Abstract
Spatial information embedded in the extracellular matrix establishes the dorsoventral polarity of the Drosophila embryo through the ventral activity of a serine protease cascade. Pipe is a Golgi-localized protein responsible for generating this spatial information during oogenesis through sulfation of unknown glycans. Although Pipe has sequence homology to glycosaminoglycan 2-O-sulfotransferases, its activity and authentic substrates have not been demonstrated and genetic evidence has argued against a role for glycosaminoglycans in dorsoventral polarity establishment. Here, direct examination of matrix glycosaminoglycans demonstrates that pipe-mutant matrix shows decreased tri-sulfated heparan sulfate compared to wild-type matrix, with correspondingly increased 2-O-sulfated heparan sulfate. Chondroitin sulfate was not detected in this matrix. These results suggest that Pipe promotes 6-O- and/or N-sulfation of heparan sulfate but is not required for heparan sulfate 2-O-sulfation. We discuss the possible significance of these unexpected findings and how they might be reconciled with the genetic data.
Keywords: pipe, sulfotransferase, extracellular matrix, heparan sulfate, disaccharide composition, drosophila, dorsoventral polarity
Introduction
During development of the Drosophila embryo, the dorsoventral axis is established by signaling through the receptor Toll downstream of the proteolytic activation of Spätzle, its NGF-related ligand (reviewed in refs. 1 and 2). Spätzle activation only occurs on the ventral side of the embryo, within the perivitelline space lying between the embryo plasma membrane and the eggshell. The activation of Spätzle occurs at the end of a four-component serine proteolytic cascade in which the first two enzymes appear to be uniformly activated around the circumference of the embryo, while the fourth enzyme is only activated ventrally. Ventral activity of the cascade relies on instructional information deposited within the perivitelline space during oogenesis. The molecular nature of this instructional cue and how it interacts with components of the proteolytic cascade are poorly understood and represent the major questions remaining in understanding this developmental pathway.
A key gene involved in synthesis of the instructional cue is pipe, whose expression is limited to a ventral zone within the follicle cell epithelium that surrounds the developing oocyte and synthesizes the eggshell.3 The major pipe splice isoform expressed in the ovary (Pipe-ST2, box 10, PA) encodes a Type II transmembrane protein that is localized to the Golgi apparatus and shows sequence similarity to mammalian uronic acid 2-O-sulfotransferases such as mouse heparan sulfate 2-O-sulfotransferase (HS2OST; 26% identity) and human dermatan/chondroitin sulfate hexuronic acid 2-O-sulfotransferase (29% identity).3-6 Pipe has been postulated to modify a secreted glycan that becomes stably fixed in the extracellular matrix surrounding the oocyte and thus available to locally stimulate the protease cascade in the embryo.3
The sulfotransferase activity of Pipe has been inferred from a co-requirement in dorsoventral patterning for the activities of 3’-phosphoadenosine 5’-phosphosulfate (PAPS) synthetase and a cytoplasm-to-Golgi PAPS transporter.7,8 However, in vitro assays of Pipe expressed in COS-7 cells or purified from bacterial expression have failed to demonstrate Pipe-mediated sulfation of heparan sulfate (HS), chondroitin sulfate (CS), or dermatan sulfate (DS) glycosaminoglycan (GAG) acceptor substrates; parallel studies of the Drosophila HS2OST, Sd (Segregation Distorter), showed activity against HS but not DS or CS GAGs.6,9 Furthermore, genetic studies have shown that sugarless, encoding a Drosophila UDP-glucose-6 dehydrogenase required for HS and CS/DS GAG biosynthesis, fringe connection, encoding a Golgi transporter required for the uptake of nucleotide sugars involved in synthesis of HS, and sulfateless, encoding a Drosophila N-deacetylase/N-sulfotransferase required for heparan sulfation, are not required in either germline or somatic cells of the ovary for proper dorsoventral patterning.7,10 These studies together have provided strongly suggestive evidence that GAG synthesis in the ovary is not involved in dorsoventral patterning, and further suggest that Pipe may be modifying a distinct and as-yet-unknown carbohydrate.
However, the carbohydrate composition of the matrix surrounding the oocyte has not been directly examined to identify potential targets of Pipe modification, and differences between wild-type and pipe-mutant matrix. Here we have subjected to direct GAG compositional analysis a washed matrix fraction previously shown to be highly enriched in eggshell proteins and substantially free of yolk and other components stored in the egg that could otherwise mask eggshell-specific carbohydrate modifications (Fig. 1).11 Two oocyte surface proteoglycans, Nudel12 and Nasrat,13 involved in patterning were also detected in this matrix preparation,11 suggesting that other fixed components of the perivitelline space such as the Pipe target will also be retained in this matrix.
Figure 1.
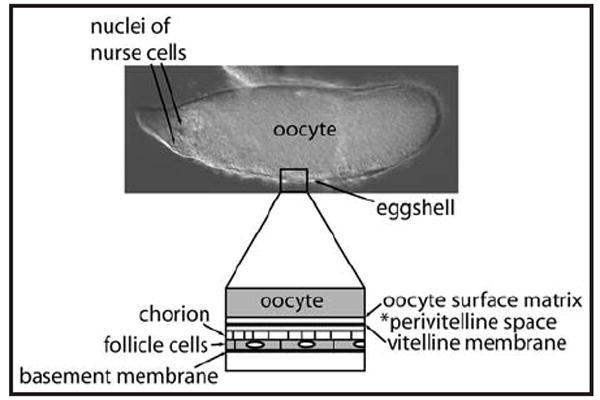
Matrix layers highly enriched in the washed extracellular matrix fraction. At top, a Stage 12 egg chamber consists of a large yolk-filled oocyte, degenerating germ-line nurse cells, and a thin layer of somatic follicle cells that synthesize the eggshell. The lower diagram illustrates the oocyte surface matrix and vitelline membrane layer of the eggshell that bound the perivitelline space in which the dorsoventral protease cascade acts. Fixed components of this space, as well as the outer chorion layer of the eggshell and the basement membrane underlying the follicle cells, are highly enriched in the matrix preparations used in this study, while yolk, other cytoplasmic components and nuclear components are excluded.11
Results
The total amount of GAGs isolated from the wild-type and pipe-mutant eggshell matrix preparations were 40 and 26 μg, respectively, as measured by carbazole assay. Although fewer pipe-mutant ovaries were used compared to wild-type ovaries (1480 versus 1900 ovary pairs), the pipe-mutant ovaries are larger and typically yield at least 20% more total protein per ovary pair in the eggshell matrix preparations (Sukumari-Ramesh S and LeMosy EK, unpublished). Thus, it is possible that there is a reduction in total GAGs associated with the eggshell matrix in the pipe mutant background; however, further quantitative studies would be required to establish this finding with certainty. The wild-type and pipe-mutant GAG samples showed the same average molecular weight and polydispersity (D) when examined by 22% PAGE (Fig. 2), which were 11,000 Da and 1.53, respectively.
Figure 2.
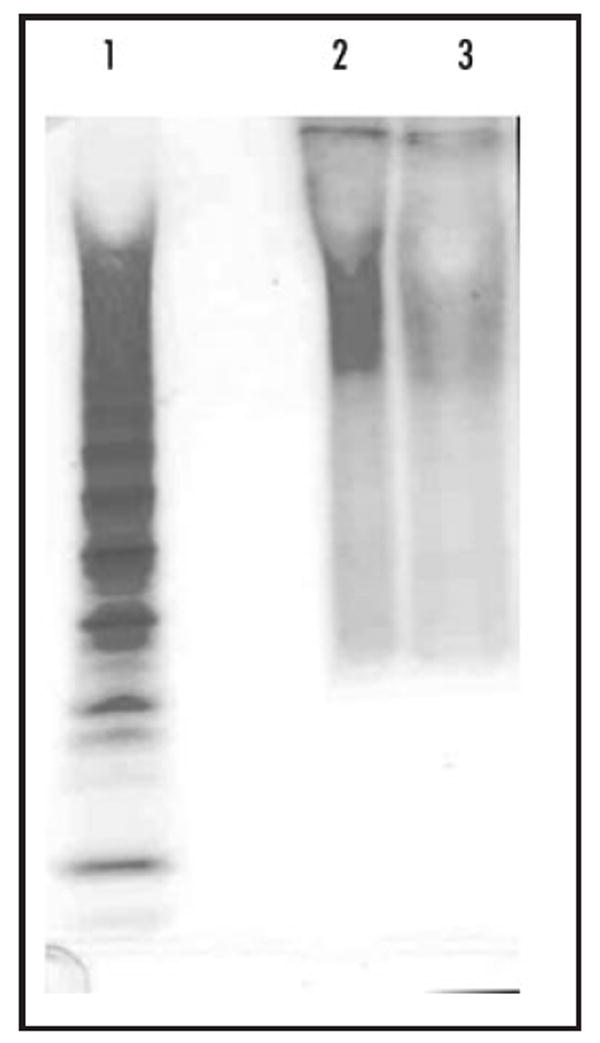
22% PAGE of wild-type and pipe-mutant GAGs. Lanes: 1, heparin oligosaccharide standard; 2, wild-type sample (300 ng); 3, pipe-mutant sample (130 ng).
For disaccharide compositional analysis, the isolated GAGs were subjected to sequential enzymatic depolymerization of CS/DS and HS/Heparin (Hp) GAGs with recovery of these pools for LC-MS analysis. No CS/DS disaccharides were detected by LC-MS (not shown). Two peaks were observed by total ion chromatography (TIC) of the HS/Hp disaccharide samples (Fig. 3A and B). The peak at 11 minutes is ΔUA2S-GlcNAc, labeled as 2S, which was identified by retention time compared to HS disaccharide standards (Fig. 3C) and by its m/z of 458 in MS spectrum (Fig. 3D). Another disaccharide, ΔUA-GlcNAc6S (6S), has a close but different retention time in TIC (Fig. 3C), though it has same m/z in MS spectrum with that of 2S. The peak observed at 42 min is ΔUA2S-GlcNS6S, labeled as triS. TriS was identified by retention time and unique m/z (576) in MS spectrum (Fig. 3E).
Figure 3.
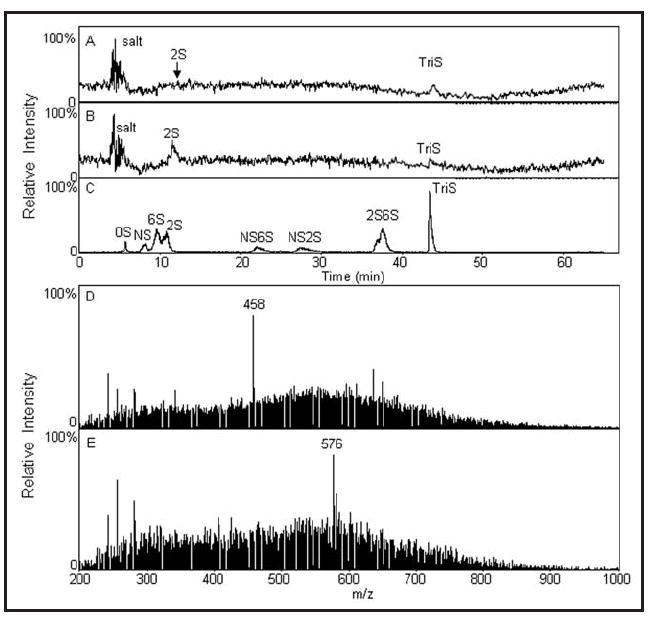
LC-MS analysis of HS/Hp disaccharide composition in wild-type and pipe-mutant samples. (A) Total ion chromatography (TIC) of HS/Hp disaccharide composition in wild-type sample. (B) TIC of HS/Hp disaccharide composition in pipe-mutant sample. (C) TIC of HS disaccharide standards. (D) Mass spectrum of 2S peak in wild-type sample (same result in pipe-mutant sample is not shown). (E) Mass spectrum of triS peak in wild-type sample (same result in pipe-mutant sample is not shown).
The ratios of the 2S and triS peaks in wild-type and pipe-mutant samples are significantly different (cf. Fig. 3A and B, and quantified in Table 1), with the pipe-mutant sample showing a marked shift from triS to the less-sulfated 2S disaccharide.
Table 1.
Hp/HS disaccharide analysis
| 0S | NS | 6S | 2S | NS6S | NS2S | 2S6S | triS | |
|---|---|---|---|---|---|---|---|---|
| WT | 34.3% | 65.7% | ||||||
| Mutant | 81.1% | 18.9% |
Discussion
The HS disaccharide composition of 65.7% triS and 34.3% 2S seen for the wild-type eggshell matrix sample differs markedly from that reported for whole ovaries,17 where triS represented 5.2% of total HS GAGs and 2S was not detected. This likely results from our use of a pre-fractionated and washed extracellular matrix preparation free of yolk and other stored components of the eggs, which allowed us to focus mainly on GAGs made by the follicle cells and deposited into the matrix. The striking difference in ratios of 2S and triS disaccharides between wild-type and pipe-mutant matrix suggests that Pipe promotes the 6-O- and/or N- sulfation of the amino sugar, glucosamine, involved in generating triS heparan sulfate, while it is not required for 2-O-sulfation of uronic acid residues in HS despite its sequence similarity to HS2OST.3 Pipe could function directly as a sulfotransferase, e.g., as a 6-O-sulfotransferase since these enzymes are more closely related to 2-O-sulfotransferases than are the N-deacetylase/N-sulfotransferases,18 or could act more indirectly, e.g., by modulating expression, functional complex formation, or substrate specificity of 6-O-sulfotransferases or N-deacetylase/N-sulfotransferases.19
The apparent involvement of Pipe in HS modification is surprising in light of genetic data that appear to rule out a role for GAGs in pipe action and in dorsoventral patterning (described in the Introduction).7,10 We suggest two general possibilities for reconciling the available data. First, Pipe could modulate the sulfation of carbohydrate substrates in addition to GAGs, and it is one of these non-GAG substrates that is involved in embryonic patterning. Such a non-GAG substrate for Pipe has been proposed to explain the persistence of Alcian blue staining of salivary gland lumens in embryos lacking both maternal and zygotic function of the sulfateless, sugarless and fringe connection genes required for HS biosynthesis, while this staining is absent in pipe-null embryos.10 In this case, our findings would be interesting with regard to Pipe function but not directly relevant to dorsoventral patterning.
Second, it is possible that the biology either of GAG biosynthesis and modification in the fly ovary or of the serine protease cascade interacting with the instructional cue has caused a role for HS to be missed in the genetic approaches. For example, how much of a correctly modified Pipe target is required for the instructional cue to function? The sulfateless, sugarless and fringe connection mutants tested have P element insertions in the 5’ promoter regions of the respective genes,7,10,20-22 so any small amount of transcript that is produced would likely encode a functional protein; also, there could be perdurance of a small amount of these enzymes in the Golgi of homozygous mutant cells descended from heterozygous cells following mitotic clone induction. If a correctly modified Pipe target mainly serves as a marker of where the protease cascade can act, it is conceivable that there could be sufficient compensatory feedback regulation within the serine protease cascade to overcome a quantitative deficit in this target.23-26 Such a mechanism would have to be very robust, however, as no dorsoventral defects were observed among thousands of eggs examined.7 Alternatively, could a HSPG created elsewhere be endocytosed by follicle cells, as can occur for yolk protein from the hemolymph,27,28 and further modified by Pipe in the Golgi? One such example of sulfation by ovarian follicle cells of a protein made in the fat body has been described.29 It is also possible that there could be functional redundancy, e.g., of other Golgi transporters for Fringe Connection or of another UDP-glucose dehydrogenase for Sugarless; however, such redundancy would have to be specific to follicle cells or to the Pipe target, as fringe connection and sugarless are strictly required for embryonic segmentation.7
We undertook the direct biochemical analysis of GAGs in wild-type and pipe-mutant matrix because of uncertainties in whether GAG biosynthetic pathways in insects are identical to those characterized in mammals. This analysis has provided further evidence that Pipe promotes sulfation of carbohydrate targets, and we suggest that the striking alteration in HS disaccharide composition in the pipe-mutant matrix warrants a re-examination of the question of whether GAGs could be involved in dorsoventral patterning.
Materials and Methods
Materials
Actinase E, a nonspecific protease derived from Streptomyces griseus, was from Kaken Biochemicals (Tokyo, Japan). Chondroitin sulfate was from Seikagaku (Tokyo, Japan). Polyacrylamide, urea, CHAPS, Alcian blue dye, 2-cyanoacetamide, and tetra-n-butyl ammonium hydrogen sulfate were from Sigma Chemical Company (St. Louis, MO). All other chemicals were of reagent grade. Vivapure Q Mini H columns were from Vivascience (Edgewood, NY).
Unsaturated disaccharide standards of chondroitin sulfate (Di-0S, ΔUA-Gal; Di-4S, ΔUA-Gal4S; Di-6S, ΔUA-Gal6S; Di-UA2S, ΔUA2S-Gal; Di-diSB,ΔUA2S-Gal4S; Di-diSD,ΔUA 2S-Gal6S; Di-diSE,ΔUA-Gal4S6S; Di-triS, ΔUA2S-Gal4S6S) and unsaturated disaccharide standards of Hep/HS (Di-0S, ΔUA-GalNAc; Di-4S, ΔUA-GalNAc4S; Di-6S, ΔUA-GalNAc6S; Di-UA2S, ΔUA2S-GalNAc; Di-diSB, ΔUA2S-GalNAc4S; Di-diSD, ΔUA 2S-GalNAc6S; Di-diSE, ΔUA-GalNAc4S6S; Di-triS, ΔUA2S-GalNAc4S6S) were purchased from Seikagaku Corporation (Japan). Chondroitinase ABC and ACII were purchased from Seikagaku Corporation (Japan). Heparinase I, II and III were recombinant Flavobacterial enzymes expressed in E. coli and were a generous gift from Dr. Jian Liu.
Preparation of eggshell matrix
Wild-type flies were the Oregon R strain, while the pipe-mutant flies were pipeZH1/Df(3L)pipeA13 (FBab0028445) physically lacking the box 7 and box 10 pipe isoforms normally expressed in the ovary.3,4 Young females were mated in groups of 15–20 to 3–4 males in heavily yeasted vials for 2–3 days to stimulate egg production. Ovaries were harvested by hand dissection in groups of 50 ovary pairs, and stored overnight at 4°C prior to fractionation; a total of 1900 wild-type ovary pairs and 1480 pipe-mutant ovary pairs were used in this analysis. Eggshell matrix fractions were prepared as previously described for LC-MS/MS analysis,11 including three homogenizations in lysis buffer and nuclease treatment, except that only two rather than four washes in low-salt wash buffer were performed after nuclease treatment. The final pellets were combined in glass vials and stored at -70°C.
Isolation and purification of GAGs
Water (500 μL water/~300 ovary pairs) and actinase E (50 μL/~300 ovary pairs; 100 μg, from 20 mg/mL stock solution) were added into the eggshell matrix samples, and incubated at 55°C overnight. After the proteolysis, dry urea and dry CHAPS were added to each sample (final 2 wt % in CHAPS and 8 M in urea). The resulting cloudy solutions were clarified by passing through a syringe filter containing a 0.2 μm membrane. The following Vivapure spin column procedure is based on 500 μL water/~300 ovary pairs. A Vivapure Q Mini H spin column (quaternary ammonium anion exchange membrane) was equilibrated with 400 μL 8 M urea containing 2% CHAPS (pH 8.3). The clarified filtered samples were loaded onto and run through the Vivapure Q Mini H spin column under centrifugal force (2000 × g). The column was washed with 400 μL 8 M urea, 2% CHAPS at 2000 × g, and then washed with 100 mM NaCl five times (400 μL/each time) at 2000 × g. GAGs were eluted by 16% NaCl 4 times (200 μL/each time) at 2000 × g. Methanol was added to afford an 80 vol% solution and the mixture was equilibrated at 4°C for 18 h. The resulting precipitate was recovered by centrifugation (2500 × g) for 20 min. The precipitate was re-dissolved in water and residual salt was removed using a microcon filter (YM-3, 3000 MWCO, Millipore, Bedford, MA). GAGs were stored frozen for further analysis.
Quantification of GAGs by carbazole assay
The isolated GAGs were subjected to carbazole assay to quantify the amount of GAG in each sample using heparan sulfate as standard.14
Polyacrylamide gel electrophoresis (PAGE) analysis
Twenty-two percent (22%) Polyacrylamide gel electrophoresis (PAGE) was applied to analyze the molecular weight and polydispersity of each sample. The purified GAG samples were subjected to electrophoresis against a standard composed of heparin oligosaccharides prepared enzymatically from bovine lung heparin, and the gel was visualized with Alcian blue followed by silver staining.15 The gel was then digitized with UN-Scan-it software (Silk Scientific, 220 UT, USA) and the average MW of the GAGs was calculated based on the heparin oligosaccharide standard.16
Disaccharide analysis using LC/MS
Enzymatic depolymerization of GAGs: The GAG samples (20 μg/5 μL) were incubated with chondroitinase ABC (10 munits) and ACII (5 munits) at 37°C for 10 h. The enzymatic products were recovered by centrifugal microcon filtration. CS/DS disaccharides, passed through the filter, were freeze-dried and ready for LC-MS analysis. Next, heparinase I, II and III (5 munits each) were added into the remainder and incubated at 37°C for 10 h. The products were also recovered by centrifugal filtration and the heparin/HS disaccharides were similarly collected and freeze-dried and ready for LC-MS analysis. The chondroitin and heparin lyases used in this protocol are pure, and other protein-linked carbohydrates, such as O-linked mucins, are not released and thus are retained by the filter. Furthermore, although GalNAc and GlcNAc have identical molecular weights, disaccharides containing these sugars would have distinct retention times in the subsequent LC chromatography step.
The analysis was performed on a LC-MS system (Agilent, LC/MSD trap MS). Solution A and B for HPLC were 15 and 70% acetonitrile, respectively, containing the same concentration of 37.5 mM NH4HCO3 and 11.25 mM tributylamine. The pH values of the buffers were adjusted to 6.5 with acetic acid. The flow rate was 10 μL/min. The separation was performed on a C-18 column (Agilent) using solution A for 20 min, followed by a linear gradient from 20 to 45 min of 0 to 50% solution B. The column effluent entered the source of the ESI-MS for continuous detection by MS. The electrospray interface was set in negative ionization mode with the skimmer potential -40.0 V, capillary exit -120.5 V and a source of temperature of 325°C to obtain maximum abundance of the ions in a full scan spectra (150–1,500 Da, 10 full scans/s). Nitrogen was used as a drying (5 l/min) and nebulizing gas (20 p.s.i.).
Acknowledgments
We thank members of the LeMosy lab for assisting with ovary dissections, and David Stein for providing fly strains and helpful feedback. This work was supported by NIH grants GM067738 to Ellen K. LeMosy and GM38060 to Robert J. Linhardt.
Abbreviations
- HS
heparan sulfate
- CS
chondroitin sulfate
- GAG
glycosaminoglycan
- HS2OST
heparan sulfate 2-O-sulfotransferase
- PAPS
3’-phosphoadenosine 5’-phosphosulfate
References
- 1.LeMosy EK. Proteolytic regulatory mechanisms in the formation of extracellular morphogen gradients. Birth Defects Res C Embryo Today. 2006;78:243–55. doi: 10.1002/bdrc.20074. [DOI] [PubMed] [Google Scholar]
- 2.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo—shaping and transducing a morphogen gradient. Curr Biol. 2005;15:887–99. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–81. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 4.Sergeev P, Streit A, Heller A, Steinmann-Zwicky M. The Drosophila dorsoventral determinant PIPE contains ten copies of a variable domain homologous to mammalian heparan sulfate 2-sulfotransferase. Dev Dyn. 2001;220:122–32. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1094>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Sen J, Goltz JS, Konsolaki M, Schüpbach T, Stein D. Windbeutel is required for function and correct subcellular localization of the Drosophila patterning protein Pipe. Development. 2000;127:5541–50. doi: 10.1242/dev.127.24.5541. [DOI] [PubMed] [Google Scholar]
- 6.Habuchi H, Habuchi O, Kimata K. Sulfation pattern in glycosaminoglycan: does it have a code? Glycoconj J. 2004;21:47–52. doi: 10.1023/B:GLYC.0000043747.87325.5e. [DOI] [PubMed] [Google Scholar]
- 7.Zhu X, Stevens LM, Stein D. Synthesis of the sulfate donor PAPS in either the Drosophila germline or somatic follicle cells can support embryonic dorsal-ventral axis formation. Development. 2007;134:1465–9. doi: 10.1242/dev.003426. [DOI] [PubMed] [Google Scholar]
- 8.Lüders F, Segawa H, Stein D, Selva EM, Perrimon N, Turco SJ, Häcker U. Slalom encodes an adenosine 3’-phosphate 5’-phosphosulfate transporter essential for development in Drosophila. EMBO J. 2003;22:3635–44. doi: 10.1093/emboj/cdg345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D, Song D, Pedersen LC, Liu J. Mutational study of heparan sulfate 2-O-sulfotransferase and chondroitin sulfate 2-O-sulfotransferase. J Biol Chem. 2007;282:8356–67. doi: 10.1074/jbc.M608062200. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Sen J, Stevens L, Goltz JS, Stein D. Drosophila Pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans. Development. 2005;132:3813–22. doi: 10.1242/dev.01962. [DOI] [PubMed] [Google Scholar]
- 11.Fakhouri M, Elalayli M, Sherling D, Hall JD, Miller E, Sun X, Wells L, LeMosy EK. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev Biol. 2006;293:127–41. doi: 10.1016/j.ydbio.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turcotte CL, Hashimoto C. Evidence for a glycosaminoglycan on the nudel protein important for dorsoventral patterning of the Drosophila embryo. Dev Dyn. 2002;224:51–7. doi: 10.1002/dvdy.10081. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez G, Gonzalez-Reyes A, Casanova J. Cell surface proteins Nasrat and Polehole stabilize the Torso-like extracellular determinant in Drosophila oogenesis. Genes Dev. 2002;16:913–8. doi: 10.1101/gad.223902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–4. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 15.al-Hakim A, Linhardt RJ. Electrophoresis and detection of nanogram quantities of endogenous and exogenous glycosaminoglycans in biological fluids. Appl Theor Electrophor. 1991;1:305–12. [PubMed] [Google Scholar]
- 16.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–7. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 17.Toyoda H, Kinoshita-Toyoda A, Selleck SB. Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J Biol Chem. 2000;275:2269–75. doi: 10.1074/jbc.275.4.2269. [DOI] [PubMed] [Google Scholar]
- 18.Hammerich S, Verdugo D, Rath VL. Strategies for drug discovery by targeting sulfation pathways. Drug Discovery Today. 2004;9:967–75. doi: 10.1016/S1359-6446(04)03261-1. [DOI] [PubMed] [Google Scholar]
- 19.Presto J, Thuveson M, Carlsson P, Busse M, Wilén M, Eriksson I, Kusche-Gullberg M. Heparan sulfate biosynthesis enzymes EXT1 and EXT2 affect NDST expression and heparan sulfate sulfation. Proc Natl Acad Sci USA. 2008;105:4751–6. doi: 10.1073/pnas.0705807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–4. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- 21.Häcker U, Lin X, Perrimon N. The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development. 1997;124:3565–73. doi: 10.1242/dev.124.18.3565. [DOI] [PubMed] [Google Scholar]
- 22.Selva EM, Hong K, Baeg GH, Beverly SM, Turco SJ, Perrimon N. Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signaling events. Nature Cell Biol. 2001;3:809–15. doi: 10.1038/ncb0901-809. [DOI] [PubMed] [Google Scholar]
- 23.Dissing M, Giordano H, DeLotto R. Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J. 2001;20:2387–93. doi: 10.1093/emboj/20.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeMosy EK. Spatially dependent activation of the patterning protease, Easter. FEBS Lett. 2003;580:2269–72. doi: 10.1016/j.febslet.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeMosy EK, Tan YQ, Hashimoto C. Activation of a protease cascade involved in patterning the Drosophila embryo. Proc Natl Acad Sci USA. 2001;98:5055–60. doi: 10.1073/pnas.081026598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra S, Hecht P, Maeda R, Anderson KV. Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development. 1998;125:1261–7. doi: 10.1242/dev.125.7.1261. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth FM, Burdes VS, Bownes M. Mutant yolk proteins lead to female sterility in Drosophila. Dev Biol. 1992;154:182–94. doi: 10.1016/0012-1606(92)90058-o. [DOI] [PubMed] [Google Scholar]
- 28.Yan YL, Postlethwait JH. Vitellogenesis in Drosophila: sequestration of a yolk polypeptide/invertase fusion protein into developing oocytes. Dev Biol. 1990;140:281–90. doi: 10.1016/0012-1606(90)90078-w. [DOI] [PubMed] [Google Scholar]
- 29.Giorgi F, Falleni A, Cecchettini A, Gremigni V. A fat body derived protein is selectively sulfated in the stick insect ovary by transcytosis through the follicular epithelium. Biol Cell. 1998;90:183–97. doi: 10.1016/s0248-4900(98)80339-0. [DOI] [PubMed] [Google Scholar]


