Abstract
Bicyclic radester analogues have been synthesized and evaluated for Hsp90 inhibitory activity. These analogues induce concentration-dependent degradation of Hsp90-dependent client proteins with the six-membered bicyclic analogues manifesting increased activity versus the five-membered counterparts.
Keywords: Hsp90, Geldanamycin, Radicicol, Anti-cancer
The 90 kDa heat shock proteins (Hsp90) are well characterized molecular chaperones responsible for the conformational maturation of nascent polypeptides and the rematuration of denatured proteins.1,2 Inhibition of Hsp90 disrupts the protein folding process, resulting in client protein degradation via the ubiquitin-proteasome pathway. Previous studies have demonstrated that the Hsp90 multiprotein complex prevalent in tumor cells exhibits a higher affinity for N-terminal ligands than the homodimeric form present in non-transformed cells. It is well known that malignant cells are highly dependent upon the Hsp90 protein folding machinery for cell survival due to the over expression of proteins needed for continual growth in hostile environments.3 Consequently, inhibition of Hsp90 results in combinatorial disruption of multiple signaling pathways that are essential to tumor cell survival and represents a promising strategy for the development of therapeutics.4
Hsp90 is an ATP-dependent protein comprised of two nucleotide-binding domains, one of which is located at the N-terminus and the other at the C-terminus.5 ATP hydrolysis at the N-terminus provides the requisite energy necessary for folding nascent polypeptides into their biologically active, three-dimensional conformations. Thus, disruption of this ATPase activity results in destabilization of the heteroprotein complex, which leads to the subsequent degradation of clients.3
N-terminal inhibitors of Hsp90 manifest their activity by binding competitively to the N-terminal ATP-binding site and preventing Hsp90-catalyzed hydrolysis of ATP. To date, several natural products have been identified as N-terminal inhibitors. Geldana-mycin (GDA)6 and radicicol (RDC, Fig. 1)6 are two prominent examples previously reported. RDC is the most potent of these inhibitors in vitro, however, in vivo it is rapidly converted into metabolites that exhibit little or no affinity for Hsp90.7 In contrast, GDA is less potent than RDC in vitro, but in cellular assays manifests greater affinity for the Hsp90 heteroprotein complex prevalent in malignant cells.3 Although two derivatives of GDA have entered clinical trials for the treatment of several cancers, toxicity and formulation difficulties remain.
Figure 1.
Known inhibitors of Hsp90.
The co-crystal structures of GDA and RDC bound to Hsp908 have been solved and reveal important binding interactions manifested by these natural products. In contrast to the extended conformation of GDA found in solution, the natural product binds Hsp90 in a bent, C-shaped conformation that contains an unusual cis-amide bond. Consequently, it appears the process of GDA binding to Hsp90 results in an entropic penalty, which has been studied in some detail.9 In contrast, RDC exists in the same bent conformation bound or unbound to Hsp90, producing favorable entropy upon binding. As a result of these thermodynamic data, it has been proposed that GDA analogues that contain conformational rigidity would yield compounds that exhibit enhanced Hsp90 affinity through minimization of entropic penalties.10–14
Recently, chimeric Hsp90 inhibitors were disclosed in which pharmacophores from both natural products were combined to provide a novel scaffold. Our laboratory has developed15–17 this class of chimeric analogues that result from hybridization of the resorcinol ring of RDC and the quinone of GDA. These compounds bind to the Hsp90 N-terminus and prevent ATP hydrolysis, similar to the natural products.18 Moreover, radester (Fig. 1) exhibits more potent ATPase inhibition than GDA.16
Overcoming the entropic barrier associated with the conformational rearrangement observed by N-terminal ligands upon binding Hsp90 is an important consideration for the development of new inhibitory scaffolds. Analyses of co-crystal structures of RDC and radester bound to Hsp90 revealed the ester carbonyl in radester to be more planar than the same moiety in RDC. The planarity of this carbonyl potentially allows for greater electron delocalization between the ester carbonyl and the phenols, resulting in an electron rich carbonyl and more acidic phenols. High electron density at this carbonyl is predicted to be important due to its mediation of hydrogen bonding interactions with Asp 79, Gly 83 and Thr 171. Thus, inhibitors existing in a bent conformation that exhibit a planar carbonyl are hypothesized to minimize the entropic penalty upon binding to Hsp90 and exhibit higher affinity due to increased utilization of the hydrogen bonding network. Therefore we proposed a series of conformationally constrained chimeric analogues exhibiting both a bent conformation and conformationally constrained carbonyls (Fig. 2).
Figure 2.

Proposed conformationally constrained analogues.
Recently, Duerfeldt et al.19 utilized the chimera radamide as a model to demonstrate that conformationally constrained cis-amide analogues demonstrate improved affinity. Radester provides an additional opportunity to probe conformational constraints through manipulation of the resorcinol motif. Preliminary docking studies which overlay the designed inhibitors 1 and 2 with RDC indicate the carbonyl of the bicyclic analogues to exhibit greater planarity than RDC (Fig. 3). Defining the plane of the resorcinol as 0° allowed rotation from this plane to be measured for compounds 1 and 2. As depicted in Figure 3, the radicicol carbonyl exhibits an angle of ∼45°, whereas 1 exhibits an angle of ∼9°, and compound 2 deviates ∼8°, supporting the conformational constraint of the carbonyl moieties. Herein, we present the design, syntheses, and biological evaluation of chimeric Hsp90 inhibitors containing a conformationally constrained bicyclic ring system.
Figure 3.

Overlay of compounds 1 (orange) and 2 (green) with RDC in the Hsp90 N-terminal ATP-binding domain. The resorcinol plane is depicted as a purple line.
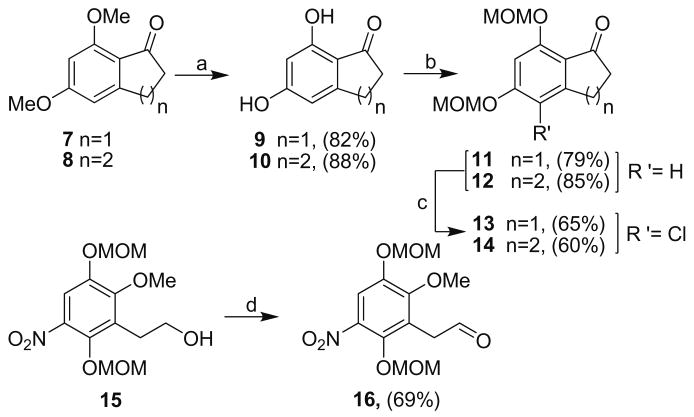
Retrosynthetically, analogues 1–6 were envisioned to be constructed via an aldol condensation between nitro-aldehyde 16 and the homologated bicyclic ketones, 13–14 (Scheme 1). Aldehyde 16 was proposed to originate from alcohol 15, which can be prepared in three steps from commercially available methoxyhy-droquinone. Cyclic ketones 13–14 were to be prepared from bicyclic ketone compounds, 7–8, which have been previously synthesized from commercially available 3,5-dimethoxybenzaldehyde.20,21
Scheme 1.
Retrosynthetic analysis.
Commencing with the synthesis of intermediates 13–14 and 16, as illustrated in Scheme 2, ketones 7–8 were constructed following known procedures.20,21 Cleavage of the methyl ethers afforded phenolic compounds 9–10. Resorcinol 9 was obtained by treating 7 with boron tribromide at −78 °C and 10 was prepared by reaction of 8 with hydrobromic acid in acetic acid at 110 °C. Treatment of compounds 9–10 with sodium hydride and methoxy methyl chloride gave the corresponding MOM-protected phenols 11–12 in good yields. Mild conditions enlisting calcium hypochlorite afforded the chlorinated aromatics, 13–14. Quinone precursor 16 was prepared by PCC oxidation of alcohol 15, which was readily available via literature procedures.15
Scheme 2.
Reagents and conditions: (a) BBr3, −78 °C to rt, 5 h or HBr, AcOH, 110 °C, 48 h; (b) NaH, DMF, MOMCl, 0°C to rt, 2 h; (c) Ca(OCl)2, acetone, AcOH/H2O (1:10), rt, 2 h; (d) PCC, DCM, MS (4 Å), rt, 2 h.
Synthesis of compounds 1–6 was accomplished by following the sequence of reactions depicted in Scheme 3. Aldol condensation between ketones 13–14 and aldehyde 16 followed by dehydration of the incipient secondary alcohols enlisting phosphorus oxychloride in pyridine, provided alkenes 17–18 in good yields. Simultaneous reduction of the nitro and alkene moieties of 17–18 employing palladium-carbon under a hydrogen atmosphere provided anilines 19–20. To mimic the amide functionality present in GDA, the aniline was converted to the corresponding formamide, 21–22, upon treatment with phenylformate. Removal of the methoxymethyl (MOM) protecting groups in situ with trimethylsilyl iodide resulted in formation of the corresponding hydroquinones, 1–2,22,23 which were subsequently oxidized to the desired quinones 3–4 with Frémy's salt (potassium nitrosodisulfonate) in buffered solution.24,25
Scheme 3.
Reagents and conditions: (a) (i) LDA, −78 °C to rt, THF,16, 2 h, (ii) POCl3, pyridine, rt, 6 h; (b) H2, Pd/C, EtOAc, 6 h; (c) PhOCHO, DCM, 40 °C, 12 h; (d) Zn, AcOH, DCM, rt, 10 min; (e) NaI, TMSCl, DCM/CH3CN, rt, 1 h; (f) Frémy's salt, acetone, rt, 15 min R = MOM for all compounds in this scheme.
Selective reduction of the nitro groups was required to access alkenes 5–6, which was problematic due to competing redox-sensitive groups (Scheme 3). The best results obtained utilize zinc metal and acetic acid in dichloromethane, in which case anilines 23–24 were subsequently converted to the corresponding formamides, 25–26, following reaction with phenylformate. Quinones 5–6 were then generated utilizing the same oxidation conditions used to afford 3–4.26,27
Upon completion of their syntheses, chimeric analogues 1–6 were evaluated for their anti-proliferative activity against MCF7 and SKBr3 breast cancer cell lines. As seen in Table 1, the six-membered analogues exhibited more potent activity than the corresponding five-membered analogues, and exhibited similar IC50 values against both cancer cell lines. In contrast, the five-membered analogues exhibited approximately three-old higher activity against MCF7 cells than SKBr3 cells.
Table 1.
Anti-proliferation activity of conformationally constrained analogues 1–6
In order to confirm the growth inhibitory activity manifested by these compounds was a consequence of Hsp90 inhibition, compounds 1 and 2 were subjected to Western blot analyses. As expected (Fig. 4), compounds 1 and 2 resulted in dose-dependent client protein degradation. Similarly, N-terminal inhibitory activity was evidenced by the concentration-dependant induction of heat shock response as demonstrated by increasing Hsp70 levels. Both, client protein degradation and heat shock induction at similar concentrations are indicative of Hsp90 N-terminal inhibition.
Figure 4.

Western blot analysis (a) for compound 1 and (b) for 2.
In conclusion, we have designed, synthesized and evaluated six new conformationally constrained analogues of radester and tested them against two breast cancer cell lines. Western blot analyses confirmed Hsp90 inhibition as evidenced by concentration-dependent degradation of Hsp90 clients and simultaneous induction of heat shock response. In general, six-membered analogues were found to be more effective than their five-membered counterparts. However, the activities of these compounds remain comparable to radester, indicating the introduction of conformationally constrained carbonyls does not play a significant role in organization of the N-terminal ATP-binding site.
Acknowledgments
The authors gratefully acknowledge NIH/NCI CA 109265, the Madison and Lila Self Graduate Fellowship (A.S.D.) and the American Foundation of Pharmaceutical Education for financial support (A.S.D.).
References and notes
- 1.Blagg BSJ, Kerr TD. Med Res Rev. 2006;26:310. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 2.Walter S, Buchner J. Angew Chem Int Ed. 2002;41:1098. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. Nature. 2003;425:407. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 4.Neckers L, Lee YS. Nature. 2003;425:357. doi: 10.1038/425357a. [DOI] [PubMed] [Google Scholar]
- 5.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. J Biol Chem. 2000;275:37181. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 6.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Proc Natl Acad Sci USA. 1994;91:8324. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng X, Yang ZQ, Danishefsky SJ. Synlett. 2004:1325. [Google Scholar]
- 8.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. J Med Chem. 1999;42:260. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 9.Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. Mol Cancer Ther. 2003;2:123. [PubMed] [Google Scholar]
- 10.Jez JM, Chen JCH, Rastelli G, Stroud RM, Santi DV. Chem Biol. 2003;10:361. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Onuoha SC, Mukund SR, Coulstock ET, Sengerova B, Shaw J, McLaughlin SH, Jackson SE. J Mol Biol. 2007;372:287. doi: 10.1016/j.jmb.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 12.Onodera H, Kaneko M, Takahashi Y, Uochi Y, Funahashi J, Nakashima T, Soga S, Suzuki M, Ikeda S, Yamashita Y, Rahayu ES, Kanda Y, Ichimura M. Bioorg Med Chem Lett. 2008;18:1588. doi: 10.1016/j.bmcl.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MQ, Gaisser S, Nur-E-Alam M, Sheehan LS, Vousden WA, Gaitatzis N, Peck G, Coates NJ, Moss SJ, Radzom M, Foster TA, Sheridan RM, Gregory MA, Roe SM, Prodromou C, Pearl L, Boyd SM, Wilkinson B, Martin CJ. J Med Chem. 2008;51:5494. doi: 10.1021/jm8006068. [DOI] [PubMed] [Google Scholar]
- 14.Hadden MK, Blagg BSJ. J Org Chem. 2009;74:4697. doi: 10.1021/jo900278g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen G, Wang M, Welch TR, Blagg BSJ. J Org Chem. 2006;71:7618. doi: 10.1021/jo061054f. [DOI] [PubMed] [Google Scholar]
- 16.Shen G, Blagg BSJ. Org Lett. 2005;7:2157. doi: 10.1021/ol050580a. [DOI] [PubMed] [Google Scholar]
- 17.Clevenger RC, Blagg BSJ. Org Lett. 2004;6:4459. doi: 10.1021/ol048266o. [DOI] [PubMed] [Google Scholar]
- 18.Immormino RM, Metzger LE, Reardon PN, Dollins DE, Blagg BSJ, Gewirth DT. J Mol Biol. 2009;388:1033. doi: 10.1016/j.jmb.2009.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerfeldt AS, Brandt GEL, Blagg BSJ. Org Lett. 2009;11:2353. doi: 10.1021/ol900783m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadeer G, Rama NH, Shah SJH. ARKIVOC. 2007;12 [Google Scholar]
- 21.El-Feraly FS, Cheatham SF, McChesney JD. Can J Chem. 1985;63:2232. [Google Scholar]
- 22.Analytical data for 1: 1H NMR (DMSO-d6, 400 MHz) δ 11.12 (s, 1H), 10.14 (s, 1H) 9.74 (d,J = 1.65 Hz, 1H), 8.82 (s, 1H), 8.36 (s, 1H), 8.17 (d,J = 2.1 Hz, 1H) 7.18 (s, 1H), 6.41 (s, 1H), 3.68 (s, 3H), 3.17 (dd,J = 7.9, 17.6 Hz, 1H), 2.70–2.63 (m, 3H), 2.53–2.50 (m, 1H), 1.96–1.89 (m, 1H), 1.45–1.38 (m, 1H); 13C NMR (DMSO-d6, 125 MHz) δ 203.8, 160.1, 156.0, 153.8, 143.6, 142.8, 142.7, 137.6, 124.3, 122.5, 116.8, 107.5, 107.3, 102.1, 59.9, 48.6, 46.9, 31.6, 21.9; IR (film) vmax 3307–3101, 2954, 2925, 1647, 1618, 1490, 1452, 1363, 1228 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C19H17ClNO7: 406.0694, found: 406.0686.
- 23.Analytical data for 2: 1H NMR (methanol-d4, 400 MHz) δ 8.18 (s, 1H), 7.07 (s, 1H) 6.28 (s, 1H), 3.78 (s, 3H), 3.16–3.10 (m, 1H), 2.88–2.76 (m, 3H), 2.57–2.47 (m, 1H), 2.32–2.26 (m, 1H), 2.15–2.08 (m, 1H), 1.95–1.87 (m, 1H), 1.73–1.65 (m, 1H); 13C NMR (methanol-d4, 125 MHz) δ 206.9, 165.2, 162.1, 161.7,145.4, 145.3, 144.6, 140.4, 126.3, 123.5, 112.5, 112.2, 109.1, 102.5, 61.4, 47.2, 30.7, 28.2, 27.6, 23.1; IR (film) vmax 3209, 2997, 2848, 1602, 1436, 1234 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C20H19ClNO7: 420.0850, found: 420.0839.
- 24.Analytical data for 3: 1H NMR (CDCl3, 500 MHz) δ 8.99 (s, 1H), 8.58 (s, 1H), 8.27 (s, 1H), 7.36 (s, 1H), 6.46 (s, 1H), 6.16 (s, 1H), 4.17 (s, 3H), 3.30 (dd, J = 7.75, 18.24 Hz, 1H), 2.83 (dd, J = 4.63, 18.05 Hz, 1H), 2.77–2.72 (m, 1H), 2.66–2.56 (m, 2H), 2.06–2.00 (m, 1H), 1.67–1.60 (m, 1H); 13C NMR (CDCl3, 125 MHz) δ 208.4, 182.5, 181.3, 159.5, 159.4, 159.3, 157.7, 156.5, 152.0, 114.2, 108.5, 102.3, 102.1, 101.1, 61.7, 47.3, 32.0, 29.7, 20.7; IR (film) vmax 3305–3103, 2997, 2927, 2856, 1654, 1649 1500, 1363, 1228 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C19H15ClNO7: 404.0537, found: 404.0543.
- 25.Analytical data for 4: 1H NMR (DMSO-d6, 500 MHz) δ 12.89 (s, 1H), 11.42 (s, 1H), 10.30 (s, 1H), 8.51 (s, 1H), 8.32 (s, 1H), 6.35 (s, 1H), 4.01 (s, 3H), 3.06 (dt, J = 4.99, 18.07 Hz, 1H), 2.84–2.77 (m, 1H), 2.64–2.56 (m, 1H), 2.26–2.20 (m, 1H), 1.98–1.93 (m, 1H), 1.86–1.78 (m, 2H), 1.54–1.46 (m 2H); 13C NMR (DMSO-d6, 125 MHz)) δ 204.6, 182.8, 182.3, 162.9, 162.4, 162.3, 160.1, 156.0, 143.7, 127.9, 112.6, 110.5, 110.3, 101.1, 61.2, 44.6, 27.5, 25.90, 25.12, 19.94; IR (film) vmax 3309–3143, 2995, 2918, 2848, 1604, 1569, 1438, 1369, 1224 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C20H17ClNO7: 418.0694, found: 418.0694.
- 26.Analytical data for 5: 1H NMR (DMSO-d6, 500 MHz) δ 11.73 (s, 1H), 10.45 (s, 1H) 10.36 (s, 1H), 8.59 (s,1H), 7.29 (s,1H), 6.52 (s, 1H), 6.40 (t,J = 8.0 Hz, 1H), 4.12 (s, 3H), 3.72 (s, 2H), 3.40 (d,J = 8.0 Hz, 2H); 13C NMR (DMSO-d6, 125 MHz) δ 188.8, 183.6, 182.3, 162.3, 160.1, 156.9, 156.1, 149.6, 138.3, 137.6, 129.8, 124.3, 118.7, 112.7, 107.5, 102.3, 61.4, 28.9, 23.1; IR (film) vmax 3201–3100, 2918, 2848, 1608, 1458, 1448, 1219 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C19H13ClNO7: 402.0381, found: 402.0367.
- 27.Analytical data for 6: 1H NMR (methanol-d4, 400 MHz) δ 8.51 (s, 1H), 7.92 (s,1H), 7.35 (s,1H), 6.70 (t, J = 7.9 Hz, 1H), 6.32 (s, 1H), 4.18 (s, 3H), 3.46 (d, J = 7.93 Hz 2H), 3.08 (t, J = 6.5 Hz, 2H), 2.94 (t, J = 6.9 Hz, 2H); 13C NMR (aceton-d6, 125 MHz) δ 192.0, 184.3, 180.2, 165.6, 164.7, 162.2, 160.8, 158.1, 144.8, 144.4, 135.8, 130.9, 113.9, 102.6, 102.5, 102.4, 62.1, 27.2, 25.2, 22.8; IR (film) vmax 3070, 2987, 2850, 1573, 1475, 1436, 688 cm−1; HRMS (ESI-) m/z [M–H]− calcd for C20H15ClNO7: 416.0537, found: 416.0534.