Abstract
Objective
To determine long term health benefits of non-ablative bone marrow transplantation for severe combined immunodeficiency (SCID), we investigated our cohort of 161 related donor bone marrow transplanted SCID patients. Only 16 (10%) had HLA-identical donors.
Study design
All 124 survivors were sent questionnaires about their current clinical statuses. Details from clinic visits were also compiled. One hundred eleven patients (90%) were reached. We compared outcomes of patients transplanted before and after 3.5 months of life and by molecular defect.
Results
The overall survival rate is 77%, but the rate for the 48 infants transplanted in the first 3.5 months of life is 94%, compared with 70% for the 113 transplanted after 3.5 months (p=0.002). Twenty-eight (76%) of the 37 deceased patients died from viral infections present at diagnosis. One or more clinical problems were reported to have been present in the past two years in 71 (64%) of the survivors, although 95 (86%) are considered healthy by their families.
Conclusions
Most patients with SCID transplanted with related donor marrow without pre-transplant chemotherapy have done well long-term, but those transplanted at <3.5 months of age had a superior survival rate, a lower rate of clinical problems, less need for booster transplants and better nutritional status.
Severe combined immunodeficiency (SCID) is a syndrome characterized by profound deficiencies in T- and B-lymphocytes and, in some cases, NK cell function.1 The disease is universally fatal in the first two years of life without immune reconstitution by hematopoietic stem cell transplantation or by gene therapy. Ideally, stem cells are obtained from HLA-identical siblings. When this is not an option, rigorously T-lymphocyte depleted haploidentical (half-matched) parental marrow can be successful in effecting immune reconstitution.1, 2 In either case, such transplants can be performed without pre-transplant chemotherapy, because the infants do not have T-lymphocytes and thus cannot reject the transplanted cells. When rigorous T-lymphocyte depletion of transplant is used, the need for post-transplantation against graft verus host disease (GVHD) prophylaxis also is avoided. Infants transplanted with T-lymphocyte-depleted marrow typically develop circulating T-lymphocytes of donor origin that are phenotypically and functionally normal by 90 to 120 days after transplantation.3
There have been several reports on the long term immunologic outcomes of patients with SCID but few have addressed the long term clinical outcome of these patients. Borghans et al reported an increased incidence of infections and autoimmune disorders late after transplantation.4 Honig et al5 reported that SCID patients with adenosine deaminase deficiency had a high incidence of CNS complications such as mental retardation, motor dysfunction, and sensorineural hearing deficits. Mazzolari et al6 and Slatter et al7 analyzed the long-term follow up of 36 and 40 patients, respectively, with severe T-lymphocyte immunodeficiency. These groups found relatively high incidences of endocrine, autoimmune and behavioral problems, as well as human papilloma virus infections post-transplantation. Neven et al 8 reported the long term outcomes of 90 surviving patients with SCID from 149 transplanted in Paris, France from 1972 to 2004. Overall survival was 63%. Chronic GVHD was found in 11%, 13% had autoimmune or inflammatory complications, and 26% had HPV infections, 9 cases of which were severe. Of 105 children with primary immunodeficiency after transplantation, there was an increased risk of long term cognitive difficulties and associated emotional and behavioral difficulties, particularly in those with ADA-deficient SCIDs.9 All six of these reporting groups followed patients who had received pre-transplant chemotherapy and post-transplant GVHD prophylaxis. Only one longterm follow-up study has been reported on non-conditioned bone marrow transplants in SCID infants and, in that study, 15 of 25 (60%) recipients were surviving from 10 to 26 years post-transplantation.10 We sought to determine the long-term clinical outcome in our cohort of 161 bone marrow transplanted SCID patients who did not receive pre-transplant chemotherapy or post-transplant GVHD prophylaxis, some of whom are in their third decade post-transplantation. We have previously reported that stem cell transplantation for SCID in the neonatal period leads to superior thymic output, earlier immune function and improved survival.11 We therefore compared the clinical outcome of patients transplanted before 3.5 months of age with that in those transplanted after 3.5 months of life. We also compared the clinical outcome of the patients based on their molecular defect. The longterm immunologic reconstitution of this large group of patients is reported elsewhere.12
METHODS
We analyzed data on 161 consecutive patients with SCID who received bone marrow transplants at Duke University Medical Center, Department of Pediatrics, Division of Allergy and Immunology, from May 19, 1982, to August 15, 2008. One hundred forty-five of these patients received rigorously T-lymphocyte depleted haploidentical parental marrow, and sixteen received HLA identical related marrow. No patient received pre-transplant chemotherapy or post-transplant GVHD prophylaxis. Donor marrow was depleted of T cells by agglutination with soybean lectin and two cycles of rosetting with sheep erythrocytes treated with aminoethylisothiuronium bromide, as previously described.3
All 124 survivors and their families were sent a detailed questionnaire (Appendix; available at www.jpeds.com) about their current clinical statuses. The patients were followed with phone calls if the questionnaires were not returned. One hundred eleven patients (90%) were reached for follow up evaluation. Table I outlines the demographics of this group. Eighty-two of the 111 patients whose families completed the questionnaire (Appendix) were also seen in the clinic for follow up in the two year period before the study ended. During the follow up evaluation, clinical validity was sought to assess the accuracy of the responses to the questionnaire. Of the 29 patients whose families only completed the questionnaire, four were reached by telephone. Time was spent discussing the questionnaire and the answers given. Minor additions were made in 2 patients, but no changes in the answers to the questions were needed.
Table 1.
Demographics of 111 patients with SCID Followed Long-term
| Sex | |
| Male | 88 |
| Female | 23 |
| Race or Ethnicity | |
| Caucasian | 90 |
| African-American | 10 |
| Hispanic | 7 |
| American Indian | 2 |
| Asian | 1 |
| Arabic | 1 |
| Molecular Type of SCID | |
| X-linked (γc-deficiency) | 53 |
| ADA-deficiency | 16 |
| Il-7Rα-deficiency | 15 |
| Autosomal recessive, SCID defect unknown | 8 |
| RAG1 or RAG2-deficiency | 6 |
| JAK3 deficiency | 6 |
| CD3 chain-deficiency | 4 |
| CD45-deficiency | 1 |
| Cartilage hair hypoplasia with SCID | 1 |
| Unknown molecular cause | 1 |
Statistical analysis
Patient survival was analyzed by means of Kaplan-Meier survival curves and 8-year survival rates were calculated. Survival between patients transplanted before 3.5 months of life and those transplanted after 3.5 months of life was compared using the log-rank test. Comparison of these two groups with respect to other clinical outcomes was performed using the χ2 test and Fisher test. Relationship between survival and age of transplant as a continuous variable was analyzed using the Cox proportional hazards regression model. In all analyses, a p-value<0.05 was considered statistically significant.
RESULTS
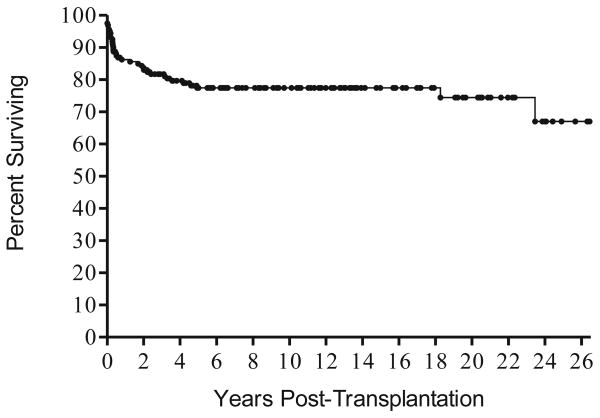
The overall survival of the entire cohort of 161 patients is 77% (Figure 1). The survival rate for those who received haploidentical transplants is 75%, and 100% of the 16 who received HLA identical related transplants survive. Twenty-eight or 25% percent of the entire cohort of 161 patients required one or more booster transplants. Four of the patients underwent gene therapy elsewhere (2 with ADA-deficiency in Italy,13 2 with γcDef SCID at the NIH14). Gene therapy was successful in three of the four but unsuccessful in one γcDef patient who subsequently received a matched unrelated donor (MUD) transplant following reduced intensity conditioning. Four ADA-deficient patients are currently receiving polyethylene glycol modified bovine adenosine-deaminase (PEG-ADA), and one received a MUD bone marrow transplant elsewhere. One Artemis-deficient patient received a MUD bone marrow transplant elsewhere. The length of follow up ranged from 6 months to 26 years post-transplantation (median follow up 8.7 years, 25th percentile 2.9 years, 75th percentile 14.1 years). Median follow up time was 9.2 years (25th percentile 5.0 years, 75th percentile 13.4 years) for patients who received their transplants in the first 3.5 months of life, compared with 8.5 years (25th percentile 2.3 years, 75th percentile 14.9 years) for patients who were transplanted after 3.5 months of age. Survival was superior (8-year Kaplan-Meier survival 96%, 95% CI: 84%–99%) in the 48 patients who received their transplants in the first 3.5 months of life, only 3 of whom failed to survive. By contrast, 8-year Kaplan-Meier survival was only 70% (95% CI: 60%–77%) in the 113 who were transplanted after 3.5 months of age. This difference in survival is statistically significant with a log rank p value of 0.0017. Patients transplanted after 3.5 months of life have a lower survival rate (p-value=0.0049), with a hazard ratio of 1.032 per 10-day increase in age at transplant (95% CI: 1.010–1.056)
Figure 1.
Kaplan Meier survival curve for all 161 patients with SCID transplanted over a 26 year period. Twenty-two of the patients died in the first year and 13 more between 1 and 5 years post-transplantation, and 28 (75%) of the 37 patients who died succumbed to viral infections present at the time of transplant.
Thirty-seven of the entire cohort of 161 patients have died, with 22 succumbing in the first year and 13 more between 1 and 5 years post-transplantation. There were two late deaths; one was a patient with SCID and ADA-deficiency being treated with PEG-ADA who died at age 18 years with pulmonary hypertension. The second was a patient with X-linked SCID who required IGIV, was non-compliant with medications and died of lung disease. Twenty-eight (75%) died from viral infections present at the time of diagnosis that continued chronically. Eight died of cytomegalovirus infection, 8 more with adenovirus infection, 6 with EBV lymphoproliferative disease, 4 with enterovirus infections, 3 with parainfluenza 3 infection, 2 with varicella, 1 with herpes simplex infection and 1 with RSV infection. Some had more than one viral infection. Four died of pulmonary disease, 2 from Candida bloodstream infection, 1 from an unrelated mitochondrial defect, 1 with the nephrotic syndrome following chemotherapy given prior to referral, and 1 of venocclusive disease. No patient died of graft-versus-host disease.
Of the 124 survivors, 111 (90%) could be reached for clinical follow up. The demographics of the group are presented in Table I. Almost one-half of patients had SCID due to mutations in the gene encoding the common gamma chain (an X-linked disorder). One patient had SCID of unknown molecular cause.
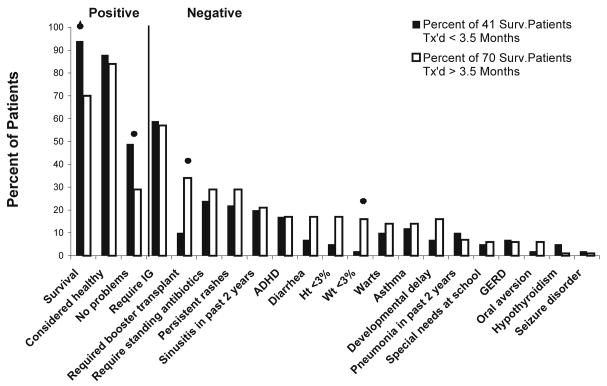
Overall, most patients reported in this follow up study are doing well clinically (Figures 2 and 3; available at www.jpeds.com). Ninety-five (86%) of the 111 patients are considered by their family to be healthy. Replacement immunoglobulin intravenous (IGIV) is being administered to 64 (58%) of the 111 patients, and standing antibiotics are given to 30 (27%) of the 111 patients. The latter includes 21 patients recently transplanted who continue to receive Pneumocystis prophylaxis. Forty (36%) of the 111 patients reported no health problems in the past two years. Others reported sinusitis (20%) or otitis media (5%) as their only clinical problem over the past two years. Pneumonia was reported in 8%. One patient developed cryptococcal osteomyelitis that was successfully treated. Asthma was reported in 14% of the patients. Autoimmune manifestations are rare in this cohort of patients. Only two patients (2% of population) experience intermittent hemolytic anemia, and one of these patients also has intermittent thrombocytopenia with associated anti-platelet antibodies. Endocrine abnormalities also are unusual. Four patients have hypothyroidism, which was congenital in these patients. Serious neurologic problems are also rare in our population. Eleven (10%) of the 111 patients have developmental delay. Only 2% of the patients have a seizure disorder. Cerebral palsy is present in 2%, and 21% of the patients have ADHD.
Figure 2.
A comparison of clinical features reported as percentages of 41 surviving patients with SCID who were transplanted prior to 3.5 months of age with the same features reported as percentages of 70 surviving patients with SCID who were transplanted after 3.5 months of age and followed long-term for up to 26 years after transplantation.
Gastrointestinal manifestations include 14% with diarrhea and 6% with GERD. Oral aversion is present in 5% of the population.. Two patients underwent successful liver transplantion: one an ADA-deficient SCID with a life-long hepatitis C infection, and one an IL7Rα-deficient SCID who had acute GVHD after a maternal T-lymphocyte-depleted marrow graft but received a maternal segmental liver transplant and is now healthy and receiving no immunosuppression. Skin problems include 25% with persistent rashes, with 12% of the population having human papilloma virus (HPV) infection in the past two years. Molluscum and tinea also contribute to a significant proportion of these rashes. The HPV, tinea and molluscum in the vast majority of patients are intermittent but tend to recur. One patient has a severe problem with persistent HPV infection. GVHD limited to the skin is present in four patients. The rash is chronic in only two of the patients. One patient with X-linked SCID also has eosinophilic fasciitis.
A majority of the patients have had satisfactory growth and development after transplantation. Only 13 (12%) of the 111 patients are currently below the 3rd percentile in weight and height. Only 3% of the population requires special schooling. Ten of the patients have graduated from or are attending college, with one currently in medical school and another in graduate school. Two males and one female have parented healthy children.
Of the 71 patients with health problems, 40 had two or fewer problems, and 31 had more than two problems. Sixteen of these patients had more than three problems and were the only patients in the follow-up cohort of 111 patients not considered healthy by their families.
Twenty-five patients reached for follow up had required booster transplants. Of the 16 patients considered not healthy by their families, 10 required booster transplants because of poor T-lymphocyte function. The other 15 patients who required boosters are doing well and considered healthy. Six patients who are not considered healthy did not receive booster transplants. Two are receiving PEG-ADA. Two others have good T-lymphocyte function, but have other problems that are not thought to be due to their transplants. Two others received their first transplants less than six months ago.
The clinical outcome for patients transplanted in the first 3.5 months of life (early) was superior to that of those transplanted after 3.5 months of life (late) (Figure 2). Need for booster transplants also was related to age at transplant (Fisher exact test p-value=0.018). Only 10% of the patients transplanted early required booster transplants, whereas 34% of those transplanted late required boosters. No clinical problems in the previous two years were reported in 49% of the patients transplanted early versus 29% in those transplanted late (χ2 p-value=0.037). Episodes of sinusitis, pneumonia and asthma were similar among the two groups, as was the use of antibiotics. More patients transplanted late experienced problems with diarrhea, oral aversion, and persistent rashes including HPV, and ADHD although these differences were not statistically significant. A discrepancy is also noted in the growth percentiles. Only 5% of the patients transplanted early are less than the 3rd percentile for height compared with 17% of those transplanted late (difference not significant), and 2% of those transplanted early compared with 17% of those transplanted late (Fisher exact test p-value=0.029) are less that the 3rd percentile for weight.
The clinical outcome differed among groups of patients with SCID with different molecular defects (Table II). Overall, patients with X-linked SCID, IL-7 receptor alpha chain deficiency, JAK3 deficiency and CD3 chain deficiencies had the best clinical outcomes, and the vast majority are considered healthy by their families.. Survival was highest in patients with RAG mutations, JAK3 deficiency and CD3 chain deficiency. The need for booster transplantation was highest in patients with RAG mutations, followed by those with CD3 chain and JAK3 deficiency. Only 22% of patients with X linked SCID required a booster transplant. The majority of patients with X-linked SCID, ARSCID, and RAG deficiency require IGIV replacement, and only 27% of patients with IL-7 receptor alpha chain deficiency require IGIV. Clinically, 67% of patients with IL-7 receptor alpha chain deficiency report no problems in the past two years. This is in contrast to only 13% of patients with ADA deficiency and 17% of patients with RAG mutations who have no problems. Hypothyroidism, autoimmune disease, and developmental delay are more common in patients with ADA deficiency and RAG mutations. The highest percentage of ADHD was found in patients with ADA deficiency. HPV infection is most common in patients with X-linked SCID.
Table 2.
Features of 111 patients with SCID According to Molecular Type
| Clinical Features | X-linked | ADA Deficiency | IL7Rα Deficiency | Autosomal Recessive, Unknown Defect | JAK3-Deficiency | RAG1 or RAG2-Deficiency | CD3 ChainDeficiency |
|---|---|---|---|---|---|---|---|
| Positive | |||||||
| Survival | 57/74 (77%) | 18/23 (78%) | 17/24 (71%) | 10/12 (83%) | 8/9 (89%) | 6/7 (86%) | 4/4 (100%) |
| Considered Healthy | 48/53 (92%) | 11/16 (69%) | 15/15 (100%) | 7/8 (88%) | 5/6 (83%) | 1/6 (17%) | 3/4 (75%) |
| No Problems | 17/53 (32%) | 2/16 (13%) | 10/15 (67%) | 3/8 (38%) | 1/6 (17%) | 1/6 (17%) | 2/4 (50%) |
| Negative | |||||||
| Sinusitis | 16/53 (30%) | 2/16 (13%) | 2/15 (13%) | 2/8 (25%) | 1/6 (17%) | 1/6 (17%) | 0/4 |
| Persistent Rashes | 14/53 (26%) | 5/16 (31%) | 1/15 (7%) | 2/8 (25%) | 3/6 (50%) | 3/6 (50%) | 0/4 |
| Need for Booster | 12/53 (26%) | 2/16 (13%) | 3/15 (20%) | 3/8 (38%) | 2/6 (33%) | 5/6 (83%) | 2/4 (50%) |
| On Standing Antibiotics | 11/53 (21%) | 6/16 (38%) | 2/15 (13%) | 4/8 (50%) | 0/6 | 5/6 (83%) | 2/4 (50%) |
| Warts | 11/53 (21%) | 2/16 (13%) | 0/15 | 0/8 | 0/6 | 0/6 | 0/4 |
| Asthma | 8/53 (15%) | 1/16 (6%) | 2/15 (13%) | 0/8 | 0/6 | 1/6 (17%) | 1/4 (25%) |
| ADHD | 8/53 (15%) | 8/16 (50%) | 3/15 (20%) | 1/8 (13%) | 1/6 (17%) | 1/6 (17%) | 1/4 (25%) |
| Diarrhea | 6/53 (11%) | 1/16 (6%) | 0/15 | 2/8 (25%) | 0/6 | 2/6 (33%) | 0/4 |
| Developmental Delay | 6/53 (11%) | 3/16 (19%) | 1/15 (7%) | 0/8 | 2/6 (33%) | 3/6 (50%) | 0/4 |
| Weight <3% | 5/53 (9%) | 3/16 (19%) | 0/15 | 1/8 (13%) | 0/6 | 2/6 (33%) | 1/4 (25%) |
| Height <3% | 4/53 (8%) | 4/16 (25%) | 0/15 | 2/8 (25%) | 0/6 | 2/6 (33%) | 1/4 (25%) |
| Pneumonia | 4/53 (8%) | 1/16 (6%) | 0/15 | 3/8 (38%) | 0/6 | 0/6 | 0/4 |
| Autoimmune Disease | 1/53 (2%) | 1/16 (6%) | 0/15 | 0/8 | 0/6 | 1/6 (17%) | 0/4 |
| Hypothyroidism | 0/53 | 4/16 (25%) | 0/15 | 0/8 | 0/6 | 1/6 (17%) | 0/4 |
| Immunologic | |||||||
| IGIV Therapy | 35/53 (66%) | 4/16 (25%) | 4/15 (27%) | 6/8 (75%) | 3/6 (50%) | 5/6 (83%) | 2/4 (50%) |
| IgG Mean±SEM | 808±50 | 1001±76 | 924±77 | 879±250 | 990±201 | 912±127 | 1001±91 |
| IgA Mean±SEM | 33±9 | 75±14 | 79±19 | 17±11 | 111±54 | 34±29 | 89±37 |
| IgM Mean±SEM | 71±8 | 73±17 | 136±19 | 55±22 | 130±53 | 14±13 | 58±16 |
| PHA Mean±SEM | 126,858±8,600 | 88,262±21,524 | 140,662±16,940 | 96,264±28,073 | 154,187±24,101 | 49,751±12,151 | 62,822±21,279 |
DISCUSSION
Our study is a long-term clinical follow-up of patients with SCID transplanted at a single center. The unique aspects of this patient population are that only 16 infants had transplant from HLA-identical donors, the rest received rigorously T-lymphocyte depleted haploidentical parental bone marrow, and the transplants were done without pre-transplant chemoablation or post-transplant prophylaxis for GVHD. One hundred eleven patients (90%) were reached for follow-up from 6 months to 26 years after transplantation. Overall, most patients are doing well clinically. Only 16 patients are considered not healthy by their families, having more than three health problems. The clinical problems reported as occurring in greater than 10 % of the survivors included: persistent rashes in 25%, ADHD in 21%, sinusitis in 20%, asthma in 14%, diarrhea in 14%, warts in 12% and height and weight below the 3rd percentile in 12%. Because a subgroup of these patients has not done well, all children with SCID should have lifelong, careful follow up by immunologic and clinical monitoring to detect complications.
There are well validated health-related quality of life questionnaires available. However, these questionnaires are not disease specific. We developed a SCID-specific questionnaire that based on problems identical in our clinic and other problems reported in the literature in relation to long term outcome in SCID. The questionnaire was not validated, nor were quality of life measures formally assessed. It is possible that the wording of the questions could have resulted in biased responses, but the questions were worded as simple yes/no answers to a variety of clinical issues, may make that less likely. The question of the child’s overall health relates to the parent’s perception of how the child is faring in life. The vast majority of our patients’ parents perceive their children as healthy despite the problems they face.
Outcome varied considerably based on age at transplant. Those patients transplanted before 3.5 months of age had better long term outcomes than those transplanted after 3.5 months of age. Survival was significantly higher in those patients transplanted early. This suggests that the thymus does not have the ability to mature stem cells as well in older infants with SCID when compared with this ability in the younger infants, possibly as a result of viral infections. Most patients transplanted after 3.5 months of life already have had one or more persistent viral infections that led to the diagnosis of SCID. Other clinical problems such as developmental delay, diarrhea, ADHD, and failure to thrive as well as need for booster transplants were significantly lower in the patients transplanted early. This emphasizes the critical need for newborn screening for SCID. If all patients with SCID could be diagnosed shortly after birth, the outcome would be far superior to the current state with most patients diagnosed after a serious viral illness.11
Outcome also varied based on molecular defect. Patients with defects in the common gamma chain, IL-7 receptor alpha, and JAK3 did best clinically, and those with defects in ADA and RAG genes fared worst. ADA deficient patients in our cohort had an increased incidence of neurological problems such as developmental delay and ADHD compared with the patients with SCID of other molecular types. Honig et al followed the long term clinical outcome of 12 patients with ADA deficiency, 7 of whom received HLA-identical transplants without conditioning.5 Half of their patients had severe neurologic abnormalities and required special schooling. Four patients (33%) had hyperactivity. Four of our 16 patients with ADA deficiency (25%) have developmental delay, with 2 patients having only mild delay. Only two patients require special schooling. There are also increased incidences of hypothyroidism and autoimmune disease in our patients with SCID due to ADA deficiency and RAG mutations. However, these problems are infrequent, and the hypothyroidism was congenital in 3 of our 4 cases.
Mazzolari et al report endocrine abnormalities in 17.5% of their cohort of 40 patients.6 Growth insufficiency (17.5%) and short stature (12.5%) also were more common in that cohort. A report by Slatter et al notes a 10.8% incidence of thyroid dysfunction after bone marrow transplantation for primary immunodeficiency in their cohort of 83 patients.15 The higher percentages of endocrine abnormalities and growth insufficiency in those two patient populations likely reflect the use of chemotherapeutic agents which are known to cause endocrine abnormalities and growth insufficiency.
Most of our patients with ADA deficiency and RAG mutation survive despite the high percentage of problems that they face. Eighty-six percent of our patients with RAG deficiency and 78% with ADA deficiency are alive. This is in contrast to 35% survival in a large European cohort of 56 patients with B-lymphocyte negative SCID (B−SCID).16 Gennery et al17 reported that 3 of their 5 patients (60%) with B−SCID survived, but only one had full engraftment after first bone marrow transplant. Antoine et al examined the long-term survival of patients with SCID in Europe and found that patients with ADA deficiency had an 81% three year survival for HLA-matched transplants and a 29% 3-year survival for HLA-mismatched transplants.18 B−SCID was associated with lower survival in the latter study and our study reveals that there are also more clinical problems in those who do survive. These comparisons, however, are limited by the relatively small numbers of patients in each report, the heterogeneity of the groups and conditioning done, and also the lack of genotyping information. The use of chemotherapy in the European cohort may account for the lower survival in their patients, as this can be harmful in patients who have existing viral infection.
Although 58% of our patients require IGIV replacement, the percentage requiring it varies based on molecular defect. Only 27% of the patients with IL-7 receptor alpha deficient require IGIV, and 83% of patients with RAG deficiency and 72% of patients with X-linked SCID require replacement. It has been suggested that this high IGIV requirement is due to lack of conditioning resulting in lack of donor B-lymphocyte engraftment. However, we find donor B-lymphocyte engraftment in approximately one-third of our patients with X-linked SCID despite the lack of conditioning.2, 19 In addition, IGIV is required in some percentage of patients from reported cohorts who received conditioning prior to transplant, albeit in lower percentages than ours. Stephan et al20 and Haddad et al21 reported that 35% and 45% of survivors, respectively, required immunoglobulin substitution despite conditioning. Clearly, conditioning does not always result in B-lymphocyte function, and the associated risks may not justify the chance to achieve normal B-lymphocyte function.
Altogether, our study shows that most patients with SCID transplanted with rigorously T-lymphocyte depleted haploidentical parental marrow without pre-transplant chemotherapy or post-transplant GVHD prophylaxis do well long-term. Most have normal childhoods, attend regular school, and experience normal childhood illnesses without difficulty. However, in view of the superior survival rate, the lower rate of clinical problems, the lower number requiring booster transplants and the better nutritional status of those transplanted early in life, the outcome of all patients with SCID would be improved significantly if all could be detected by newborn screening before they are exposed to common viral pathogens.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Allergy and Infectious Diseases (AI042951 and AI47605) and a grant from the National Center for Research Resources (NCRR) (# 1 UL1 RR024128-01), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 3.Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136:2398–407. [PubMed] [Google Scholar]
- 4.Borghans JA, Bredius RG, Hazenberg MD, Roelofs H, Jol-van der Zijde EC, Heidt J, et al. Early determinants of long-term T-cell reconstitution after hematopoietic stem cell transplantation for severe combined immunodeficiency. Blood. 2006;108:763–9. doi: 10.1182/blood-2006-01-009241. [DOI] [PubMed] [Google Scholar]
- 5.Honig M, Albert MH, Schulz A, Sparber-Sauer M, Schutz C, Belohradsky B, et al. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109:3595–602. doi: 10.1182/blood-2006-07-034678. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolari E, Forino C, Guerci S, Imberti L, Lanfranchi A, Porta F, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J Allergy Clin Immunol. 2007;120:892–9. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Slatter MA, Brigham K, Dickinson AM, Harvey HL, Barge D, Jackson A, et al. Long-term immune reconstitution after anti-CD52-treated or anti-CD34-treated hematopoietic stem cell transplantation for severe T-lymphocyte immunodeficiency. J Allergy Clin Immunol. 2007;121:361–7. doi: 10.1016/j.jaci.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, et al. Long-term outcome after haematopoietic stem cell transplantation of a single-centre cohort of 90 patients with severe combined immunodeficiency: Long-term outcome of HSCT in SCID. Blood. 2009 doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 9.Titman P, Pink E, Skucek E, O’Hanlon K, Cole TJ, Gaspar J, et al. Cognitive and behavioural abnormalities in children following haematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood. 2008;112:3907–13. doi: 10.1182/blood-2008-04-151332. [DOI] [PubMed] [Google Scholar]
- 10.Patel NC, Chinen J, Rosenblatt HM, Hanson IC, Brown BS, Paul ME, et al. Long-term outcomes of nonconditioned patients with severe combined immunodeficiency transplanted with HLA-identical or haploidentical bone marrow depleted of T cells with anti-CD6 mAb. J Allergy Clin Immunol. 2008;122:1185–93. doi: 10.1016/j.jaci.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 12.Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, Sempowski GD, Buckley RH. Thymic output, T cell diversity and T cell function in long-term human SCID chimeras. Blood. 2009 doi: 10.1182/blood-2009-01-199323. In Press. Available as a First Edition Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 14.Chinen J, Davis J, De Ravin SS, Hay BN, Hsu AP, Linton GF, et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood. 2007;110:67–73. doi: 10.1182/blood-2006-11-058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slatter MA, Gennery AR, Cheetham TD, Bhattacharya A, Crooks BN, Flood TJ, et al. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant. 2004;33:949–53. doi: 10.1038/sj.bmt.1704456. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand Y, Landais P, Friedrich W, Gerritsen B, Morgan G, Fasth A, et al. Influence of severe combined immunodeficiency phenotype on the outcome of HLA non-identical T cell-depleted bone marrow transplantation. J Pediatr. 1999;134:740–8. doi: 10.1016/s0022-3476(99)70291-x. [DOI] [PubMed] [Google Scholar]
- 17.Gennery AR, Dickinson AM, Brigham K, Barge D, Spickett GP, Curtis A, et al. CAMPATH-1M T-cell depleted BMT for SCID: long-term follow-up of 19 children treated 1987–98 in a single center. Cytotherapy. 2001;3:221–32. doi: 10.1080/146532401753174052. [DOI] [PubMed] [Google Scholar]
- 18.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003:361. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 19.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Ann Rev Immunol. 2004;55:625–56. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 20.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, de Saint-Basile G, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 cases. J Pediatr. 1993;123:564–72. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 21.Haddad E, Deist FL, Aucouturier P, Cavazzana-Calvo M, Blanche S, Basile GD, et al. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: A single-center study of 22 patients. Blood. 1999;94:2923–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.