Abstract
BACKGROUND
Converging evidence has implicated endogenous neurotensin (NT) in the pathophysiology of brain processes relevant to schizophrenia. Prepulse inhibition of the startle reflex (PPI) is a measure of sensorimotor gating and considered to be of strong relevance to neuropsychiatric disorders associated with psychosis and cognitive dysfunction. Mice genetically engineered to not express NT display deficits in PPI that model the PPI deficits seen in schizophrenia patients. NT1 receptors have been most strongly implicated in mediating the psychosis relevant effects of NT such as attenuating PPI deficits. To investigate the role of NT1 receptors in the regulation of PPI, we measured baseline PPI in wildtype (WT) and NT1 knockout (KO) mice. We also tested the effects of amphetamine and dizocilpine, a dopamine agonist and NMDA antagonist, respectively, that reduce PPI as well as the NT1 selective receptor agonist, PD149163, known to increase PPI in rats.
METHODS
Baseline PPI and acoustic startle response were measured in WT and NT1 knockout KO mice. After baseline testing, mice were tested again after receiving intraperatoneal (IP) saline or one of three doses of amphetamine (1.0, 3.0 and 10.0 mg/kg), dizocilpine (0.3, 1.0 and 3.0 mg/kg) and PD149163 (0.5, 2.0 and 6.0 mg/kg) on separate test days.
RESULTS
Baseline PPI and acoustic startle response in NT1 KO mice were not significantly different from NT1 WT mice. WT and KO mice exhibited similar responses to the PPI-disrupting effects of dizocilpine and amphetamine. PD149163 significantly facilitated PPI (P < 0.004) and decreased the acoustic startle response (P < 0.001) in WT but not NT1 KO mice.
CONCLUSIONS
The data does not support the regulation of baseline PPI or the PPI disruptive effects of amphetamine or dizocilpine by endogenous NT acting at the NT1 receptor, although they support the antipsychotic potential of pharmacological activation of NT1 receptors by NT1 agonists.
Keywords: schizophrenia, neurotensin, animal model, PD149163, amphetamine, dizocilpine
INTRODUCTION
Neurotensin (NT) is a neuropeptide that modulates neurotransmitter systems in brain areas that are highly relevant to the pathophysiology of schizophrenia (Hokfelt et al., 1984; Manberg et al., 1982). Evidence suggests that endogenous NT may exert antipsychotic-like effects on brain processes relevant to schizophrenia and that perturbations in the endogenous NT system may contribute to the manifestation of symptoms of this disorder. For example, several clinical studies have demonstrated that a large number of untreated schizophrenia patients exhibit significantly decreased CSF levels of NT and that these levels are inversely related to the severity of psychosis (Garver et al., 1991; Sharma et al., 1997). There is also some evidence that increases in the level of endogenous NT in the brain mediates some of the therapeutic effects of antipsychotic drugs. For example, converging evidence from human and animal studies have shown that antipsychotic drug treatment elevates NT levels in the brain (Kinkead and Nemeroff, 2002) (Garver et al., 1991; Lindström et al., 1988; Nemeroff et al., 1989; Sharma et al., 1997; Widerlöv et al., 1982). It has also been suggested that endogenous NT may mitigate the effects of psychotomimetic drugs on the brain (Binder et al., 2001; Kinkead and Nemeroff, 2002).
Animal models provide a useful tool to investigate the role that perturbation in the NT system may play in the manifestation of schizophrenia and the potential of NT agonists to ameliorate those manifestations. Since it is not possible to model the symptoms of schizophrenia, for example psychosis, directly in animals, investigators measure biological features that are relevant to schizophrenia. One such feature is prepulse inhibition (PPI) of the startle reflex. PPI describes the normal suppression of the startle response, when a startle-eliciting stimulus, for example a sudden loud noise, is immediately preceded by a weak lead stimulus, such as a soft auditory click. PPI is considered an operational measure of sensorimotor gating, a brain-based process that is involved in filtering irrelevant environmental information (Braff and Geyer, 1990; Swerdlow and Geyer, 1998). PPI is of great interest to schizophrenia researchers because unmedicated schizophrenia patients exhibit reduced PPI compared to normal subjects (Braff and Geyer, 1990) and there is converging evidence that antipsychotics, particularly atypical antipsychotics, remediate this PPI deficiency in these patients (Swerdlow et al., 2006).
In rodents, schizophrenia-like reduced levels of PPI are produced by administration of psychotomimetic drugs such as dopamine agonists, e.g., D-amphetamine and apomorphine (Mansbach et al., 1988) or non-competitive N-methyl-D-aspartate (NMDA) antagonists, e.g., phencyclidine and dizocilpine. There are also strains of rodents, natural or genetically engineered, that exhibit schizophrenia-like PPI deficits (Geyer et al., 2001; Swerdlow and Geyer, 1998). Schizophrenia-relevant genetically modified animal models that exhibit PPI deficits have been used to further study the biology of candidate genes for this disorder (Clapcote et al., 2007; Fradley et al., 2005; Kelly et al., 2007; Kelly et al., 2009; Stefansson et al., 2002). In addition, antipsychotics tend to increase PPI levels in rodents with natural or drug-induced deficits in PPI (Feifel et al., 2007; Feifel et al., 2004; Geyer et al., 2001; Olivier et al., 2001) making this a model with high predictive validity for the therapeutic effects of antipsychotics in schizophrenia patients (Geyer et al., 2001). Therefore, it is a potentially powerful tool for the screening and identification of novel therapeutic targets (Kanes et al., 2007).
Kinkead et al. (2005) examined PPI levels in mice deficient in endogenous NT due to an engineered genetic deletion (i.e. NT peptide knockout mice) and found that these mice had reduced PPI compared to their WT counterparts. In contrast to their WT counterparts, they were not sensitive to the PPI disrupting effects of amphetamine, suggesting that endogenous NT plays a role in maintaining baseline levels of PPI and that endogenous NT is necessary for effects of amphetamine on PPI.
There are three NT receptor subtypes, NT1, NT2 and NT3 that have been identified to date and evidence suggests that additional subtypes exist (Li et al., 2005; Mazella and Vincent, 2006). It is not known which receptors mediate the effects of NT on PPI but converging evidence most strongly implicates NT1 receptors. For example, our lab has reported that the NT1 agonist, PD149163, blocks PPI deficits produced by amphetamine and dizocilpine (Feifel et al., 1999), as well as the naturally occurring PPI deficits in Brattleboro rats (Feifel et al., 2007; Feifel et al., 2004). In addition, Caceda et al (2005) found that over expression of NT1 receptors in the nucleus accumbens also attenuated amphetamine-induced PPI deficits in rats. The availability of mice lacking NT1 receptors due to genetic deletion (‘NT1 knockouts’) provides a useful tool to further investigate the role of the NT1 receptor in the regulation of baseline and psychotomimetic-induced sensorimotor gating. We hypothesized that NT1 receptor knockout (NT1 KO) mice would have lower baseline PPI levels and be more sensitive to the disrupting effects of psychotomimetic drugs compared to wild type (WT) mice. To test this hypothesize, we measured PPI in WT and in mice deficient in NT1 receptors due to a homozygous deletion of NT1 receptor, at baseline and after treatment with amphetamine and the NMDA antagonist, dizocilpine. In addition, we tested the effects, in these two strains, of the NT1 selective agonist PD149163, a modified analog of the c-terminal hexapeptide fragment of NT that penetrates the blood brain barrier and has been shown to increase PPI in rats after systemic administration (Feifel et al., 2007; Feifel et al., 2004; Feifel et al., 1999).
METHODS
Animals
Forty-seven WT and 60 NT1 KO male and 44 WT and 41 NT1 KO female mice were bred at the UCSD breeding facility, San Diego and housed in groups of 2 – 5 in clear plastic chambers in a climate controlled room on a 12:12 hour light/dark cycle (lights on 7:00 AM– 7:00 PM). They were allowed free access to food and water for the extent of the study. All mice were 2 – 5 months old at testing. Behavioral testing was performed between 8:30 AM and 3:00 PM. All studies described in this publication were carried out in accordance with The Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Generation of Knockout Mice
Generation of NT1 deficient mice was carried out at Roche Pharmaceuticals, Palo Alto, CA, using a strategy described previously (Mechanic et al, 2009). A targeting vector was introduced into C57BL/6-derived Bruce-4 mouse ES cells. Homologous recombination resulted in the deletion of 1.4 kb NT1 genomic sequence, including the first ATG-containing exon that encodes the N-terminal 238 amino acids. Two of the ES clones were injected into blastocysts to generate chimeras, which were mated with C57Bl/6J mice to produce N1 heterozygotes. To produce a purer genetic background, one additional backcross was performed to generate the N2 heterozygotes. Homozygote NT1 KO and WT mice (F1) were generated from N2 heterozygote × heterozygote mating. These NT1 KO and WT mice were used to establish the KO and WT colonies, respectively. NT1 KO and WT mice from the F3 generation were used in subsequent experiments.
Polyermase Chain Reaction (PCR)
All NT1 KO and WT breeders were genotyped to confirm their identities. DNA was extracted from a 0.5 mm section of tail from each mouse using the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Subsequently, DNA from each sample was amplified in a thermal cycler (MJ Research, Waltham, MA) under the following conditions: 94 C for 3 min, 35 cycles of the following 94 C, 45 sec, 58 C, 45 sec 72 C, 45 sec and 72 C, 7 min. These PCR conditions were developed empirically at Roche Pharmaceutical (Palo Alto, CA). Three primers synthesized by Qiagen (Chatsworth, CA) were used to amplify the DNA of interest – forward primer NT1-GT2 5′ CAGGAGTGCAGACCAACCACAG 3′, reverse primer NT1-GT3 5′ GTTCACGTCCAGGTTGCTGTT 3′ and a primer specific to the TK-neo construct in the KO mice Neo- GT1 5′CCTTCTTGACGAGTTCXTTCTGAG 3′. PCR products for each sample were run on 1 % agarose gels via gel electrophosis. DNA was visualized with ethidium bromide on an UV illumination box (Fotodyme, Hartland, WI), photographed and each sample genotyped. The expected sizes for the WT and KO alleles were 488 bp and 351 bp, respectively.
Drugs
Amphetamine, dizocilpine (Sigma Chemicals, St. Louis, MO), and PD149163 (NIMH chemical synthesis program, RTI International, Research Triangle Park, NC) were dissolved in saline and injected intraperatoneally (IP).
Animal Testing
Behavioral testing was conducted at UCSD. Mice were placed in startle chambers and tested for baseline PPI and acoustic startle reflex (ASR). Four groups with similar PPI levels that were created based upon baseline PPI matching (independent of sibling status), were used in the amphetamine study. Animals were then tested two more times separated by a minimum of one week. Before each test, animals were counterbalanced based on their previous drug treatments. In the first drug study, mice were administered IP saline or one of three doses of amphetamine (1.0, 3.0, or 10.0 mg/kg). One week later animals received either IP saline or one of three doses of dizocilpine (0.3, 1.0 or 3.0 mg/kg). In the third test, they received either IP saline or PD149163 (0.5, 2.0, or 6.0 mg/kg). For all three tests, animals were placed in startle chambers twenty minutes after drug administration.
Startle Testing
Startle testing was performed in four identical startle chambers obtained from San Diego Instruments (San Diego, CA). Each chamber consisted of a clear non-restrictive Plexiglass cylinder resting on a Plexiglass platform inside a ventilated and illuminated enclosure housed in a sound-attenuated room. A continuous background noise of 65 dB, as well as the various acoustic stimuli were produced within each chamber by a high-frequency loudspeaker (Radio Shack Supertweeter, San Diego, CA). The whole-body startle response of each animal produced vibrations of the Plexiglass cylinder which were transduced into analog signals by a piezoelectric unit mounted underneath the Plexiglass platform (Mansbach et al., 1988). These analog signals were then digitized and stored by an interface unit connected to a microcomputer. Amplitude of ASR was defined as the degree of motion detected by the piezoelectric unit.
Test Sessions
Animals were tested in startle chambers (San Diego Instruments, San Diego, CA) 20 minutes after drug administration. Animals were then subjected to a 10 minute acclimation to the 65 dB background noise, which continued throughout the session. The acclimation was followed by a 14 minute PPI test session. Five trial types were presented during the test session: a 40 msec 120 dB startle pulse (PULSE-ALONE), a 20 msec prepulse (4, 8 or 16 dB above background) preceded the PULSE-ALONE by 100 msec, and a NO-STIMULUS trial. All trial types were presented in pseudo-random order separated by an average of 15 seconds. In addition, four PULSE-ALONE trials that were not used in the calculation of PPI values, were presented at the beginning and at the end of the test session (Bakshi and Geyer, 1998).
Data and Statistical Analysis
A startle response was recorded for all the PULSE-ALONE, and all prepulse trials. From these data, PPI measures and startle magnitude were calculated for each animal. PPI was calculated as a percentage of the pulse-alone ASR magnitude using the following formula: [1− (startle magnitude after prepulse-pulse pair/startle magnitude after pulse only] × 100.
ASR data was analyzed using ANOVA with Gender, Genotype and, except for baseline data, Drug dose (amphetamine, dizocilpine or PD149163) as between subjects factors. ANOVAs for PPI data also included Prepulse Intensity as within subjects factor. Because our a priori interest was limited to potential differences between WT and NT1 KO mice, data was collapsed across any factor that did not have a significant interaction effect with Genotype for the primary analysis. Significant Genotype main effects or interaction effects that included Genotype were followed by a two-tailed Dunnett’s post-hoc test to compare each dose with saline. If there was no significant main or interaction Genotype effect revealed in the ANOVA, data were not collapsed across Genotype, rather planned pairwise comparisons of corresponding WT and NT1 KO groups were conducted using independent subjects t-tests, corrected for multiple comparisons with the Bonferonni method. Though not the focus of this report, results of analysis of significant effects that didn’t interact with Genotype are presented, for informational purposes, in table form (Table 1).
Table 1.
Effects of Amphetamine and Dizocilpine across prepulse intensities.
| 4 db | 8 db | 16 db | |
|---|---|---|---|
| Male/Female Combined | |||
| Saline | 38.99±2.68 | 51.8±3.06 | 62.59±2.93 |
| Amph (1 mg/kg) | 37.42±2.75 | 54.03±2.79 | 70.64±2.58 |
| Amph (3 mg/kg) | 17.77±2.73** | 36.38±2.95** | 61.38±2.42 |
| Amph (10/mg/kg) | 8.77±2.70** | 25.50±3.17** | 50.16±2.79** |
| Male | |||
| Saline | 32.73±3.99 | 45.41±4.53 | 57.69±4.84 |
| Dizocilpine (0.3 mg/kg) | 12.54±3.27** | 25.50±4.24** | 52.28±3.60 |
| Dizocilpine (1.0 mg/kg) | 10.47±3.62** | 19.38±3.31** | 41.76±4.01* |
| Dizocilpine (3.0 mg/kg) | 15.96±4.02** | 24.67±3.90** | 43.95±4.30 |
| Female | |||
| Saline | 39.21±3.79 | 52.15±3.92 | 62.72±3.80 |
| Dizocilpine (0.3 mg/kg) | 2.23±5.36** | 34.83±4.34** | 53.31±3.97 |
| Dizocilpine (1.0 mg/kg) | 9.53±3.38** | 16.49±4.61** | 36.47±5.32** |
| Dizocilpine (3.0 mg/kg) | 12.18±2.68** | 20.19±2.24** | 37.81±4.04** |
There was a significant Prepulse × Amphetamine interaction and a Prepulse intensity × Dizocilpine × Gender interaction. Therefore, male and female data are shown separately for dizocilpine but combined for amphetamine. Means ± SEM. Significantly different from saline at the same prepulse intensity with two tailed Dunnett’s post hoc tests, represented by
P < 0.05,
P < 0.01.
Prior to any data analysis any mouse with extremely low P120 values (< 10) was eliminated from the analysis. We had established this ASR threshold parameter in our previous work (Shilling and Feifel, 2008). This resulted in the elimination of sixteen WT mice treated with PD149163 (low = 5, mid = 3, and high dose = 8). After this elimination there were insufficient numbers of female mice in the PD149163 study to conduct a meaningful analysis, therefore female mice were not included in the analysis .
RESULTS
Baseline Testing
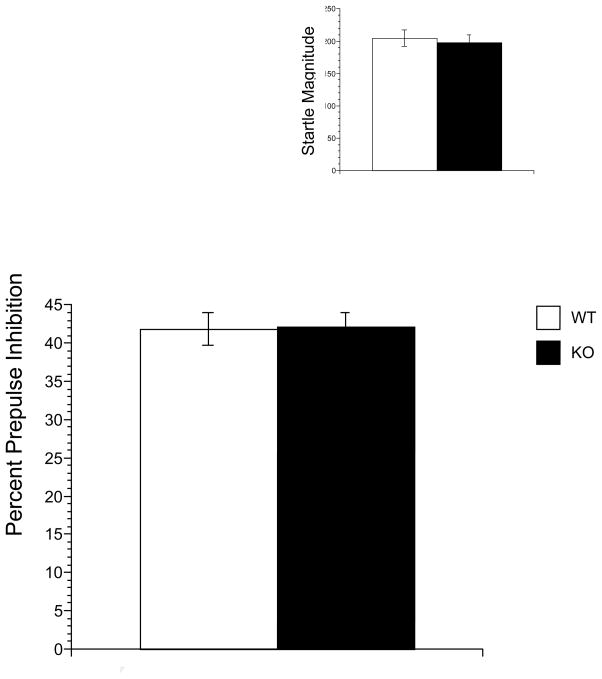
Figure 1 illustrates the baseline PPI (Main Figure) and pulse-alone ASR magnitude (Inset) results. A-two factor ANOVA applied to baseline PPI data revealed that, as expected, there was a significant main effect of Prepulse Intensity [F(2,380)= 367.023, P < 0.001] since stronger prepulses produced greater PPI. There no significant effect of Gender or Genotype nor any interaction among the three factors. For ASR data, there was no significant effect of Gender or Genotype and no interaction effect.
Figure 1.
Baseline prepulse inhibition (Main) and startle magnitude (Inset) in NT1 WT and NT1 KO mice. Bars represent represent the mean ± SEM (n= 91/101). There were no significant between group differences.
Amphetamine Testing
Figure 2 illustrates the PPI (Main Figure) and ASR (Inset) results from amphetamine testing. For PPI data there was a main effect of Prepulse Intensity [F(1,360) = 468.470, P < 0.001], and Drug [F(3,180) = 22,893 , P< 0.001] but no main effect of Genotype. There was a significant Prepulse × Drug interaction [F(6,360) = 8.817, P < 0.001] as amphetamine produced greater disruption of PPI induced by stronger prepulses (see Table 1). There were, however, no other significant interaction effects.
Figure 2.
The effects of amphetamine on prepulse inhibition (Main) and startle magnitude (Inset) in WT and NT1 KO mice. Bars represent the mean ± SEM (n=21–26).
Amphetamine significantly different from saline, represented by * P < 0.05, ** P< 0.01 by Bonferonni corrected t-tests.
ASR data analysis revealed a main effect of Gender F[(1,187)=54.758, P < 0.001], but not Genotype or Drug. There was a significant Gender × Genotype interaction [F (3,187) = 6.921, P < 0.01] as WT males, but not females, exhibited a significantly higher ASR than their KO counterparts [t(102) = 2.849, P < 0.01]. There were no other significant interaction effects.
Dizocilpine Testing
Figure 3 illustrates the PPI (Main Figure) and ASR magnitude (Inset) results from dizocilpine testing. Three-factor ANOVA applied to PPI data revealed a main effect of Prepulse Intensity [F(2,356) = 152.266, P < 0.001] and Drug [F(3,178) = 25.339, P < 0.001]] but not Genotype or Gender. There was a significant Prepulse × Drug [F(6,356) = 7.322, P < 0.001] and Prepulse × Drug × Gender interaction [F((6, 356)=2.56, P<0.05] (See Table 1). There were no other significant interaction effects.
Figure 3.
The effects of dizocilpine on prepulse inhibition (Main) and startle magnitude (Inset) in WT and NT1 KO mice. Bars represent the mean ± SEM (n=18–26). Dizocilpine significantly different from saline, represented by * P < 0.05, ** P< 0.01. by Bonferonni corrected t-tests.
For ASR data, there was a main effect of Drug [F(3,185) = 10.152, P < 0.001], as dizocilpine tended to increase ASR at the low dose of dizocilpine. There was also a main effect of Genotype [F(1,185) = 3.939, P < 0.05] reflected in a higher startle magnitude in WT mice. The Drug × Genotype interaction was not significant.
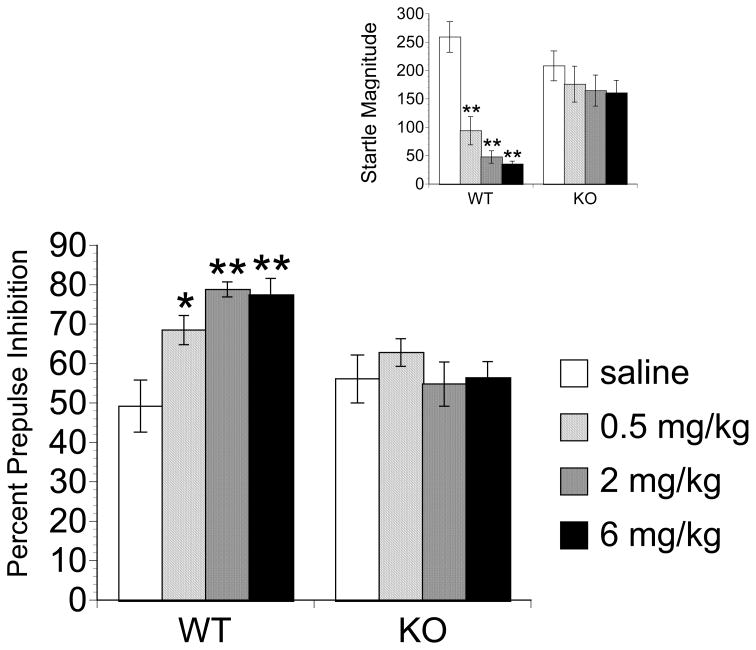
PD149163 Testing
Figure 4 illustrates the PPI (Main Figure) and ASR magnitude (Inset) results from PD149163 testing. PPI data revealed a main effect of Prepulse Intensity [F(2,166) = 131.800, P < 0.001], Genotype, [F(1,83) =6.726, P < 0.05] and Drug [F(3,83)=3.450, P < 0.05]. There was also a significant Genotype × Drug interaction [F(3,83) = 3.397, P < 0.05] and Prepulse × Drug × Genotype interaction [F(6,166)=2.311, P < 0.05] as all the dose of PD149163 elevated PPI in WT but not KO mice and this was most apparent at lower prepulse intensities (4dB and 8 dB) where baseline PPI (saline-treated) was the lowest. No other interactions were significant. Effects of PD149163 at each prepulse data are displayed in Table 2.
Figure 4.
The effects of PD149163 on prepulse inhibition (Main) and startle magnitude (Inset) in WT and NT1 KO mice.. Bars represent the mean ± SEM (n=5–16). PD149163 significantly different from saline, represented by * P < 0.05, ** P< 0.01 by Dunnet’s two-tailed post-hoc test.
Table 2.
Effects of PD149163 (PD) on Wild Type (WT) and Knockout (KO) mice (males and females combined): % PPI means ± SEM at 4 db, 8 db and 16 db.
| WT | NT1 KO | |||||
|---|---|---|---|---|---|---|
| 4 db | 8 db | 16 db | 4 db | 8 db | 16 db | |
| Saline | 35.21±6.21 | 48.58±7.13 | 63.75±6.95 | 43.96±5.49 | 57.49±7.13 | 66.98±6.16 |
| PD (1.0 mg/kg) | 56.67±6.48 | 70.64±3.99 | 78.27±2.93 | 49.16±4.08 | 65.30±3.86 | 73.81±3.38 |
| PD (2.0 mg/kg) | 72.00±3.50** | 81.78±2.67** | 82.56±2.97 | 41.34±5.99 | 53.21±6.31 | 70.00±5.49 |
| PD (3.0 mg/kg) | 64.75±5.74* | 80.25±5.41** | 87.26±2.22* | 43.83±5.83 | 53.81±5.30 | 71.70±3.31 |
Significantly different from saline at the same prepulse intensity using a two-tailed Dunnett’s test represented by
P < 0.05,
P < 0.01.
For ASR data, there was a main effect of Genotype [F (1,83)=9.536, P < 0.01] and Drug [F(3,83) = 9.885, P < 0.001]. In addition, there was a significant Drug × Genotype interaction [F(3,83) = 4.257, P < 0.01] as all doses of PD149163 decreased ASR in WT but not NT1 KO mice.
DISCUSSION
The lack of significant differences in baseline PPI or ASR between NT1 KO and NT1 WT mice does not support a role for NT1 receptors in the regulation of baseline PPI or startle. This finding is consistent with the finding that overexpression of NT1 receptors in the brain does not significantly alter baseline PPI or ASR in mice (Caceda et al, 2005). However, Kinkead et al. reported that mice deficient in NT exhibit reduced PPI and increased ASR compared to WT mice, suggesting a role for endogenous NT in regulation of baseline PPI and startle by endogenous NT (Kinkead et al., 2005). Several explanations may reconcile these findings. First, endogenous NT may regulate baseline PPI and startle via either another non-NT1 receptor. In this respect, the function of NT2 receptors has been extensively investigated (Maeno et al., 2004; Yamauchi et al., 2007). However, to the best of our knowledge, there have not been any published reports on the role of NT2 receptor or NT3 receptors in the regulation of baseline PPI and startle. To better understand which NT receptors regulate the effects of endogenous NT on PPI and startle, additional studies will need to be performed. Another possible explanation to reconcile the differences in our findings and Caceda et al’s from with those of Kinkead et al. may be that congenital absence of NT1 receptors induces compensatory mechanisms that result in maintenance of normal levels of baseline PPI in adulthood. Depletion of NT may not induce these compensatory mechanisms and, therefore, NT1 receptors in NT deficient mice may maintain a role in the regulation of baseline PPI levels. Studies on conditional NT1 receptor knockout mice, whose NT1 receptor deficiency is induced in mature animals would be useful in resolving these possible explanations.
Amphetamine and dizocilpine reduced PPI with comparable efficacy in both WT and NT1 KO mice. These findings do not support the contention that endogenous NT acting via NT1 receptors inhibits the effects of amphetamine on neural circuits and processes relevant to psychosis (Caceda et al., 2005; Kinkead et al., 2005). This stands in contrast to the evidence that exogenously administered NT or NT agonits can block PPI reduction induced by amphetamine and dizocilpine via activation of the NT1 receptor (Feifel et al, 2009) or that overexpression of NT1 receptors in the brain by genetic manipulation can block amphetamine-induced PPI disruption (Caceda et al, 2005). It is possible that supra-physiological levels of NT1 receptor activation, such as produced by pharmacological or genetic means, produces antipsychotic drug-like reversal of psychotomimetic-inuced PPI disruption, whereas the degree of NT1 activation produced under normal conditions by endogenous NT, the effect absent in NT1 KO mice, plays no major role in modulating the PPI effects of psychotomimetcs.
PD149163 treatment significantly increased PPI and decreased startle in WT mice, which is consistent with its effects in rats where it reverses PPI disruption induced by psychotomimetic drugs and increases PPI in Brattleboro rats, a strain of rat with naturally low PPI (Feifel et al., 2007; Feifel et al., 2004; Feifel and Priebe, 2001). Although it was necessary to eliminate many low startling animals from the PD-treated WT groups in this study, the findings are consistent with our findings in rats, suggesting that these findings are not an artifact of any potential selection bias due to the elimination of these mice. To the best of our knowledge this is the first report on the PPI and startle effects of a NT1 agonist in mice. Increases in baseline PPI and decreases in baseline startle magnitude of C57 mice are produced by established antipsychotics (Olivier et al., 2001; Ouagazzal et al., 2001) and thus the effects of PD149163 are consistent with the effects of antipsychotic drugs. Consistent with a role for NT1 receptor mechanisms in the antipsychotic-like effects of NT agonists, Mechanic et al. (2009) using another strain of NT1 KO mice, recently reported that the antipsychotic-like effects of NT agonists on climbing behavior were blocked in mice lacking NT1 receptors.
In contrast to its effects in WT mice, PD149163 had no significant effect on PPI or ASR in NT1 KO mice. The lack of an effect of PD149163 on PPI and ASR in NT1 KO mice confirms that PD149163 produces its effect on PPI and ASR via NT1 receptors. The ability of PD149163 to increase baseline PPI in WT mice and Brattleboro rats suggests that NT1 receptors are able to modulate baseline PPI, at least in animals with low baseline PPI levels. However, the effects of PD149163 reveal only that supra-physiological activation of NT1 receptors produces increases in baseline PPI. Thus, not withstanding the possibility of developmental compensation discussed above, the effects of PD149163, together with the absence of baseline PPI differences in NT1 KO compared to WT mice may suggest that pharmacological activation of NT1 receptors can increase PPI, whereas, activation of NT1 receptors by endogenous levels of NT does not contribute to the baseline PPI “tone”.
In summary, PD149163 facilitated PPI in WT but not NT1 KO mice providing further support for the antipsychotic-like effects of NT agonists and the mediation of these effects via NT1 receptors. In addition, the deletion of the NT1 gene had no effect on baseline PPI or PPI reduction induced by amphetamine or dizocilpine, suggesting that under normal physiological condition, NT1 receptors do not modulate the baseline PPI tone nor the PPI effects of dopamine agonists and NMDA receptor antagonists..
Acknowledgments
This research was supported by NIMH grant MH080910 to DF.
Footnotes
Disclosures: DF is a consultant and holds stock options in Argolyn Biosciences, and Tenzia, biopharmaceutical companies developing neurotensin agonists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. Journal of Neuroscience. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Kilts CD, Nemeroff CB. Enhanced neurotensin neurotransmission is involved in the clinically relevant behavioral effects of antipsychotic drugs: evidence from animal models of sensorimotor gating. J Neurosci. 2001;21:601–608. doi: 10.1523/JNEUROSCI.21-02-00601.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies [see comments] Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Caceda R, Kinkead B, Owens MJ, Nemeroff CB. Virally mediated increased neurotensin 1 receptor in the nucleus accumbens decreases behavioral effects of mesolimbic system activation. J Neurosci. 2005;25:11748–11756. doi: 10.1523/JNEUROSCI.4282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;81:278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Priebe K. Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry. 2001;50:425–433. doi: 10.1016/s0006-3223(01)01100-3. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;288:710–713. [PubMed] [Google Scholar]
- Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Garver DL, Bissette G, Yao JK, Nemeroff CB. Relation of CSF neurotensin concentrations to symptoms and drug response of psychotic patients. American Journal of Psychiatry. 1991;148:484–488. doi: 10.1176/ajp.148.4.484. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Everitt BJ, Theodorsson-Norheim E, Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol. 1984;222:543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: a specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–246. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, Houslay MD, Kanes SJ, Abel T. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32:577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, Esposito MF, Wand G, Kanes SJ, Abel T. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry. 2009;14:398–415. 347. doi: 10.1038/mp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J Pharmacol Exp Ther. 2005;315:256–264. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- Kinkead B, Nemeroff CB. Neurotensin: an endogenous antipsychotic? Curr Opin Pharmacol. 2002;2:99–103. doi: 10.1016/s1471-4892(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Li JH, Sicard F, Salam MA, Baek M, LePrince J, Vaudry H, Kim K, Kwon HB, Seong JY. Molecular cloning and functional characterization of a type-I neurotensin receptor (NTR) and a novel NTR from the bullfrog brain. J Mol Endocrinol. 2005;34:793–807. doi: 10.1677/jme.1.01709. [DOI] [PubMed] [Google Scholar]
- Lindström LH, Widerlöv E, Bisette G, Nemeroff C. Reduced CSF neurotensin concentration in drug-free schizophrenic patients. Schizophrenia Research. 1988;1:55–59. doi: 10.1016/0920-9964(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Maeno H, Yamada K, Santo-Yamada Y, Aoki K, Sun YJ, Sato E, Fukushima T, Ogura H, Araki T, Kamichi S, Kimura I, Yamano M, Maeno-Hikichi Y, Watase K, Aoki S, Kiyama H, Wada E, Wada K. Comparison of mice deficient in the high- or low-affinity neurotensin receptors, Ntsr1 or Ntsr2, reveals a novel function for Ntsr2 in thermal nociception. Brain Res. 2004;998:122–129. doi: 10.1016/j.brainres.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Manberg PJ, Nemeroff CB, Iversen LL, Rosser MN, Kizer JS, Prange AJ., Jr Human brain distribution of neurotensin in normals, schizophrenics, and Huntington’s choreics. Annals of the New York Academy of Sciences. 1982;400:354–367. doi: 10.1111/j.1749-6632.1982.tb31581.x. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Mazella J, Vincent JP. Functional roles of the NTS2 and NTS3 receptors. Peptides. 2006;27:2469–2475. doi: 10.1016/j.peptides.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Widerlov E, Beckmann H, Gerner R, Manberg PJ, Lindstrom L, Prange AJ, Jr, Gattaz WF. Neurotensin-like immunoreactivity in cerebrospinal fluid of patients with schizophrenia, depression, anorexia nervosa-bulimia, and premenstrual syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 1989;1:16–20. doi: 10.1176/jnp.1.1.16. [DOI] [PubMed] [Google Scholar]
- Olivier B, Leahy C, Mullen T, Paylor R, Groppi VE, Sarnyai Z, Brunner D. The DBA/2J strain and prepulse inhibition of startle: a model system to test antipsychotics? Psychopharmacology (Berl) 2001;156:284–290. doi: 10.1007/s002130100828. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Janicak PG, Bissette G, Nemeroff CB. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 1997;154:1019–1021. doi: 10.1176/ajp.154.7.1019. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. The neurotensin-1 receptor agonist PD149163 blocks fear-potentiated startle. Pharmacol Biochem Behav. 2008;90:748–752. doi: 10.1016/j.pbb.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophrenia Bulletin. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Widerlöv E, Lindström LH, Besev G, Manberg PJ, Nemeroff CB, Breese GR, Kizer JS, Prange AJ., Jr Subnormal CSF levels of neurotensin in a subgroup of schizophrenic patients: normalization after neuroleptic treatment. American Journal of Psychiatry. 1982;139:1122–1126. doi: 10.1176/ajp.139.9.1122. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]