Abstract
Understanding the mechanisms of fear extinction has become increasingly important for treating a number of disorders, particularly post-traumatic stress disorder. Conditioning of rabbit heart rate (HR) is an established model for studying fear, yet little is known about procedures for extinguishing it other than repeated presentations of cue(s) associated with the fear-inducing event. The following study examined the effects of conditioned stimulus (CS) alone, unconditioned stimulus (US) alone, unpaired CS/US presentations, continued CS-US pairings, or no further stimulation on rabbit HR following HR conditioning. We have previously shown the rabbit HR response to the US can change as a function of learning when measured in the absence of the CS, a phenomenon referred to as conditioning-specific reflex modification (CRM). More specifically, the HR exhibits a deceleration in response to the US reminiscent of the conditioned bradycardia that develops to the CS. Consequently, the following study also examined the effects of extinction treatments on HR CRM. For HR conditioned responses (CRs), CS-alone and unpaired CS/US presentations were the most successful extinction treatments. For HR CRM, all conditions led to a reduction in CRM except for a subset of rabbits that maintained high levels following unpaired extinction, indicating a dissociation between extinction of HR CRs and CRM. The findings highlight the parameters of HR extinction, the transient nature of HR CRM, vagal involvement in both acquisition and extinction of HR CRM, and suggest that HR CRM cannot be fully explained as a CR that has generalized from the CS to the US.
Keywords: classical conditioning, heart rate conditioning, rabbit, fear conditioning, reflex modification, extinction
Introduction
The usefulness of the rabbit heart rate (HR) response for studying conditioned autonomic changes was evident as early as 1943 when rabbits were shown to develop conditioned HR deceleration to a bell conditioned stimulus (CS) following pairings of the bell with the unconditioned stimulus (US) ammonia (Kosupkin and Olmsted 1943). Since then, rabbit HR conditioning has become a widely used model for studying classical conditioning and in particular, fear conditioning [6,10,20,38,47,48,51,78]. More recently, abnormalities in heart rate conditioning have been associated with human fear disorders, including post-traumatic stress disorder (PTSD) [22].
In a typical rabbit HR experiment, a tone serves as the CS and electrical stimulation serves as the US. The innate response of the restrained adult rabbit to a tone is a slowing of the heart, or bradycardia, an orienting or alpha response that can be habituated with repeated presentations of the tone by itself [54,61]. The reaction to electrical stimulation is typically a quickening of the heart, or tachycardia, a measure of the rabbit's defense reaction to a stressful experience [16,55,56,59]. Following habituation of HR to the tone, pairings of the tone CS and US result in conditioned deceleration to the CS, an effect that is not observed in rabbits receiving explicitly unpaired presentations of the CS and US [21,40,51,61,65]. A consistent observation with HR conditioning is that, depending on parameters, it develops rapidly in comparison to conditioning of somatomotor response systems, such as the rabbit nictitating membrane response (NMR), and then wanes with prolonged CS-US pairings [51,60,70,79]. In addition, the training parameters that support HR conditioning and NMR conditioning are distinct, with HR conditioning superior at long CS-US intervals and NMR conditioning favored at shorter intervals [32,34,51,60]. When intermediate parameters are utilized, the acquisition functions for HR versus NMR conditioning are inversely related [51,60].
The neural mechanisms underlying HR conditioning have been widely researched in rabbits and other species including humans, with studies concentrating on vagal-mediated, parasympathetic cardiovascular changes [12,18,19,29,30,42,46,62], sympathetic-mediated changes [1,31], emotional/affective learning components involving the amygdala and prefrontal cortex [10,47,50,52,58], contributions of the cerebellar vermis [5,74], and involvement of the hippocampus in encoding the memory trace [39,40]. The CNS contributions to HR conditioning have also been assessed by detailed analysis of the electrocardiogram, including measurement of the intervals between the P and Q or Q and T deflections [42,43]. Nijsen and colleagues were the first to show that conditioned heart rate (HR) changes were correlated with a change in PQ interval, and by manipulating the vagus with atropine, showed that PQ interval increases were mediated by the vagal nerve [42]. The presumed origin of these changes in the PQ interval are the medial geniculate neurons that project to the amygdala which, in turn sends projections to the dorsal motor nucleus of the vagus [77].
Previous HR conditioning studies have mainly focused on mechanisms responsible for the CS-mediated modification of HR, but recent work in our laboratory has shown that the HR conditioning procedure can also modify unconditioned HR responses to the US that, like the HR conditioned response (CR), are parasympathetically mediated by the vagus as indexed by changes in the PQ interval [65,70]. We refer to these learning-related changes in the unconditioned response (UR) as conditioning-specific reflex modification (CRM) [8,63]. CRM is defined as an associative learning phenomenon in which responding to a US is modified following repeated pairings of a CS and US. Some unique properties of CRM are that it is observed in the absence of the CS, does not occur following explicitly unpaired presentations of the CS and US, and comes to resemble aspects of the CR [8,63,67]. To date, CRM has been found in two different behavioral response systems in rabbits, the NMR [67] and the HR response [65]. In the case of NMR CRM, a simple blink is transformed into a larger and more complicated response, similar in many aspects to the conditioned NMR. In the case of HR CRM, HR responses to the US exhibit a deceleration very reminiscent of the conditioned deceleration that develops to the CS. While NMR CRM has been confirmed by several different laboratories [25,76] and has well-established behavioral laws [7,8,66,68,73], less is known about the specific properties of HR CRM.
Because responses to the US following conditioning show similarities to the CR, one hypothesis is that CRM is a generalization of the CR from the CS to the US. To test this hypothesis for NMR CRM, an experiment was conducted to examine whether manipulations designed to extinguish the CR would also extinguish CRM [68]. Unpaired CS/US presentations and CS-alone presentations were the most successful in extinguishing the CR whereas US-alone presentations were the least successful. In contrast, extinction of CRM was best accomplished by US-alone presentations, followed by unpaired CS/US presentations, and then CS-alone presentations. These findings showed that unpaired extinction was best able to extinguish both CRs and CRM concurrently, but also suggested that the generalized CR hypothesis could not fully account for the phenomenon of NMR CRM.
The purpose of the present experiment was to explore the nature of the extinction of HR CRs and CRM by conducting an experiment in which rabbits received HR conditioning and CRM testing followed by CS-alone, US-alone, explicitly unpaired CS/US presentations, further CS-US presentations or no further stimulus presentations while remaining in their home cage. Heart rate responses were measured as a change in interbeat interval (IBI), and in addition, the PQ interval of the rabbit electrocardiogram (ECG) was measured to index vagal-mediated parasympathetic function [42,43]. Because an increase in PQ interval corresponds to the development of HR CRM [65], it was expected that reductions in PQ interval would accompany extinction of HR CRM.
Materials and Methods
Subjects
The subjects were 58 male, New Zealand White rabbits (Oryctolagus cuniculus) weighing approximately 2.0-2.2 kg upon delivery from the supplier (Harlan, Indianapolis, IN). The rabbits were housed in individual cages on a 12 hour light-dark cycle and given ad libitum access to food and water. They were maintained in accordance with guidelines issued by the National Institutes of Health, and the research was approved by the West Virginia University Animal Care and Use Committee.
Apparatus
The apparatus has been detailed elsewhere [64,71] and is modeled after those developed and described by Gormezano [14,23]. Briefly, rabbits were restrained in a Plexiglas box placed inside a sound-attenuating, ventilated chamber (Coulborn Instruments, Allentown, PA; Model E10-20). Inside the chambers, a stimulus panel containing a speaker and houselight (10-W, 120 V) was mounted at a 45° angle 15 cm anterior and dorsal to the rabbit's head. An exhaust fan created a constant ambient noise level of 65 dB inside the chamber. Periorbital electrical stimulation (ES) served as a US, which was delivered by a programmable two-pole stimulator (Colbourn Instruments, Model E13-35) via stainless steel Autoclip wound clips (Stoelting, Wood Dale, IL) that were positioned 10 mm ventral and 10 mm posterior to the dorsal canthus of the right eye.
For the recording of heart rate, three wound clips were placed in shaved skin, two on either side of the breast bone (20 mm lateral of the apex) and one on the right shoulder. For each rabbit, the three clips were coupled to an individual custom amplifier providing a 10,000-fold amplification of the ECG signal which was then routed to an analog-to-digital converter (1-ms sampling rate) and stored on a trial-by-trial basis for subsequent inspection and analysis. A custom circuit isolated the US from the ECG amplifier during US presentations to prevent high-voltage artifacts and subsequent electronic ringing from saturating the system. The custom circuit was crucial for resolving components of the ECG waveform during trials where the US was presented, allowing detection of all heartbeats with the exception of those during or immediately following the US. Stimulus delivery, data collection, and analysis were all accomplished using the LabVIEW software system (National Instruments, Austin, TX).
Procedure
One week after arrival, rabbits received one session per day starting with adaptation, US pretesting (Pretest), two sessions of HR conditioning, US post testing (Post1), three sessions of extinction, US post testing (Post2), and a CS alone test. For adaptation, subjects were prepared for ES delivery ECG recording and then adapted to the training chambers for an amount of time equivalent to subsequent training sessions (80 min). For pretest and post tests, subjects received 80 trials of US presentations with an average inter-trial interval (ITI) of 60 s (range 50-70 s). Each US presentation was one of 20 combinations of ES intensity (0.1, 0.25, 0.5, 1.0, or 2.0 mA) and duration (10, 25, 50, or 100 ms), and these 20 unique USs were presented in four separately randomized sequences with the restriction that the same intensity or duration could not occur more than 3 times in succession during the session. For HR conditioning, paired trials consisted of presentations of a 1000 ms, 1 kHz, 82 dB tone CS followed by a 100 ms, 2 mA ES US (1000 ms interstimulus interval, 0 ms trace) that were interspersed with CS-alone test trials following every 9th paired trial. For the first session of HR conditioning, 20 CS-alone trials were presented in order to habituate the bradycardic orienting response that occurs to a novel tone. The remaining 60 trials of the first session and all 80 trials of the second session were paired trials interspersed with CS-alone trials, yielding a total of 54 and 72 paired CS-US presentations on the first and second days of conditioning, respectively. Average ITIs for HR conditioning sessions and all other sessions were the same as US testing, 60 s (range 50-70 s).
For the extinction sessions, rabbits were divided into five groups that received either US-alone presentations (US Ext, n=12), CS-alone presentations (CS Ext, n=12), unpaired CS/US presentations (UP Ext, n=12), continued CS-US pairings (PD, n=10), or no further stimulus presentations while remaining in home cages (Sit, n=12). For all extinction conditions and sessions, the CS and US were identical to those presented during the HR conditioning sessions. For US-alone and CS-alone extinction, sessions consisted of 80 presentations of the ES US or tone CS, respectively. For unpaired extinction, 80 CS-alone and 80 US-alone presentations were explicitly unpaired and delivered on average, every 30 s. Sessions of continued CS-US pairings were identical to the second session of HR conditioning, and the CS-alone test (CS Test) that all subjects received as the final session was identical to a CS-alone extinction session. While Post1 was meant to assess the level of HR CRM, Post2 and the CS Test assessed the effects of extinction on HR CRM and HR conditioning, respectively.
The experiment was conducted in three separate replications. Assignment of extinction groups took place following Post1 in order to match groups based on levels of HR conditioning and HR CRM prior to extinction. Total subject numbers in each group were analyzed by analysis of variance (ANOVA; (SPSS 14.0) with violations of the sphericity assumption corrected with Greenhouse-Geisser. Planned and follow up comparisons were Bonferroni corrected for the number of comparisons unless otherwise noted.
Heart Rate Data Collection and Analysis
For all sessions, the ECG signal was recorded for a total of 6 s per trial. For paired trials and CS-alone trials, heartbeats were recorded starting 2200 ms prior to the onset of the CS, and for US-alone trials, 2200 ms prior to the onset of the US. Details for detection of heartbeats and components of the ECG signal have been described in detail elsewhere [69]. Briefly, heartbeats were detected in the filtered ECG signal with a template-matching algorithm created in LabVIEW software. Visual inspection of the data corrected any false positives or negatives as a result of artifacts such as those related to movement. Heart beat detection was relatively straightforward because the R component of the PQRST waveform of the ECG had amplitudes approaching 1 mV, typically 10-20 times greater than the noise level. Data were expressed as a change in interbeat interval (IBI) to the CS or US from the pre-stimulus baseline. There is continued debate in the field of neurophysiology about the appropriate measurement of HR change, but we have selected to measure and report on change in IBI because it is consistent with our previously published studies and a good deal of the HR conditioning literature [40,49,65,69,70,74]. Additional individual components of the ECG -such as the P component, which at amplitudes of 20 to 40 μV have a lower signal to noise ratio, were better resolved with a peak detection algorithm (Origin Software, Origin Lab, MA). The distance between the P and Q components (PQ interval) was then calculated. The measurement of the PQ interval has been used in previous animal ECG studies [26,42,65,70] and represents the vagal-mediated parasympathetic contribution to HR [42].
In addition to IBI and PQ measurements, topographies of the HR responses during US testing and HR conditioning were also generated to examine timing of the HR response and to determine the appropriate post-stimulus time frames for analysis of CRs to the CS and CRM to the US. Topographies were plotted as average change in IBI from a pre-stimulus baseline.
Results
Timing and Shape of Heart Rate Responses
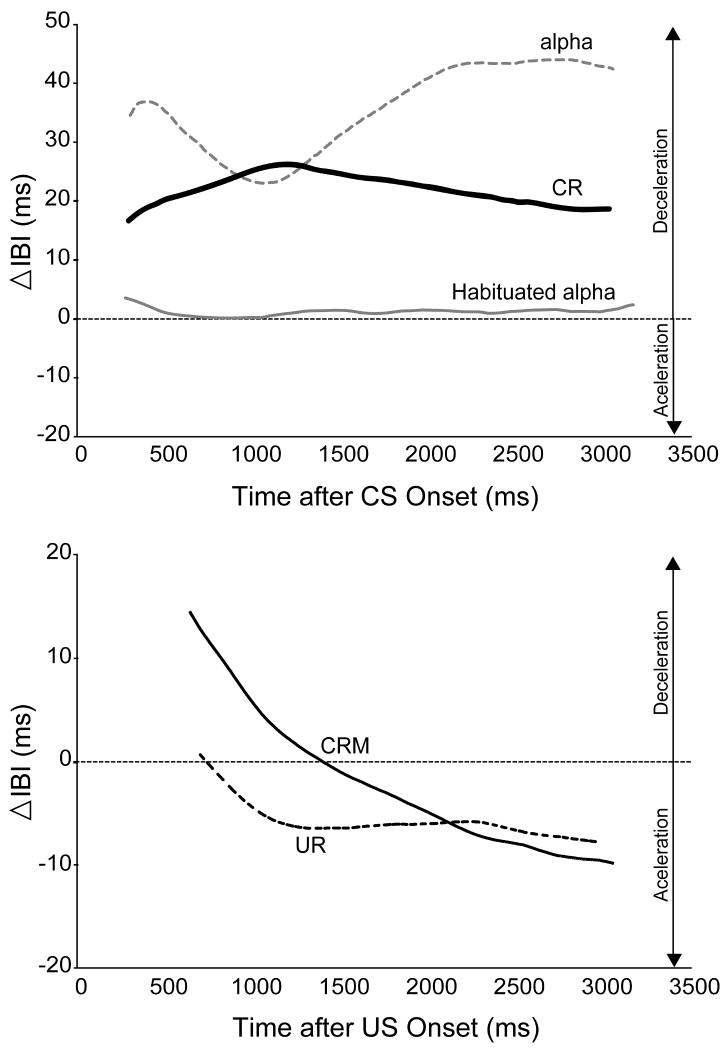
The HR topographies in Figure 1 illustrate the distinct timing and shape of HR responses to the CS (top panel) and US (bottom panel) before and after HR conditioning. While the maximal conditioned HR deceleration to the CS was found to take place in the first 2000 ms following CS onset, the conditioning-specific changes to the US (CRM) relative to the pre-conditioning UR occurred within the first one or two heart beats post-US, as has been established in other published HR CRM studies [65,70]. A 1000 ms post-US time period was therefore found to capture these initial changes better than a longer post-stimulus period. Based on these findings, analyses of HR responses included the first 2000 ms following CS onset or the first 1000 ms following US onset with either a 2000 ms or 1000 ms pre-stimulus baseline, respectively. Also, the CS topographies in the top panel of Figure 1 clearly document the progression of the CS-evoked response from a decelerative orienting response (alpha) with a very distinct topography that habituates to baseline levels (Habituated alpha) to a conditioned deceleration with its own unique topography (CR). This confirms that the HR CR is not simply the reinstatement of a previously habituated alpha response (i.e. alpha conditioning).
Figure 1.
Averaged heart rate (HR) response topographies of the mean change in inter-beat interval (IBI) from baseline to the conditioned stimulus (CS, top panel) and unconditioned stimulus (US, bottom panel) illustrating the effect of conditioning on the shape and timing of HR responses to the CS and US. Averaged CS topographies include the alpha (gray dotted line) and habituated alpha (gray solid line) response to the first and last CS-alone presentation during Habituation, respectively, and the averaged CS-alone probes during the first day of HR conditioning (CR, solid black line). Averaged US topographies include the HR unconditioned response (UR) to a 2.0 mA US during both Pretest (dotted black line) and during the first Post Test following HR conditioning, which represents conditioning-specific reflex modification (CRM). The topographies were generated with respect to the time point at which all subjects had at least one post-stimulus heart beat and therefore do not start at time point zero.
Heart Rate Responses to the Tone CS
HR Conditioning
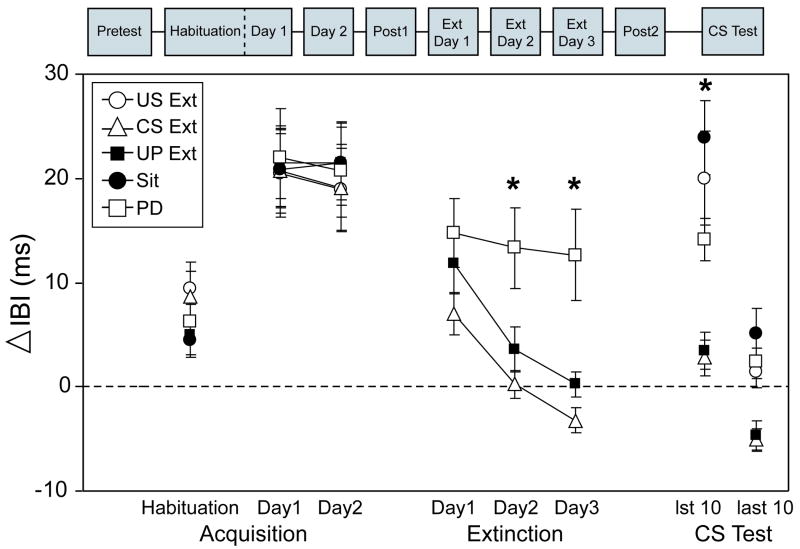
The left side of Figure 2 depicts the mean IBI change from baseline to the tone CS during the 20 CS-alone habituation trials of the first session of HR conditioning (Habituation) and CS-alone probe trials on the first and second HR conditioning sessions (Day1 and Day2). Analyses revealed no group differences in HR responses during acquisition, including baseline responding. As can be seen for all groups in Figure 2, there was a slight increase in mean change IBI from baseline during habituation, reflecting the HR orienting response (deceleration) to the novel tone CS. With pairings of the CS and shock US, mean IBI change increased from habituation (reflecting conditioned HR deceleration), reaching a maximal change on Day1 (21.1 ms) which was maintained across Day2. A repeated measures analysis of variance confirmed the development of conditioned deceleration, indicating a main effect of Session [F(1.73, 91.75) = 63.95, p < 0.001], with post hoc comparisons indicating that there was significantly more deceleration on Day1 and Day2 compared to Habituation (p < 0.001).
Figure 2.
Heart rate (HR) conditioning to the tone conditioned stimulus (CS) was established in all groups and subsequently extinguished by CS- alone and unpaired CS and unconditioned stimulus (US) presentations. Data are shown as mean change (± SEM) in inter-beat interval (IBI) from baseline to the CS during CS-alone habituation trials, CS-alone probe trials on Day1 and Day2 of HR conditioning, extinction days 1-3 of HR extinction, and the first 10 and last 10 trials of the CS Test. For HR extinction, data pictured are only from groups receiving CS presentations during extinction, including the CS-alone probes from the group receiving continued CS-US pairings (PD, open box, n=10) and CS-alone presentations from the CS-alone extinction group (CS Ext, open triangle, n=12) and unpaired CS/US extinction group (UP Ext, black box, n=12). Other abbreviations: US-alone extinction (US Ext, open circle, n=12), sit control group remaining in home cages (Sit, black circle, n=12). Asterisks indicate significant differences between groups at p < 0.05.
Effects of Extinction on Heart Rate Conditioning
Analyses of the mean IBI change to the CS during extinction were restricted to the groups receiving CS-alone presentations (CS Ext), unpaired CS/US presentations (UP Ext), and continued pairings of the CS and US with CS-alone probes every 9th trial (PD). As shown in the middle of Figure 2, conditioned deceleration was abolished in the CS Ext and UP Ext groups across the three days of extinction (Ext Day1, Ext Day2, and Ext Day3) but to a much lesser degree in the PD group whose mean IBI change did not drop below habituation levels. A repeated measures ANOVA of the three days of extinction indicated a significant main effect of Extinction Day [F(2, 62) = 34.92, p < 0.001], Group [F(2, 31) = 7.36, p < 0.001], as well as an interaction of Extinction Day × Group [F(4, 62) = 4.54, p < 0.004]. Post hoc comparisons for each extinction day indicated that the CS Ext and UP Ext groups significantly diverged from the PD group on the second and third day of extinction without differing from one another (all p's< 0.03). When the analyses focused on within group changes from the second day of conditioning (Day2) across each day of extinction, follow up comparisons on a main effect of Session [CS Ext: F(1.24, 13.59) = 27.75, p < 0.001; UP Ext: F(1.56, 17.16) = 44.67, p < 0.001] further revealed that HR responses had significantly dropped from conditioning levels on the first day of extinction for both the CS Ext (p < 0.05) and UP Ext groups (p < 0.01). Throughout extinction, the PD group also exhibited a reduction in conditioned HR deceleration but not to the extent of CS Ext and UP Ext groups, as indicated by a main effect of Session [F(3, 27) = 3.48, p < 0.03)] for which there were no significant follow-up comparisons after Bonferroni correction. This finding is consistent with other studies showing that HR conditioning asymptotes after 2 days of pairings and begins to gradually wane with additional training sessions [51,60,70,79]. In summary, both CS alone and unpaired CS/US presentations were successful at completely extinguishing conditioned HR deceleration, whereas a marginal level of HR deceleration remained in the group receiving continued CS-US pairings.
Analysis of the CS Test given as the final session was used to assess the effects of extinction on conditioned HR responses in all groups, which included the group receiving US-alone presentations (US Ext) and the home cage sit control group that received no further stimulus presentations (Sit). Because CS alone presentations were shown to extinguish conditioned HR responses in the CS Ext group, the analysis was focused on the first 10 CS presentations during the CS Test to avoid the confounding effects of extinction resulting from additional CS presentations. As shown in the right side of Figure 2, conditioned HR responses were intact in the US Ext and Sit groups, with the highest level in the Sit group. The HR responses in the CS Ext, UP Ext, and PD groups during the CS Test were found to be similar to the performance on the third day of extinction, with a small suggestion of recovery. An ANOVA conducted for the first 10 trials of the CS Test indicated a significant effect of Group [F(4,53) = 11.39, p < 0.001] with post hoc comparisons (Tamehane's T2) confirming that US Ext, PD, and Sit groups showed significantly more conditioned deceleration relative to the CS Ext and UP Ext groups (p's < 0.04). Analyses focused on within-group comparisons of Day2 with the CS Test indicated that HR responses on the first 10 trials of the CS Test were not significantly different than the second session of HR conditioning, suggesting that conditioned HR responses were not extinguished after US alone presentations or after a three-day wait period in home cages. Corroborating the effectiveness of CS alone presentations on extinguishing conditioned HR responses, the last 10 trials of the CS Test are also pictured in the right side Figure 2 and illustrate a decrease in conditioned deceleration, particularly in the US Ext, Sit, and PD groups, as confirmed by a significant interaction of Group and Trial Block (1st 10 trials versus last 10) in a repeated measures ANOVA [F(4,53) = 4.15, p < 0.006].
Heart Rate Responses to the Shock US
HR CRM
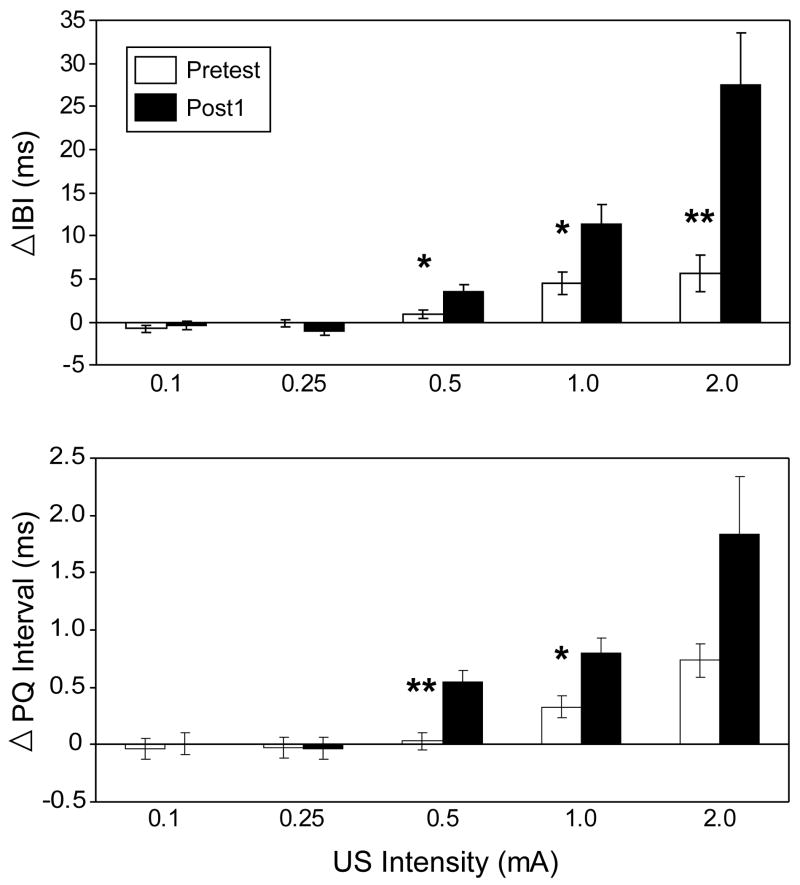
In replication of previous HR CRM studies [65,70], examination of the mean IBI change during the first 20 trials of the US Pretest and the first Post Test (Post1) for all groups combined confirmed the development of HR CRM, manifested as an increase in mean IBI (HR deceleration) to the higher intensity USs during Post1 that was not initially present during Pretest (see Figure 3, top panel). Repeated measures ANOVA corroborated this finding, indicating a significant interaction of Test × US Intensity [F(1.22, 64.77) = 11.69, p < 0.002] with planned comparisons indicating that there was significantly more HR deceleration at Post1 compared to Pretest at 0.5 mA (p < 0.03), 1.0 mA (p < 0.03), and 2.0 mA (p < 0.006). The analysis did not indicate any main effect or interaction involving Group, confirming that prior to the extinction manipulations, all groups were showing equivalent levels of HR CRM.
Figure 3.
Conditioning-specific reflex modification of heart rate as indexed by a Pretest to Post Test increase in interbeat interval (IBI, top panel) and PQ interval (bottom panel) at the highest intensities of the unconditioned stimulus (US). Data are shown as the mean change (± SEM) in inter-beat interval (IBI) from baseline during the first 20-trial block of US presentations of the Pretest and first Post Test (Post1) at the five different US intensities (0.1 mA, 0.25 mA, 0.5 mA, 1.0 mA, and 2.0 mA), collapsed across duration and averaged across subjects (n=58). Single and double asterisks indicate significant differences between groups at p < 0.05 and p < 0.01, respectively.
HR CRM: PQ Interval
Also in replication of previous studies [65,70], there was an increase in PQ interval in correspondence with the increase in mean IBI change observed at the higher intensity US trials at Post1. The bottom panel of Figure 3 depicts the mean change in PQ interval from baseline following the presentation of the US at Pretest and Post1 at each US intensity. A repeated measures ANOVA on the mean PQ interval change during Pretest and Post1 indicated a significant Test × Intensity interaction [F(1.61, 85.37) = 3.36, p < 0.05] with planned comparisons confirming that the PQ interval was significantly elevated at Post1 compared to Pretest at 0.5 mA (p < 0.006), and 1.0 mA (p < 0.04), intensities at which HR CRM was observed. Although Figure 3 indicates that the PQ interval also showed a Post1 increase at 2.0 mA, the comparison was not significant with the Bonferroni correction. The correspondence between the mean changes in IBI and PQ was confirmed by a significant correlation at 1.0 mA [r(58) = 0.62, p < 0.001] and 2.0 mA [r(58) = 0.30, p < 0.03].
Effects of Extinction on HR CRM
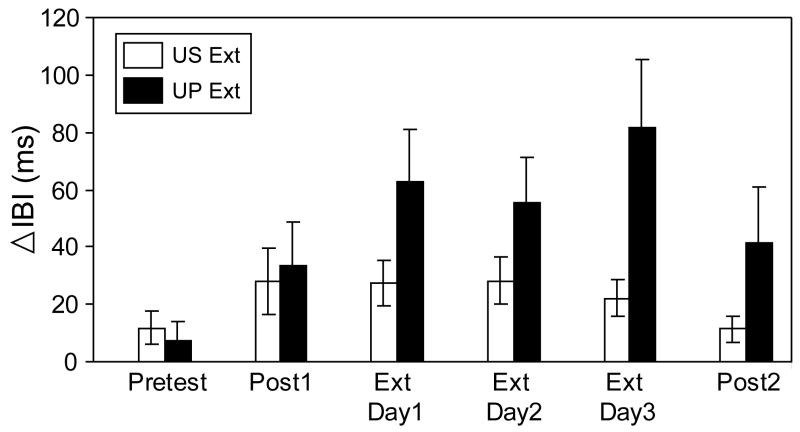
Analyses of the mean IBI change to the US during the three extinction sessions were restricted to the groups receiving US presentations (US Ext, UP Ext). Responses to the US in the PD group were not included because the US was not presented alone as in US Ext and UP Ext but was always preceded by the CS, making it difficult to separate the UR from the CR. Figure 4 illustrates HR responses to the US during extinction and for comparison, also includes HR responses during Pretest, Post1, and Post2 to the 2mA US, the same intensity used throughout HR conditioning and extinction sessions. Examination of Figure 4 shows that HR CRM (at the 2.0 mA intensity) for the US Ext group remained at levels similar to Post1 and began to drop by the third day of extinction, with a return to Pretest levels by Post2. In contrast, the UP Ext group maintained HR CRM, even showing a potentiation of HR deceleration to the US, peaking at extinction Day3 and remaining elevated at levels similar to Post1 at Post2. Repeated measures ANOVA for the three extinction sessions indicated that there was a main of effect of Group [F(1, 22) =4.30, p = 0.05], confirming the higher amount of HR deceleration in the UP Ext group compared to the US Ext group, but there was no significant Group × Day interaction.
Figure 4.
Mean change (± SEM) in inter-beat interval (IBI) from baseline to the 2.0 mA unconditioned stimulus (US) during the first block of 20 trials of the US Pretest and first Post Test (Post1), three days of extinction (Ext Day1-3), and the first 20-trial block of the second Post Test (Post2). Data shown are from the US-alone (US Ext, white bars, n=12) and unpaired CS/US (UP Ext, black bars, n=12) extinction groups and illustrate that in contrast to the US Ext group, the UP Ext group continued to show conditioning-specific reflex modification throughout extinction and Post2.
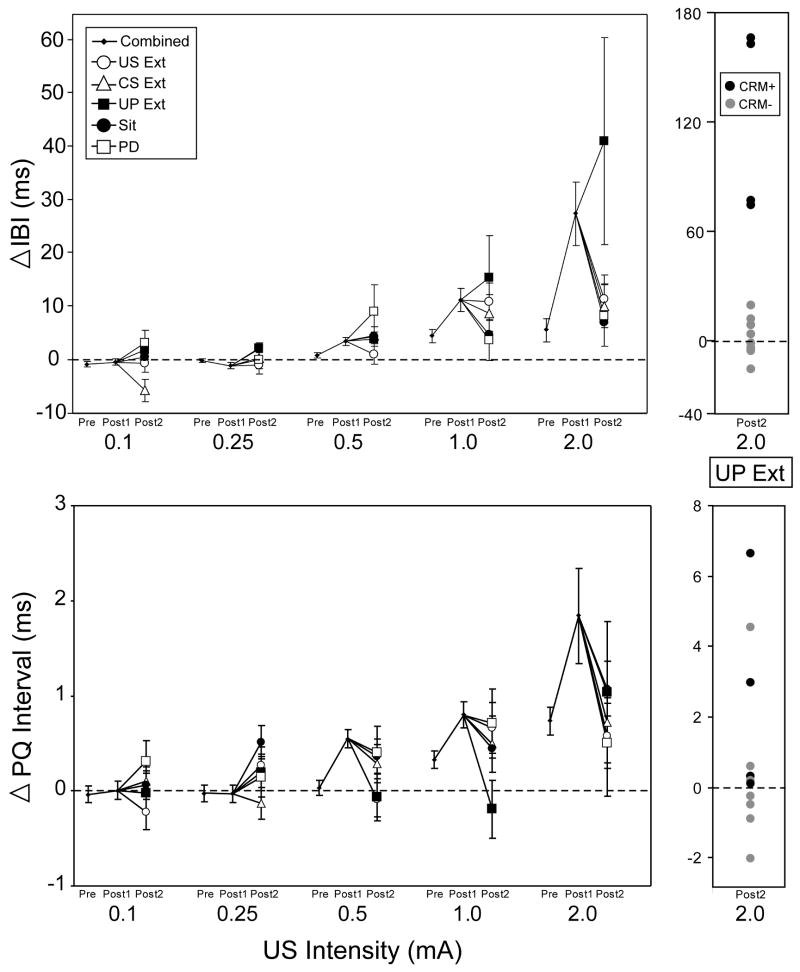
Figure 5, top panel, illustrates the effects of extinction on HR CRM for all groups as measured at Post2 following the extinction manipulations. Responses at each of the five US intensities (0.1 mA, 0.25 mA, 0.5 mA, 1.0 mA, 2.0 mA) collapsed across duration are shown as a combined score on Pretest and Post1 and are shown for the separate groups on Post2. For the higher US intensities, the majority of groups showed a decrease in HR CRM at Post2 compared to Post1 with the exception of the UP group which continued to exhibit HR CRM at 1.0 mA and 2.0 mA. Although a repeated measures ANOVA revealed no significant effects of Group, there was significant Test × Intensity interaction [F(1.44, 76.12) = 4.21, p < 0.04], and when the UP Ext group and the remaining groups were analyzed separately, there was a significant Test × Intensity interaction in the remaining groups [F (1.39, 58.41) = 6.83, p < 0.007] with planned comparisons indicating that the mean change IBI was significantly reduced following extinction for HR responses at the 2.0 mA intensity (p < 0.05). Analysis of the UP Ext group indicated a significant main effect of Intensity [F(1.09, 11.96) = 5.26, p < 0.04] with no effects involving Test, showing that responding in the UP Ext group at Post2 remained at Post1 levels following extinction. In summary, HR CRM was shown to be reduced by US-alone and CS-alone extinction but also waned following continued paired presentations or after three days of no further stimulus presentations. In contrast, HR CRM remained elevated in the UP Ext group.
Figure 5.
Effects of extinction on conditioning-specific reflex modification of heart rate as indexed by mean change (± SEM) in inter-beat interval (top panel) and PQ interval (bottom panel) from baseline during the first 20-trial block of unconditioned stimulus (US) presentations during the US Pretest, first Post Test (Post1), and the Post Test following extinction (Post2) at the five different US intensities (0.1 mA, 0.25 mA, 0.5 mA, 1.0 mA, and 2.0 mA), collapsed across duration. Data shown are shown averaged across all subjects for Pretest and Post1 and shown as separate extinction groups for Post2. Unlike the other groups, the unpaired extinction group (UP Ext, black box, n=12) did not show reductions in mean IBI change at the 2 mA US intensity, largely the result of four rabbits (CRM+, black dots) whose individual IBI and PQ responses along with the responses of the remaining rabbits in the UP Ext group (CRM-, gray dots) are shown to the right of the top and bottom panels. Other abbreviations: US-alone extinction (US Ext, open circle, n=12), CS-alone extinction (CS Ext, open triangle, n=12), continued CS-US pairings (PD, open box, n=10), sit control group remaining in home cages (Sit, black circle, n=12).
HR CRM Extinction: PQ Interval
Because an increase in PQ interval has been found in this and several other publications to correspond to an increase in mean change IBI that represents HR CRM, a question of interest was whether PQ interval would also correspond to the changes in HR CRM resulting from the extinction manipulations. Figure 5, bottom panel, illustrates the change in PQ interval across the different US intensities collapsed across duration at Pre and Post1 for all groups combined and for all groups separately at Post2. The figure illustrates that for the US Ext, CS Ext, PD, and Sit groups, a decrease in PQ interval paralleled the decrease in mean change IBI at 2.0 mA. However, there was a lack of correspondence between the PQ interval and mean IBI change for the UP group, with the PQ interval reduced at Post2 despite the IBI remaining elevated. Repeated measures ANOVA comparing the PQ interval at Post1 and Post2 indicated a significant effect of Test × Intensity [F(1.81, 95.94) = 4.84, p < 0.02] and no significant effects involving Group. Planned comparisons of Post1 and Post2 indicated that there was a significant Post1 to Post2 decrease in mean PQ interval at 0.5 mA (p < 0.03) and decreases at 0.25 mA, 1.0 mA, and 2.0 mA that did not remain significant after Bonferroni correction. Additionally, there was a significant correlation of mean IBI change and change in PQ interval at 0.1 mA [r(58) = 0.30, p < 0.03], 0.25 mA [r(58) = 0.287, p < 0.03], 0.5 mA [r(58) = 0.374, p < 0.005), and 2 mA [r(58) = 0.470, p < 0.001]. These findings suggest that a decrease in PQ interval partially corresponds with the decrease in HR CRM following the extinction manipulations, with the strongest correlation between IBI and PQ at the 2 mA intensity.
Individual Subject Differences during Unpaired Extinction
Although the HR CRM in the UP Ext group persisted and appeared to be potentiated, the large error bars shown in the top panel of Figure 5 suggested that the effect may not have been consistent across all subjects. An examination of the individual rabbit responses revealed that the effect was largely due to four out of 12 rabbits. The individual IBI and PQ responses to the 2 mA US at Post2 for the rabbits in the UP Ext group are pictured to the right of the top and bottom panels of Figure 5. An analysis of mean IBI change to the 2.0 mA US during US testing and extinction was conducted to compare the four rabbits with persistent HR CRM at Post2 (CRM+) with the eight remaining rabbits in the UP Ext group (CRM-). A repeated measures ANOVA indicated a significant interaction of Group (CRM+ vs CRM-) × Session [F(2.53, 25.31) = 6.20, p < 0.005] with post hoc comparisons at each session indicating that the CRM+ rabbits had significantly larger decelerative HR responses at Post 1, Post2, and all three extinction sessions (p's < 0.006). However, analyses revealed no differences within the UP group on HR CRs during acquisition, extinction, or the CS test, and no differences in baseline HR throughout all training sessions. These findings indicated that the CRM+ rabbits were initially showing superior HR CRM prior to extinction, suggesting that the effects of UP extinction were specific to the initial level of HR CRM. However, the UP Ext group was not unique in containing a small population of rabbits with superior CRM at Post1, as all extinction groups contained an average of three superior CRM rabbits. But unlike the UP Ext group, these subpopulations did not show CRM extinction resistance. As shown in the bottom panel, right side of Figure 5, only two of the CRM+ rabbits exhibited a corresponding elevation in PQ-interval, indicating that the vagal contribution to the persisting HR CRM was inconsistent across CRM+ subjects.
Discussion
To our knowledge, the data presented here constitute the first comprehensive study of extinction of rabbit HR conditioning. The most successful extinction treatments were found to be conventional CS-alone presentations and explicitly unpaired presentations of the CS and US, whereas HR CRs remained stable in rabbits receiving US-alone presentations or no further stimulus presentations while remaining in home cages. For HR CRM, all treatments reduced responding except for a subset of rabbits in which unpaired extinction resulted in persistent CRM. The corresponding increases and decreases in the PQ interval of the heart ECG with CRM acquisition and extinction suggested a common vagal-mediated neural basis for both. Overall, these findings highlighted a dichotomy between HR CRs and CRM, showing that HR CRM decays over time, even under circumstances in which HR CRs remain intact. Therefore, CRM cannot be fully explained as a CR that has generalized from the CS to the US.
There is currently high interest in the behavioral and neural mechanisms responsible for extinction of conditioned fear due to their importance to disorders stemming from failures of fear extinction, such as phobias, anxiety disorders, and PTSD [28,41,53]. Despite the large number of extinction studies examining common fear paradigms including conditioned freezing to discrete cues and context [3,11,35], fear-potentiated startle [15,27,36], and NMR conditioning [17,44,57], significantly less information exists about extinction of HR CRs. Of the HR conditioning studies that have included extinction, the main treatment was CS-alone presentations [45,75,79,80]. Results presented here are in agreement with these earlier HR studies, demonstrating that CS-alone presentations reduce HR CRs. In addition, these results document that explicitly unpaired extinction is an equally successful treatment, consistent with extinction studies in other fear paradigms [7,33]. Presentations of the US-alone, however, were not shown to produce any significant decrements in HR CRs. Importantly, rabbits that did not receive any extinction treatment still showed robust HR CRs, indicating that HR CRs can be maintained several days following conditioning. This finding confirms that the reduction in CRs seen in the CS-alone and unpaired treatments was the result of extinction and not a passive decrease in responding over time. The length of retention of HR CRs if training stops at the peak of HR conditioning has not been thoroughly explored, but this study suggests that it is retained for at least 4 days.
Regarding extinction of CRM, several differences exist between previously published results for CRM of rabbit NMR and the CRM of HR presented here. Extinction of CRM of the rabbit NMR was found to be best accomplished by US-alone presentations, followed by unpaired presentations, and then CS-alone presentations, with the latter two being most successful in extinguishing NMR CRs [7]. The optimal extinction treatment for concurrently decreasing NMR CRM and CRs was therefore determined to be unpaired presentations, presumably because it combined the successful extinction treatments for both, CS-alone and US-alone presentations. For the current study, it was predicted that similar results would be found for HR CRM. However, with the exception of a subset of rabbits receiving unpaired presentations, all treatments were shown to reduce HR CRM, including sitting in the home cage with no further stimulus presentations. An alternative explanation, however, is that the US-alone presentations that all subjects received during the first Post Test following HR conditioning were sufficient to extinguish HR CRM. In support of this possibility is the observation in this and previous studies that HR CRM exhibits within session declines during post testing [65].
Several possible mechanisms may be responsible for the persistent CRM that some rabbits exhibit following unpaired presentations (despite extinction of HR CRs). One possibility is that context conditioning may be contributing to the maintenance of CRM, with the context serving as an anticipatory cue for the presence of the shock US. Arguing against a primary role for context in HR CRM are results from previous HR CRM studies showing that unpaired training in naïve animals does not lead to HR CRM and that a strong correlation exists between the level of HR conditioning and CRM [65]. Another argument is that in this study, rabbits receiving US-alone extinction, which maintains the US-context association, did not exhibit persisting CRM. In addition, baseline HR analyses, which could provide a measurement of contextual fear [2,9], did not show any differences among the various extinction conditions even when the subset of high CRM rabbits in the unpaired group were analyzed separately. Another possibility is that the rabbits with persistent CRM represent a unique population that is extinction-resistant. This speaks to the importance of examining individual subject responses in extinction studies, particularly those modeling failures of fear extinction in humans that only occur in a small percentage of individuals exposed to fearful or stressful situations, as in the case of PTSD [13]. However, one might expect that the sub-population of resistant subjects would therefore exist within all extinction groups, but this was not found to be true. These results therefore suggest there may be something unique about the switch from paired to unpaired training that may trigger greater extinction resistance. A qualitative observation about rabbits receiving unpaired extinction is that they exhibit more resistance to restraint and engage in frustration-type behaviors, such as chewing on restrainers and transport containers, compared to rabbits receiving other extinction treatments.
A closer look at the nature of HR CRs and CRM may also add important insight. Fear is typically associated with an increase in HR or tachycardia [16,55,56,59], whereas HR CRs and CRM in the restrained rabbit both involve conditioned deceleration (bradycardia). The development of conditioned bradycardia under restraint is thought to be an adaptive mechanism to restore internal homeostasis since the animal cannot engage fight or flight mechanisms [77]. The question then is whether extinction of HR CRM represents a loss of fear itself (and therefore a loss of the need to have to restore HR homeostasis) or instead represents a return to a maladaptive heart rate response (tachycardia). This leaves open the possibility that the subset of rabbits in the unpaired extinction group with persistent CRM are actually better adapted than those for which CRM was extinguished. Recent work on extinction-induced despair, an animal model of depression, indicates that subjects resistant to extinction are less prone to develop learned helplessness-type behaviors such as immobility [72]. Therefore, in some situations it may be better to hold on to conditioned behaviors than to replace them with behaviors that constitute “giving up” that could have a negative impact on health and survival.
In replication of previous experiments [65,70], the HR CRM observed in this study was characterized by an increase in mean change IBI (deceleration) to US intensities equal to or approaching the intensity utilized during HR conditioning. A parallel increase in PQ interval of the ECG further solidified evidence that HR CRM is in part vagally-mediated and under the control of the parasympathetic autonomic nervous system [65,70]. New findings from this study have shown that the PQ intervals also decrease in parallel with extinction of CRM, suggesting a common vagal-involvement for both the acquisition and extinction of HR CRM. The less than perfect correlations between IBI and PQ interval, however, suggest that the IBI changes related to CRM acquisition and extinction could additionally be under sympathetic control [1,31] and influenced by other central and peripheral contributors to the control of heart rate [4] including potential intrinsic intra-ganglionic cardiac interactions [24]. Recent work by Thompson and colleagues has shown that for the rabbit NMR, distinct neural pathways may exist for acquisition versus extinction of CRs [57]. Because the vagal outputs may be argued to be the final motor pathway for HR CRs [37] and CRM, it is possible that upstream sites may be distinct for acquisition versus extinction, warranting future studies examining the neural substrates of HR CRM.
Acknowledgments
The present research was supported in part by funds from the National Institutes of Health RO1MH081159, the Blanchette Rockefeller Neurosciences Institute, and West Virginia University Bridge Funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren B. Burhans, Email: lburhans@brni.org.
Carrie Smith-Bell, Email: cbell@brni.org.
Bernard G. Schreurs, Email: bschreurs@hsc.wvu.edu.
References
- 1.Albiniak BA, Powell DA. Peripheral autonomic mechanisms and Pavlovian conditioning in the rabbit (Oryctolagus cuniculus) J Comp Physiol Psychol. 1980;94:1101–1113. doi: 10.1037/h0077747. [DOI] [PubMed] [Google Scholar]
- 2.Antoniadis EA, McDonald RJ. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res. 1999;101:1–13. doi: 10.1016/s0166-4328(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 3.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basal amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Berntson GG, Hart S, Sarter M. The cardiovascular startle response: anxiety and the benzodiazepine receptor complex. Psychophysiology. 1997;34:348–357. doi: 10.1111/j.1469-8986.1997.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 5.Bradley DJ, Ghelarducci B, Spyer KM. The role of the posterior cerebellar vermis in cardiovascular control. Neurosci Res. 1991;12:45–56. doi: 10.1016/0168-0102(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan SL, Thompson RH. Mediodorsal thalamic lesions and Pavlovian conditioning of heart rate and eyeblink responses in the rabbit. Behav Neurosci. 1990;104:912–918. doi: 10.1037//0735-7044.104.6.912. [DOI] [PubMed] [Google Scholar]
- 7.Buck DL, Seager MA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: generality and nature of the phenomenon. Behav Neurosci. 2001;115:1039–1047. [PubMed] [Google Scholar]
- 8.Burhans LB, Smith-Bell C, Schreurs BG. Conditioning-specific reflex modification of the rabbit's nictitating membrane response and heart rate: Behavioral rules, neural substrates, and potential applications to posttraumatic stress disorder. Behav Neurosci. 2008;122:1191–1206. doi: 10.1037/a0013599. [DOI] [PubMed] [Google Scholar]
- 9.Carrive P. Conditioned fear to environmental context: cardiovascular and behavioral components in the rat. Brain Res. 2000;858:440–445. doi: 10.1016/s0006-8993(00)02029-1. [DOI] [PubMed] [Google Scholar]
- 10.Chachich M, Powell DA. Both medial prefrontal and amygdala central nucleus lesions abolish heart rate classical conditioning, but only prefrontal lesions impair reversal of eyeblink differential conditioning. Neurosci Lett. 1998;257:151–154. doi: 10.1016/s0304-3940(98)00832-5. [DOI] [PubMed] [Google Scholar]
- 11.Chang CH, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16:62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen DH, Pitts LH. Vagal and sympathetic components of conditioned cardioacceleration in the pigeon. Brain Res. 1968;9:15–31. doi: 10.1016/0006-8993(68)90255-2. [DOI] [PubMed] [Google Scholar]
- 13.Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 14.Coleman SR, Gormezano I. Classical conditioning of the rabbit's (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. J Comp Physiol Psychol. 1971;77:447–455. doi: 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- 15.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 16.Duan YF, Winters RW, McCabe PM, Green EJ, Huang Y, Schneiderman N. Cardiorespiratory components of defense reaction elicited from paraventricular nucleus. Physiol Behav. 1997;61:325–330. doi: 10.1016/s0031-9384(96)00410-6. [DOI] [PubMed] [Google Scholar]
- 17.Dudeney JE, Olsen KN, Kehoe EJ. Time-specific extinction and recovery of the rabbit's (Oryctolagus cuniculus) conditioned nictitating membrane response using mixed interstimulus intervals. Behav Neurosci. 2007;121:808–813. doi: 10.1037/0735-7044.121.4.808. [DOI] [PubMed] [Google Scholar]
- 18.Dykman RA, Gantt WH. The parasympathetic component of unlearned and acquired cardiac responses. J Comp Physiol Psychol. 1959;52:163–167. doi: 10.1037/h0047073. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald RD, Martin GK, O'Brien JH. Influence of vagal activity on classical conditioned heart rate in rats. J Comp Physiol Psychol. 1973;83:485–491. doi: 10.1037/h0034656. [DOI] [PubMed] [Google Scholar]
- 20.Ghelarducci B, Sebastiani L. Role of the medial prefrontal cortex in the development of conditioned bradycardia in rabbits with lesions of the cerebellar vermis. Exp Brain Res. 1999;129:185–190. doi: 10.1007/s002210050888. [DOI] [PubMed] [Google Scholar]
- 21.Ghelarducci B, Sebastiani L. Classical heart rate conditioning and affective behavior: the role of the cerebellar vermis. Arch Ital Biol. 1997;135:369–384. [PubMed] [Google Scholar]
- 22.Ginsberg JP, Ayers E, Burriss L, Powell DA. Disruption of bradycardia associated with discriminative conditioning in combat veterans with PTSD. Neuropsychiatr Dis Treat. 2008;4:635–646. doi: 10.2147/ndt.s2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental methods and instrumentation in psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- 24.Gray AL, Johnson TA, Ardell JL, Massari VJ. Parasympathetic control of the heart. II. A novel intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol. 2004;96:2273–2278. doi: 10.1152/japplphysiol.00616.2003. [DOI] [PubMed] [Google Scholar]
- 25.Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Exp Brain Res. 1995;104:431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- 26.Hayes E, Pugsley MK, Penz WP, Adaikan G, Walker MJA. Relationship between QaT and RR intervals in rats, guinea pigs, rabbits, and primates. J Pharmacol Toxicol Methods. 1994;32:201–207. doi: 10.1016/1056-8719(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 27.Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata J, LeDoux JE. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav Neurosci. 1988;102:66–76. doi: 10.1037//0735-7044.102.1.66. [DOI] [PubMed] [Google Scholar]
- 30.Katcher AH, Solomon RL, Turner LH, LoLordo V, Overmier JB, Rescorla RA. Heart rate and blood pressure responses to signaled and unsignaled shocks: effects of cardiac sympathectomy. J Comp Physiol Psychol. 1969;68:163–174. [PubMed] [Google Scholar]
- 31.Kazis E, Milligan WL, Powell DA. Autonomic-somatic relationships: blockade of heart rate and corneo-retinal potential responses. J Comp Physiol Psychol. 1973;84:98–110. doi: 10.1037/h0035027. [DOI] [PubMed] [Google Scholar]
- 32.Kehoe EJ, McCrea RA. Classical conditioning of the rabbit nictitating membrane can be fast or slow: implications for Lennartz and Weinberger's (1992) two-factor theory. Psychobiology. 1994;22:1–4. [Google Scholar]
- 33.Kehoe EJ, White NE. Extinction revisited: similarities between extinction and reductions in US intensity in classical conditioning of the rabbit's nictitating membrane response. Anim Learn Behav. 2002;30:96–111. doi: 10.3758/bf03192912. [DOI] [PubMed] [Google Scholar]
- 34.Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: support for two factors, implications for behavior and neurobiology. Psychobiology. 1992;20:93–119. [Google Scholar]
- 35.Leung HT, Westbrook RF. Spontaneous recovery of extinguished fear responses deepens their extinction: a role for error-correction mechanisms. J Exp Psychol Anim Behav Process. 2008;34:461–474. doi: 10.1037/0097-7403.34.4.461. [DOI] [PubMed] [Google Scholar]
- 36.Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by D-cycloserine is blocked by proteasome inhibitors. Neuropsychopharmacology. 2008;33:3085–3095. doi: 10.1038/npp.2008.30. [DOI] [PubMed] [Google Scholar]
- 37.McCabe PM, Schneiderman N, Jarrell TW, Gentile CG, Teich AH, Winters RW, Liskowsky DR. Central pathways involved in classical differential conditioning of heart rate responses in rabbits. In: Gormezano I, Wasserman EA, editors. Learning and memory: the behavioral and biological substrates. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1992. pp. 321–346. [Google Scholar]
- 38.McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Air puff versus shock unconditioned stimuli in rabbit heart rate conditioning. Physiol Behav. 1992;51:195–199. doi: 10.1016/0031-9384(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 39.McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 42.Nijsen MJ, Croiset G, Diamant M, Stam R, Delsing D, deWied D, Wiegant VM. Conditioned fear-induced tachycardia in the rat: vagal involvement. Eur J Pharmacol. 1998;350:211–222. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 43.Nijsen MJMA, Croiset G, Diamant M, de Wied D, Wiegant VM. CRH signalling in he bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 44.Nolan BC, Freeman JH., Jr Purkinje cell loss by OX7-saporin impairs acquisition and extinction of eyeblink conditioning. Learn Mem. 2006;13:359–365. doi: 10.1101/lm.168506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Notterman JM, Schoenfeld WN, Bersh PJ. A comparison of three extinction procedures following heart rate conditioning. J Abnorm Psychol. 1952;47:675–677. [PubMed] [Google Scholar]
- 46.Obrist PA, Wood DM, Perez-Reyes M. Heart rate during conditioning in humans: effects of UCS intensity, vagal blockade, and adrenergic block of vasomotor activity. J Exp Psychol. 1965;70:37–42. doi: 10.1037/h0022033. [DOI] [PubMed] [Google Scholar]
- 47.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 48.Pascoe JP, Supple WF, Kapp BS. Learning and memory: Vertebrate models. In: Martinez JL, Kesner RP, editors. Learning and Memory: A Biology View. San Diego: Academic Press; 1991. pp. 359–407. [Google Scholar]
- 49.Powell DA, Churchwell J, Burriss L. Medial prefrontal lesions and Pavlovian eyeblink and heart rate conditioning: effects of partial reinforcement on delay and trace conditioning in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2005;119:180–189. doi: 10.1037/0735-7044.119.1.180. [DOI] [PubMed] [Google Scholar]
- 50.Powell DA, Ginsberg JP. Single unit activity in the medial prefrontal cortex during Pavlovian heart rate conditioning: Effects of peripheral autonomic blockade. Neurobiol Learn Mem. 2005;84:200–213. doi: 10.1016/j.nlm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Powell DA, Levine-Bryce D. A comparison of two model systems of associative learning: heart rate and eyeblink conditioning in the rabbit. Psychophysiology. 1988;25:672–682. doi: 10.1111/j.1469-8986.1988.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 52.Powell DA, Watson K, Maxwell B. Involvement of subdivisions of the medial prefrontal cortex in learned cardiac adjustments in rabbits. Behav Neurosci. 1994;108:294–307. doi: 10.1037//0735-7044.108.2.294. [DOI] [PubMed] [Google Scholar]
- 53.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raskin DC. Orienting and defensive reflexes and conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 269–289. [Google Scholar]
- 55.Raskin DC, Kotses H, Bever J. Autonomic indicators of orienting and defensive reflexes. J Exp Psychol. 1969;80:423–433. doi: 10.1037/h0027491. [DOI] [PubMed] [Google Scholar]
- 56.Richter A, Schumann NP, Zweiner U. Characteristics of heart rate fluctuations and respiratory movements during orienting, passive avoidance and flight-fight behavior in rabbits. Int J Psychophysiol. 1990;10:75–83. doi: 10.1016/0167-8760(90)90048-i. [DOI] [PubMed] [Google Scholar]
- 57.Robleto K, Thompson RF. Extinction of a classically conditioned response: red nucleus and interpositus. J Neurosci. 2008;28:2651–2658. doi: 10.1523/JNEUROSCI.4781-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rorick-Kehn LM, Steinmetz JE. Amygdalar unit activity during three learning tasks: eyeblink classical conditioning, Pavlovian fear conditioning, and signaled avoidance conditioning. Behav Neurosci. 2005;119:1254–1276. doi: 10.1037/0735-7044.119.5.1254. [DOI] [PubMed] [Google Scholar]
- 59.Schadt JC, Hasser EM. Hemodynamic effects of acute stressors in the conscious rabbit. Am J Physiol Regul Integr Comp Physiol. 1998;274:R814–R821. doi: 10.1152/ajpregu.1998.274.3.R814. [DOI] [PubMed] [Google Scholar]
- 60.Schneiderman N. Response system divergencies in aversive classical conditioning. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 341–376. [Google Scholar]
- 61.Schneiderman N, Smith MC, Smith AC, Gormezano I. Heart rate classical conditioning in rabbits. Psychon Sci. 1966;6:241–242. [Google Scholar]
- 62.Schneiderman N, Van Dercar DH, Yehle AL, Manning AA, Golden T, Schneiderman E. Vagal compensatory adjustment: relationship to heart rate classical conditioning in rabbits. J Comp Physiol Psychol. 1969;68:175–183. doi: 10.1037/h0027514. [DOI] [PubMed] [Google Scholar]
- 63.Schreurs BG. Classical conditioning and modification of the rabbit's (Oryctolagus cuniculus) unconditioned nictitating membrane response. Behav Cogn Neurosci Rev. 2003;2:83–96. doi: 10.1177/1534582303255014. [DOI] [PubMed] [Google Scholar]
- 64.Schreurs BG, Alkon DL. Learning and memory. In: Crockard A, Hayward R, Hoff JT, editors. Neurosurgery: the scientific basis of clinical practice, 1. Oxford: Blackwell Scientific; 1997. pp. 192–204. [Google Scholar]
- 65.Schreurs BG, Crum JM, Wang D, Smith-Bell CA. Conditioning-specific reflex modification of rabbit (Oryctolagus cuniculus) heart rate. Behav Neurosci. 2005;119:1484–1495. doi: 10.1037/0735-7044.119.6.1484. [DOI] [PubMed] [Google Scholar]
- 66.Schreurs BG, Gonzalez-Joekes J, Smith-Bell CA. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response is sensitive to context. Learn Behav. 2006;34:315–324. doi: 10.3758/bf03192886. [DOI] [PubMed] [Google Scholar]
- 67.Schreurs BG, Oh MM, Hirashima C, Alkon DL. Conditioning-specific modification of the rabbit's unconditioned nictitating membrane response. Behav Neurosci. 1995;109:24–33. doi: 10.1037//0735-7044.109.1.24. [DOI] [PubMed] [Google Scholar]
- 68.Schreurs BG, Shi T, Pineda S, III, Buck DL. Conditioning the unconditioned response: modification of the rabbit's (Oryctolagus cuniculus) unconditioned nictitating membrane response. J Exp Psychol Anim Behav Process. 2000;26:144–156. doi: 10.1037//0097-7403.26.2.144. [DOI] [PubMed] [Google Scholar]
- 69.Schreurs BG, Smith-Bell CA. Heart rate changes during conditioning-specific reflex modification of the rabbit's (Oryctolagus cuniculus) nictitating membrane response. Neurobiol Learn Mem. 2005;84:148–158. doi: 10.1016/j.nlm.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Schreurs BG, Smith-Bell CA, Darwish DS, Wang D, Burhans LB, Gonzales-Joekes J, Deci S, Stankovic G, Sparks DL. Cholesterol enhances classical conditioning of the rabbit heart rate response. Behav Brain Res. 2007;181:52–63. doi: 10.1016/j.bbr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schreurs BG, Tomsic D, Gusev PA, Alkon DL. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit's nictitating membrane response. J Neurophysiol. 1997;77:86–92. doi: 10.1152/jn.1997.77.1.86. [DOI] [PubMed] [Google Scholar]
- 72.Schulz D, Huston JP, Buddenberg T, Topic B. “Despair” induced by extinction trials in the water maze: relationship with measures of anxiety in aged and adult rats. Neurobiol Learn Mem. 2007;87:309–323. doi: 10.1016/j.nlm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 73.Seager MA, Smith-Bell CA, Schreurs BG. Conditioning-specific reflex modification of the rabbit (Oryctolagus cuniculus) nictitating membrane response: US intensity effects. Learn Behav. 2003;31:292–298. doi: 10.3758/bf03195990. [DOI] [PubMed] [Google Scholar]
- 74.Supple WF, Jr, Kapp BS. The anterior cerebellar vermis: essential involvement in classically conditioned bradycardia in the rabbit. J Neurosci. 1993;13:3705–3711. doi: 10.1523/JNEUROSCI.13-09-03705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weidemann G, Kehoe EJ. Savings in classical conditioning in the rabbit as a function of extended extinction. Learn Behav. 2003;31:49–68. doi: 10.3758/bf03195970. [DOI] [PubMed] [Google Scholar]
- 76.Wikgren J, Korhonen T. Interpositus nucleus inactivation reduces unconditioned response amplitude after paired but not explicitly unpaired treatment in rabbit eyeblink conditioning. Neurosci Lett. 2001;308:181–184. doi: 10.1016/s0304-3940(01)02000-6. [DOI] [PubMed] [Google Scholar]
- 77.Winters RW, McCabe BJ, Schneiderman N. Functional utility and neurobiology of conditioned autonomic responses. In: Moore JW, editor. A Neuroscientists Guide to Classical Conditioning. New York: Springer-Verlag; 2002. pp. 46–85. [Google Scholar]
- 78.Yehle A, Dauth G, Schneiderman N. Correlates of heart-rate classical conditioning in curarized rabbits. J Comp Physiol Psychol. 1967;64:98–104. doi: 10.1037/h0020845. [DOI] [PubMed] [Google Scholar]
- 79.Yehle AL. Divergences among rabbit response systems during three-tone classical discrimination conditioning. J Exp Psychol. 1968;77:468–473. doi: 10.1037/h0025985. [DOI] [PubMed] [Google Scholar]
- 80.Zimny GH, Stern JA, Fjeld SP. Effects of CS and UCS relationships on electrodermal response and heart rate. J Exp Psychol. 1966;72:177–181. doi: 10.1037/h0023462. [DOI] [PubMed] [Google Scholar]