Abstract
Consequences of spinal cord injury (SCI) depend on the level and extent of injury. Cervical SCI often results in a compromised respiratory system. Primary treatment of SCI patients with respiratory insufficiency continues to be with mechanical ventilatory support. In an animal model of SCI, an upper cervical spinal cord hemisection paralyzes the hemidiaphragm ipsilateral to the side of injury. However, a latent respiratory motor pathway can be activated to restore respiratory function after injury. In this review, restoration of respiratory activity following systemic administration of theophylline, a respiratory stimulant will be discussed. Pharmacologically, theophylline is a non specific adenosine receptor antagonist, a phosphodiesterase inhibitor and a bronchodilator. It has been used in the treatment of asthma and other respiratory-related diseases such as chronic obstructive pulmonary disease (COPD) and in treatment of apnea in premature infants.
However, the clinical use of theophylline to improve respiration in SCI patients with respiratory deficits is a more recent approach. This review will focus on the use theophylline to restore respiratory activity in an animal model of SCI. In this model, a C2 hemisection (C2HS) interrupts the major descending respiratory pathways and paralyzes the ipsilateral hemidiaphragm. The review also highlights involvement of central and peripheral adenosine receptors in functional restitution. Biochemical binding assays that highlight changes in adenosine receptors after chronic theophylline administration are discussed as they pertain to understanding adenosine receptor-mediation in functional recovery. Finally, the clinical application of theophylline in SCI patients with respiratory deficits in particular is discussed.
Introduction
According to the National spinal cord data base, the major cause of mortality and morbidity following spinal cord injury (SCI) is respiratory failure. The mainstay of current therapy for patients with severe respiratory deficits following upper cervical spinal cord injury is mechanical respiratory support (National Spinal Cord Injury Statistical Center, 2008). Patients tethered to mechanical ventilators feel a loss of independence and long term use of mechanical support can often lead to infections and pneumonia (Winslow and Rozovsky, 2003). There is therefore a need for alternative approaches to alleviate respiratory deficits. In order to understand the respiratory system and improve impaired respiratory function after injury, several animal models are employed in basic research. In this review, we will focus on the rat C2 hemisection model (C2HS).
In the C2HS model, an upper cervical (C2) hemisection incision interrupts the major descending bulbospinal respiratory motor pathways and effectively paralyzes the ipsilateral hemidiaphragm. However, a latent respiratory motor pathway can be activated to restore function to the paralyzed hemidiaphragm (Goshgarian, 2003). The latent respiratory motor pathway is referred to as the “crossed phrenic pathway”, (CPP). The physiological basis for the CPP was elucidated by Lewis and Brookhart (1951) who concluded that the CPP could be attenuated by artificial respiration and enhanced by conditions that increased central respiratory drive. The conclusion of Lewis and Brookhart that the amount of CPP activity was directly proportional to the intensity of central respiratory drive and independent of the state of conduction in the contralateral phrenic nerve led to studies that investigated use of pharmacologic agents that enhance central respiratory drive and activate the CPP without the need to transect the functionally intact contralateral phrenic nerve.
2. Pharmacologic Activation of the CPP
The pharmacologic approach preserves the integrity of the contralateral phrenic nerve and simultaneously induces functional recovery in the previously paralyzed ipsilateral hemidiaphragm (Nantwi et al., 1996; Goshgarian, 2003, Mitchell and Johnson, 2003). With this approach the animal is better off functionally since the two halves of the diaphragm are functional, i.e., function in the ipsilateral hemidiaphragm is induced and function in the contralateral hemidiaphragm is initially left intact. The clinical relevance of the pharmacologically-induced activity is that the entire diaphragm becomes bilaterally functional and the animal is better able to deal with respiratory distress. Drugs known to stimulate respiration and excite neuronal activity have been extensively studied in functional recovery in the C2HS model.
A commonly used xanthine, 1, 3-dimethylxanthine, (theophylline) is a respiratory stimulant. It has also been used to treat apnea in infants, asthmatic patients, and patients with “chronic obstructive respiratory disease” (COPD, Newman et al., 1994). It enhances respiratory frequency in healthy adult humans (Gorini et al., 1994), humans with compromised a respiratory function (Chevrolet et al., 1984), as well as human infants (Bona et al., 1995). The pharmacologic profile of theophylline includes non specific adenosine receptor antagonism, phosphodiesterase (PDE) inhibition and bronchodilation (Ribeiro et al., 2003).
Theophylline was used in one of the earliest studies that employed the pharmacologic approach to activate the CPP and induce respiratory recovery without transecting the contralateral phrenic nerve (Nantwi et al., 1996).
2.1 Acute Theophylline administration
Theophylline administered systemically 24 hr after C2HS induces recovery in adult female retired Sprague-Dawley rats. In their first study, Nantwi and colleagues (1996) assessed the respiratory-related effects of theophylline in three groups of animals. In the first group of animals, activation of the CPP was induced (by asphyxia) and thereafter, varying doses (2.5-15 mg/kg) of theophylline were systemically administered in order to generate a dose response curve under standardized electrophysiologic recording conditions: the stroke volume of the small animal ventilator was set at 60 breaths/min, tidal volume was 2.5 ml, end tidal CO2 was set at 25 mm Hg and the rats were paralyzed with pancuronium bromide (0.5 mg/kg). In a second group of hemisected animals, activity in the ipsilateral and contralateral phrenic nerves was monitored continuously before and after systemic administration of theophylline in spontaneously-breathing C2 hemisected adult female retired Sprague-Dawley rats. In a third group of animals, respiratory activity in both the ipsilateral phrenic nerve and the ipsilateral hemidiaphragm were assessed under spontaneous breathing conditions (Fig 1).
Figure 1.
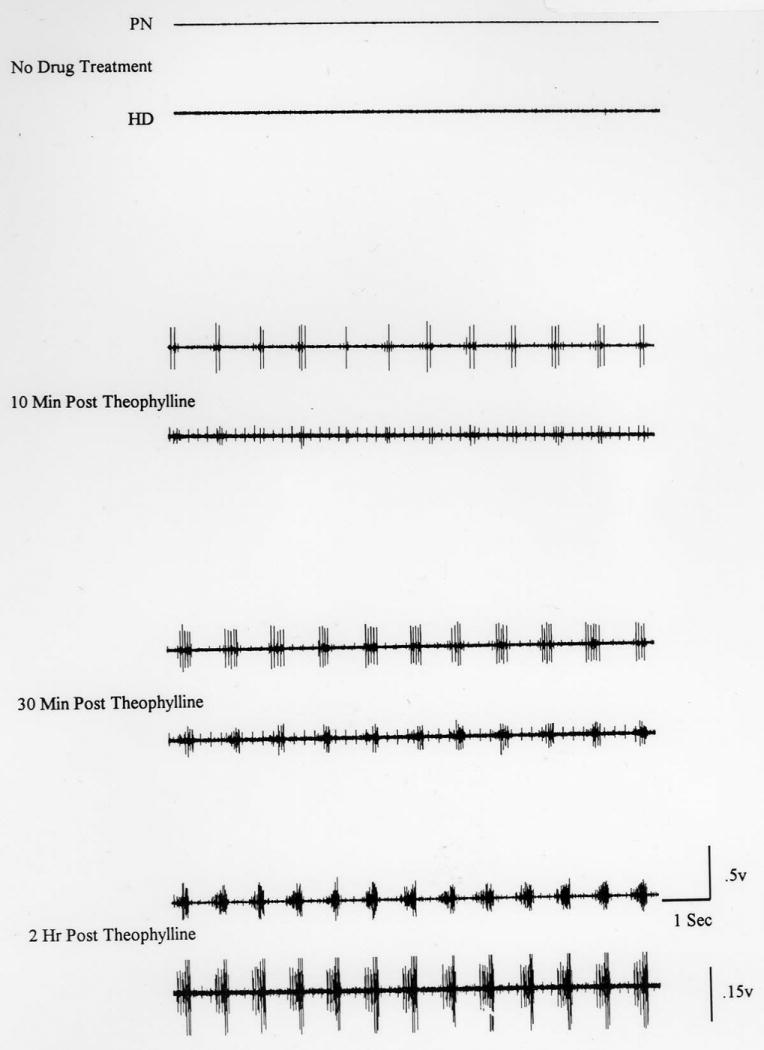
A representative neurogram from a hemisected and spontaneously breathing animal showing respiratory-related activity in the nerve (PN) and hemidiaphragm (HD) ipsilateral to hemisection. In the upper two traces, the total absence of activity in both the nerve and hemidiaphragm is indicative of a complete hemisection. However, 10 min after administration of theophylline (third and fourth traces), activity in the left nerve and left hemidiaphragm becomes evident. The induced respiratory-related activity in the nerve and hemidiaphragm becomes progressively enhanced. 30 min after the drug (fifth and sixth traces), induced activity is more distinct. At 2 h after drug administration, (seventh and eighth traces), the induced respiratory-related activity in both the nerve and hemidiaphragm is very similar (synchronous) with normal activity (Reprinted from Nantwi et al., 1996). Note the similarity in timing and duration of activity in both the nerve and hemidiaphragm.
The optimal dose was 15 mg/kg and the onset of drug effects was evident in 5-10 min post administration and persisted for the 2-3 h that electrophysiologic recordings of respiratory activity was monitored. In this study, Nantwi and colleagues demonstrated that functional recovery could be attained without the need to transect the contralateral phrenic nerve to enhance central respiratory drive to activate the CPP. This was a significant advancement in SCI research since never before had so much motor recovery been achieved in a mammalian model. Previous studies had demonstrated that in rats subjected to phrenicotomy, systemic administration of aminophylline a more soluble form of theophylline improved ventilation (Nachazel and Palecek, 1987). Aubier and colleagues suggested that improved ventilation in dogs after aminophylline infusion was due to enhanced diaphragmatic contraction and not to CNS stimulation (1983). The findings by Nantwi and colleagues confirmed conclusions by other investigators that theophylline/aminophylline stimulates respiration probably via a centrally-mediated mechanism though not excluding a direct diaphragmatic contraction. The duration of theophylline-induced recovery observed in the study by Nantwi and colleagues supported and extended the work of Olsen and Schlitz (1983) that in spontaneously breathing dogs, theophylline enhanced respiratory frequency for up to 3 h.
At 30 mg/kg, theophylline was not effective in inducing recovery of respiratory activity and actually depressed respiration. The finding that theophylline evokes excitation at 15 mg/kg and depression at 30 mg/kg, is consistent with the demonstration that theophylline induces a biphasic response in cats and in clinical studies (Eldridge et al., 1983; Woodcock and Johnson, 1983).
At the optimal dose (15 mg/kg, iv), theophylline not only induced recovery but also enhanced respiratory frequency and contralateral phrenic nerve activity. The theophylline-induced enhanced frequency suggested involvement of both spinal and brainstem mechanisms. The stimulatory effects of theophylline on respiratory activity observed in C2HS rats were also observed in non-injured age-matched control animals. The effects of theophylline in non injured rats confirmed previous findings theophylline stimulates respiration (Eldridge et al., 1983; 1985).
In this seminal work in the C2HS model, Nantwi and colleagues (1996) demonstrated a drug-induced functional restoration of respiratory-related activity that was characterized by shortened phrenic burst interval duration and increased burst amplitude. It was concluded that theophylline increased central respiratory drive to restore respiratory function after paralysis (Nantwi et al., 1996). The results also supported the early suggestion by Lewis and Brookhart that the CPP could be enhanced and/or activated by conditions that increase central respiratory drive.
2.2 Chronic (IP) Theophylline administration
Since acute administration of theophylline induced functional recovery it was important to ascertain whether chronic drug administration would maintain functional recovery or induce tolerance similar to that shown after chronic caffeine as reported by Chou and colleagues (1985). To study the chronic effects theophylline administration, theophylline was injected (i.p. 20 mg/kg 3× d) for 3, 7, 14 and 30 days in C2HS rats. At the conclusion of the respective injection periods, C2HS rats were prepared for assessment of respiratory activity under spontaneously breathing conditions (Nantwi and Goshgarian, 1998a). Assessment of respiratory function in the hemidiaphragm and phrenic nerve was conducted and at the termination of recording respiratory activity, approximately 1 ml of blood was withdrawn via the femoral vein for serum analysis. Serum levels of drug were subsequently correlated with recovery. In this study, theophylline serum levels (mean ± SEM) of animals treated for 3 and 7 d amounted to 2.075 ± 0.54 μg/ml and 18.20 ± 12.50 μg/ml respectively. These levels were significantly lower than levels detected after administration of pro-convulsive doses of theophylline (Chu, 1981). Rats tend to tolerate higher serum levels than man. In man, the serum range of 5-20 μg/ml is therapeutically beneficial while higher levels may be deleterious (Bousey et al., 1989). At 14 and 30 d drug treatment serum levels were higher than the detection limits of the instrumentation. It was concluded that the amount of recovered function correlated positively with serum levels at 3 and 7 days. In addition, chronic theophylline administration did not induce tolerance to the effects of the drug, i.e., functional recovery was not diminished with chronic drug treatment.
Thus, theophylline induced and maintained function when administered chronically in C2 HS rats. Earlier work by Eldridge (1983, 1985) suggested that theophylline acts via adenosine receptor antagonism however; a mechanism of action had not been tested in theophylline-induced recovery in the C2HS model. In addition, theophylline is a bronchodilator and has been shown to increase diaphragm contractility in vitro (Murciano et al., 1987). At the end of the studies on chronic theophylline administration, Nantwi and Goshgarian suggested that the drug may be exerting its effects via adenosine receptor antagonism (1998a). The assertion was based on the following: (1) adenosine modulates respiratory activity (McQueen and Ribeiro, 1986; Maxwell et al., 1986; Fuller et al., 1987), (2) theophylline reverses adenosine-mediated respiratory depression (Eldridge et al., 1985), and (3) the adenosine A1 receptor is located on phrenic motoneurons and modulates respiratory activity in vitro (Dong and Feldman, 1995).
3. Mechanism(s) of Actions
3.1 Adenosine (A1) Receptors in theophylline-induced recovery
To determine the site and mechanism of action of theophylline in inducing recovery, a series of test and retest experiments were undertaken with theophylline and an adenosine A1 receptor agonist, N6 (L-2-phenylisopropyl) adenosine, (L-PIA) (Nantwi and Goshgarian, 1998b). As expected, theophylline induced recovery of respiratory activity which was attenuated and eventually blocked by L-PIA. Pretreatment with L-PIA precluded any respiratory-related activity. The study confirmed involvement of adenosine A1 receptors in theophylline-induced functional recovery (Nantwi and Goshgarian, 1998b) extending the seminal work of Eldridge in identifying adenosine receptor antagonism as a mechanism of action of theophylline (1983, 1985). However, since theophylline is a nonspecific adenosine receptor antagonist as well as a bronchodilator and PDE inhibitor, it was necessary to further identify mechanism(s) of actions by employing a variety of alkylxanthines with diverse pharmacologic profiles. It is known that theophylline and 8-Phenyltheophylline are adenosine receptor antagonists (Smellie et al., 1979). In addition to theophylline and 8-Phenyltheophylline, 3-propylxanthine (enprofylline) and 8(p-sulfophenyl)theophylline were tested for their relative effects in functional recovery. Enprofylline is a methylxanthine like theophylline but a more potent bronchodilator than theophylline (Fredholm et al., 1995). However unlike theophylline, enprofylline is not characterized as an adenosine antagonist (Persson et al., 1986). 8-Phenyltheophylline is a more potent adenosine receptor antagonist than theophylline and 8(p-sulfophenyl)theophylline is a peripherally specific adenosine receptor antagonist (Evoniuk et al., 1986; Nikodijevik et al., 1991).
In addition, the magnitude of recovered activity was assessed under standardized electrophysiologic recording conditions 24 hr after C2HS by (1) expressing recovery as a function of pre-drug activity in the right phrenic nerve or (2) expressing recovery as a function of activity in the homo-lateral nerve of non injured age-matched control animals. We utilized the integrated area under the inspiratory respiratory burst rather than the conventional peak amplitudes (Eldridge, 1976) because we rationalized that the integrated area provided a more global assessment of phrenic output than peak amplitude. Moreover, previous studies in our lab had shown that either approach results in similar outcome conclusions (Hadley et al., 1998). Expressing recovery as a function of the contralateral activity that our lab has employed consistently has also been employed by other investigators (Vinit et al., 2007). It must be stressed however, that there is currently no consensus on how to accurately quantify recovered activity after C2HS. A simple approach of using the raw or unprocessed respiratory signal of motor output (Fuller et al., 2005; Golder and Mitchell, 2005) has been used. However in an effort to offset variability inherent with this approach, the raw signal can be “normalized” to a maximum during a challenge (Fuller et al., 2005) or minimum output during baseline levels (Fuller et al., 2008; Golder et al., 2008). It is more likely that a combination of approaches will yield the most meaningful information in assessing the magnitude of recovered function after injury.
The duration of inspiratory burst, inter-burst duration and respiratory frequency before and after drug administration were all assessed. With either method of quantitation, the results demonstrated that 8-Phenyltheophylline (10 mg/kg) was more potent in inducing functional recovery than theophylline (15 mg/kg). The magnitude of recovered activity attained with 8-Phenyltheophylline was 69.3 3± 4.1% of the contralateral pre-drug levels of activity compared to 70.3± 2.5% induced by theophylline. The effects of the two alkyxanthines, theophylline and 8-Phenyltheophylline were attenuated and eventually blocked in test/retest experiments with the A1 agonist, LPIA. Although theophylline and 8-Phenyltheophylline significantly enhanced respiratory frequency, the effect of theophylline appeared to be via decreasing the inspiratory burst duration while the effect of 8-Phenyltheophylline in enhancing frequency was via decreasing the inter-burst interval. Enprofylline (2.5 -15 mg/kg) was ineffective. At 20 mg/kg (i.v.), a dose similar to that employed by Thomas and colleagues (1994) to monitor involvement of adenosine in systemic hypoxia, 8(p-sulfophenyl)theophylline, did not induce recovery.
It was therefore concluded that (1) central (spinal and brainstem) adenosine receptor antagonism underlies theophylline-induced functional recovery, (2) the effects of theophylline and 8-Phenyltheophylline are unrelated to any bronchodilatory actions, (3) peripheral adenosine receptor antagonism may not be involved and (4) the effects of theophylline and 8-Phenyltheophylline in inducing functional recovery after acute administration are unrelated to PDE inhibition since both drugs have minimal PDE inhibitory properties (Bergstrand, 1980). Perhaps the most relevant finding was that the two alkylxanthines that induce functional recovery are both non specific adenosine receptor antagonists acting at spinal and brainstem levels. This principal finding led to a series of experiments to establish the relative contribution(s) of adenosine A1 and A2 receptor antagonism in functional recovery.
Adenosine is formed inside cells or on cell surfaces and acts via receptor-mediated mechanisms. Adenosine receptors are classified into four subtypes (A1, A2A, A2B, and A3). All four receptor subtypes are coupled to G-proteins. The A1 and A3 are coupled to an inhibitory G-protein (Gi) while the A2A and A2B are coupled to a stimulatory G-protein (Gs). The four receptor subtypes have been cloned and pharmacologically characterized (Fredholm et al., 2005). Of the four adenosine receptors, the A1, A2A, and A2B subtypes mediate the physiologic actions of endogenous adenosine (Fredholm et al., 2005). The expression and distribution of adenosine receptors is wide (see Table 1). Upon activation, the receptors may exert their distinct effects by acting with different G-proteins that in turn activate enzyme systems (adenyl cyclase) to alter intracellular levels of cyclic AMP. In addition signal transduction pathways may be involved in adenosine signaling (Dip, 2009). The role of the signaling pathways will not be covered in the present review (see Fredholm, 2007 for a more comprehensive review). In C2HS rats the question that had to be resolved was the contribution of specific adenosine receptor subtype(s) in respiratory recovery.
TABLE 1.
Distribution of Adenosine Receptors in Mammalian Systems
| A1 Receptor | A2A Receptor | A2B Receptor | A3 Receptor | Reference |
|---|---|---|---|---|
|
High Expression Brain (cortex, cerebellum, hippocampus); dorsal horn of spinal cord, pons, medulla, eye, adrenal gland, atria |
Spleen, thymus, leucocytes (lymphocytes and granulocytes), blood platelets, GABAergic neurons (in caudate putamen, nucleus accumbens, tuberculum olfactorium), olfactory bulb | Caecum, colon, bladder | Testes (rat) Mast Cells (rat) |
Fredholm et al. 2000 Gaytan et al., 2006 |
|
Intermediate Expression Other brain regions, skeletal muscle, liver, kidney adipose tissue, salivary glands, esophagus, colon, antrum, testis |
Heart, lung, blood vessels | Lung, blood vessels, eye, median eminence, mast cells | Cerebellum (human?) Hippocampus (human?) Lung, spleen (sheep) Pineal |
Fredholm et al. 2000 Fozard et al., 2003 |
3.2 Adenosine A1 and A2 specific compounds in functional recovery
Previous studies from several investigators had established that adenosine itself modulates respiratory activity through activation of central and peripherally mediated mechanisms (Fuller et al., 1987; Reid et al., 1991). It is known that the A1 receptor has a higher affinity for the endogenous ligand than the A2 receptor (Latini et al. 1996). The A2 receptor is subdivided into the A2A which is localized primarily in the hippocampus and the low affinity A2B receptor localized throughout the CNS. In a series of studies, Nantwi and Goshgarian (2002) assessed the actions of specific adenosine receptor agonist and antagonists alone and in combination in order to evaluate possible interactions of the specific adenosine subtypes in functional recovery. Specific adenosinergic compounds were selected based on their known central and peripheral actions (see Table 2). The selective adenosine A1 compounds chosen were the specific A1 receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine, (DPCPX) and A1 agonist, N6-cyclohexyladenosine (CHA). The selective adenosine A2 compounds chosen were, the specific A2 antagonist, 3,7-dimethyl-1-proparglyxanthine (DMPX) and the specific A2 agonist (2-p-(2-carboxyethyl)phenethyl-amino-5-N-ethylcarboxyamidoadenosine (CGS-21680).
TABLE 2.
Representative Adenosine Receptor Agonists and Antagonists
| A1 Receptor | A2A Receptor | A2B Receptor | A3 Receptor | Reference | |
|---|---|---|---|---|---|
| Agonists | CHA, CCPA, S-ENBA, CVT-510 | CGS-21680, HE-NECA, CVT-3146 | NECA (non selective) | IB-MECA, 2-Cl-IB-MECA, DBXRM, VT160 | Burnstock, 2007 |
| Antagonists | DPCPX, N-0840, MRS1754, N-0840, WRC-0571 | DMPX, KF17837, SCH58261, ZM241385, KW 6002 | Enprofylline, MRE2029, F20, MRS17541, MRS 1706, 8-SPT, CGS15493, CVT-5440, IDPX | MRS1220, L-268605, MRS1191, MRS1523, VUF8504 | Feoktisyov et al., 2001, Zablocki et al., 2005; Burnstock, 2007; |
CCPA= chlorocyclopentyladenosine, CPA= cyclopentyladenosine, NECA= 5′-N-ethylcarboxyamido adenosine, CHA=cyclohexlyadenosine,
It was discovered that 24 h after C2HS, systemic administration (i.v.) of DPCPX (0.05-0.2 mg/kg, i.v.) induces recovery in a dose-dependent manner while the A2 antagonist, 3,7-dimethyl-1-propargylxanthine (DMPX) was ineffective over the range of doses (0.5-2 mg/kg) tested. As with previous studies conducted under standardized recording conditions from our lab, the magnitude of recovered activity was expressed as a function of contralateral nerve activity prior to drug administration (i.v.). The effect of DPCPX confirmed and extended our previous findings that adenosine A1 receptor antagonism is the mechanism that underlies functional recovery induced with theophylline (Nantwi and Goshgarian, 1998b, 2001). DPCPX was more potent than either theophylline or 8-Phenyltheophylline in eliciting functional recovery. At 0.1 mg/kg administered (i.v.) the magnitude of recovered activity induced by DPCPX was significantly greater than that elicited by the optimal theophylline or 8-Phenyltheophylline dose. When the A1 agonist, CHA (0.1 mg/kg), was administered approximately 5 min before DPCPX, the latter was not effective in inducing recovery and furthermore, activity in the right phrenic nerve was suppressed. When DPCPX was administered first it induced respiratory activity which was attenuated and eventually blocked by subsequent (within 10 min) CHA administration. After 20 min all respiratory activity ceased. This confirmed that DPCPX-mediated recovery was receptor mediated.
When the optimal dose of DPCPX was administered in non injured age-matched control rats under similar experimental conditions, significant changes in inspiratory burst, frequency and amplitude of both the right and left phrenic nerves were detected. The finding confirmed and extended the previous biochemical studies that DPCPX is selective for the A1 receptor site(s) and penetrates into the CNS (Bisserbe et al., 1992). The principal finding that adenosine A1 receptor antagonist, DPCPX mediates functional recovery is extremely important because of the potential clinical implications.
In experiments with the A2 receptor agonist, CGS-21680 we discovered that systemic administration (0.5-2 mg/kg; i.v.) elicited enhanced tonic activity but not functional recovery in the majority (6/8) of C2HS rats 24 h following injury. In order to ascertain that enhanced tonic activity was induced by A2 receptor activation, the A2 antagonist, DMPX was administered 5-10 min after enhanced tonic activity was detected. Administration of the antagonist, DMPX blocked the CGS-2180-induced tonic activity. Under similar experimental conditions, respiratory excitation was elicited in age-matched non injured control animals after systemic administration of CGS-21680. Induced excitation lasted longer than 30 min. In particular, the amplitude, respiratory burst duration and inter-burst interval were significantly changed. Specifically, burst duration and burst interval were enhanced and reflected as decreased respiratory frequency. It was concluded from these experiments that activation of A2 receptors induces changes in respiratory activity, characterized by enhanced amplitude of respiratory bursts in the phrenic nerves and decreases in inspiratory burst duration and inter burst interval (Nantwi and Goshgarian, 2002). These findings were intriguing since the kinetics of CGS-21680 may preclude its access into the CNS (Hutchinson et al., 1990; Nikodijevik et al. 1991).
In a recent study from Golder and colleagues (2008) it has been shown that spinal administration of CGS-21680 elicits a long-lasting facilitation of phrenic motor activity by strengthening short term latency. There was no significant effect on respiratory frequency and the authors suggested that supra-spinal mechanisms may not be involved in mediating the effects of the drug after spinal administration. In the same study the authors showed that systemic administration (0.1 mg/kg, i.p.) of the drug in freely behaving rats did enhance respiratory frequency via mechanisms that are currently unknown. Perhaps more importantly the studies demonstrated that systemic activation of A2a receptors stimulates ventilation with a magnitude and time course similar to phrenic motor facilitation. The authors suggested that while spinal mechanisms are involved in mediating long term facilitation, putative involvement of adenosine receptors in the carotid body may also be involved (Golder et al., 2008). The assertion is in agreement with previous findings from our lab (Bae et al., 2005; James and Nantwi, 2006). In our lab we observed that systemic administration (0.5-2.0 mg/kg, i.v.) of CGS-21680 in anesthetized non-injured rats significantly enhanced phrenic motor output monitored characterized by the area under integrated inspiratory waveform in the phrenic nerves. Interestingly, respiratory frequency decreased after drug administration (Nantwi and Goshgarian, 2002). It is noteworthy that Nantwi and Goshgarian (2002) employed a dose range of (0.5-2.0 mg/kg, IV) while Golder and colleagues employed a dose of 0.1 mg/kg (i.p.). Experimental differences in route and dose of administration do not alter the conclusions from the studies. However, they emphasize that involvement of adenosine A2a receptors in respiratory recovery is an area that requires further studies to more thoroughly characterize putative influence(s) of adenosine A2a receptor activation on respiratory function.
Out of the four specific adenosinergic compounds tested, DPCPX and CGS-2168 induced detectable respiratory related changes. To confirm interactions/cross talk between the A1 and A2 receptors in C2HS as previously demonstrated in other studies, (Lopes et al., 1999), we employed a combinatorial approach of the two adenosine receptor specific drugs. The goal was to assess possible interactions and the extent of any such interaction by selective simultaneous activation of adenosine receptors.
The combinatorial approach demonstrated that prior administration of CGS-21680 before DPCPX significantly enhanced the magnitude of DPCPX-induced recovery. In contrast, when CGS-21680 was administered after DPCPX, the magnitude of DPCPX-induced recovery was not enhanced further. This suggested that activation of A2 receptors was necessary to subserve A1 receptor mediated effects only but may not be necessary to maintain the recovery. Another important observation with the combinatorial approach was that although the amount of DPCPX-induced recovery was not enhanced by a later CGS-21680 administration, the amplitude of the respiratory waveform in the right phrenic nerve was significantly enhanced (Nantwi and Goshgarian, 2002). We explained this observation as follows: In C2HS rats, the ipsilateral phrenic nerve is served by only the “crossed phrenic pathway” whereas in the intact or right phrenic nerve, the major descending bulbospinal respiratory pathway mediates activity. Other investigators (Sieck and Fournier 1986) have demonstrated that there is a reserve capacity in the intact animal, thus it is plausible that in the intact right phrenic nerve, the enhanced amplitude reflects a drug-induced ability to access this greater reserve.
An important caveat to the findings with the combinatorial approach is that it reinforces a previous suggestion by Ribeiro (1999) that adenosine-related medicines that combine A1 receptor blockade with A2 receptor activation may be clinically beneficial. The findings indicate that combined application of adenosine receptor compounds can be a useful clinical strategy in drug interactions to elicit maximum beneficial actions. The effects observed with CGS-21680, coupled with the kinetics of the drug that it may be more peripherally specific (Nikodijevik et al., 1991) and findings that adenosine A2 receptors are located in the carotid bodies suggested that the apparent influence of CGS-21680 on respiratory-related activity may be mediated by A2 receptors located in the carotid bodies. This led to the next series of studies to delineate the putative contribution(s) of adenosine receptors localized in the carotid bodies to respiratory activity.
3.3 Adenosine Receptors in the Carotid bodies
Peripherally located chemoreceptors modulate respiration (Fuller et al., 1987). Adenosine receptors that modulate respiration are located in the carotid bodies of several mammalian species including man (Monteiro and Ribeiro; 1987; Watt and Routledge, 1985). It is also established that adenosine receptors that mediate respiratory excitation are of the A2 subtype while the A1 receptor subtype mediates depression (Monteiro and Ribeiro 1987; Carley and Radulovacki, 1999).
Adenosine A1 receptor activation can reduce centrally mediated respiratory disturbances that occur during sleep (Carley and Radulovacki, 1999; Monti et al., 1996). Bae and colleagues (2005) tested the hypothesis that adenosine A1 receptors localized in the carotid bodies can be specifically activated to modulate recovered respiratory activity in C2 hemisected rats. The hypothesis was predicated on previous studies which demonstrated that while theophylline induced respiratory function it also significantly enhanced respiratory frequency (Nantwi et al., 1996; Nantwi and Goshgarian, 1998; Nantwi and Goshgarian, 2001). Bae and colleagues predicted that in the clinical use of theophylline, the enhanced respiratory rate could over time lead to untoward effects i.e., diaphragm muscle fatigue. In order to preserve the potential therapeutic benefit of theophylline and concurrently minimize any potential of respiratory muscle fatigue, the authors designed a series of experiments using specific adenosine receptor compounds to differentially target adenosine receptors localized in peripheral sites of action.
Adenosine A1 receptors were targeted with a specific agonist in C2 hemisected animals with intact or excised carotid bodies. We employed the specific A1 agonist, N6-p-sulfophenyladenosine (p-SPA) that does not cross the blood brain barrier and is classified as peripherally specific (Gleeson and Zwillich, 1992). Carotid body excision was conducted according to the method of Olson and colleagues (Olson et al., 1988). Using immunohistochemistry, Bae and colleagues demonstrated positive immunoreactivity for adenosine receptors in the carotid body confirming that adenosine A1 and A2 receptors are located in the carotid bodies (Gauda, 2000). Next the investigators demonstrated that in C2 hemisected animals with the carotid body intact, theophylline-induced recovery was significantly reduced to near basal discharge levels by concurrent administration of p-SPA (0.25 mg/kg, i.v.) (Bae et al., 2005). Activation of adenosine A1 receptors by the specific agonist, p-SPA was effective in attenuating theophylline-induced respiratory rate back to near normal levels without losing the recovered function. In animals with carotid bodies excised however, administration of the same dose of p-SPA that was effective in carotid body intact animals was ineffective, i.e., enhanced respiratory frequency persisted.
Thus studies targeting peripherally localized adenosine A1 receptors led to the following conclusions: (1) carotid body excision by itself does not adversely affect the expression of theophylline-induced functional recovery; (2) adenosine A1 and A2 receptors are located in the carotid bodies as demonstrated by immunohistochemistry; (3) activation of peripherally located adenosine A1 receptors with p-SPA can modulate theophylline-induced enhanced frequency back to normal discharge rates and (4) a novel approach of selective activation of peripheral adenosine A1 receptors combined with blockade of central adenosine A1 receptors can be employed to attain the desired pharmacologic action of recovered function and maintain basal rates to near normal levels (Bae et al., 2005).
3.4. Peripheral adenosine A2 receptors and functional Recovery
The study of Bae and colleagues (2005) confirmed and extended previous studies that adenosine A1 and A2 receptor are localized in the carotid bodies (Fuller et al., 1987; McQueen and Ribeiro; 1983; Gauda, 2000).
In light of the discovery that the adenosine A1 receptor antagonist, DPCPX elicited robust recovery we rationalized that a concurrent administration of the A2 agonist, CGS-21680 and A1 antagonist, DPCPX may evoke an even greater amount of recovery than had been realized with the A1 antagonist alone. We extended the study of Bae and colleagues (2005) in which the carotid bodies were intact (H-CBI) or excised (H-CBE) in C2 hemisected rats. Briefly, H-CBI and H-CBE rats were tested following systemic administration DPCPX alone or a combination of DPCX and CGS-21680. In cases of concurrent administration of the two drugs, we examined the effects based on the order of drug administration, i.e., whether CGS-21680 was administered before or after DPCPX.
The results showed that carotid body excision did not preclude the capability of DPCPX to elicit recovery in C2 hemisected animals confirming the findings from a previous study (Bae et al., 2005). Secondly, prior administration of CGS-21680 in H-CBI rats elicited a significantly greater amount of recovery than that elicited by DPCPX alone (Fig 2). However, the apparent sub-serving effect of CGS-21680 to subsequent DPCPX action was eliminated by excision of the carotid bodies confirming that the site of enhanced activation resides in the carotid bodies. We concluded from this study that activation of adenosine A2 receptors localized in the carotid bodies can modulate DPCPX-mediated functional recovery. The study confirmed a previous observation (Nantwi and Goshgarian, 2002) and secondly demonstrated that the A2 receptors that augment the magnitude of DPCPX-induced recovery are localized in the carotid bodies (James and Nantwi, 2006). A schematic of the interactions of peripherally located adenosine A2 receptor activation and central adenosine A1 receptor antagonism is shown in figure 3. Once again our findings extended the suggestion of Ribeiro (1999) that in therapy of adenosine-related medicines combination of central A1 receptor blockade with peripheral A2a receptor activation may be clinically beneficial.
Figure 2.
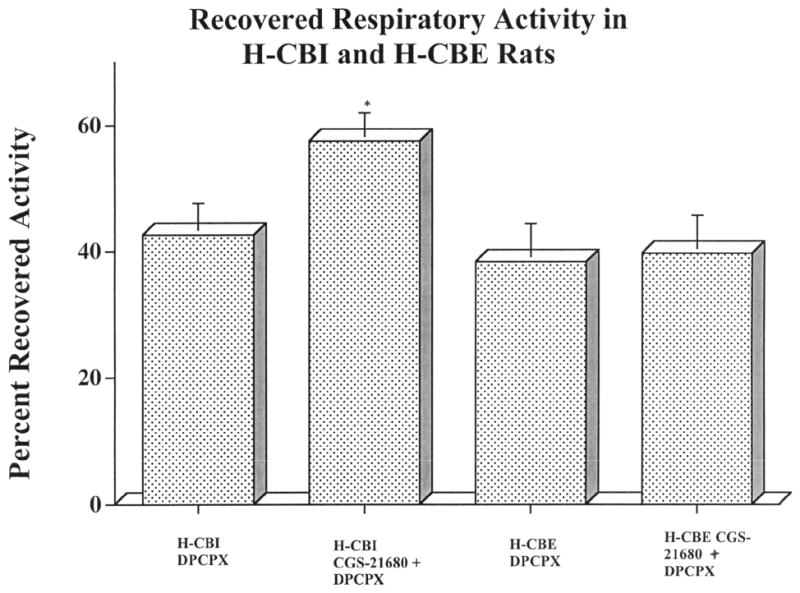
A summary of the quantitative analysis of the magnitude of recovered activity in the LPN in H-CBI and H-CBE after administration of DPCPX (only) or sequential administration of CGS-21680 followed by DPCPX is shown in this histogram. In both H-CBI and H-CBE animals, DPCPX induced recovery of respiratory-related activity in the LPN. The magnitude of recovery in H-CBI animals (42.6 ± 4.6%), whereas greater than that in H-CBE animals (38.4 ± 4.2%), was not statistically significant. However, the magnitude of DPCPX-induced recovery in H-CBI animals after prior CGS-21680 administration was significantly (P < 0.05) enhanced (compare H-CBI DPCPX only and H-CBI CGS-21680 + DPCPX). In contrast, the magnitude of DPCPX-induced recovery was not enhanced further by prior CGS-21680 administration in H-CBE animals. Statistical significance is shown by *P < 0.05 (Newman-Keuls comparison test). (Reprinted from James and Nantwi, 2006).
- LPN= Left Phrenic Nerve
- H-CBI= C2HS carotid bodies intact
- H-CBE= C2HS carotid bodies excised
Figure 3.
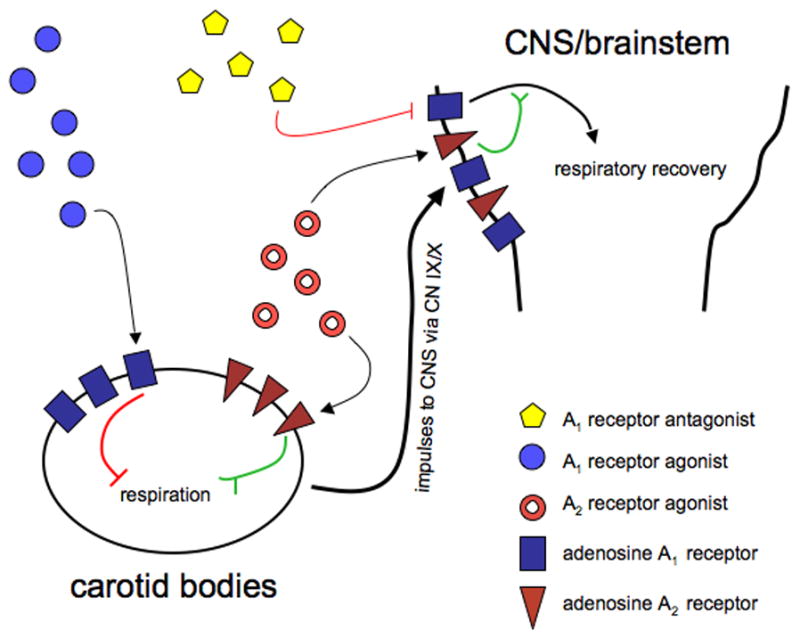
This is a schematic that illustrates interaction of peripheral and central adenosine receptor in respiratory recovery. Activation of peripherally located A2A receptors in the carotid bodies excites respiratory activity which is transmitted to the CNS via the IX/X cranial nerves. On the other hand, activation of peripheral adenosine A1 receptors inhibits respiration and can dampen induced recovery. Central blockade of adenosine A1 receptors releases the inhibitory effect of A1 receptor activation on respiration and the result is disinhibition or excitation. Simultaneous administration of the adenosine A1 receptor antagonist DPCPX and peripherally acting A2a agonist, CGS-21680 can therefore elicit enhanced recovery via an apparently additive effect through a direct excitation combined with a disinhibitory effect.
3.5 Interaction of adenosine A1 receptors and Serotonin 2A/C Receptors
It is known that 5-hydroxytryptamine (5HT) is a neuromodulator that excites spinal motoneurons including neurons in the phrenic nucleus (Mitchell et al., 1992). More recently, it has been demonstrated that activation of the latent respiratory motor pathway can be attained by serotonin (5HT) receptor activation (Zhou, et al., 2001). Since the phrenic motoneurons represent a critical area for the expression of the CPP and A1 receptor antagonism is the mechanism of theophylline-induced functional recovery, we hypothesized that interaction(s) of serotoninergic compounds and adenosinergic compounds may induce additive effects in functional restoration if both compounds are administered concurrently. Zhou and colleagues (2001) demonstrated that activation of the serotonin 5-HT2A/2C receptor by the selective agonist, (±)-1(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DO-I) induces recovery in the phrenic nerve ipsilateral to hemisection most likely via the 5HT2A receptor.
Since both DO-I (via 5-HT2A/2C) and theophylline (via A1 receptor antagonism) induce functional recovery we investigated the effects of concurrent administration of both drugs in functional recovery. In addition, in order to produce further understanding of where the effects of systemic theophylline might be modulating phrenic nerve activity, immunocytochemical and in situ hybridization analysis of phrenic motoneurons was conducted to identify adenosine A1 receptor immunoreactivity and mRNA expression respectively.
It was discovered in this study that the degree of theophylline-induced recovered activity was enhanced significantly by concurrent activation of serotonin 2A/2C receptors with DO-I (1 mg/kg, i.v.). The significant enhancement was evident in the respiratory burst amplitude of both the left and right phrenic nerves and not rate suggesting that both drugs may be focally linked to phrenic motoneurons (Basura et al., 2002). The enhancement of theophylline-induced recovery by DO-I was attenuated by subsequent administration of the 5-HT2 receptor antagonist, ketanserin (2 mg/kg), confirming that 5HT 2 receptors mediate the DO-I actions in augmenting theophylline-induced recovery. Prior administration of ketanserin did not prevent functional recovery by subsequent theophylline administration; it only precluded enhancement of theophylline-induced recovery by subsequent DO-I.
The physiologic significance of the apparent enhanced burst amplitude is that tidal volume is improved (Eldridge, 1976). Secondly, for the first time, a co-localization of adenosine A1 receptor immunoreactivity and mRNA expression with phrenic motoneurons in C3-C6 spinal cord segments was demonstrated (Fig 4). Basura and colleagues (2002) concluded that theophylline may induce recovery of respiratory function in hemisected rats likely at post synaptic adenosine A1 receptors located at the level of the phrenic nucleus in the spinal cord. The authors also concluded that 5-HT2 receptors located in phrenic motoneurons may be capable of increasing phrenic motoneurons excitement over and beyond that of theophylline alone.
Figure 4.
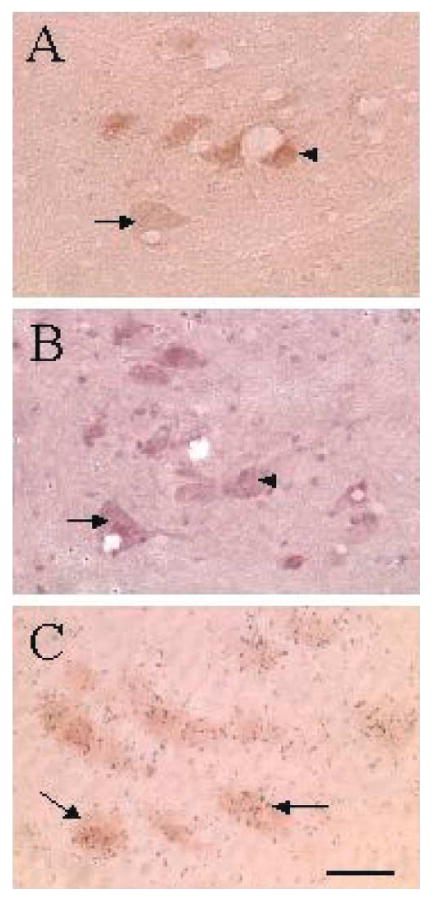
Bright-field photomicrograph of fluoro-labeled, DAB-positive phrenic motoneurons (A) and adenosine A1 receptor immunoreactivity (B) in the ventral horn of the cervical spinal cord. Sections pictured in (A, B) are 12 μm apart and matching arrows and arrowheads from each panel represent the same cell being positive for fluorogold-DAB (A) and adenosine A1 receptor immunoreactivity in (B). Arrows in (C) show extensive aggregation of adenosine A1 receptor mRNA-hybridization signals (silver grains) over fluorogold-labeled, DAB-positive phrenic motoneurons. Calibration bar = 50 μm. (Reprinted from Basura et al. 2002).
The immunocytochemistry data extended previous findings that adenosine A1 receptors involved in mediating theophylline-induced recovery are located in phrenic motoneurons (Nantwi and Goshgarian, 1998; 2001). Augmentation by serotonin receptor stimulation of theophylline-induced recovery of respiratory activity may offer a possible clinical approach to improve respiratory function in SCI.
4. Adenosine A1 mRNA Expression
Thus far, our studies have implicated central and peripheral adenosine receptors in functional recovery of respiratory-related activity after injury (Nantwi et al., 1996; Nantwi and Goshgarian, 1998; 2001; 2002; Nantwi et al., 2003; Bae et al., 2005; James and Nantwi, 2006). The immunocytochemistry study of Basura and colleagues (2002) that demonstrated a positive co-localization of adenosine A1 receptor mRNA and immunoreactivity with phrenic motoneurons suggested the need to ascertain putative effects of chronic theophylline on expression of adenosine A1 mRNA.
The study of Basura and colleagues (2002) confirmed and extended the work of Fastbom and Fredholm who showed autoradiographic evidence for increased A1 receptors in the rat brain after chronic theophylline treatment (1990). Based on evidence that theophylline-induced recovery is mediated by central A1 receptors it was important to assess whether A1 mRNA expression was altered following chronic theophylline administration and secondly whether changes in message levels correlated with the degree of recovered function. To directly test A1 mRNA involvement we sought to ascertain whether A1 mRNA expression is altered after repeated administrations of theophylline. This was necessary since up to this point, all of the studies described have addressed adenosine receptor involvement following acute (i.e. 24 h after hemisection) administration of theophylline.
In the study that sought to test involvement of A1 mRNA we showed that when theophylline is administered orally (20 mg/kg, 3× daily) to C2HS rats, the magnitude of recovered activity assessed under standardized recording conditions correlates positively (over a theophylline serum range of 1.2-1.9 μg/ml) for 3, 7, 12, or 30 days. The serum range detected here was less than that from a previous study perhaps due to the route of administration (Oral gavage compared to i.p.). Perhaps most importantly, when animals were weaned from the drug (for 7, 12 or 30 days) after only 3 d of administration, the beneficial effects, i.e., recovered respiratory activity persisted (Nantwi et al., 2003b). This was the first finding in which a drug-induced effect persisted when the initial drug stimulus had been withdrawn and is an example of drug-induced plasticity. The finding that functional recovery persisted even after weaning from the drug suggested that chronic administration may not be necessary to sustain the therapeutic benefit of theophylline.
The mechanism(s) that underlie the apparent drug-induced plasticity described above remains to be clarified. We rationalized that changes in adenosine A1 receptor mRNA located in phrenic motoneurons may underlie theophylline-induced apparent persistent recovery. To address this question, a series of experiments were conducted in which theophylline was administered for various periods in C2HS rats. At the end of each administration period, adenosine A1 mRNA levels were analyzed by situ hybridization and immunohistochemical methods. Analysis of in situ hybridization and immunohistochemical data did not reveal any significant changes in A1 mRNA expression after chronic theophylline treatment (Nantwi et al., 2003a, 2003b). This latter finding is in agreement with other investigators who assessed the long term effect(s) of caffeine, a methylxanthine with a pharmacologic profile similar to theophylline that adenosine A1 mRNA expression is unchanged after chronic caffeine treatment in mice (Johansson et al., 1993; 1997). Based on the observation that A1 mRNA expression did not change after chronic theophylline treatment it is likely that changes in A1 mRNA after chronic administration of methylxanthines occur post translationally as suggested previously (Johansson et al., 1993; 1997, Basura et al., 2003). Thus, based on the demonstration of Basura and colleagues (2002) of a positive immunoreactivity of A1 receptors in the phrenic motoneurons and the observations that there were no significant changes in A1 mRNA levels over prolonged theophylline treatment, we sought to ascertain whether changes in adenosine receptors occurred at the protein level. In order to accomplish this, we first assessed biochemical changes, receptor affinity and/or receptor numbers in cervical spinal cord segments (C3-C6) where the phrenic motoneurons are located (Goshgarian, 2003).
5. Biochemical Binding Assays after chronic theophylline
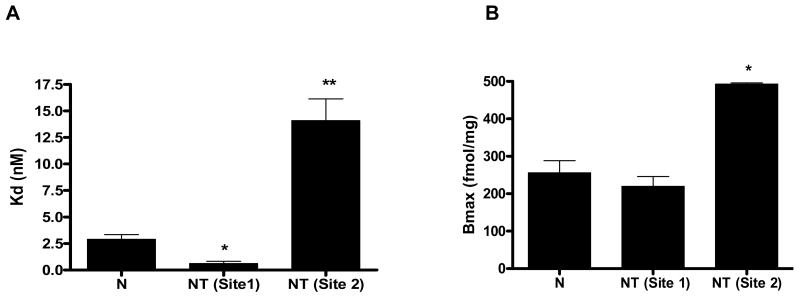
We also rationalized that changes in adenosine receptors after chronic theophylline administration may induce biochemical changes in receptor numbers and/or affinity at the specific receptor binding sites. In subsequent biochemical studies our lab, employed a radioligand binding filtration assay to characterize adenosine A1 receptors before and after chronic theophylline administration. The study demonstrated that after 3 days of oral theophylline administration, the receptor profile of adenosine A1 located in the phrenic motoneurons (C3-C6) is altered (Saharan and Nantwi, 2006). Specifically, the binding assays showed that the specific adenosine A1 receptor antagonist, [3H]-DPCPX detects one binding site with a Bmax of 492.00 ± 32.13 fmol/mg and Kd of 2.89± 0.45 nmol/L in naïve untreated animals. However after chronic administration of theophylline, [3H]-DPCPX detects two binding sites; one site is identical to the single site detected in naïve non treated animals and the second is an unknown site. The Bmax and Kd after theophylline were: 219.00 ± 26.31 fmol/mg with a Kd of 0.60 ± 0.21 nmol/L. respectively for one site. For the second site the Bmax and Kd were: 492.6 ± 3.15 fmol/mg and 14.09 ± 2.06 nmol/L respectively (Fig 5). The second site may be due to binding the A2 receptor(s) since DPCPX has been shown to have affinity for the A2 site at higher concentrations (Klotz, 2000, McAdoo, 2000). Alternatively, the unmasked site may represent a conformational change in the A1 receptor.
Figure 5.
A: A quantitative summary of the kinetic profiles characterizing binding affinity in naïve (N) and theophylline-treated (NT) animals. In the histogram, the Kd value in the naïve group was 2.89 ± 0.45 (nM) and the theophylline-treated group was 0.60 ± 0.21 (nM). The Kd value for the second site was 14.09 ± 2.06nmol/L. The histogram was generated from saturation binding studies. Displacement of 1nM [3H]-DPCPX binding, to rat spinal cord (C3-C5) homogenate from naïve rats and theophylline treated rats by 2.7pM-27mM theophylline. Values represent the mean specific binding as a percentage increase above basal binding (± SEM) of at least three experiments each performed in triplicate. All values are corrected for non specific binding (NSB) defined with 100μM adenosine. B: Histogram showing the respective Bmax values in naïve and theophylline-treated animals. Bmax for the naïve amounted to 256.00 ± 32.13 (fmol/mg protein) and the theophylline-treated was 219.00 ± 26.3 for site 1 which appears to be the same in both groups. More importantly however, a second binding site with a Bmax of 492.6 ± 3.15 (fmol/mg protein) is unmasked and persisted when drug was weaned for 12 days. (Reprinted from Saharan and Nantwi, 2006).
We chose to assess changes in receptor numbers and/or affinity with the A1 antagonist because the use of antagonists in binding assays generally allows for the detection of all receptors (Williams and Jacobson, 1990). In contrast, the use of agonists in such assays does not allow for the differentiation between increases in receptor numbers and an increase in coupling to the G protein either as a result of enhanced number of receptors or increased function as a result of up-regulation. It should be noted however, that other investigators have assessed adenosine receptor numbers and affinity after chronic administration of methylxanthines like theophylline and caffeine and reported no significant changes in A1 receptors (Holtzman et al., 1991; Kaplan et al., 1993; Georgiev et al., 1993). In their study, Holtzman and colleagues employed the adenosine A1 receptor agonist 3[H]CHA and as explained earlier, this approach may not allow for the detection of all receptors. These authors assessed adenosine receptor levels in mice brain. Differences in species and areas studied may account for the differences in our findings compared with the findings reported by the authors.
The clinical relevance of changes in adenosine A1 receptor profile reported by Saharan and Nantwi (2006) may support the findings of Varani and colleagues that altered adenosine receptor numbers and/or affinity may be involved in respiratory dysfunction (Varani et al., 2006). The findings of Saharan and Nantwi (2006) may offer insights into the potential use of adenosine compounds in respiratory deficits. If the unmasked site is characterized as an A2 receptor, then it would suggest that activation of A2 receptors may be an important therapeutic target for improving respiratory impairment after injury as suggested (Nantwi, 2008). A more recent study by Golder and colleagues (2008) demonstrates that selective activation of A2a receptors elicits long term facilitation, a form of respiratory plasticity. The findings suggest a therapeutic potential of adenosine A2a agonists in respiratory deficits in SCI (Golder et al., 2008).
6. Theophylline administration and Spontaneous Recovery of Respiratory Activity
An important strategy towards the pharmacological application in functional recovery after injury is the duration of sustained injury. To address the issue of duration post injury to initiate theophylline therapy, we conducted a series of studies in which theophylline was administered in chronic C2HS rats (4-16 weeks post injury). The study was predicated on a previous finding which has since been confirmed and extended by other investigators that spontaneous recovery of respiratory function occurs after chronic C2HS (Nantwi et al., 1999; Golder et al., 2001; Fuller et al., 2005). Clinical observations have also indicated the occurrence of spontaneous recovery (McKinley, 1996).
Since theophylline had been shown in all our earlier studies to induce recovery of respiratory activity in C2HS rats and enhance respiratory activity in non injured animals, we rationalized that the drug would further improve spontaneously recovered (acting synergistically with mechanisms that underlie spontaneous recovery) respiratory activity in C2 hemisected animals after prolonged post injury periods. We tested the effects of the drug in C2 hemisected animals after prolonged post injury periods (4-16 weeks). Contrary to our expectations, theophylline (over a range of 2.5-15 mg/kg, i.v.) rather unexpectedly did not enhance respiratory activity but instead blocked spontaneous recovery (Nantwi et al., 2003b). In the same study, we did not detect any significant differences in adenosine A1 mRNA expression up to 4 months post injury. We therefore concluded that (1) A1 receptor plasticity is not activated by chronic injury and (2) the therapeutic benefits of theophylline depend on post injury periods, i.e., how soon after injury to administer the drug. The ineffectiveness of theophylline to improve respiratory activity after prolonged post injury periods may be related to the enhanced affinity of adenosine A1 receptors after prolonged post injury periods (Nantwi and Kizy, 2007).
On the other hand, based on the biochemical profile(s) of adenosine receptors after chronic theophylline administration, we speculated that after prolonged post injury periods, it may be therapeutically more beneficial to activate adenosine receptors, in particular the A2 receptor subtype with specific agonists, i.e., rather than using antagonists. This speculation was also predicated on (1) previous observations that administration of the specific A2 agonist, CGS-21680 enhances respiratory activity in non-injured animals (Nantwi and Goshgarian, 2002; Golder et al., 2004), (2) spinal activation of A2a receptors by CGS-21680 improves respiratory activity in C2HS rats (Golder et al., 2008), (3) CGS-21680 enhances the magnitude of DPCPX-induced recovery C2HS rats (James and Nantwi, 2006), (4) A2 receptor activation improves ventilation (Bonneau et al., 2006;) and (5) adenosine A2 receptor activation excites respiration and may improve respiration (Bonneau et al., 2006).
Thus far we have observed that systemic administration of CGS-21680 in chronic (4-12 weeks) C2HS rats may improve further the magnitude of recovery that occurs spontaneously and perhaps equally important, A2 receptor activation appears to accelerate the onset of spontaneous recovery (Nantwi, 2008).
7. Adenosine A1 and A2 receptor mRNA/protein levels after chronic theophylline
In our previous studies on the putative involvement of adenosine A1 mRNA in functional recovery, we concluded that theophylline-induced recovery may not be due to alterations at the message level since we did not observe any significant changes as indicated above (Nantwi et al., 2003a, 2003b). Petrov and colleagues (2007) reasoned that changes in mRNA and or protein levels in the A2 receptor may be involved in the persistent and sustained actions of theophylline. Petrov and colleagues sought to ascertain whether adenosine receptor proteins may be altered by C2 hemisection and subsequent theophylline administration. The investigators assessed expression of adenosine A1 and A2 proteins in C3-C6 spinal cord segments containing phrenic motoneurons in non-injured, C2HS (only), C2HS rats treated with theophylline for 3 days, and C2HS rats treated with theophylline for 3 days followed by 12 drug-free days.
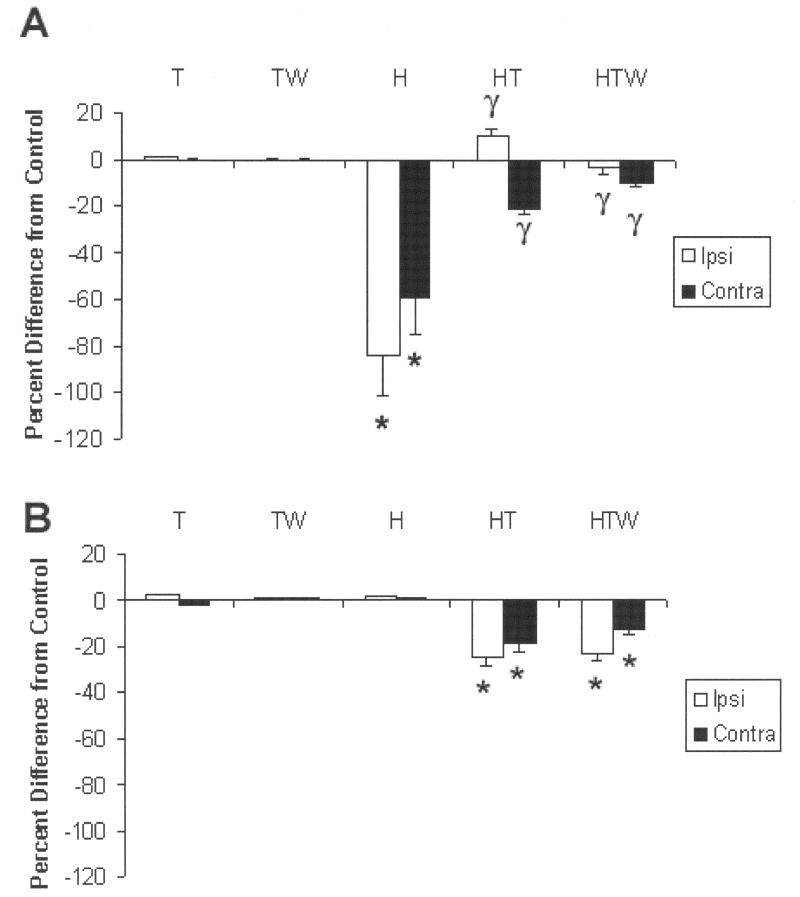
Briefly, Petrov and colleagues showed that 24 h following C2HS, adenosine A1 and A2 receptors were differentially altered, i.e., A1 receptors were decreased significantly while the A2 receptors remained unchanged. The mean (+SEM) of A1 positive immuno-reactive cells counted in non injured control rats amounted to 20 ± 2.1. The numbers were changed significantly by C2 hemisection to: 3.5 ± 0.8 (ipsilateral) and 7.0 ± 1.0 (contralateral) representing an overall decrease of approximately 80%. However, after chronic administration of theophylline for 3 days, the initial decrease in A1 receptors was increased to 24.0± 1.3 and 13.0 ± 1.8 respectively. Weaning from theophylline for 12 days resulted in 20.0± 0.7 and 17.0± 0.7 immunoreactive cells respectively (Fig 6). Thus, the trend in reversing the initial decrease of A1 receptors persisted even after cessation of theophylline administration.
Figure 6.
A. In noninjured animals administered theophylline with or without interruption (T,TW) adenosine A1 receptor immunoreactivity was unaltered compared with naïve noninjured animals. However, C2HS rats (H) demonstrated a significant (* P<0.05) decrease in protein expression (number of labeled cells) compared with noninjured animals (the decrease was apparent ipsilateral as well as contralateral to injury). Although the ipsilateral decrease appeared more pronounced than contralateral, this was not significant. After a 3-day administration of theophylline (HT) the decrease in protein levels was significantly attenuated (γ P< 0.05 on the ipsilateral and contralateral sides. When theophylline was withdrawn for 12 days prior to assessment (HTW), the number of A1 immuno-reactive cells was still significantly lower compared with H (injury alone). (B). In marked contrast to the decrease in A1 protein levels after C2 hemisection, A2A protein levels were not altered compared with controls (T, TW) and with naïve, noninjured animals. However, after theophylline administration, protein levels significantly decreased (P<0.05) ipsilateral as well as contralateral to injury. Although the decrease appeared more pronounced ipsilaterally, (a trend similar to that observed for A1 as shown above) it was not significant. Withdrawal of theophylline for 12 days prior to assessment did not alter the decrease in protein levels, i.e., theophylline-induced decrease in A2A protein levels persisted. *=significant from control, γ= significant from hemisection.
Adenosine A2 receptor protein levels were not changed by C2HS. Theophylline administration significantly decreased basal levels, a decrease that persisted after theophylline withdrawal for 12 days. Basal levels of A2 protein amounted to 70 ± 1.0 in non-injured age-matched control rats. Theophylline treatment resulted in a decrease in intensity in immunoreactivity to: 60 ± 2.8 and 51.0 ± 1.6 ipsilateral and contralateral respectively. Weaning from theophylline resulted in levels of 62.0 ± 1.5 and 55.0 ± 0.8 (Fig 6). Petrov and colleagues concluded that theophylline mitigates the effects of C2 hemisection by attenuating the C2 hemisection-induced decrease in A1 protein levels and that A2 receptor protein levels are unaltered by C2HS but decrease after theophylline withdrawal (Petrov et al., 2007). The author is aware of the limitations of quantitation by counting immuno-labeled cells. It should be emphasized that milder changes in immunochemically labeled cells would not be detected by the cell counting approach employed. In this regard, future studies will employ Western blot analysis to obtain a more quantitative assessment of protein levels.
The significance of the findings from this study is that the decrease in A1 receptors may dampen the known inhibitory actions of endogenous adenosine and could contribute to the hyperexcitability of motoneurons which may underlie spasticity after spinal cord injury. In addition, it also suggests that altered levels of adenosine A2 receptor expression may at least contribute to the apparent persistent actions of theophylline in functional recovery after drug withdrawal. It further suggests that if A2 receptors are down regulated after theophylline cessation, then activation of the same receptors may be a tool to further improve recovery.
To summarize, Petrov and colleagues used immunocytochemistry and semi-quantitative densitometry and showed for the first time (1) differential changes in expression of adenosine A1 and A2 receptor proteins in C2 hemisected animals, (2) changes in adenosine A1 and A2 receptor levels after continuous chronic (3 days) administration of theophylline by oral gavage and (3) changes in the receptor protein levels after weaning from theophylline for 12 days (Petrov et al., 2007). This was an important study towards identifying whether levels of adenosine A1 and A2 receptor protein changes correlate with persistent functional recovery even after drug cessation as had been previously demonstrated (Nantwi et al., 2003a). It appears that changes in adenosine A1 and A2 receptor protein changes reported by Petrov and colleagues (2007) support the findings reported by Saharan and Nantwi (2006) of the unmasking of a second site similar in pharmacologic profile with the A2 receptor. Ongoing studies will characterize the functional significance of A2 receptors to identify the putative role(s).
8. Clinical Studies
Since our basic research studies had demonstrated the effectiveness of theophylline in inducing recovery after hemidiaphragmatic paralysis and theophylline has been used clinically for many years we suggested the therapeutic potential of the drug in ventilatory dependent SCI patients (Nantwi et al. 1996; Nantwi and Goshgarian, 1998, 2001). Two case studies demonstrated that theophylline administration increased respiratory motor output after SCI (Ferguson et al., 1999; Bascom et al., 2005). The drug enhanced central respiratory drive and inspiratory muscle force in one patient with C5-C7 injury and a history of respiratory insufficiency (Ferguson et al., 1999). In another case study, a patient with a C5-C6 injury was weaned from ventilator support after aminophylline (a more soluble form of theophylline) treatment (Bascom et al., 2005). This particular case was very promising in clinical use of theophylline in ventilatory dependent patients since the patient had developed respiratory insufficiency after prolonged bouts of pneumonia and septicemia which had to be cleared first. After resolution of the infections, treatment with theophylline enabled weaning from ventilator support. In a third case study, Schulz-Stubner, demonstrated that small doses of theophylline were successfully used in three SCI patients with persistent bradycardia (Schulz-Stubner 2005). In one of the three patients, the dose of theophylline used increased respiratory rate and improved minute ventilation (Schulz-Stubner 2005). After 6 weeks, treatment with theophylline was discontinued with no further episodes of bradycardia. However, the apparent promise shown in the case studies mentioned above has not been documented in a larger patient population.
In a double-blind placebo controlled cross over study in 10 patients with chronic tetraplegia, theophylline administration appeared to improve respiratory function in 40% of the patients, however, the drug did not significantly improve functional outcome in the patient pool (Tzelepis et al., 2006). The outcome from this study may have been due to the small population of patients and also the fact that the patients did not present respiratory insufficiency. Future studies will thoroughly examine clinical effects of theophylline in selected SCI patients with respiratory deficits, in particular insufficiency. It is anticipated that a more rigorous screening of patients, with a particular focus on duration of injury and other underlying conditions can provide reliable results.
Conclusion
We have provided a summary of theophylline-mediated actions in functional recovery in the C2 hemisection model of SCI. The mechanism of action of theophylline has been shown to be via adenosine receptors. Although blockade of the A1 receptor subtype has been emphasized, we have also underscored the role of the A2 receptors with a particular emphasis on the contribution of activation of A2 receptor localized in the carotid body to improved respiratory activity. The basic research that has led to the clinical application of theophylline in SCI patients has been emphasized. It has been shown that while theophylline induces recovery after acute C2 hemisection, it is ineffective after chronic C2HS. As stated earlier, clinical case studies have shown that in chronic SCI patients, theophylline is beneficial (Ferguson et al., 1999; Bascom et al., 2005; Schulz-Stubner 2005). As far as the author is aware, theophylline has not been used in acute clinical SCI cases so far. However, based on our basic science observation of the effects of theophylline in acute versus chronic injury, we surmise that in SCI, early treatment with theophylline may provide a more likely positive response. In chronic injury, the development of plasticity within the respiratory system may alter adenosine receptor profile(s) to an extent that beneficial effects of the drug may not be realized. The reasons for the apparent dichotomy are unclear. However, we have provided evidence that changes in adenosine receptor profile may be involved.
The following aspects of adenosine receptor targeting drugs need to be emphasized:
Theophylline acting via blockade of adenosine A1 receptor induces a persistent recovery even after cessation of theophylline treatment
Concurrent application of adenosine and serotonin compounds can have additive effects of drug induced recovery
Therapeutic strategy using adenosinergic approach of central adenosine A1 receptor blockade and peripheral A2 receptor activation offers a therapeutic strategy in adenosinergic compounds to improve respiratory activity
Unmasking of a second adenosine receptor, a putative A2 site may provide a therapeutic target for intervention
Theophylline administration induces a differential expression of adenosine A1 and A2a protein after injury
Activation of adenosine A2 receptors localized in the carotid body may improve respiratory activity
In an attempt to identify molecular targets that may underlie the apparent persistent theophylline-induced effects, we recently conducted a series of molecular studies that targeted neurotrophic factors known to be involved in respiratory plasticity. Thus far, it appears that in theophylline-treated animals there is an up-regulation of brain derived neurotrophic factor (BDNF) mRNA expression (Nantwi and Singh, unpublished observations). Although a direct positive correlation between enhanced BDNF levels and persistent recovery has yet to be confirmed, initial observations are intriguing and may shed light on molecular targets involved in drug-induced plasticity. Secondly, identification of such targets may suggest therapeutic strategies to harness the inherent plasticity within the respiratory system to improve respiratory function after upper cervical spinal cord injury.
Acknowledgments
This work was supported by National Institute of Child and Human Development Grants 35766 and 31550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubier M, Murciano D, Vires N, Lecocguic Y, Palacios S, Pariente R. Increased ventilation caused by improved diaphragmatic efficiency during aminophylline infusion. Am Rev Res Dis. 1983;127:148–154. doi: 10.1164/arrd.1983.127.2.148. [DOI] [PubMed] [Google Scholar]
- Bae H, Nantwi KD, Goshgarian HG. Recovery of respiratory function following C2 hemi and carotid body denervation in adult rats: influence of peripheral adenosine receptors. Exp Neurol. 2005;191:94–103. doi: 10.1016/j.expneurol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127(2):658–61. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Nantwi KD, Goshgarian HG. Theophylline-induced respiratory recovery following cervical spinal cord hemisection is augmented by serotonin 2 receptor stimulation. Brain Res. 2002;956:1–13. doi: 10.1016/s0006-8993(02)03097-4. [DOI] [PubMed] [Google Scholar]
- Baussart B, Stamegna JC, Polentes J, Tadié M, Gauthier P. A new model of upper cervical spinal cord contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis. 2006;22:562–574. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Bergstrand H. Phosphodiesterase inhibition and theophylline. Eur J Respir Dis. 1980;61(109):37–44. [PubMed] [Google Scholar]
- Bisserbe JC, Pascal O, Deckert J, Mazière B. Potential use of DPCPX as a probe for in vivo localization of brain adenosine A1 receptors. Brain Res. 1992;599:6–12. doi: 10.1016/0006-8993(92)90845-z. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Ped Res. 1995;38(2):312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Bonneau O, Wyss D, Ferretti S, Blayton C, Stevenson SC, Trifilieff A. Effect of adenosine A2A activation in murine models of respiratory disorders. Am Lung Cell Mol Mol Physiol. 2006:L1036–L1043. doi: 10.1152/ajplung.00422.2005. [DOI] [PubMed] [Google Scholar]
- Boussey HA. Basic and Clinical Pharmacology: Bronchodilators and other agents used in treatment of asthma. Appleton and Lange; Norwalk CT: 1989. p. 247. [Google Scholar]
- Burnstock G. Purine and Pyrimidine Receptors. Cell Mol Life Sci. 2007;204:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carley DW, Radulovacki M. Role of peripheral adenosine A1 receptors in regulation of sleep apneas in rats. Exp Neurol. 1999;159:545–550. doi: 10.1006/exnr.1999.7167. [DOI] [PubMed] [Google Scholar]
- Chevrolet JC, Reverdin A, Suter PM, Tschopp JM, Junod A. Ventilatory Dysfunction resulting from bilateral anterolateral high cervical cordotomy. Chest. 1983;84(1):112–115. doi: 10.1378/chest.84.1.112. [DOI] [PubMed] [Google Scholar]
- Choi H, Liao WL, Newton KM, Onario RC, Knig AM, Desilets FC, Woodward EJ, Eichler ME, Frontera WR, Sabharwal S, Teng YD. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005;25(18):4550–4559. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu NS. Caffeine- and aminophylline-induced seizures. Epilepsia. 1981;22:85–94. doi: 10.1111/j.1528-1157.1981.tb04335.x. [DOI] [PubMed] [Google Scholar]
- Dong XW, Feldman JL. Modulation of inspiratory drive to phrenic motoneurons by presynaptic adenosine A1 receptors. J Neurosci. 1995;15:3458–3467. doi: 10.1523/JNEUROSCI.15-05-03458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Killey JP. Antagonism by theophylline of respiratory inhibition induced by adenosine. J Appl Physiol. 1985;59(5):1428–1433. doi: 10.1152/jappl.1985.59.5.1428. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG, Killey JP. Mechanism of respiratory effects of methylxanthines. Respiration Physiology. 1983;53:239–261. doi: 10.1016/0034-5687(83)90070-1. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Quantification of electrical activity in the phrenic nerve in the study of ventilatory control. Chest. 1976;70:154–157. doi: 10.1378/chest.70.1_supplement.154. [DOI] [PubMed] [Google Scholar]
- Evoniuk G, Von Borstel RW, Wurtman RJ. Antagonism of the cardiovascular effects of adenosine by caffeine or 8-(-p-Sulfophenyl)theophylline. J Pharmacol Exp Ther. 1986;240(20):428–432. [PubMed] [Google Scholar]
- Fastbom J, Fredholm BB. Effects of long term theophylline treatment on adenosine A1-receptors in the rat brain: autoradiographic evidence for increased receptor number and altered coupling to G-proteins. J Neurosci. 1990;507:195–199. doi: 10.1016/0006-8993(90)90272-d. [DOI] [PubMed] [Google Scholar]
- Fersuson GT, Narendra K, Lattin CD, Goshgarian HG. Clinical effects of theophylline therapy on inspiratory muscle in tetraplegia. Neurorehab Neural Repair. 1999;13(3):191–197. [Google Scholar]
- Feoktisyov L, Garland EM, Goldstein AE, Zeng D, Belardinelli L, Wells JN, Biaggioni I. Inhibition of human mast cell activation with the selective adenosine A(2B) receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IDPX)(2) Biochem Pharmacol. 2001;62:1163–1173. doi: 10.1016/s0006-2952(01)00765-1. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Baur F, Wobler C. Antagonist pharmacology of adenosine A2B receptors from rat, guinea pig, and dog. Eur J Pharmacol. 2003;475:79–84. doi: 10.1016/s0014-2999(03)02078-8. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn-Sch Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Ann Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Maxwell DL, Conradson TBG, Dixon CMS, Barnes PJ. Circulatory and respiratory effects of infused adenosine in conscious man. Br J Clin Pharmacol. 1987;24:309–17. doi: 10.1111/j.1365-2125.1987.tb03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal cord hemisection in rats. J Appl Physiol. 2005;100:800–806. doi: 10.1152/japplphysiol.00960.2005. [DOI] [PubMed] [Google Scholar]
- Gauda E. Expression and localization of A2A- and A1-adenosine receptor genes in the carotid body and petrosal ganglia. A2A and A1-adenosine receptor m RNA in the carotid body. Adv Exp Med Biol. 2000;475:549–558. [PubMed] [Google Scholar]
- Gaytan SP, Saadani-Makki F, Bodineau L, Frugiere A, Larnicol N, Pasaro R. Effect of postnatal exposure to caffeine on the pattern of adenosine A1 receptor distribution in the respiration-related nuclei of the rat brainstem. Aut Neurosci Basic and Clinical. 2006:126–127. 339–346. doi: 10.1016/j.autneu.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Georgiev V, Johansson B, Fredholm BB. Long term caffeine treatment leads to a decreased susceptibility to NMDA-induced clonic seizures in mice without changes in adenosine A1receptor number. Brain Res. 1993;612:271–277. doi: 10.1016/0006-8993(93)91672-f. [DOI] [PubMed] [Google Scholar]
- Gleeson K, Zwellich CW. Adenosine stimulation, ventilation and arousal from sleep. Am Rev Respir Dis. 1992;145:453–457. doi: 10.1164/ajrccm/145.2_Pt_1.453. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Fuller DD, Davenport PW, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory output. J Neurosci. 2003;23(6):2494–501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol. 2001;91:2451–2458. doi: 10.1152/jappl.2001.91.6.2451. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94(2):795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Gorini M, Duranti R, Misuri G, Valenza T, Spinelli A, Goti P, Gigliotti F, Scano G. Aminophylline and respiratory muscle interaction in normal humans. Am J Physiol. 1994:1227–1234. doi: 10.1164/ajrccm.149.5.8173763. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Mante S, Minneman KP. Role of adenosine receptors in caffeine tolerance. J Pharamcol Exp Ther. 1991;256:62–68. [PubMed] [Google Scholar]
- James E, Nantwi KD. Involvement Of Peripheral Adenosine A2 Receptors in Adenosine A1-Receptor Mediated Recovery Of Respiratory Motor Function Following Upper Cervical Spinal Cord Hemisection. J Spinal Cord Med. 2006;29(1):57–66. doi: 10.1080/10790268.2006.11753857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Ahlberg S, van der Ploeg I, Brene S, Lindefors N, Peterson H, Fredholm BB. Effects of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Nauny-Sch Arch Pharmacol. 1993;347:407–414. doi: 10.1007/BF00165391. [DOI] [PubMed] [Google Scholar]
- Johansson B, Georgiev V, Lindstrom K, Fredholm BB. A1 and A2 adenosine receptors and A1 mRNA in mouse brain: effect of long term caffeine treatment. Brain Res. 1997;762:153–164. doi: 10.1016/s0006-8993(97)00378-8. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Kent MA, Cotreau-Bibbo MM. Caffeine treatment and withdrawal in mice: relationships between dosage, concentrations, locomotor activity and A1 adenosine receptor binding. J Pharmacol Exp Ther. 1993;266:1563–1572. [PubMed] [Google Scholar]
- Klotz KN. Adenosine receptors and their ligands. Naunyn Schmiedelbergs Arch Pharmacol. 2000;362(4):382–91. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors. Their presence and neuromodulatory role in the central nervous system. Gen Pharmacol. 1996;27:925–33. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Brookhart JM. Significance of the crossed phrenic phenomenon. J Neurophysiol. 1951;166:241–254. doi: 10.1152/ajplegacy.1951.166.2.241. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2 adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- McKinley WO. Late return of diaphragm function in a ventilator-dependent patient with a high cervical tetraplegia: Case report and interactive review. Spinal Cord. 1996;34:626–629. doi: 10.1038/sc.1996.112. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Pharmacological characterization of the receptor involved in chemoexcitation induced by adenosine. Br J Clin Pharmacol. 1986;88:615–20. doi: 10.1111/j.1476-5381.1986.tb10242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael W, Jacobson KA. Characterization of the Receptor and its Binding Site. In: Michael W, editor. Adenosine and Adenosine Receptors, Section 2: Radioligand Binding Assays for Adenosine Receptors. Humana; 1990. [Google Scholar]
- Mitchell GS, Sloan HE, Jiang C, Miletic V, Hayashi F, Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneuron discharge in spinalized rats. Neurosci Lett. 1992;141:75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Invited Review: Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Monteiro EC, Ribeiro JA. Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Nauyn-Schmiedeberg Arch Pharmacol. 1987;335:143–148. doi: 10.1007/BF00177715. [DOI] [PubMed] [Google Scholar]
- Monti D, Carley DW, Radulovacki M. p-SPA, a peripheral adenosine A1 analog, reduces sleep apnea in rats. Pharmacol Biochem Behav. 1996;53(2):341–345. doi: 10.1016/0091-3057(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Nachazel J, Palecek F. Aminophylline enhances ventilation in phrenicotomized rats. Eur Respir J. 1990;3:311–317. [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Goshgarian HG. Actions of systematic theophylline on hemidiaphragm recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimser GW, Reier PJ, Goshgarian HG. Spontaneous Functional Recovery in a Paralyzed Hemidiaphragm Following Upper Cervical Spinal cord Injury in Adult Rats. Neurorehab Neural Repair. 1999;13(4):225–234. [Google Scholar]
- Nantwi KD, Goshgarian HG. Effects of chronic systematic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res. 1998a;789:126–129. doi: 10.1016/s0006-8993(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats. Contribution of adenosine receptors. Neuropharmacology. 1998b;37:113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Alkylxanthine-induced recovery of respiratory function following cervical spinal cord injury in adult rats. Exp Neurol. 2001;168(1):123–134. doi: 10.1006/exnr.2000.7581. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Basura JG, Goshgarian HG. Adenosine A1 mRNA expression and the effects of systemic theophylline on respiratory function four months after C2 hemisection. J Spinal Cord Med. 2003a;26(4):364–371. doi: 10.1080/10790268.2003.11753707. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Basura JG, Goshgarian HG. Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol. 2003b;182:232–239. doi: 10.1016/s0014-4886(03)00109-2. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 compounds in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29(10):915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Nantwi KD. Activation of adenosine A2 receptors improves spontaneous recovery in C2 hemisected rats. Soc Neurosci Abstr. 2008;371 [Google Scholar]
- Nantwi KD, Kizy T. Biochemical Profiles of Adenosine A1 Receptors after prolonged Post Cervical (C2) Hemisection Periods. J Spinal Cord Med. 2007;30(4):394. doi: 10.1080/10790268.2007.11753948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Spinal Cord injury: facts and figures at a glance. J Spinal Cord Med. 2006;29:89–90. [Google Scholar]
- Newman D, Tamir J, Speedy L, Newman JP, Ben-Dov I. Physiological and neuropsychological effects of theophylline in chronic obstructive pulmonary disease. Isr J Med Sci. 1994;30:811–817. [PubMed] [Google Scholar]
- Nikodijevic O, Sarges R, Daly JW, Jacobson KA. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists: Evidence for synergism and antagonism. J Pharmacol Exp Ther. 1991;259(1):286–294. [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. AM j Physiol. 1988;64(2):666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- Persson CGA, Anderson KE, Kjellin G. Effects of enprofylline and theophylline may show the role of adenosine. Life Sciences. 1986;38(12):1057–72. doi: 10.1016/0024-3205(86)90241-9. [DOI] [PubMed] [Google Scholar]
- Petrov T, Alilain WJ, Kreipke C, Nantwi KD. Differential expression of adenosine A1 and A2A receptors after upper cervical (C2) spinal cord hemisection in adult rats. J Spinal Cord Med. 2007;30(4):331–7. doi: 10.1080/10790268.2007.11753948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid PG, Watt AH, Penny WJ, Newby AC, Smith AP, Routledge PA. Plasma adenosine concentrations during adenosine-induced respiratory stimulation in man. Eur J Clin Pharmacol. 1991;40:175–80. doi: 10.1007/BF00280073. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A. Participation of adenosine receptors in neuroprotection. Drug News Perspect. 2003;16(2):80–6. doi: 10.1358/dnp.2003.16.2.740246. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–45. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Slack SR, Shucart W. Respiration dysfunction associated with traumatic injury to the central nervous system. Clinics in Chest Med. 1994;15(4):739–749. [PubMed] [Google Scholar]
- Smellie FW, Davis CW, Daly JW, Wells JN. Alkylxanthines: Inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979;24:2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Thomas T, Elnazir BK, Marshall JM. Differentiation of the peripherally mediated from the centrally mediated influences of adenosine in the rat during systemic hypoxia. Exp Physiol. 1994;79:809–22. doi: 10.1113/expphysiol.1994.sp003809. [DOI] [PubMed] [Google Scholar]
- Varani K, Caramori G, Vincenzi F, Adcock I, Casolari P, Leung E, Maclennan S, Gessi S, Morello S, Barnes PJ, Ito K, Chung KF, Cavallesco G, Azzena G, Papi A, Borea PA. Alteration of adenosine receptors in patients with obstructive pulmonary disease. Am J Crit Care Med. 2006;173:398–406. doi: 10.1164/rccm.200506-869OC. [DOI] [PubMed] [Google Scholar]
- Watt AH, Routledge PA. Adenosine stimulates respiration in man. Br J Pharmacol. 1985;20:503–506. doi: 10.1111/j.1365-2125.1985.tb05108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Arch Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Woodcock AA, Johnson MA, Geddes DM. Theophylline prescribing, serum concentrations and toxicity. Lancet. 1983;2:610–613. doi: 10.1016/s0140-6736(83)90691-8. [DOI] [PubMed] [Google Scholar]
- Zablocki J, Kalla R, Perry T, Palle V, Varkhedkar V, Xiao D, Piscopio A, Maa T, Gimbel A, Hao J, Chu N, Leung K, Zeng D. The discovery of a selective, high affinity A(2B) adenosine receptor antagonist for the potential treatment of asthma. Bioorg Med Chem Lett. 2005;15:609–612. doi: 10.1016/j.bmcl.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Basura GJ, Goshgarian HG. Involvement of Serotonin 2 receptors in respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91(6):2665–2673. doi: 10.1152/jappl.2001.91.6.2665. [DOI] [PubMed] [Google Scholar]