Abstract
We explored the role of 20-hydroxy-5Z, 8Z, 11Z, 14Z-eicosatetraenoic acid (20-HETE) in oxygen-induced vasoconstriction in a normal renin form of hypertension [the 1 kidney-1 clip Goldblatt hypertensive rat (1K1C)] and a high renin form of hypertension [the 2 kidney-1 clip Goldblatt hypertensive rat (2K1C)]. A silver clip was placed around the left renal artery of adult Sprague-Dawley males. The right kidney was removed in the 1K1C group and left intact in the 2K1C group. Arteriolar responses to elevation of O2 concentration in the superfusion solution from 0% O2 to 21% O2 were determined in the in situ cremaster muscle before and after inhibition of cytochrome P450 4A ω-hydroxylase (CYP450 4A) with N-methyl-sulfonyl-12, 12-dibromododec-11-enamide (DDMS). Arteriolar constriction to elevated PO2 was enhanced in the chronic 1K1C but not the acute 1K1C or 2K1C. DDMS eliminated O2-induced arteriolar constriction in the 9 week 1K1C, but had no effect in the 2 wk 1K1C, and only partially inhibited O2-induced constriction of arterioles in the 4 wk 2K1C rat. These findings indicate that although the CYP4A/20-HETE system contributes to arteriolar constriction in response to elevated PO2 in the established stage of 1K1C renovascular hypertension, physiological alterations in other mechanisms are the primary determinants of O2-induced constriction of arterioles in the early and developing stages of 1K1C and 2K1C hypertension.
Keywords: renovascular hypertension, 20-HETE, cytochrome P450 ω-hydroxylase, oxygen, cremaster muscle
INTRODUCTION
Arterioles of many vascular beds constrict when exposed to elevated oxygen levels (Bak et al., 2007; Gilmore et al., 2005; Kolbitsch et al., 2002; Mak et al., 2002; Rossi and Boussuges, 2005; Rousseau et al., 2007; Sirinyan et al., 2006; Tsai et al., 2003; Yamazaki, 2007). While multiple chemical mediators have been proposed to contribute to vascular responses to changes in O2 availability (Cabrales et al., 2006; Drenjancevic-Peric et al., 2003; Frisbee et al., 2001; Frisbee et al., 2002; Jackson et al., 1986; Mak et al., 2002; Messina et al., 1994; Rubanyi and Vanhoutte, 1986; Sirinyan et al., 2006), a number of recent reports suggest that 20-hydroxy-5Z, 8Z, 11Z, 14Z-eicosatetraenoic acid (20-HETE), a vasoactive metabolite of arachidonic acid produced by the enzymatic activity of the cytochrome P450-4A ω-hydroxylase (CYP450 4A) family of enzymes, is a likely candidate to serve as a tissue oxygen sensor (Harder et al., 1996; Kunert et al., 2001a; Kunert et al., 2001b; Lombard et al., 2004; Lombard et al., 1999).
There are multiple reports of enhanced vasoconstrictor sensitivity to elevated PO2 in arterioles of animals with genetic and experimentally-induced hypertension compared to their respective normotensive controls (Drenjancevic-Peric et al., 2003; Kunert et al., 2001b; Lombard et al., 1984; Lombard et al., 1986; Lombard and Stekiel, 1988; Rafi and Boegehold, 1993). Because angiotensin II (ANG II) has been proposed to increase the formation and release of 20-HETE (Alonso-Galicia et al., 2002; Carroll et al., 1997; Carroll et al., 1996; Croft et al., 2000), the present study investigated the role of 20-HETE in O2-induced constriction of cremasteric arterioles in non-genetic models of normal renin hypertension ([the 1 kidney-1 clip Goldblatt hypertensive rat (1K1C)] and high-renin hypertension [the 2 kidney-1 clip Goldblatt hypertensive rat (2K1C)].
MATERIALS AND METHODS
Experimental Animal Groups
Male Sprague Dawley rats were anesthetized with sodium pentobarbital (60mg/kg). The left kidney was accessed through a left lateral incision and a 0.20 mm silver clip was placed around the left renal artery. In the 1K1C rat, the right kidney was accessed through a right lateral incision and removed after tying off the renal artery and vein. 1K1C rats and their sham operated controls (SOC) were studied 2 wks post surgery (1K1C, 11±0.2 wks, 311±7 g, n=17 and SOC, 11±0.3 wks, 317±7 g, n=18) and 9 weeks post surgery (1K1C 19±0.4 wks, 413±10 g, n=23 and SOC 18±0.3 wks, 423±7 g, n=18). 2K1C rats and their SOC were studied 4 weeks post clipping (2K1C 11±0.4 wks, 349±9 g, n=13 and SOC 12±0.4 wks, 364±9 wks, n=13). All rats were housed with free access to food and water in an animal care facility at the Medical College of Wisconsin (MCW), which is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the MCW IACUC.
In Vivo Experiments
On the day of the experiment, the rats were anesthetized with sodium pentobarbital (60mg/kg, i.p.), and an in situ transilluminated cremaster muscle was prepared for measurement of internal diameters of third-order arterioles via television microscopy, as described previously (Baez, 1973; Hill et al., 1990). The tissue was superfused at 35°C at a rate of 3–5ml/minute with a bicarbonate-buffered (pH 7.35) physiological salt solution (PSS), equilibrated with 0% O2, 5% CO2, 95% N2 gas mixture, to ensure that O2 delivery to the tissue was controlled entirely by the microcirculation and that no O2 was delivered from the superfusate. Under these conditions, PO2 in the rapidly flowing layer of the superfusate is 3–5mmHg, while tissue PO2 is higher, due to O2 supply from the microcirculation. Arterioles for study were selected by indentifying a second-order arteriole in a clearly visible region of the cremaster muscle and tracking along its length to find a third order arteriolar branch that was located in a region of the muscle that was away from any incision, had clearly discernible vessel walls, a brisk flow velocity, and active tone, as verified by the occurrence of a brisk dilation following topical application of 10−4 M adenosine.
Evaluation of Vascular O2 Sensitivity
After a 30 minute to 1 hour equilibration period, control measurements of arteriolar diameter and mean arterial pressure (carotid artery cannula) were obtained each minute for 5 minutes during 0% O2 superfusion. Arteriolar responses to increased O2 availability were then tested by measuring arteriolar diameters for 10 minutes after equilibrating the superfusion solution with a 21% O2, 5% CO2, 74% N2 gas mixture. This gas mixture causes a significant elevation of tissue and periarteriolar PO2, although not to the same extent as the elevation in superfusate PO2 (Duling and Berne, 1970), and has been used in previous studies testing arteriolar O2 sensitivity (Frisbee et al., 2000). In those studies, arteriolar responses to smaller elevations in superfusate oxygen concentration (5% O2 and 10% O2) were also potentiated in animals with reduced renal mass hypertension, and were sensitive to inhibition of 20-HETE production (Frisbee et al., 2000). After exposure to the 21% O2 solution, the superfusate was re-equilibrated with the control (0% O2) gas mixture until vessel diameters recovered to their control values. The preparation was then superfused for 30 minutes at 0.33 mL per minute with warmed PSS containing a 50 µM solution of the selective cytochrome P450 4A ω-hydroxylase inhibitor, N-methylsulfonyl-12, 12-dibromododec-11-enamide (DDMS) (Alonso-Galicia et al., 1997) or its vehicle (a 0.1% solution of absolute ethanol added to PSS), followed by continuous superfusion with PSS containing a 1 µM maintenance concentration of DDMS (DDMS treated animals only) for the remainder of the experiment. After application of the DDMS, the preparation was superfused again with 0% O2 solution at the control rate of 3–5 ml/min, after which arteriolar responses to elevated PO2 were re-evaluated. Vessel responses to 10−7 M norepinephrine were also tested to verify the ability of the arteriole to respond to vasoconstrictor stimuli, e.g., in vessels where inhibition of the CYP4A system eliminated O2-induced constriction of the arterioles.
Statistical Analysis
In order to determine the influence of the treatment factor and the surgical factor on the results, data was statistically analyzed with a two way ANOVA with repeated measures and a Bonferroni post hoc test (Figure 2 and Figure 3, GraphPad Prism) and were summarized as means ±SEM. Data for Figure 1 (arteriolar constriction to 21% oxygen in all groups before any treatment) was statistically analyzed with a one way ANOVA with a Student Newman-Keuls post hoc test. A p < 0.05 was considered to be statistically significant.
Figure 2.
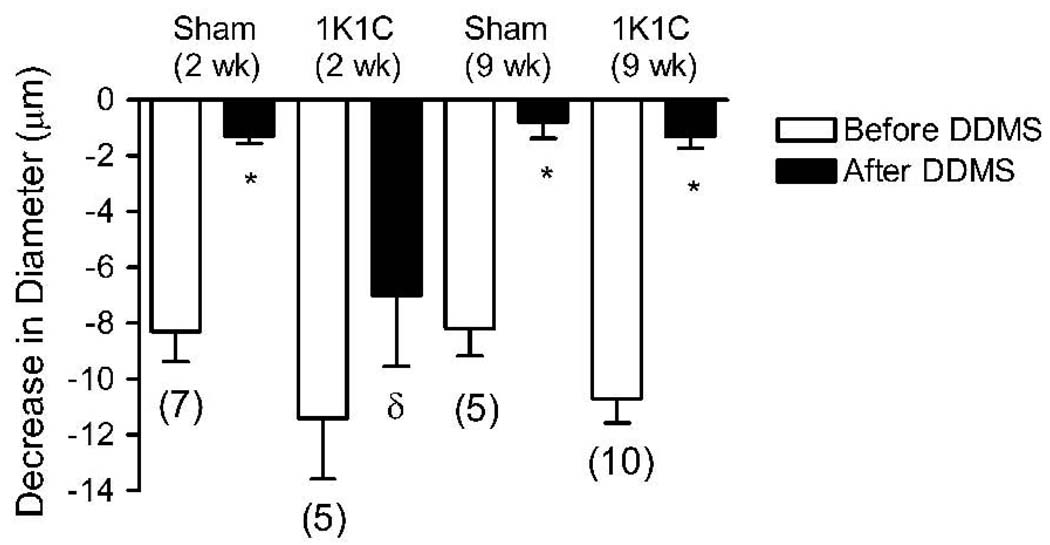
Decrease in cremasteric arteriolar diameter during superfusion with 21% O2 PSS before (open bars) and after (black solid bars) the application of DDMS in 1K1C rats 2 weeks post clipping and 9 weeks post clipping versus their respective sham-operated controls (open and slant-lined bar, respectively). Parentheses indicate number of animals in each group; * = within group, significantly different (p<0.01) from the response prior to treatment with DDMS; δ=between groups, significantly different (p<0.05) from sham-operated control with same treatment.
Figure 3.
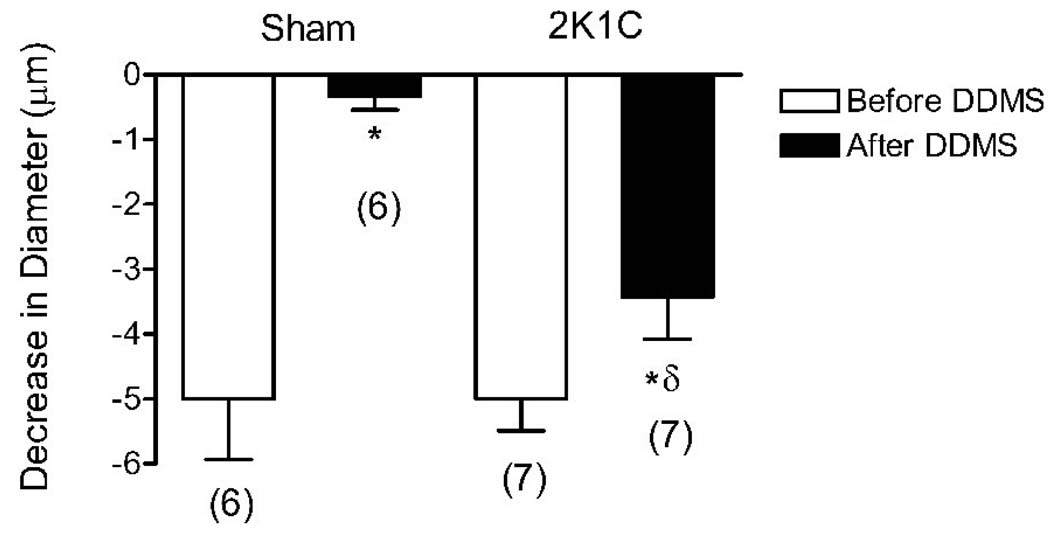
Decrease in cremasteric arteriolar diameter during superfusion with 21% O2 PSS before and after application of DDMS in 2K1C rats 4 weeks after clipping the left renal artery versus their sham-operated controls. Parentheses indicate number of animals in each group; * = within group, p<0.05 (2K1C); p<0.001(sham), significantly different from response prior to treatment with DDMS, δ= p<0.01, between groups, significantly different from sham with same treatment.
Figure 1.
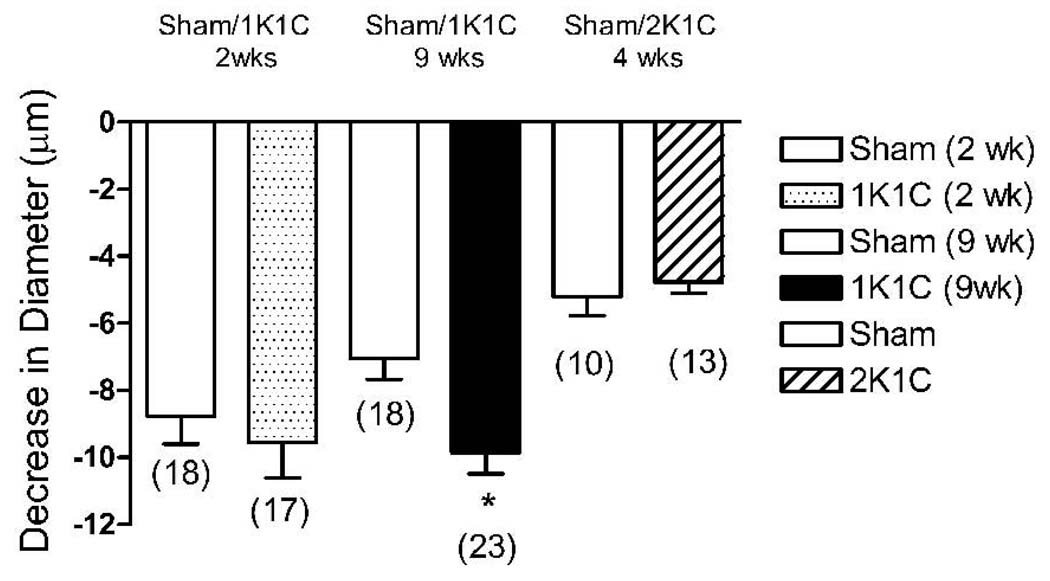
Decrease in cremasteric arteriolar diameter from control (0% O2 superfusion) in response to superfusion with 21% O2 PSS before any treatment in all 1K1C rats 2 weeks post clipping (stippled bar), all 1K1C rats 9 weeks post clipping (black solid bar) and all 2K1C rats 4 weeks post clipping (slanted line bar) versus all their respective sham-operated controls (open bars). Parentheses indicate number of animals in each group; * = p<0.05, significantly different from matched sham prior to any treatment.
RESULTS
Arterial blood pressures in the 1K1C (2 and 9 wk) and 2K1C (4 week) rats were significantly higher than those of their respective sham-operated controls (Table 1). Treatment with vehicle only or DDMS (Table 1) had no effect on the resting diameters (0% O2 superfusion) of arterioles in the sham or experimental rats, 2, 9 or 4 weeks post surgery (Table 1). Treatment with vehicle alone also had no effect on arteriolar responses to elevated PO2 in any of the experimental groups (data not shown for clarity).
Table 1. Mean arterial pressures and arteriolar diameters in 1K1C and 2K1C rats.
First Column: Mean arterial pressure in 1K1C rats (2 and 9 weeks post clipping) and 2K1C rats (4 weeks post clipping) versus mean arterial pressure in their respective sham-operated controls. The pressures represent averages of blood pressure measurements taken via the carotid artery in each anesthetized rat throughout the experimental protocol. Brackets indicate number of animals in each group * = p<0.05, mean arterial pressure significantly different from sham-operated controls. Third through Fifth Column: Diameter (µm) in cremasteric arterioles of 1K1C, 2 and 9 weeks post clipping, 2K1C, 4 weeks post clipping and in their respective sham-operated controls at rest (initial post-equilibration diameter) and before and after the application of DDMS with 0% oxygen in the superfusion solution (PSS). Vehicle alone had no effect on resting diameters (data not shown to improve clarity).
| Mean Arterial Pressure (mmHg±SE) [“n”] |
Animal Group | Arteriolar Diameter (0% O2 Superfusion) |
||
|---|---|---|---|---|
| Initial Diameter (µm±SE) |
Before DDMS (µm±SE) |
After DDMS (µm±SE) |
||
| 160±6 [17] | 1K1C (2 wk) | 24±0.5 | 25 ±1.0 | 26±1.0 |
| 121±4 [17] | Sham (2 wk) | 25±0.7 | 24±1.0 | 25±1.0 |
| 166±3 [23] | 1K1C (9 wk) | 24±0.6 | 24±0.7 | 24±1.0 |
| 129±4 [18] | Sham (9 wk) | 23±0.6 | 23±1.0 | 23±1.0 |
| 160±3 [23] | 2K1C (4 wk) | 20±1.3 | 20±1.3 | 20±1.3 |
| 132±5 [21] | Sham (4 wk) | 20±0.9 | 19±1.4 | 20±1.3 |
Arteriolar constriction in response to elevated PO2 in 1K1C rats (prior to vehicle or any treatment) was similar to sham-operated controls 2 weeks post-clipping, but was significantly larger in the hypertensive animals 9 weeks post-clipping compared to their age-matched sham-operated controls (Figure 1). DDMS eliminated arteriolar constriction during 21% O2 superfusion in the 1K1C rats 9 weeks post clipping and in all sham operated controls, but had no effect on O2-induced constriction of arterioles in 1K1C rats 2 weeks post clipping (Figure 2). The alternative CYP-4A inhibitor 17-octadecynoic acid (17-ODYA, 10 µM) had identical effects on arteriolar O2 responses in both groups of 1K1C rats and their sham operated controls, verifying that the failure of DDMS to eliminate O2-induced constriction in arterioles of 2 week 1K1C rats was not due to ineffectiveness of the inhibitor (data not shown). In 2K1C rats, arteriolar constriction in response to elevated PO2 was similar to that in the sham-operated controls (Figure 1). DDMS abolished arteriolar constriction in response to 21% O2 superfusion in the sham-operated controls, and reduced, but did not eliminate O2-induced constriction of arterioles in the 2K1C rats 4 weeks post-clipping (Figure 3).
DISCUSSION
Plasma renin activity (PRA) is normal in 1K1C hypertensive rats after a brief rise immediately post clipping (Ayers et al., 1977; Murphy et al., 1984), but is substantially elevated (2.5–7 times normal) 4 weeks after clipping in the 2K1C model (Amiri and Garcia, 1997; Murphy et al., 1984; Russell et al., 1983; Welch et al., 2007; Wilcox et al., 1996). Therefore, the design of the present study can provide insight into the possible influence of the renin-angiotensin system on vascular responses to elevated PO2, and the ability of cytochrome P450 inhibition to modify that response in 1K1C and 2K1C renovascular hypertension. This is important because circulating ANG II levels have been shown to play a crucial role in maintaining normal vascular relaxation in response to reduced PO2 and other vasodilator stimuli (Lombard et al., 2003; McEwen et al., 2009; Phillips and Lombard, 2005).
In this study, arteriolar responses to elevated PO2 were enhanced in 1K1C rats 9 weeks after clipping (Figure 1). However, cremasteric arterioles of 2 week 1K1C (Figure 1) and 4 week 2K1C (Figure 2) rats did not show an enhanced O2 response compared to their sham-operated controls, even though arterial blood pressure was significantly elevated in both models of hypertension (Table 1). The lack of an enhanced response to elevated PO2 in these groups is surprising, and contrasts with the enhanced constriction to elevated PO2 in multiple models of hypertension including SHR with developing hypertension (Kunert et al., 2001b; Lombard et al., 1986), Dahl SS rats (Rafi and Boegehold, 1993), and rats in the early and established stages of reduced renal mass (RRM) hypertension (Lombard et al., 1989). To the best of our knowledge, this is the first documented case of a hypertensive rat model in which O2-induced constriction of arterioles is not enhanced early in hypertension. While the reason for this difference is not clear, it is possible that the neural, hormonal, or local mechanisms that potentiate O2-induced constriction of arterioles in other forms of hypertension are not altered sufficiently to enhance arteriolar O2 responses in either the 1K1C or 2K1C rats.
In contrast to sham operated controls and 1K1C rats 9 weeks post clipping, inhibition of the CYP450 4A/20-HETE system with DDMS did not affect O2-induced constriction of arterioles in the 2 week 1K1C rat (Figure 2) and only partially inhibited O2 induced constriction of arterioles in the 4 week 2K1C rats (Figure 3). These observations suggest that factors other than the CYP-4A/20-HETE system contribute to O2-induced vasoconstriction of arterioles in the earlier phase of 1K1C or 2K1C Goldblatt hypertension, while this metabolic pathway eventually plays a role in the enhanced response to elevated PO2 in the established stage of 1K1C Goldblatt hypertension. This finding is similar to that of a previous study showing that inhibition of cytochrome P-450 4A ω-hydroxylase with two mechanistically different inhibitors, 17-octadecynoic acid (17-ODYA) and N-methyl-sulfonyl-12, 12-dibromododec-11-enamide (DDMS) attenuated arteriolar constriction to elevated PO2 in mature (12 week old) SHR, but not in young (6 week old) SHR (Kunert et al., 2001b), suggesting that 20-HETE mediates the oxygen-induced constriction of arterioles in SHR with established hypertension, but not in young SHR prior to the development of established hypertension.
The differential response to 20-HETE inhibition after different durations of 1K1C hypertension appears to be related to vascular alterations occurring during the development of hypertension, rather than age per se, because cytochrome P450 inhibition eliminated O2-induced constriction of arterioles in age-matched sham-operated controls for all the groups (Figure 2 and Figure 3). This reduced contribution of CYP-4A enzymes and 20-HETE to arteriolar constriction in the 2 week 1K1C rats and the 2K1C rats may involve mechanisms similar to those reported by Marvar et al. (Marvar et al., 2007), who found that chronic exposure to elevated dietary salt intake led to a reduction in arteriolar sensitivity to elevated PO2 that was mediated by a reduced responsiveness of the arterioles to 20-HETE.
Since plasma renin activity is normal in the early 1K1C rat but high in the 4 week post clipping 2K1C rat (Amiri and Garcia, 1997), plasma renin levels do not seem to explain the differential response to inhibiting 20-HETE production in these models. The latter finding is consistent with studies of SS rats versus consomic and congenic controls, where normalization of PRA and circulating ANG II levels by introgression of the Brown Norway (Drenjancevic-Peric et al., 2005) or Dahl R renin gene (Drenjancevic-Peric et al., 2004) does not affect vascular oxygen responses or prevent the enhanced constriction of cremasteric arterioles in response to elevated PO2 in salt-fed animals (even though it restores normal vascular relaxation in response to acetylcholine).
In summary, the CYP450 4A/20-HETE system plays a role in the enhanced O2-induced vasoconstriction in 1K1C rats with established hypertension. However, O2-induced vasoconstriction is not enhanced in the acute phases of the 1K1C (normal renin) and the 2K1C (high renin) models of renovascular hypertension. Inhibition of cytochrome P450 ω-hydroxylase does not affect O2-induced constriction of skeletal muscle arterioles of rats in the acute phase of 1K1C renovascular hypertension and only partially inhibits arteriolar constriction in response to elevated PO2 in animals with 4 weeks of 2K/1C hypertension, but eliminates arteriolar O2 responses in the established phase of 1K1C hypertension. Taken together, these findings indicate that 20-HETE plays a role in the oxygen response during steady state renovascular hypertension, but that other factors dominate during the transition to a stabilized hypertensive state.
Acknowledgments
support: K01NR000105 NINR; HL29587; HL65289; HL72920
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research performed
Medical College of Wisconsin, Physiology Department, 8701 Watertown Plank Rd, Milwaukee Wisconsin 53226
References
- Alonso-Galicia M, et al. Inhibition of 20-HETE Production Contributes to the Vascular Responses to Nitric Oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, et al. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- Amiri F, Garcia R. Renal angiotensin II receptor regulation in two-kidney, one clip hypertensive rats: effect of ACE inhibition. Hypertension. 1997;30:337–344. doi: 10.1161/01.hyp.30.3.337. [DOI] [PubMed] [Google Scholar]
- Ayers CR, et al. Intrarenal renin-angiotensin-sodium interdependent mechanism controlling postclamp renal artery pressure and renin release in the conscious dog with chronic one-kidney Goldblatt hypertension. Circ Res. 1977;40:238–242. doi: 10.1161/01.res.40.3.238. [DOI] [PubMed] [Google Scholar]
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Bak Z, et al. Human cardiovascular dose-response to supplemental oxygen. Acta Physiol (Oxf) 2007;191:15–24. doi: 10.1111/j.1748-1716.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- Cabrales P, et al. Nitric oxide regulation of microvascular oxygen exchange during hypoxia and hyperoxia. J Appl Physiol. 2006;100:1181–1187. doi: 10.1152/japplphysiol.01105.2005. [DOI] [PubMed] [Google Scholar]
- Carroll MA, et al. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- Carroll MA, et al. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol. 1996;271:R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- Croft KD, et al. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- Drenjancevic-Peric I, et al. Skeletal muscle arteriolar reactivity in SS.BN13 consomic rats and Dahl salt-sensitive rats. Hypertension. 2003;41:1012–1015. doi: 10.1161/01.HYP.0000067061.26899.3E. [DOI] [PubMed] [Google Scholar]
- Drenjancevic-Peric I, et al. Arteriolar responses to vasodilator stimuli and elevated P(O2) in renin congenic and Dahl salt-sensitive rats. Microcirculation. 2004;11:669–677. doi: 10.1080/10739680490517695. [DOI] [PubMed] [Google Scholar]
- Drenjancevic-Peric I, et al. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am J Physiol Heart Circ Physiol. 2005;289:H188–H195. doi: 10.1152/ajpheart.00504.2004. [DOI] [PubMed] [Google Scholar]
- Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27:669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, et al. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H1517–H1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, et al. Role of prostanoids and 20-HETE in mediating oxygen-induced constriction of skeletal muscle resistance arteries. Microvasc Res. 2001;62:271–283. doi: 10.1006/mvre.2001.2341. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, et al. Integration of hypoxic dilation signaling pathways for skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol. 2002;283:R309–R319. doi: 10.1152/ajpregu.00741.2001. [DOI] [PubMed] [Google Scholar]
- Gilmore ED, et al. Retinal arteriolar diameter, blood velocity, and blood flow response to an isocapnic hyperoxic provocation. Am J Physiol Heart Circ Physiol. 2005;288:H2912–H2917. doi: 10.1152/ajpheart.01037.2004. [DOI] [PubMed] [Google Scholar]
- Harder DR, et al. Identification of a putative microvascular oxygen sensor. Circ Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- Hill MA, et al. Altered cremaster muscle hemodynamics due to disruption of the deferential feed vessels. Microvasc Res. 1990;39:349–363. doi: 10.1016/0026-2862(90)90048-v. [DOI] [PubMed] [Google Scholar]
- Jackson RM, et al. Production of arachidonic acid metabolites by endothelial cells in hyperoxia. J Appl Physiol. 1986;61:584–591. doi: 10.1152/jappl.1986.61.2.584. [DOI] [PubMed] [Google Scholar]
- Kolbitsch C, et al. The influence of hyperoxia on regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV) and cerebral blood flow velocity in the middle cerebral artery (CBFVMCA) in human volunteers. Magn Reson Imaging. 2002;20:535–541. doi: 10.1016/s0730-725x(02)00534-9. [DOI] [PubMed] [Google Scholar]
- Kunert MP, et al. Cytochrome P-450 omega-hydroxylase: a potential O(2) sensor in rat arterioles and skeletal muscle cells. Am J Physiol Heart Circ Physiol. 2001a;280:H1840–H1845. doi: 10.1152/ajpheart.2001.280.4.H1840. [DOI] [PubMed] [Google Scholar]
- Kunert MP, et al. Differential effect of cytochrome P-450 omega-hydroxylase inhibition on O2-induced constriction of arterioles in SHR with early and established hypertension. Microcirculation. 2001b;8:435–443. doi: 10.1038/sj/mn/7800114. [DOI] [PubMed] [Google Scholar]
- Lombard JH, et al. Evaluation of cytochrome P450-4A omega-hydroxylase and 20-hydroxyeicosatetraenoic acid as an O2 sensing mechanism in the microcirculation. Methods Enzymol. 2004;381:140–165. doi: 10.1016/S0076-6879(04)81009-7. [DOI] [PubMed] [Google Scholar]
- Lombard JH, et al. Neural and local control of arterioles in SHR. Hypertension. 1984;6:530–535. doi: 10.1161/01.hyp.6.4.530. [DOI] [PubMed] [Google Scholar]
- Lombard JH, et al. Enhanced response of arterioles to oxygen during development of hypertension in SHR. Am J Physiol. 1986;250:H761–H764. doi: 10.1152/ajpheart.1986.250.5.H761. [DOI] [PubMed] [Google Scholar]
- Lombard JH, et al. Hemodynamics and microcirculatory alterations in reduced renal mass hypertension. Hypertension. 1989;13:128–138. doi: 10.1161/01.hyp.13.2.128. [DOI] [PubMed] [Google Scholar]
- Lombard JH, et al. Cytochrome P-450 omega-hydroxylase senses O2 in hamster muscle, but not cheek pouch epithelium, microcirculation. Am J Physiol. 1999;276:H503–H508. doi: 10.1152/ajpheart.1999.276.2.H503. [DOI] [PubMed] [Google Scholar]
- Lombard JH, Stekiel WJ. Active tone and arteriolar responses to increased oxygen availability in the mesoappendix of spontaneously hypertensive rats. Microcirc Endothelium Lymphatics. 1988;4:339–353. [PubMed] [Google Scholar]
- Lombard JH, et al. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- Mak S, et al. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414–H2421. doi: 10.1152/ajpheart.00947.2001. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, et al. High dietary salt reduces the contribution of 20-HETE to arteriolar oxygen responsiveness in skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1507–H1515. doi: 10.1152/ajpheart.00754.2006. [DOI] [PubMed] [Google Scholar]
- McEwen ST, et al. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation. 2009;16:220–234. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina EJ, et al. Increases in oxygen tension evoke arteriolar constriction by inhibiting endothelial prostaglandin synthesis. Microvasc Res. 1994;48:151–160. doi: 10.1006/mvre.1994.1046. [DOI] [PubMed] [Google Scholar]
- Murphy WR, et al. Effects of graded renal artery constriction on blood pressure, renal artery pressure, and plasma renin activity in Goldblatt hypertension. Hypertension. 1984;6:68–74. doi: 10.1161/01.hyp.6.1.68. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Lombard JH. Chronic At1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol. 2005;46:706–712. doi: 10.1097/01.fjc.0000184118.76188.8c. [DOI] [PubMed] [Google Scholar]
- Rafi JA, Boegehold MA. Microvascular responses to oxygen and muscle contraction in hypertensive Dahl rats. Int J Microcirc Clin Exp. 1993;13:83–97. [PubMed] [Google Scholar]
- Rossi P, Boussuges A. Hyperoxia-induced arterial compliance decrease in healthy man. Clin Physiol Funct Imaging. 2005;25:10–15. doi: 10.1111/j.1475-097X.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- Rousseau A, et al. Hyperoxia decreases cutaneous blood flow in high-perfusion areas. Microvasc Res. 2007;74:15–22. doi: 10.1016/j.mvr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Russell GI, et al. Hemodynamic changes induced by reversal of early and late renovascular hypertension. Am J Physiol. 1983;245:H734–H740. doi: 10.1152/ajpheart.1983.245.5.H734. [DOI] [PubMed] [Google Scholar]
- Sirinyan M, et al. Hyperoxic exposure leads to nitrative stress and ensuing microvascular degeneration and diminished brain mass and function in the immature subject. Stroke. 2006;37:2807–2815. doi: 10.1161/01.STR.0000245082.19294.ff. [DOI] [PubMed] [Google Scholar]
- Tsai AG, et al. Microvascular oxygen distribution in awake hamster window chamber model during hyperoxia. Am J Physiol Heart Circ Physiol. 2003;285:H1537–H1545. doi: 10.1152/ajpheart.00176.2003. [DOI] [PubMed] [Google Scholar]
- Welch WJ, et al. Roles of vasoconstrictor prostaglandins, COX-1 and -2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2644–H2649. doi: 10.1152/ajpheart.00748.2007. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, et al. AT1 and TxA2/PGH2 receptors maintain hypertension throughout 2K,1C Goldblatt hypertension in the rat. Am J Physiol. 1996;271:R891–R896. doi: 10.1152/ajpregu.1996.271.4.R891. [DOI] [PubMed] [Google Scholar]
- Yamazaki F. Hyperoxia attenuates endothelial-mediated vasodilation in the human skin. J Physiol Sci. 2007;57:81–84. doi: 10.2170/physiolsci.SC011006. [DOI] [PubMed] [Google Scholar]