Abstract
Purpose
To determine the prognostic significance of a multi-marker assay incorporating expression levels of three molecular markers in primary cutaneous melanoma.
Experimental Design
We assessed expression levels of NCOA3, SPP1, and RGS1 using immunohistochemical analysis in a tissue microarray cohort of 395 patients. For each marker, we identified optimal cut-points for expression intensity to predict disease-specific survival (DSS) and, as a secondary endpoint, sentinel lymph node (SLN) status. The cumulative over-expression of all three markers was embodied in a multi-marker index, and its prognostic impact on DSS and SLN status was assessed using Cox regression, Kaplan-Meier analysis, and logistic regression. The prognostic impact of this multi-marker assay on DSS was assessed in an independent cohort of 141 patients, in which marker expression levels were scored using immunohistochemical analysis of stained tissue sections.
Results
Increasing multi-marker index scores were significantly predictive of reduced DSS and increased SLN metastasis in the 395-patient cohort. Multivariate logistic regression analysis revealed multi-marker expression scores as an independent predictor of SLN status (P=0.001). Multivariate Cox regression analysis showed the independent impact of the multi-marker index on DSS (P<0.001). The multi-marker index was the most significant factor predicting DSS, when compared to other clinical and histological factors, including SLN status (P=0.002). Multi-marker expression scores were also the most significantly predictive of DSS in the independent cohort (P=0.01).
Conclusions
These results describe a multi-marker assay with independent prognostic impact on the prediction of survival associated with melanoma in two distinct cohorts.
Introduction
Melanoma is estimated to be the fifth most common cancer in the United States in 2009 (1). The unpredictable behavior of melanoma has prompted the search for prognostic factors to better predict its outcome. The vertical thickness of the primary tumor consistently emerges as a dominant prognostic factor for melanoma, but does not account adequately for its heterogeneity. Numerous clinical and histological prognostic factors have been examined for their ability to predict melanoma progression (2). Ulceration was included in the American Joint Cancer Committee (AJCC) staging classification for cutaneous melanoma because of its independent impact on melanoma survival (3,4). Despite this development, further advances in the prognostic assessment of melanoma are still essential to improve prognostic predictions for all patients diagnosed with melanoma.
One such approach to improving the prognostic assessment of cancer is the use of molecular markers. In the genomic era, the sequencing of the human genome and the availability of genome-wide approaches to interrogate the malignant phenotype have raised the promise that molecular markers will be routinely incorporated into the clinical assessment of cancer patients. Recent studies have shown the efficacy of multi-marker prognostic assays for several malignancies, including breast cancer, lung cancer, and B-cell lymphomas (5–7). To date, no molecular markers are routinely used in the prognostic assessment of melanoma patients. Gene expression profiling analyses of melanoma have identified a plethora of putative biomarkers (8–10). However, the prognostic significance of these gene signatures has not been validated. Three markers (NCOA3, SPP1, and RGS1) derived from a cDNA microarray study conducted by our group have been shown to play an independent prognostic role, when analyzed separately in a cohort of melanomas with defined histology and follow up (11–13). NCOA3 (also known as AIB1 or SRC-3) is a member of the steroid receptor coactivator 1 family. SPP1 (also known as osteopontin) is a secreted integrin-binding protein implicated in the progression of several cancers. RGS1 (regulator of G protein signaling 1) is a GTPase activating protein and a member of the regulator of G-protein family.
In this study, we both assess the predictive efficacy of a multi-marker prognostic assay combining the impacts of these three biomarkers, drawn from a tissue microarray cohort of 395 patients with primary cutaneous melanoma, and evaluate its efficacy in an independent cohort of 141 patients.
Materials and Methods
Study Population
We previously assessed expression levels of NCOA3, SPP1, and RGS1, separately, on a primary melanoma tissue microarray obtained from a retrospective cohort of UCSF patients with at least two years of follow-up or documented relapse or following SLN biopsy. All patients underwent wide excision of their primary melanoma and where indicated, SLN biopsy. This study focuses on the 395 of these patients on whom marker expression data were available. According to the REMARK guidelines (14), the breakdown of tumor thickness within this tissue microarray cohort was as follows: T1 (< 1.0 mm)-5%; T2 (1.01–2.0 mm)-33%; T3 (2.01–4.0 mm)-28%; T4 (> 4.0 mm)- 34%. The median age of this cohort was 53, with males comprising 65% of the patients. An updated dataset was utilized for this analysis, with mean and median follow up times of 68 and 57 months, respectively.
The prognostic impact of the multi-marker assay was separately evaluated in an independent cohort of 141 patients collected by the Skin Cancer Unit, German Cancer Research Center in Heidelberg, and the Department of Dermatology, University of Kiel, Germany (recorded using Achiever Medical, a web-based electronic medical database and tissue management and retrieval system), who also had at least two years of follow up or documented relapse. The breakdown of tumor thickness within this 141-patient cohort was as follows: T1-27%; T2-28%; T3-30%; T4-15%. The median age of the cohort was 63, with males comprising 60% of the patients. The mean and median follow-up times were 50 months and 43 months, respectively. The molecular prognostic factor analyses performed herein were approved by the appropriate ethics boards both at UCSF and in Germany.
Immunohistochemistry
Tissue microarray construction and immunohistochemical staining of NCOA3, SPP1, and RGS1 were carried out in the 395-patient cohort as previously described (11–13). In the case of the 141-patient Heidelberg/Kiel tissue set, immunohistochemical analysis was performed on 5 μM tissue sections, and the following primary antibodies used: mouse monoclonal anti-NCOA3 IgG (Abcam, Cambridge, MA, 1:10 dilution); rabbit polyclonal anti-SPP1 IgG (Abcam, 1:200 dilution); and chicken anti-RGS1 IgG (GeneTex, San Antonio, TX, 1:50 dilution). All of the testing was performed on paraffin-embedded tissues, requiring no special storage or handling.
Evaluation of Immunohistochemical Staining
For both tissue microarrays and tissue sections, in cases in which marker expression was homogeneous, the region(s) of most intense staining was scored for each tissue specimen. In the event of heterogeneity of marker expression within a tumor, the largest region of homogeneous marker expression was scored. Marker expression was graded using the following scale: no staining (0), weak staining (1), moderate staining (2), and intense staining (3). All tissue specimens in both tissue sets were scored by the same pathologist (R.W.S.) blinded to the identity of the cases, as previously described (11–13).
Imaging Analysis of Immunohistochemical Staining
In the 395-patient cohort, mean densitometric intensity for each marker was calculated for the specimen cores on tissue arrays stained using the Carl Zeiss Mirax Scan and Axiovision 4.5 image processing system as previously described (15).
Statistical Analysis
Statistical methods used to assess the individual impact of various prognostic factors on melanoma outcomes were previously described in detail (11–13,16,17), and the coding both for clinical and for pathological attributes was performed exactly as described by the AJCC staging committee for melanoma (3). Mitotic rate was coded as ≤ 4 vs. > 4, which represented the optimal cut-point for this factor in the UCSF dataset. To assess the joint impact of the combination of the three biomarkers on melanoma progression, a prognostic index was developed using cut-points for over- or under-expression for each outcome measure (i.e., DSS and SLN status) that provided the best prediction for that outcome measure. We used univariate Cox regression to determine the optimal cut-points for DSS and univariate logistic regression to determine the optimal cut-points for SLN status. Each marker was given a score of +1 or −1 for each lesion on the basis of whether the marker was over- or under-expressed (i.e., above or below the cut-point) for the given outcome measure. A score of 0 was given whenever no specific degree of expression could be determined by the pathologist (e.g., insufficient tissue). Then, for each lesion, a prognostic index was calculated that reflected the net score (i.e., the sum of the scores of the three individual markers), resulting in a seven-point scale ranging from −3 (all markers below their cut-points for the given outcome measure) to +3 (all markers above their cut-points for the outcome measure). The prognostic efficacy of the multi-marker index with respect to SLN status was then assessed via both univariate and multivariate logistic regression analyses. The prognostic efficacy of the multi-marker prognostic index with respect to DSS was assessed via both univariate and multivariate Cox regression analyses and via Kaplan-Meier analysis. For Kaplan-Meier analysis, a high-risk group was defined as all patients in the 395-patient cohort with positive net multi-marker index scores, and a low-risk group as all patients with negative net multi-marker scores. The significance of the difference in 5-year DSS rates between high-risk and low-risk patients was assessed via the Fisher exact test. In the digital imaging analysis, optimal cut-points for marker over- or under-expression using mean densitometric intensity were identified by maximizing the average of specificity and sensitivity of each separate marker in the prediction of 5-year DSS. Then, the multi-marker index was created exactly as described above, using data from 353 patients possessing sufficient data to construct this index. None of our conclusions depends specifically on the linearity of our multi-marker index.
In the analysis of the 141-patient Heidelberg/Kiel cohort, indices of combined marker over- or under-expression were constructed in exactly the same way as described above for the 395-patient cohort. Data recording mitotic rate and SLN status were not available in the Heidelberg/Kiel cohort, precluding the inclusion of these factors in both univariate and multivariate models. All P values reported are two-sided.
Results
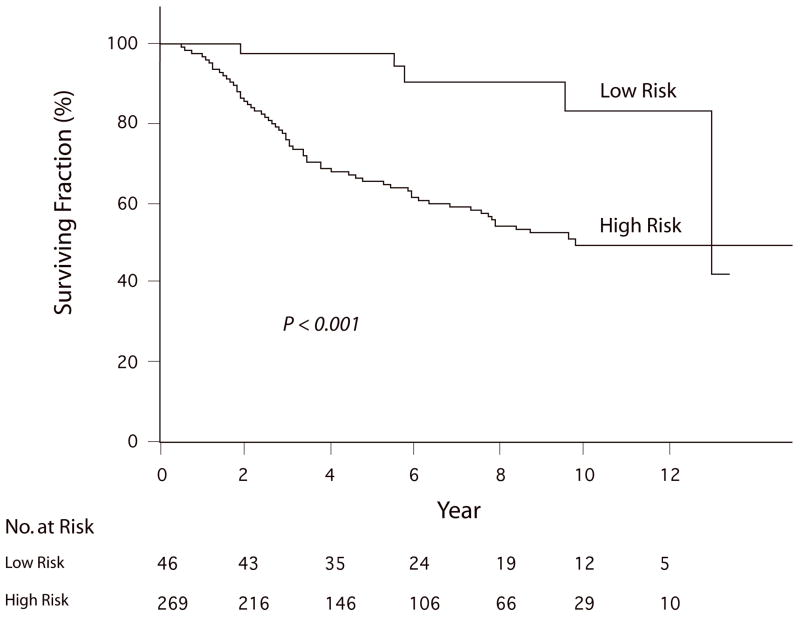
Given the significant prognostic impact of three molecular markers (NCOA3, SPP1, and RGS1), when analyzed individually, a multi-marker index was constructed to combine the separate information provided by each marker. The cumulative impact of multi-marker over-expression was evaluated relative to DSS (the primary endpoint) and, where possible, sentinel lymph node (SLN) status. Initially, the impact of the multi-marker index (using the entire seven-point scale) on melanoma outcome was examined using univariate analysis. By Cox regression analysis, the multi-marker index was significantly predictive of reduced DSS (P<0.001). Increasing multi-marker index scores were significantly predictive of SLN metastasis by logistic regression analysis (P<0.001). A high-risk group was identified as all patients in the 395-patient cohort with positive net multi-marker index scores, and a low-risk group as all patients with negative net multi-marker scores. Patients in the high-risk group had a significantly higher prevalence of SLN positivity (31.6%) than patients in the low-risk group (5.0%, P<0.001, Fisher exact test), as well as significantly reduced DSS (P<0.001, Log-rank test, Fig. 1) by Kaplan-Meier analysis. The 5-yr DSS of patients in the low-risk group was 96%, compared with 60% in the high-risk group (P<0.001, Fisher exact test).
Figure 1.
Kaplan-Meier analysis of DSS in the UCSF cohort according to multi-marker expression level, comparing the low-risk group (N=46) with the high-risk group (N=269).
Next, we analyzed the impact of the multi-marker index on melanoma outcome by multivariate analyses. In these multivariate models, the six factors analyzed by the AJCC staging committee for melanoma were included (i.e., tumor thickness, ulceration, Clark level of invasion, primary tumor site, sex, and age). All of the results presented follow the conventions set by the AJCC staging committee (3), in which the relative importance of prognostic factors was measured by Chi-square values since their interpretation is unrelated to the coding of any covariate, while the comparison of risk ratios depends critically on the particular manner in which each covariate is separately coded. Multivariate logistic regression analysis revealed the multi-marker index to be independently and significantly predictive of SLN status (P=0.001, Table 1), following decreasing age, but with a significance greater than tumor thickness.
Table 1.
Multivariate logistic regression analysis of the impact of various factors on SLN metastasis (N=274)
| Prognostic factor | Chi-square | Odds Ratio | P value |
|---|---|---|---|
| Age | 16.42 | 0.66 | <0.001 |
| Multi-marker index | 10.46 | 1.47 | 0.001 |
| Tumor thickness | 6.90 | 1.76 | 0.009 |
| Sex | 2.72 | 1.73 | 0.10 |
| Clark level | 1.55 | 1.37 | 0.21 |
| Ulceration | 0.94 | 1.39 | 0.33 |
| Site | 0.20 | 0.87 | 0.66 |
Then, several multivariate Cox regression analyses of DSS were performed. The first analysis again included the multi-marker index and the six AJCC factors. The multi-marker index was independently and significantly predictive of reduced DSS (P<0.001), emerging as the most significant predictor of DSS (Table 2). In addition, a multivariate analysis was performed to include mitotic rate, an important histologic prognostic factor that will be included in the next version of the AJCC staging classification for melanoma. The multi-marker expression index remained the top factor predicting DSS in this analysis, surpassing tumor thickness, ulceration, and mitotic rate (data not shown).
Table 2.
Multivariate Cox regression analysis of the impact of various factors on DSS-impact of the multi-marker index (N=340)
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Multi-marker index | 13.33 | 1.30 | <0.001 |
| Clark level | 9.13 | 1.59 | 0.003 |
| Ulceration | 8.63 | 1.82 | 0.003 |
| Site | 2.29 | 1.35 | 0.13 |
| Tumor thickness | 1.12 | 1.16 | 0.29 |
| Age | 0.06 | 1.01 | 0.80 |
| Sex | 0.03 | 1.04 | 0.86 |
Subsequently, a multivariate analysis was performed incorporating SLN status in addition to the six AJCC prognostic factors. In this analysis, the multi-marker index remained the most significant factor predicting DSS (P=0.002, Table 3). While ulceration and SLN status were still significantly predictive of DSS, tumor thickness was no longer significant. In order to achieve a legitimate comparison of risk ratios between the multi-marker assay and SLN status, this particular multivariate analysis was repeated with the seven-point multi-marker index re-scaled and re-entered into the model as a dichotomous variable, representing the high-risk vs. low-risk groups. The dichotomized multi-marker index still emerged as the most significant factor in the revised analysis, with a higher risk ratio (3.27) than that observed for SLN status (1.75).
Table 3.
Multivariate Cox regression analysis of the impact of various factors on DSS-impact of SLN status (N=277)
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Multi-marker index | 9.31 | 1.29 | 0.002 |
| Ulceration | 6.84 | 1.88 | 0.009 |
| SLN status | 4.87 | 1.71 | 0.03 |
| Clark level | 3.33 | 1.39 | 0.07 |
| Tumor thickness | 2.81 | 1.34 | 0.09 |
| Sex | 0.90 | 1.27 | 0.34 |
| Age | 0.64 | 1.06 | 0.42 |
| Site | 0.42 | 1.16 | 0.52 |
Given that the multi-marker expression index combines the impacts of three molecular markers, we compared its prognostic efficacy to that of AJCC stage, an analogous eleven-point multi-marker prognostic index that combines the impacts of the T, N, and M stages. Cox regression analysis of DSS showed both the AJCC stage (P<0.001) and the multi-marker expression score (P=0.006) to be independently and significantly predictive of DSS.
The prognostic impact of this multi-marker assay on DSS was further evaluated in this cohort by substituting digital imaging analysis of marker intensity for pathologist scoring. The stained specimen cores were scanned digitally, and mean densitometric intensity was calculated for each marker, separately. A densitometrically-derived multi-marker index was significantly and independently predictive of DSS (P<0.001) in multivariate Cox regression analysis (Table 4). The multi-marker expression index was also independent of AJCC stage in the prediction of DSS in multivariate Cox regression analysis (data not shown).
Table 4.
Multivariate Cox regression analysis of the impact of various factors on DSS-impact of the digital multi-marker index (N=255)
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Multi-marker index | 10.63 | 1.34 | 0.001 |
| SLN status | 9.12 | 2.09 | 0.003 |
| Clark level | 6.80 | 1.66 | 0.009 |
| Ulceration | 6.38 | 1.91 | 0.01 |
| Sex | 1.83 | 1.43 | 0.18 |
| Tumor thickness | 1.10 | 1.21 | 0.29 |
| Age | 0.95 | 1.08 | 0.33 |
| Site | 0.85 | 1.25 | 0.36 |
Finally, the prognostic impact of the cumulative over-expression of NCOA3, SPP1, and RGS1 was further evaluated in tissues obtained from 141 patients collected independently by the Skin Cancer Unit of the German Cancer Research Center in Heidelberg and the University of Kiel, Germany. In this cohort, immunohistochemical analysis of the three markers was performed on tissue sections, and a multi-marker index was constructed exactly as before to examine the impact of combined marker over-expression on survival. The multi-marker expression index was significantly predictive of DSS (P=0.01) by univariate Cox regression analysis. Multivariate Cox regression analysis of the multi-marker index (P=0.002), tumor thickness (P=0.001), and ulceration (P=0.07) revealed the multi-marker index to be independent of both tumor thickness and ulceration in the prediction of DSS. Finally, a multivariate model that incorporated the multi-marker expression index and the six AJCC factors revealed the multi-marker index as independently predictive of DSS (P=0.01) in this cohort (Table 5).
Table 5.
Multivariate Cox regression analysis of the impact of various factors on DSS-the Heidelberg/Kiel cohort (N=102)
| Prognostic factor | Chi-square | Risk Ratio | P value |
|---|---|---|---|
| Multi-marker index | 5.93 | 1.34 | 0.01 |
| Tumor thickness | 4.77 | 2.17 | 0.03 |
| Age | 3.00 | 0.69 | 0.08 |
| Site | 2.69 | 0.41 | 0.10 |
| Ulceration | 2.46 | 2.19 | 0.12 |
| Sex | 1.20 | 1.85 | 0.27 |
| Clark level | 0.10 | 0.86 | 0.76 |
Discussion
To our knowledge, this is the first description of an independently predictive molecular prognostic assay for primary melanoma. Moreover, it is the first study to replicate the independent prognostic impact of any molecular markers (i) in a data set drawn from a completely different patient population (the Heidelberg/Kiel cohort); (ii) across different tissue platforms (tissue microarray in the initial study cohort vs. tissue sections in the replication cohort); and (iii) using different measurement techniques (pathologist scoring vs. digital imaging analysis in the initial study cohort). We examined the prognostic utility of this three-marker index by incorporating into the multivariate models several powerful and commonly used prognostic factors. In these analyses, we tested the multi-marker expression index against the six factors included in the AJCC staging committee analyses of prognostic factors for melanoma (3,4). In our 395-patient cohort, the multi-marker index was independently predictive of DSS and SLN status with the inclusion of these six factors.
The multi-marker index described here remained significantly predictive of DSS, even when SLN status was included in the multivariate model. Lymph node status in general, including SLN status, is well recognized to represent a powerful predictor of melanoma survival and has been shown to be more powerful as a predictor than most routinely coded clinical and histological factors (3,18,19). Nevertheless, the multi-marker index was independent of SLN status, tumor thickness, and ulceration, which comprise the three most important factors in the AJCC analysis of patients with localized cutaneous melanoma. We are unaware of any other histological or molecular factor that has been shown to have a more significant impact on DSS and to have a higher risk ratio than SLN status. Lymph node metastasis is a complex phenomenon - likely reflecting the sum total of numerous molecular events. Thus, it is of particular interest that the combined expression levels of these three markers could provide independent outcome information relative to SLN status, since their individual expression levels can be determined from assessment of the primary tumor, without the need for additional surgery or general anesthesia. We are unaware of studies in other solid tumors showing that expression of (as few as) three molecular markers analyzed in the primary tumor provides such powerful prognostic information.
The independent and powerful prognostic efficacy of the multi-marker expression index in predicting DSS was confirmed using a digital imaging analysis. Importantly, the prognostic significance of the multi-marker index relative to tumor thickness, ulceration, and SLN status was confirmed, substituting this densitometric analysis of individual marker expression. Taken together, these results suggest that the multi-marker assay can provide useful prognostic information beyond that provided by routine clinical and histological factors, including SLN status or AJCC stage.
The impact of the multi-marker assay on DSS was separately evaluated for 141 patients drawn from a completely different population (the Heidelberg/Kiel cohort). Univariate and multivariate Cox regression analyses demonstrated a statistically significant impact of the multi-marker expression index on DSS in this distinct cohort, independent of tumor thickness and ulceration. By multivariate analysis, the multi-marker index was found to independently predict DSS, thereby providing important confirmation of its prognostic significance. To our knowledge, this is the first replication of the prognostic impact of any molecular markers in primary cutaneous melanoma in a distinct patient population, further substantiating the prognostic role of these markers in predicting melanoma outcome. Moreover, while the multi-marker assay was initially developed in our 395-patient cohort using a tissue microarray, the replication study performed on the 141-patient cohort was based on tissue sections, demonstrating the prognostic efficacy of the multi-marker assay across these two platforms.
It is important to note that the two cohorts examined in this study were not identical in their composition. The initial study cohort included few patients with thin (≤ 1.0 mm, or T1) melanoma, in part due to the cohort being enriched for patients undergoing SLN biopsy. However, this was not the case with the Heidelberg/Kiel cohort, which included a higher proportion of T1 patients. Despite these differences, the multi-marker assay was significantly and independently predictive of DSS in both cohorts. Also, both of these cohorts were analyzed retrospectively, which could result in a selection bias. Thus, further studies of this multi-marker assay in prospectively-collected, population-based series are warranted in order to assess its more broad-based role in the prognostic assessment of distinct subsets of melanoma patients.
Many individual biomarkers (reviewed in reference 20) have been suggested as molecular prognostic factors for melanoma, including Ki67 and p16, which have been shown to have independent prognostic significance in distinct cohorts (21). However, to date, none of these factors has been shown to have independent prognostic significance with the inclusion of a powerful factor such as SLN status, and none has been validated in an independent cohort. Moreover, these markers were not differentially expressed in our microarray analyses, thereby precluding their inclusion in the current analysis. However, whether addition of Ki-67, p16, or other markers could improve the prognostic efficacy of the current multi-marker assay could be the subject of future studies. The multi-marker assay described here incorporated three novel markers identified by gene expression profiling of melanoma (8). While transcriptome analysis has shown promise to radically alter current approaches to cancer classification and prognosis, significant challenges have prevented the routine translation of gene expression signatures into clinically useful assays. The multi-marker assay presented in our study thus provides a model for the development and validation of immunohistochemical prognostic assays for human cancer derived from gene expression profiles.
In addition to their role as biomarkers, the three markers analyzed here have plausible roles in promoting melanoma progression. The genes encoding NCOA3 and RGS1 reside on chromosomal loci with gains or amplifications in human melanoma (22). SPP1 and NCOA3 may promote melanoma cell growth and metastasis by virtue of activation of the nuclear factor κB signaling pathway (23,24). The RGS family has been shown to be involved in regulating signaling pathways relevant to melanoma progression, including Wnt and Rho (25). We have previously shown that SPP1 expression correlated with tumor thickness, Clark level and mitotic index (12), whereas RGS1 expression correlated with tumor thickness, mitotic index and vascular involvement (13), suggesting mechanisms by which these markers may contribute their prognostic impact.
The practical significance of biomarkers that demonstrate independent prognostic information lies in their potential to affect treatment decisions. Knowledge of the expression levels of RGS1, NCOA3, and SPP1 may be useful in various clinical scenarios for patients diagnosed with primary cutaneous melanoma in which routine clinical and histological factors fail to inform such decisions. One scenario in which this multi-marker assay could be used is in the selection of patients to undergo SLN biopsy (19). Our results suggest that the multi-marker assay described here could be used as an adjunct to tumor thickness in the selection of patients to undergo SLN biopsy, given its significant prediction of SLN status by multivariate logistic regression analysis. It will be important to replicate the independent prognostic role of this multi-marker assay on SLN status in additional cohorts, given that we were unable to examine this in the Heidelberg/Kiel cohort.
Secondly, these markers can identify patients at higher risk for death due to metastatic melanoma, who would then be candidates for adjuvant therapy. Significant controversy exists regarding the patient population benefiting and the magnitude of benefit derived from interferon alpha-2b (IFN), the only FDA-approved therapy for high-risk melanoma (26–30). Our results suggest the potential utility of this multi-marker assay to identify high-risk patient populations to undergo IFN therapy, given its independent ability to predict DSS, even with the inclusion of SLN status or AJCC stage. Whether the multi-marker assay is predictive of response to or benefit from IFN therapy awaits evaluation in cohorts of patients undergoing this treatment.
Acknowledgments
This work was supported by the Herschel and Diana Zackheim Endowment Fund, and National Institutes of Health (CA114337 and CA122947). We thank Loretta Chan for assistance with marker immunostaining, Rosie Casella for manuscript preparation, and Ken Pratt of Carl Zeiss for the use of the Mirax scanner and Axiovision software.
Footnotes
Conflicts of Interest
Mohammed Kashani-Sabet has ownership interest in Melanoma Diagnostics, Inc.
Statement of Translational Relevance
This manuscript describes the prognostic role of an immunohistochemical assay combining the expression levels of three molecular markers for cutaneous melanoma derived from gene expression profiling studies. The multi-marker assay described here was an independent predictor of disease-specific survival in two distinct cohorts, and could be used to identify patients at higher risk of relapse or death that may be candidates for sentinel lymph node biopsy or adjuvant therapy.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Zettersten E, Shaikh L, Ramirez R, Kashani-Sabet M. Prognostic factors in primary cutaneous melanoma. Surg Clinics N Am. 2003;83:61–75. doi: 10.1016/s0039-6109(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 5.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 6.Lossos IS, Czerwinski DK, Alizadeh AA, et al. Prediction of survival in diffuse large B-cell lymphoma based on expression of six genes. N Engl J Med. 2004;350:1828–37. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 7.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 8.Haqq C, Nosrati M, Sudilovsky D, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA. 2005;102:6092–7. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 10.Winnepenninckx V, Lazar V, Michiels S, et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–82. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- 11.Rangel J, Torabian S, Shaikh L, et al. Prognostic significance of NCOA3 overexpression in primary cutaneous melanoma. J Clin Oncol. 2006;24:4565–9. doi: 10.1200/JCO.2006.07.3833. [DOI] [PubMed] [Google Scholar]
- 12.Rangel J, Nosrati M, Torabian S, et al. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–50. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- 13.Rangel J, Nosrati M, Leong SP, et al. Novel role for RGS1 in melanoma progression. Am J Surg Pathol. 2008;32:1207–12. doi: 10.1097/PAS.0b013e31816fd53c. [DOI] [PubMed] [Google Scholar]
- 14.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–91. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani-Sabet M, Rangel J, Torabian S, et al. A multi-marker assay to distinguish malignant melanomas from benign nevi. Proc Natl Acad Sci USA. 2009;106:6268–72. doi: 10.1073/pnas.0901185106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashani-Sabet M, Shaikh L, Miller JR, 3rd, et al. NF-κB in the vascular progression of melanoma. J Clin Oncol. 2004;22:617–23. doi: 10.1200/JCO.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 17.Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR., 3rd Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–31. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- 18.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–83. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 19.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 20.Torabian S, Kashani-Sabet M. Biomarkers for Melanoma. Curr Opin Oncol. 2005;17:167–71. doi: 10.1097/01.cco.0000154039.07466.5d. [DOI] [PubMed] [Google Scholar]
- 21.Straume O, Sviland L, Akslen LA. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res. 2000;6:1845–53. [PubMed] [Google Scholar]
- 22.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 23.Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2009;276:44926–35. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- 24.Xu RC, Qin J, Hashimoto Y, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–61. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong H, Neubig RR. Regulator of G protein signaling proteins: novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837–45. [PubMed] [Google Scholar]
- 26.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 27.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–58. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 28.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–80. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 29.Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–52. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 30.Schuchter LM. Adjuvant interferon therapy for melanoma: high-dose, low-dose, no dose, which dose? J Clin Oncol. 2003;22:7–10. doi: 10.1200/JCO.2004.10.907. [DOI] [PubMed] [Google Scholar]