safety assessment
Assessment of product safety included clinical observation and monitoring of hematological and chemical parameters. Safety was evaluated by monitoring participants for local and systemic adverse reactions for 36 hours after each injection, physical exam and laboratory assessment at two weeks after each injection, and participants were followed for a total of 12 months after the first injection. Parameters assessed include 1) Local reactogenicity signs and symptoms; 2) Systemic reactogenicity signs and symptoms; 3) Laboratory measures of safety; 4) Adverse and serious adverse experiences. These assessments dictated the speed of enrollment, as well as the timing for progression from Part A to Part B of the clinical trial.
In Part A, enrollment across all participating sites for Group 1 (EP-1043, 50µg; and placebo control) and Group 2 (EP-1043, 200µg; and placebo control) were restricted to a maximum of 1 participant per day until 5 participants had been enrolled in that group. The Protocol Safety Review Team (PSRT) reviewed the safety and reactogenicity data reported for the first 72 hours post-vaccination on each of these 5 participants and then determined whether it was safe to proceed with full enrollment in that group. In addition to monitoring participant safety throughout the study period, the PSRT reviewed cumulative safety data available on all participants in each group (vaccinees and control combined) up to and including the two week visit after the second vaccination to determine the initiation of dose escalation part of the study. The PSRT had the option to consult with the HVTN Safety Monitoring Board (SMB) on an ad hoc basis for these evaluations. In addition to monitoring participant safety throughout the study period, the PSRT reviewed all cumulative safety data available from Groups 1 and 2 up to and including the 2 week visit after the second vaccination of participants in Group 2. Based on the assessment of this safety data, the PSRT makes a decision regarding the appropriateness of moving to Part B as well as the recommended (maximum safe) dose from Part A to be used in Part B.
Immunogencity assays
A validated intracellular cytokine staining assay (ICS) for IL-2 and IFNγ using an 8-color FACS analysis was used for the immunogencity assessment. IL-4 and TNFα were also measured but these components of the assay were not validated. We chose these cytokines because they are produced by HIV-specific CD4 and CD8 T cells. It is hypothesized that T cells capable of producing multiple cytokines are better associated with protection against HIV infection than those that make fewer cytokines [6].
(i) PBMC Sample Processing
PBMC were isolated and cryopreserved from whole blood within 8 hours of venipuncture [7]. PBMC were thawed and rested overnight at 37°C/5%CO2 in R10 [RPMI 1640 (GibcoBRL, NY, USA) containing 10% FCS (Gemini Bioproducts, CA), 2mM L-glutamine (GibcoBRL), 100U/ml penicillin G, 100µg/ml streptomycin sulfate] prior to stimulation. A minimum cell viability of 66% measured after overnight resting on the day following thaw was required for use in ICS assays.
(ii) In Vitro Stimulations
PBMC were assessed for ex vivo responses to pools of peptides included in each of the vaccines. Staphylococcal enterotoxin B (SEB) stimulation was used as a positive control. PBMC stimulated with peptide diluent (1% DMSO) was used as the negative control. During the six-hour stimulation, Brefeldin A (10 µg/ml, Sigma, St. Louis, MO) and the co-stimulatory antibodies CD28 and CD49d (each at 1 µg/ml, Becton Dickinson (BD) Biosciences, San Jose, CA) were added. The protein pool consists of 18 peptides contained in EP-1043, provided by Pharmexa (Pharmexa Inc., San Diaego, CA). The DNA pool consists of 21 peptides encoded by EP-1090, provided by Pharmexa. The PADRE peptide was tested individually. Only the protein pool results were obtained from all subjects as earlier assays showed only a few subjects responded to the DNA pool and PADRE stimulations.
(iii) ICS Protocol
The ICS protocol has been described previously [8]. Briefly, cells were first stained with the Violet Live/Dead Fixable Dead Cell Stain (Invitrogen/Molecular Probes, Eugene, OR) [9]. Cells were fixed and permeabilized, and then stained intracellularly for CD3 PE-TR (ECD), CD4 FITC, CD8 PerCP-Cy5.5, IFN-γ PE-Cy7, IL-2 PE, IL-4 APC, and TNFα Alexa 700. All antibodies, except the CD3 ECD, were obtained from BD Biosciences (San Jose, CA). The CD3 ECD was obtained from Beckman-Coulter (Marseille, France). All samples were acquired on an LSRII flow cytometer (BD Biosciences), collecting approximately 300,000 events. Samples were collected from 96-well plates using the High Throughput Sample (HTS, BD) device for analysis by the LSRII. All FACS analyses were performed using FlowJo® software (Treestar, Inc; OR). Positivity was based on comparisons of the percentage of T cells with positive cytokine staining between the experimental well and the negative control well [8].
Statistical methods
The number and percentage of participants experiencing each type of reactogenicity sign or symptom were tabulated by severity and vaccine regimen. For a given sign or symptom, each participant’s reactogenicity was counted once under the maximum severity for all injection visits. Adverse experiences were analyzed using Medical Dictionary for Regulatory Activities (MedDRA) preferred terms. The number and percentage of participants experiencing each specific adverse experience were tabulated by severity and by relationship to treatment. For the calculations in these tables, each participant’s adverse experience was counted once under the maximum severity or the strongest recorded causal relationship to treatment. A complete listing of serious adverse experiences for each participant was provided including severity, relationship to treatment, onset, duration and outcome.
ICS positivity was tested by a 2-by-2 contingency table and one-sided Fisher’s Exact Test with a Bonferroni adjustment for multi-group analyses. The protocol indicated visit 9 (2 weeks after the 4th vaccination) as the primary immunogenicity time point. Due to the discontinuation of vaccinations, a modified analysis plan was used. For all participants, analysis of immunogenicity was performed on specimens obtained at visit 5 (2 weeks after the 2nd vaccination, day 42). Analysis for the durability and poly-functionality of CD4+ T cell immune responses were performed on some specimens obtained at visit 9 (2 weeks after the 4th vaccination, day 182) (Table 1B). Results were expressed as box-whisker plot to indicate median and interquartile range.
RESULTS
Clinical trial design and the demographics of trial participants
The original clinical trial plan included the enrollment of a total of 120 healthy, HIV-uninfected adult subjects between ages of 18 and 50 years. They were randomized into a two-part dose-escalation and regimen comparison clinical trial. Twenty-four participants were enrolled in Part A, the dose-escalation study for the protein vaccine EP-1043. Each of groups 1 and 2 had 12 participants that were randomized to receive vaccine or control in a (10:2) ratio. In Part B, extended safety and immunogenicity were evaluated for the combination of a protein vaccine EP-1043 and a DNA vaccine EP-1090, as well as each vaccine alone. For groups in Part B, 96 participants were to be concurrently randomized to groups 3, 4 and 5: 24 participants to be randomized to group 3 according to a (vaccine:placebo)=(20:4) ratio, and 36 participants to be randomized to each of groups 4 and 5 according to a (vaccine:placebo)=(30:6) ratio (Table 1A). Although a sufficient amount of the protein vaccine was made and vialed for the clinical trial, there was an incomplete documentation of the sterilization of the vaccine vials and thus the vials were recalled. Part B was not executed as originally planned. Only 84 of planned 120 volunteers were enrolled into the actual clinical trial, 70 received vaccines, and 14 received placebo controls (Table 1B). Thus, safety and immunogenicity results on these 84 subjects are reported. We belive this problem will not likely to affect the interprestion of safety and immunogenicity data obtained from this clinical trial, nor will it alter significantly the vaccine’s potential utility for future clincial trials.
Study volunteers were enrolled from three US cities: Rochester, New York; Baltimore, Maryland; and San Francisco, California. About 2/3 of the trial participants were male (55/84), and 1/3 were female (29/84). Half of them were white, non-Hispanic (49/84), approximately 1/5 were black (17/84), followed by a small proportion of Hispanic (5/84), and Asian/Other participants (13/84). The median age of these volunteers was 35 years. The overwhelming majority of them were between 21–50 (80/84) years of age, and the rest were between the ages of 18 to 20 (4/84).
The EP-1043 vaccine is safe and well-tolerated
The vaccines were safe and well tolerated when either used alone or in combination. A total of 285 adverse events (AEs) were reported by 83% (71/84) of trial participants. The majority of them reported either mild (30/70), or moderate AEs (29/70); a small proportion of subjects suffered severe AEs (10/70). Two participants reported life-threatening events: one was hospitalized with gastric ulcer bleeding 73 days after the 3rd vaccination of the EP-1090 vaccine, and another had a significant elevation of CPK levels noted at the final visit (10 months after the last vaccination with 0.2mg of the EP-1043 vaccine). The episode of elevated CPK was considered likely due to recent weight training exercises. Neither of these events was considered related to the use of the vaccine product. Other reports of severe AEs include urinary tract infection, influenza infection, low back pain, pharyngitis, suicide attempt, acute gastroenteritis, and exacerbation of pre-existing diverticulitis (Table 2B). However, none of the severe AEs were related to the vaccine products. Most commonly reported reactogenicity symptoms were local pain and tenderness at the site of injection; systemic symptoms related to vaccine products were malaise/fatigue, myalgia, arthragia, headache, nausea, vomiting, and fever/chills (Table 2A).
Table 2.
| Table 2A. Frequency of Systemic Symptoms in Trial Participants | ||||
|---|---|---|---|---|
| Frequency (%) | ||||
| Type | ||||
| None | Mild | Moderate | Severe | |
| Malaise/Fatigue | 65.5 | 19.0 | 15.5 | 0.0 |
| Myalgia | 61.9 | 32.1 | 6.0 | 0.0 |
| Headache | 76.2 | 19.0 | 3.6 | 1.2 |
| Nausea | 83.3 | 15.5 | 1.2 | 0.0 |
| Vomiting | 98.8 | 0.0 | 1.2 | 0.0 |
| Chills | 91.7 | 6.0 | 2.4 | 0.0 |
| Arthralgia | 90.5 | 6.0 | 3.6 | 0.0 |
| Fever | 83.3 | 16.7 | 0.0 | 0.0 |
| Table 2B. Frequency of Severe Adverse Events in Trial Participants | ||
|---|---|---|
| Type | Number of Subject | Occurrence |
| Urinary tract infection | 1 | 3 |
| Influenza infection | 1 | 1 |
| Low back pain | 1 | 1 |
| Pharyngitis | 1 | 2 |
| Suicide attempt | 2 | 1 |
| Acute gastroenteritis | 1 | 1 |
| Exacerbation of pre-existing diverticulitis |
1 | 1 |
The EP-1043 vaccine elicits potent CD4+ T cell responses
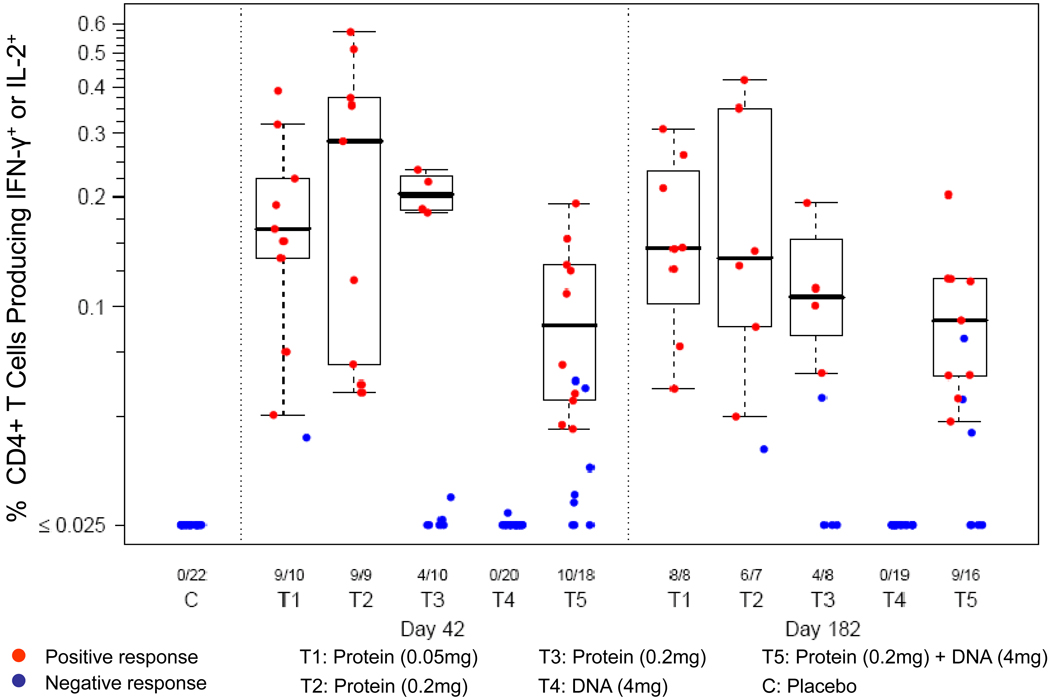
Immunogencity assessment was performed on all trial participants with PBMC samples obtained 2 weeks after the 2nd vaccination (Day 42), and some subjects after the 4th vaccination (Day 182). Figure 1 shows the sum of CD4+ T cell responses to HIV antigens above background levels for either or both cytokines (IFNγ+IL2+, IFNγ+IL2-and IFNγ-IL2+). The combined response (IL2 and/or IFNγ) is defined as the sum of the subset responses after stimulation with a pool of HTL peptides included in the EP-1043 vaccine. Participants with a positive combined cytokine response were included in the plots. The ICS assay was performed on 22 samples from 12 control subjects (from days 42 and 182), and on samples from 67 and 58 vaccine recipients at day 42 (2 weeks after the 2nd vaccination), and day 182 (2 weeks after the 4th vaccination), respectively. After 2 vaccinations (Day 42), none of the control recipients (C) had a positive response. In subjects enrolled in groups receiving the protein vaccine (T1, T2, T3 and T5), 68% (32/47) of subjects had a positive CD4+ T response, and the magnitudes of IL-2- or IFNγ-producing CD4+ T cells were as high as 0.1–0.5% of total CD4+ T cells. The levels of these responses, however, were not boosted by subsequent vaccinations, and the proportion of positive responders remained at about 70% (27/39) (Day 182). Since CD8+ T cell responses were not induced by the EP-1090 vaccine either alone or in combination with the EP1043 vaccine (groups T4 and T5), only CD4+ T cell responses elicited by the EP-1043 vaccine were further examined.
Figure 1. CD4+ T cells producing IFN-γ or IL-2 are elicited by EP-1043 vaccine.
Immunogenicity in subjects from all 5 study groups (T1-5) was evaluated at days 42 and 182 post vaccination. Placebo controls (C) were assessed at Day 42 and Day 182 (not shown). PBMC were stimulated using a single pool of peptides contained in the EP-1043 vaccine (T1-50μg, T2 and T3-200μg), the EP-1090 vaccine (T4-4mg), or both vaccines (T5-200μg+4mg), the IL-2 and/or IFNγ-producing T cells were quantified by ICS assay and FACS analysis. Results show the sum of percentage of CD4+ T cells making either IL-2 or IFNγ after peptide stimulation. Responses determined to be positive are shown in red and those negative are shown in blue. The numbers below the graph show the number of positive responses over the total number of samples in each group.
The EP-1043 vaccine elicits poly-functional CD4+ T cell responses
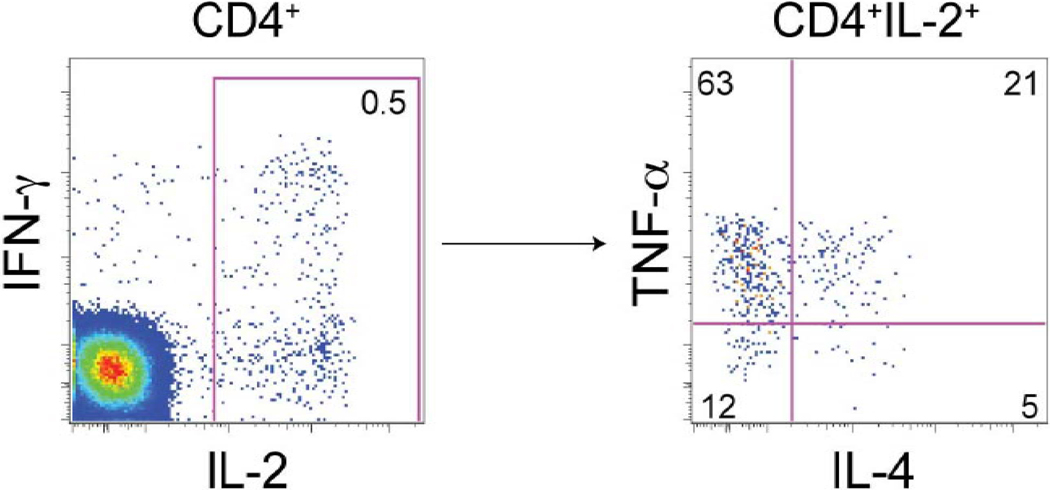
In a murine model of leishmaniasis, poly-functional CD4 T cells have been shown to be a key effector for protection [10]. In human HIV infections, these cells are hypothesized to be more important for protection than those that only produce a narrow range of cytokines [11]. We next examined whether EP-1043 could elicit poly-functional T cells using ICS assay for TNF-α and IL-4 in additional to IFN-γ and IL-2. Figure 2 shows an example of the flow cytometric staining profile for the CD4+ T cell response in one participant. In this individual, the most abundant vaccine-induced cytokine made by CD4 cells was IL-2, and of these IL-2-producing cells, 63% produce TNF-α and a smaller proportion produce IFN-γ or IL-4.
Figure 2. Flow cytometric profile of cytokine-secreting CD4+ T cells.
The poly-functionality of CD4+ T cells was examined using PBMC from in a vaccine-recipient at 2 weeks post the 4th vaccination of 0.05mg of EP-1043. Cells producing IL-2, IFN-γ, IL-4, and TNF-α were quantified by ICS assay after stimulation with peptides included in the EP1043 vaccine. CD4+ T cells producing IL-2, IFN-γ are shown on the left, and IL-2-producing CD4+ T cells that also made IL-4 and TNF-α are shown in the right. Numbers in the graphs show relative levels of each of these cytokines reported as percentage of CD4+ T cells (left) or as percentage of CD4+ T cells producing IL-2 (right).
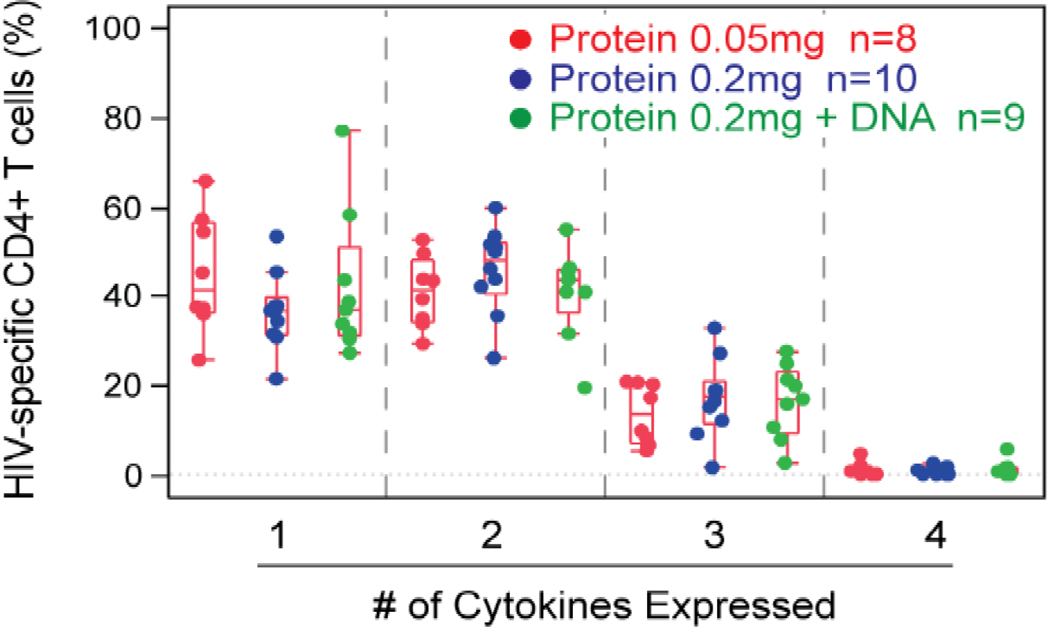
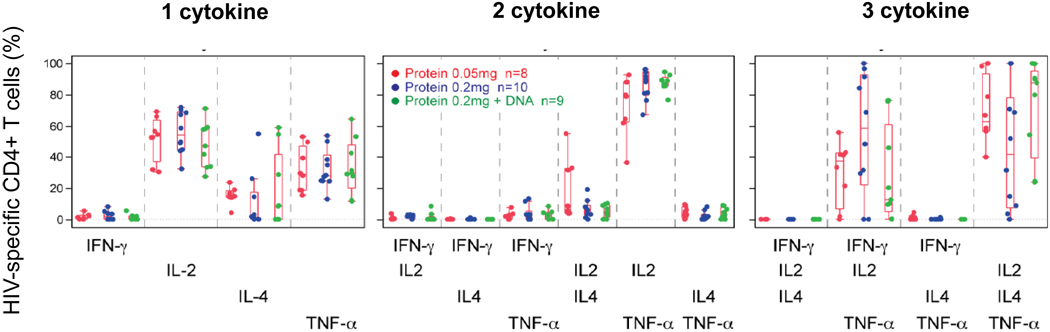
Figure 3 summarizes the poly-functionality of the vaccine-induced CD4+ T cells for 27 trial participants who received the HTL vaccine alone or in combination with the DNA vaccine at 2 weeks after the 4th vaccination (Day 182). The degree of poly-functionality is similar for the two protein doses and when DNA is co-administered. Most cells produced either one or two cytokines (40% and 45%, respectively), approximately 10% produced three, and none produced all four cytokines measured (Fig.3a). To determine which cytokine or cytokine combinations were produced, cells with each type of poly-functionality were further examined. Among cells producing one cytokine, IL-2 was most commonly produced; TNF-α or IL-4 was produced by a smaller percentage of cells; none produced IFN-γ only. Among cells producing two cytokines, IL-2 in combination with TNF-α was the dominant combination, followed by the IL-2 and IL-4 combination. Among cells producing three cytokines, only two of the four potential combinations were observed. Both included IL-2 and TNF-α, and were produced in combination with either IL-4 or IFN-γ (Fig.3b).
Figure 3. Cytokine-secreting CD4+ T cells are induced by EP-1043 vaccine.
Similar to that described in Figure 2, the poly-functional CD4+ T cells at 2 weeks post the 4th vaccination in trial participants were quantified by ICS assay. (a) For each participant with a positive response, the percentage of HIV-specific CD4+ T cells producing 1, 2, 3, or 4 cytokines is shown. Treatment group 1 is in red, group 5 in green, group 2 and 3 (blue) are combined since they both received 0.2mg of the EP-1043 vaccine. Group 4 is not shown because there no responses to the DNA vaccine. (b) The HIV-specific CD4+ T cells are further characterized by the specific cytokine or combination of cytokines produced. For each graph, the percentages plotted are relative to the HIV-specific CD4+ T cells producing 1, 2, or 3 cytokines.
DISCUSSION
We report results of a multi-site, double-blinded, Phase I human clinical trial with a novel multiepitope polypeptide composed of 18 T helper epitopes (EP-1043) and a DNA vaccine encoding 21 CTL epitopes (EP-1090). The vaccines were safe and well tolerated when either used alone or in combination. In subjects enrolled in groups receiving the protein vaccine, 68% (32/47) of subjects had a positive CD4+ T response after two vaccinations, and the responding CD4+ T cells had a diverse poly-functional cytokine profile. The DNA vaccine EP-1090 was not immunogenic for either CD4+ or CD8+ T cell responses.
A number of salient features of this current study are noted. Previous HIV vaccines designed to stimulate a CD4+ T response included the use of gp160 recombinant protein in HIV-infected subjects [12–14], and gp120 recombinant protein in HIV-uninfected people [3, 15]. In contrast, the current study used a rationally-designed vaccine of multiple HTL epitopes from Env, Gag, Pol and Vpu. Moreover, the magnitude of polyfunctional CD4+ T cell responses is similar to that seen in some HIV-infected long-term nonprogressors [16, 17]. Whether the quality and quantity of CD4+ T cell responses elicited by the EP-1043 vaccine will confer protection against HIV infection or disease progression awaits future study.
The examination of four cytokines allowed for a detailed evaluation of the poly-functionality of the vaccine-induced CD4+ T cells. This analysis revealed that only selected combinations of cytokines were produced from individual cells. Cells producing all four cytokines were not detected. When cells produced two cytokines, only one combination (IL-2 and TNF-α) was dominant. Among cells producing three cytokines, there was a polarization between two cytokine combinations, demonstrating that no cells produced IL-4 in combination with IFN-γ. These types of cytokine combinations have been observed for CD4+ T cells induced by vaccination for Hepatitis B [18]. Of note, as for the Hepatitis B vaccine, the EP-1043 HTL vaccine is also administered in aluminum hydroxide, and these cytokine profiles may be due to the effects of this adjuvant [19].
In this proof-of-concept study, we did not perform detailed assessment of CD4+ T cell responses to the individual peptide included in the EP-1043 vaccine. This may be the focus of future studies. There was no apparent synergistic effect between the EP-1043 and EP-1090 vaccines, possibly due to the weak immunogenicity of the EP-1090 vaccine [4]. Furthermore, the two vaccines were administered to different and distal sites so that the safety of each component could be monitored. Co-formulation of both vaccines into a single injection may help to better demonstrate synergy if it exists. Nonetheless, the study demonstrated immunogenicity of a novel T helper vaccine, supporting its potential utility in a multi-modality HIV vaccine.
In summary, we found that the EP-1043 vaccine is not only safe and well-tolerated, but also elicits robust poly-functional T helper responses in a majority of human volunteers after just two vaccinations. We suggest that the EP-1043 vaccine, or a modified version of it, may be included in a multi-modality HIV vaccine to provide help for the induction of neutralizing antibody and CD8+ T cell responses.
ACKNOWLEDGMENTS
We thank all past and present members of the HVTN-064 team whose names have not appeared as coauthors, but whose contributions are nonetheless critical for the success of this study. We thank all sites’ staff for recruiting volunteers and administering vaccinations. We thank all the volunteers whose participation made this clinical trial possible. We would like to acknowledge the support of the NIH/NCRR UCSF-CTSI (Grant number UL1 RR024131) in the follow-up of the San Francisco Department of Public Health study participants. We thank Drs Chris Butler and Dale Lawrence of DAIDS for their support and guidance throughout the study. This study is supported by the HVTN of NIAID, and DAIDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Mark J. Newman, Brian D. Livingston, and Denise M. McKinney were employees of the Pharmexa Inc. (San Diego, CA) at the time of the study. Other co-authors have no conflict of interest.
References
- 1.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008 Nov 29;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005 Mar 1;191(5):666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 4.Gorse GJ, Baden LR, Wecker M, Newman MJ, Ferrari G, Weinhold KJ, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008 Jan 10;26(2):215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CC, Palmer B, Southwood S, Sidney J, Higashimoto Y, Appella E, et al. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. Journal of virology. 2001 May;75(9):4195–4207. doi: 10.1128/JVI.75.9.4195-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantaleo G, Graziosi C, Fauci AS. The role of lymphoid organs in the immunopathogenesis of HIV infection. AIDS (London, England) 1993;7 Suppl 1:S19–S23. [PubMed] [Google Scholar]
- 7.Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007 Feb 28; doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007 May 31;323(1):39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006 Jun 30;313(1–2):199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 11.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med. 2004 Aug;10(8):806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 12.Kundu SK, Katzenstein D, Moses LE, Merigan TC. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11204–11208. doi: 10.1073/pnas.89.23.11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfield RR, Birx DL, Ketter N, Tramont E, Polonis V, Davis C, et al. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- 14.Valentine FT, Kundu S, Haslett PA, Katzenstein D, Beckett L, Spino C, et al. A randomized, placebo-controlled study of the immunogenicity of human immunodeficiency virus (HIV) rgp160 vaccine in HIV-infected subjects with > or = 400/mm3 CD4 T lymphocytes (AIDS Clinical Trials Group Protocol 137) J Infect Dis. 1996 Jun;173(6):1336–1346. doi: 10.1093/infdis/173.6.1336. [DOI] [PubMed] [Google Scholar]
- 15.Clements-Mann ML, Weinhold K, Matthews TJ, Graham BS, Gorse GJ, Keefer MC, et al. NIAID AIDS Vaccine Evaluation Group. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. J Infect Dis. 1998 May;177(5):1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 16.Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. Journal of Immunology (Baltimore, Md : 1950) 2002 Dec;169(11):6376–6385. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 17.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine_Diab B, Sekaly RP, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. Journal of virology. 2003 Oct;77(20):10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004 Nov 1;173(9):5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 19.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009 Apr;9(4):287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]