Abstract
Ginger, the rhizome of the plant Zingiber officinale, has received extensive attention due to its antioxidant, anti-inflammatory, and anti-tumor activities. Most researchers have considered gingerols as the active principles and have paid little attention to shogaols, the dehydration products of corresponding gingerols during storage or thermal processing. In this study, we have purified and identified eight major components including three major gingerols and corresponding shogaols from ginger extract and compared their anti-carcinogenic and anti-inflammatory activities. Our results showed that shogaols ([6]-, [8]-, and [10]-) had much stronger growth inhibitory effects than gingerols ([6]-, [8]-, and [10]-) on H-1299 human lung cancer cells and HCT-116 human colon cancer cells, especially when comparing [6]-shogaol with [6]-gingerol (IC50: ~8 µM vs. ~150 µM). In addition, we found that [6]-shogaol had much stronger inhibitory effects on arachidonic acid release and nitric oxide (NO) synthesis than [6]-gingerol.
Keywords: ginger, Zingiber officinale, [6]-gingerol, [6]-shogaol, inflammation, cancer
INTRODUCTION
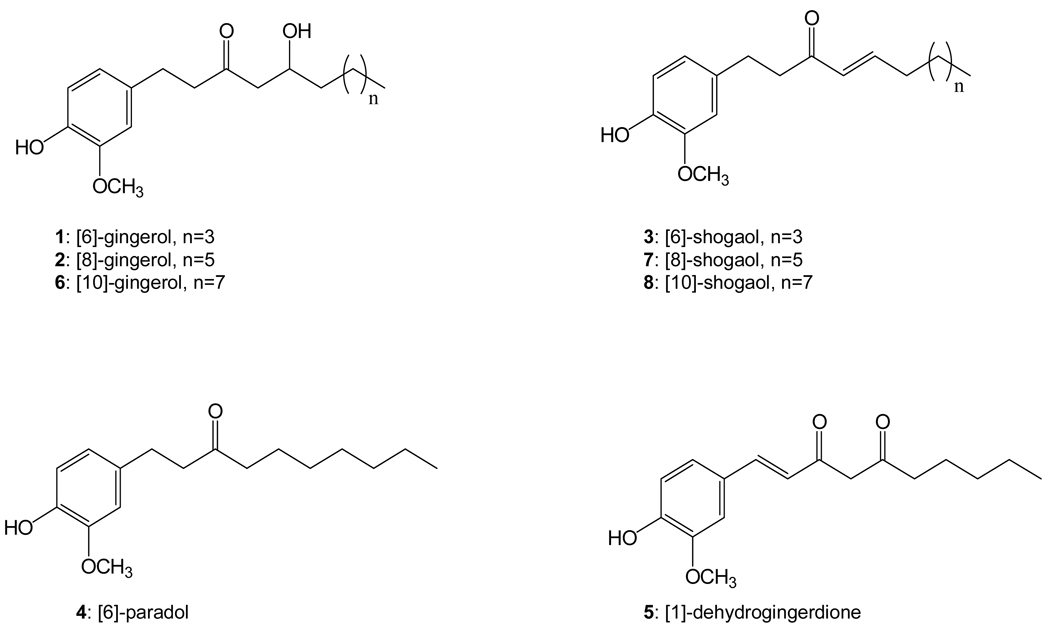
Ginger (Zingiber officinale Rosc.), a member of the Zingiberaceae family that consists of 47 genera including Zingiber and Curcuma, has been cultivated for thousands of years as a spice and for medicinal purposes. The gingerols, a series of homologs differentiated by the length of their unbranched alkyl chains, were identified as the major pungent components in the ginger oleoresin from fresh rhizome, with [6]-gingerol (Figure 1) being the most abundant (1). Gingerols are not stable during storage or thermal processing to generate the dehydration products, shogaols, which are predominant pungent constituents in the ginger oleoresin from dried ginger (1, 2). It has been reported that shogaols were minor components in fresh ginger and the ratio of [6]-shogaol to [6]-gingerol was about 1:1 in dried ginger (1, 2). Other gingerol- or shogaol-related compounds have also been reported in ginger rhizome, such as [6]-paradol, [6]- and [10]-dehydrogingerdione, [6]- and [10]-gingerdione, [4]-, [6]-, [8]-, and [10]-gingerdiol, [6]-methylgingerdiol, zingerone, [6]-hydroxyshogaol, and [6]-, [8]-, [10]-dehydroshogaol, and diarylheptanoids (1–3). However, these minor compounds only count for about 1 to 10% of those for the overall amount of gingerols and shogaols (1–3).
Figure 1.
Structures of the eight major components purified from ginger extract.
Recently, ginger has received extensive attention as a botanical dietary supplement in the USA and Europe due to its antioxidative, anti-inflammatory, and anti-tumor activities (4, 5). Although most of the animal studies with ginger extract showed anti-oxidative, anti-inflammatory, and anti-tumor activities, no report has considered the instability of gingerols during thermal process and long-term storage will affect the chemical profile of the ginger extract used in their animal studies. They either did not quantify the levels of the active components in their raw material or simply used the total levels of gingerols as the standard.
The nature of the active components in ginger has not been fully explored. It has been reported that topical application of [6]-gingerol or [6]-paradol onto shaven backs of female ICR mice prior to each topical dose of 12-O-tetradecanoylphorbol-13-acetate (TPA) significantly inhibited 7,12-dimethylbenz[α]anthracene (DMBA)-induced skin tumor incidence and tumor burden (6). The same group also found that [6]-gingerol inhibited TPA-induced cyclooxygenase-2 (COX-2) expression in mouse skin in vivo by blocking the p38 MAP kinase-NF-B signaling pathway (7). [6]-Gingerol was found to decrease the number of lung metastasis in mice implanted with B16F10 melanoma cells (8). Jeong et al. reported that [6]-gingerol effectively suppressed in vivo tumor growth in HCT-116 cancer cell-bearing nude mice (9). Several in vitro studies have found that shogaols also have anti-inflammatory and anti-cancer activities. It has been reported that [6]-shogaol significantly suppressed the expression of inducible nitric oxide sysnthesis and COX-2 in lipopolysaccharide (LPS)-induced macrophasges (10). Wei et al. found that [6]- and [10]-shogaol could significantly inhibit the growth of HL-60 human leukemia cells. Rhode et al. reported that [6]-, [8]-, and [10]-gingerol had no effect and [6]-shogaol could significantly inhibit the growth of A-2780 ovarian cancer cells (11). Pan et al. found that [6]-shogaol inhibited the growth of human colon COLO-205 cells and induced apoptosis through modulation of mitochondrial functions regulated by reactive oxygen species (12). Kim et al. reported that [6]-shogaol had much stronger growth inhibitory effects on A-549 human lung cancer cells, SK-OV-3 human ovarian cancer cells, SK-MEL-2 human skin cancer cells, and HCT-15 human colon cancer cells than [4]-, [6]-, [8]-, and [10]-gingerol (13).
In order to further study whether shogaols have better anti-inflammatory and anti-cancer activities than corresponding gingerols, we purified and identified eight major components including three major gingerols ([6]-, [8]-, and [10]-gingerol), corresponding shogaols, [6]-paradol, and [1]-dehydrogingerdione from ginger extract, and compared their growth inhibitory effects against human lung and colon cancer cells and the inhibition of arachidonic acid release and nitric oxide (NO) synthesis from LPS-stimulated RAW 264.7 cells.
MATERIALS AND METHODS
Materials
Ginger extract, which contains 20% gingerols and shogaols, was obtained from Sabinsa Corporation (Piscataway, NJ). RP C-18 silica gel, silica gel, Sephadex LH-20 gel, TLC plates (250 µm thickness, 2–25 µm particle size), CD3OD, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). HPLC-grade solvents and other reagents were obtained from VWR Scientific (South Plainfield, NJ). HPLC-grade water was prepared using a Millipore Milli-Q purification system (Bedford, MA). H-1299 human lung cancer cells, HCT-116 human colon adenocarcinoma cells, and RAW 264.7 murine macrophages were obtained from American Type Tissue Culture (Manassas, VA, USA).
NMR
1H (400 MHz), 13C (100 MHz), and all 2D NMR spectra were acquired on a Varian 400 instrument (Varian Inc., Palo Alto, CA). Compounds were analyzed in CD3OD, with TMS as the internal standard. 1H-13C HMQC (heteronuclear multiple quantum correlation) and HMBC (heteronuclear multiple band correlation) experiments were performed as described previously (14).
HPLC analysis
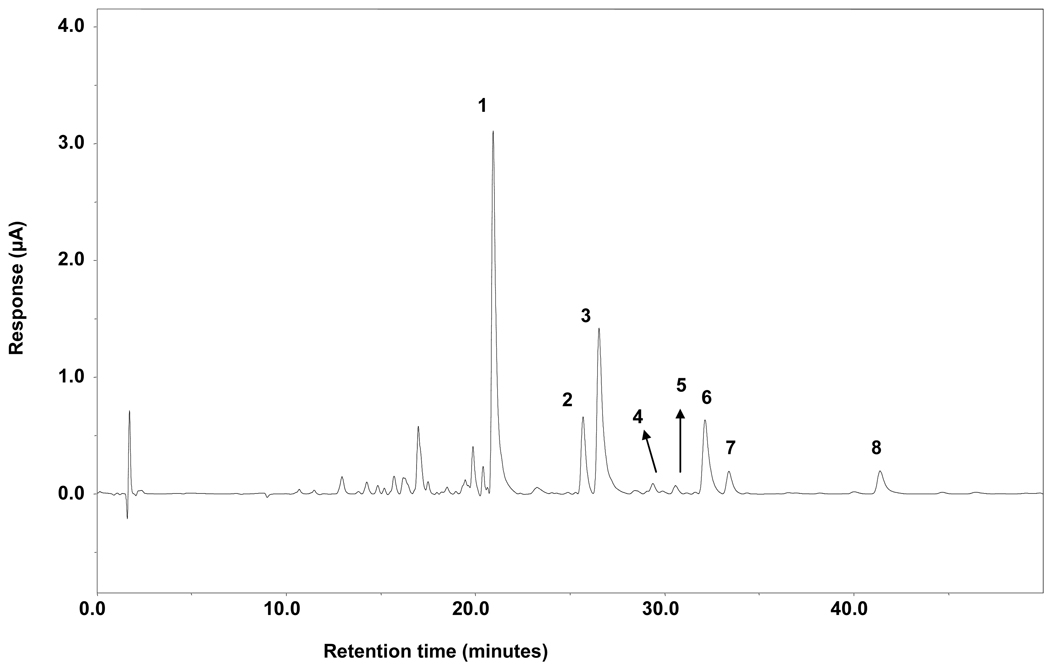
HPLC analysis was carried out with a system consisting of a Waters 717 refrigerated autosampler, a HITACHI L-6200A intelligent pump, and an ESA 5600 coulochem electrode array system (CEAS). The potentials of the CEAS were set at 0, 200, 300, and 400 mV. The column used was a 150 mm × 4.6 mm i.d., 5 µm, Supelcosil RP-18. For binary gradient elution, mobile phase A (1.75% acetonitrile and 0.12% tetrahydrofuran in 30 mM NaH2PO4, pH 3.35) and B (58.5% acetonitrile and 12.5% tetrahydrofuran in 15 mM NaH2PO4, pH 3.45) were used. The flow rate was maintained at 1 mL/min, and the mobile phase began with 100% A. It was followed by progressive linear increases in B to 65% at 15 min, and 100% at 35 min. The mobile phase was maintained at 100% B for 10 min and then was re-equilibrated to 100% A at 46 min for another run. The HPLC profile of the ginger extract displaying these eight compounds is shown in Figure 2.
Figure 2.
HPLC-ECD profiles of ginger extract: Peak 1, [6]-gingerol; 2, [8]-gingerol; 3, [6]-shogaol; 4, [6]-paradol; 5, [1]-dehydrogingerdione; 6, [10]-gingerol; 7, [8]-shogaol, and 8, [10]-shogaol.
Isolation of the major constituents in ginger extract
The ginger extract (50 g) was chromatographed on a Sephadex LH-20 column with 95% ethanol as eluant to remove the nonphenolic compounds (Fraction 1, 21.8 g) and to generate the gingerols and shogaols enriched fraction (Fraction 2, 28 g). Fraction 2 was then loaded into a Diaion HP-20 column eluted first with water to remove the water soluble compounds and then with 40% aqueous ethanol to obtain fraction A (9 g) followed by 95% aqueous ethanol to obtain fraction B (11g). Fraction A (5g) was subjected to a normal phase silica gel column with a stepwise gradient of hexane/ethyl acetate [9:1; 8:2, and 7:3] to give pure [6]- (2g), [8]- (0.5 g), and [10]-gingerol (0.4 g). Fraction B (5 g) was also subjected to a normal phase silica gel column with a stepwise gradient of hexane/ethyl acetate [9:1 and 8:2] to generate 13 fractions. Fraction B5 (1g) was subjected to a C-18 reverse phase column eluted with a stepwise gradient of methanol/water [3:2, 7:3, and 4:1] to give [6]-paradol (40 mg), [1]-dehydrogigerdione (60 mg), and [10]-shogaol (120 mg). Following a similar procedure, fraction B7 (1.5 g) gave 200 mg [8]-shogaol and 1 g of [6]-shogaol. The purification procedure was guided by TLC and HPLC analysis. The structures of these eight compounds were confirmed based on their 1H and 13C NMR analysis (Figure 1).
Growth inhibition against human lung and colon cancer cells
Cell growth inhibition was determined by the MTT assay (15). The cells were plated in 96-well microtiter plates and allowed to attach for 24 h at 37 °C. The test compounds (in DMSO) were added to cell culture medium to desired final concentrations (Final DMSO concentrations for control and treatments are 0.1%). After culturing for 24 h, the medium was aspirated, and the cells were treated with 100 µL fresh medium containing 2.41 mmol/L MTT. Following incubation for 1 – 3 h at 37 °C, the MTT-containing medium was aspirated, 100 µL DMSO was added to solubilize the formazan precipitate, and the plate was read at 550 nm on a microtiter plate reader. The reading reflected the number of viable cells, and was expressed as % viable cells in control. Both H-1299 and HCT-116 cells were cultured in McCoy’s 5A medium. All the above media were supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% glutamine, and the cells were kept in a 37 °C incubator with 95% humidity and 5% CO2.
Inhibition of arachidonic acid release from LPS-stimulated RAW 264.7 cells
To determine inhibition of arachidonic acid release, RAW 264.7 cells were plated into a 24-well plate (3 × 105 cells per well). After 24 h, the media were removed and replaced with 1 mL of serum-free DMEM media containing 0.1 µCi/mL [5,6,8,9,11,12,14, 15-3H](N) arachidonic acid (NEN Life Science, Boston, MA, USA). The cells were incubated overnight, resulting in over 90% arachidonic acid absorption, and washed twice with PBS containing 0.1% BSA. The cells were stimulated with 2 µg/mL LPS for 1 h and the media was replaced with serum-free medium containing the test compounds with desired final concentrations. After incubation for 18 h, the media were collected and centrifuged for 10 min at 12000 rpm. Radioactivity in the extracellular fluid was measured with a scintillation counter. RAW 264.7 cells were maintained in log phase growth in Dulbecco’s modified Eagles Medium.
Inhibition of nitric oxide (NO) synthesis
RAW 264.7 cells were plated in 24-well plates (3.0 × 105 cells per well) and stimulated for 1 h with 1 µg/mL LPS and 10 ng/mL interferon gamma (IFNγ). The media were then replaced with serum-free medium containing compounds with desired final concentrations and cells were cultured for 24 and 30 h. NO production was determined spectrophotometrically using previously reported methods (16).
Statistical Analysis
For simple comparisons between two groups, two-tailed Student's t-test was used. A p-value of less than 0.05 was considered statistically significant in all the tests.
RESULTS AND DISCUSSION
Purification and structure elucidation
Ginger extract was chromatographed successively on Sephadex LH-20, Diaion HP-20, normal phase silica gel, and/or RP-C18 columns to afford [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione (1 – 8) (Figure 1). Their structures were determined by comparison of their NMR and MS data with those reported in the literature (13, 17, 18). Then, we developed an HPLC method using an electrochemical array detector (ECD) to analyze those eight components (Figure 2). This method is about 50 to 100 fold more sensitive than the UV detection method reported in previous literature (19). The limit of detection is ~1 – 2 ng/mL for all eight components, and the limit of quantification is ~2 – 4 ng/mL for all eight components.
Inhibitory Effects on the proliferation of human lung and colon cancer cells
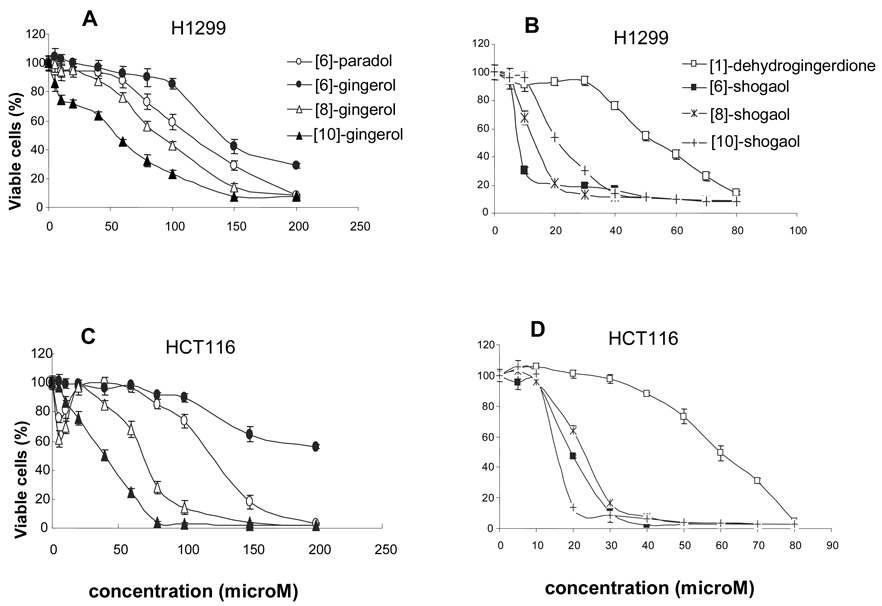
The growth inhibitory activities of compounds 1–8 were determined after treatment for 24 h in H-1299 human lung cancer cells and in HCT-116 human colon cancer cells. Our results indicated that [6]-, [8]-, and [10]-gingerol and [6]-paradol showed effective inhibition on H-1299 cells with [10]-gingerol > [8]-gingerol > [6]-paradol > [6]-gingerol. However, shogaols ([6]-, [8]-, and [10]-) had much stronger growth inhibitory effects than gingerols ([6]-, [8]-, and [10]-) on H-1299 human lung cancer cells, especially when comparing [6]-shogaol with [6]-gingerol (IC50: ~8 µM vs. ~150 µM) (Figure 3A and 3B). The inhibitory effects of [6]-, [8]-, and [10]-shogaol exhibited an order of [6]-shogaol > [8]-shogaol > [10]-shogaol, and were much stronger than the effects of [1]-dehydrogingerdione.
Figure 3.
Effects of [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione on the growth of H1299 human lung cancer cells and HCT-116 human colon adenocarcinoma cells. Each value represents the mean ± S.D. (n = 8).
Similarly, we found that shogaols ([6]-, [8]-, and [10]-) had much stronger growth inhibitory effects than gingerols ([6]-, [8]-, and [10]-) on HCT-116 human colon cancer cells (Figure 3C and 3D). HCT-116 cells were less sensitive to both [6]-shogaol and [6]-gingerol treatments than H-1299 cells.
Inhibitory effects on arachidonic acid release
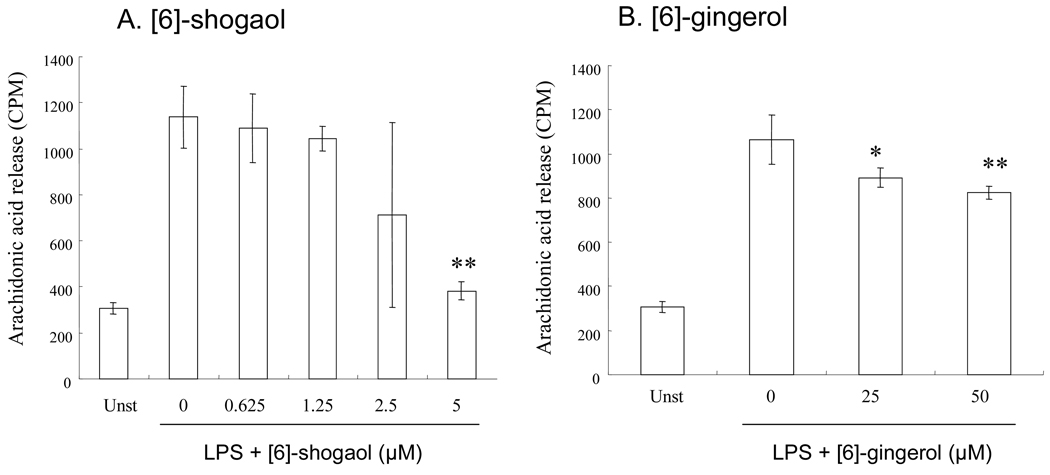
After stimulation of the cells with 2 µg/mL LPS for 1h, the release of arachidonic acid and its metabolites from RAW264.7 macrophage cells to the culture media increased ~3-fold after 18 h incubation. At 5 µM, [6]-shogaol significantly decreased the release of arachidonic acid and its metabolites during the 18 h incubation (~90% inhibition) (Figure 4A). This is much more effective than 50 µM [6]-gingerol which yielded ~30% inhibition (Figure 4B).
Figure 4.
Effects of [6]-shogaol (A) and [6]-gingerol (B) on arachidonic acid release in LPS-stimulated RAW264.7 cells. Each bar represents the mean ± SD (n = 8). *, ** Significantly different from control according to the Student's t-test (*P < 0.05; **P < 0.01).
Inhibitory effects on NO synthesis
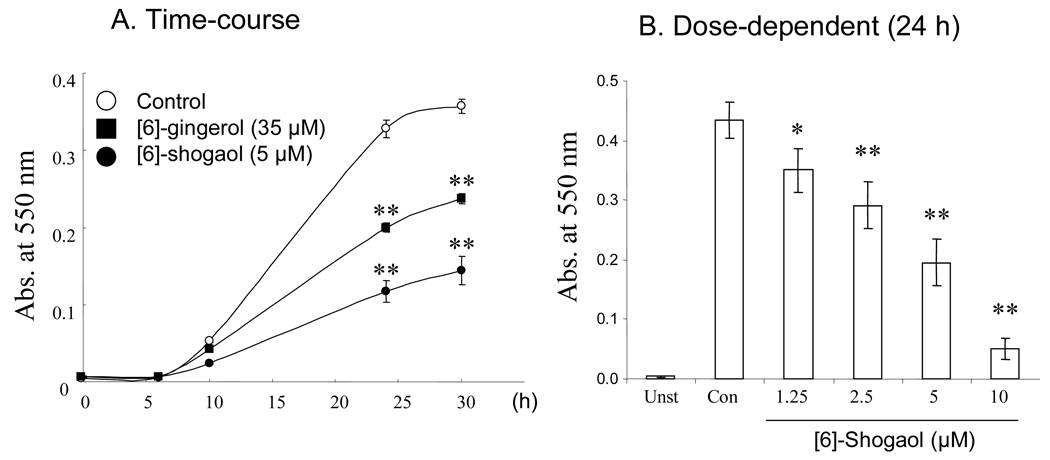
After stimulation of RAW264.7 cells with 1 µg/mL LPS and 10 ng/mL IFNγ for 1h, significant NO accumulation in culture medium was observed at 24h and much higher accumulation of NO was observed at 30h. Our results indicated that both [6]-shogaol and [6]-gingerol significantly inhibited NO accumulation (p < 0.01) (Figure 5A), whereas, [6]-shogaol at 5 µM has much stronger inhibitory effects than [6]-gingerol at 35 µM (Figure 5A). The inhibitory effect of [6]-shogaol was concentration-dependent, with significant inhibition observed at a concentration as low as 1.25 µM (Figure 5B).
Figure 5.
Effects of [6]-shogaol and [6]-gingerol on NO synthesis in LPS/IFNγ-stimulated RAW264.7 cells. The levels of NO in the culture medium of LPS/IFNγ-stimulated RAW264.7 cell were analyzed at different time points after treatment with 5 µM [6]-shogaol and 35 µM [6]-gingerol (A); The concentration-dependent effects of [6]-shogaol on NO release was analyzed after 24 h of incubation (B). *, ** Significantly different from control according to the Student's t-test (*P < 0.05; **P < 0.01). Each bar represents the mean ± SD (n = 8).
One of the challenges to study the in vivo efficacy of [6]-gingerol and [6]-shogaol is both compounds are not commercial available with affordable prices. In this study, we found that Diaion HP-20 column chromatogram is a useful tool to separate [6]-gingerol and [6]-shogaol in large quantify. We recently further polished our purification methods. We found that 1 kg Diaion HP-20 resin could load 100 g of ginger extract. [6]-gingerol with 90% purity could be eluted by 40% aqueous ethanol and [6]-shogaol could be eluted out by 75% aqueous ethanol through Diaion HP-20 column. Both [6]-gingerol and [6]-shogaol enriched fraction could be further purified using RP C-18 column to generate [6]-gingerol and [6]-shogaol with more than 95% purity. Therefore, large quantities (10 –20 g) of [6]-gingerol and [6]-shogaol can be purified from ginger extract within four weeks. Since both Diaion HP-20 resin and RP C-18 silica gel are reusable, the purification method developed in this study will be very practical to prepare large quantities of [6]-gingerol and [6]-shogaol in academic lab or botanical company to support future in vivo studies.
In this study, we purified eight major ginger components including [6]-, [8]-, and [10]-shogaols and related gingerols from ginger extract and compared their anti-carcinogenic and anti-inflammatory effects. Our results indicated that shogaols ([6]-, [8]-, and [10]-) had much stronger growth inhibitory effects than gingerols ([6]-, [8]-, and [10]-) on H-1299 human lung cancer cells and HCT-116 human colon cancer cells, especially when comparing [6]-shogaol with [6]-gingerol. This is the first study to show that both shogaols and gingerols could significantly inhibit the growth of H-1299 human lung cancer cells. It has been reported that [6]-gingerol effectively suppressed in vivo tumor growth in HCT-116 human colon cancer cell-bearing nude mice. Our results indicated that [6]-shogaol had much stronger inhibitory effects than [6]-gingerol on the growth of HCT-116 cancer cells in vitro. Therefore, it is worthwhile to further study whether [6]-shogaol has stronger inhibitory effect to suppress in vivo tumor growth in HCT-116 colon cancer cell-bearing nude mice. In addition, we found that both [6]-shogaol and [6]-gingerol were extensively metabolized under cell culture conditions (unpublished data). We did not observe any conversion between [6]-shogaol and [6]-gingerol in the cell medium after 24 h incubation. We are in the process to purify and identify the major metabolites of [6]-shogaol and [6]-gingerol. Whether their metabolites have anti-cancer activities merits further investigation.
The shogaols are the dehydration products of related gingerols during storage or thermal processing (2, 3). Therefore, the contents of shogaols and gingerols in ginger preparations can vary greatly. This may have contributed to the inconsistencies in published effects of ginger preparations. Thus, ginger extract with high levels of shogaols such as extract from dry ginger may have stronger cancer preventive effect than ginger extract with high levels of gingerols, such as extract from fresh ginger. It is important to identify the bioactive components in ginger and standardize the products for use in future laboratory studies and clinical trials. The development of a standardized and a more active ginger extract preparation will facilitate future pre-clinical and clinical studies on the health benefit of ginger extracts.
Both arachidonic acid metabolites and NO are important mediators of oxidative stress and inflammation in vivo. Many studies have shown that arachidonic acid and its metabolites as well as NO play important roles in the development of cancer (20, 21). An increasing number of studies have indicated that inhibitors of arachidonic acid cascade and NO synthesis have potential therapeutic value for cancer prevention (22–28). The present results demonstrate that [6]-shogaol is capable of inhibiting both of these processes in LPS-induced murine macrophages. The inhibition of arachidonic acid release may be caused by a blockage of phospholipase A2 activation or activity and further mechanistic studies are required. Similarly, the mechanism for the inhibition of NO synthesis by LPS/IFNγ-activated macrophages is not clear. [6]-Shogaol may inhibit either iNOS activity or LPS induction of the enzyme. Further studies are needed to determine the mechanism(s) of action of shogaols.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by NIH grant CA138277 to S. Sang.
Footnotes
SUPPORTING INFORMATION AVAILABLE: NMR (1H and 13C) data for [6]-, [8]-, and [10]-gingerol, [6]-, [8]-, and [10]-shogaol, [6]-paradol, and [1]-dehydrogingerdione. This material is available free of charge via the Internet at http://pubs.acs.org.
LITERATURE CITED
- 1.Govindarajan VS. Ginger--chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1982;17:1–96. doi: 10.1080/10408398209527343. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran PNBNK. Ginger: The Genus Zingiber. CRC Press; 2005. pp. 1–15.pp. 87–115. [Google Scholar]
- 3.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40:1091–1097. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 6.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998;129:139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 7.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, Surh YJ. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24:2558–2567. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 8.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 9.Jeong CH, Bode AM, Pugliese A, Cho YY, Kim HG, Shim JH, Jeon YJ, Li H, Jiang H, Dong Z. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69:5584–5591. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- 10.Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467–1477. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- 11.Rhode J, Fogoros S, Zick S, Wahl H, Griffith KA, Huang J, Liu JR. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement Altern Med. 2007;7:4. doi: 10.1186/1472-6882-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52:527–537. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Lee SI, Park HW, Yang JH, Shin TY, Kim YC, Baek NI, Kim SH, Choi SU, Kwon BM, Leem KH, Jung MY, Kim DK. Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch Pharm Res. 2008;31:415–418. doi: 10.1007/s12272-001-1172-y. [DOI] [PubMed] [Google Scholar]
- 14.Fang X, Qiu F, Yan B, Wang H, Mort AJ, Stark RE. NMR studies of molecular structure in fruit cuticle polyesters. Phytochemistry. 2001;57:1035–1042. doi: 10.1016/s0031-9422(01)00106-6. [DOI] [PubMed] [Google Scholar]
- 15.Momann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immun. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Ryu JH, Ahn H, Jin Lee H. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia. 2003;74:350–354. doi: 10.1016/s0367-326x(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 17.Charles R, Garg SN, Kumar S. New gingerdione from the rhizomes of Zingiber officinale. Fitoterapia. 2000;17:716–718. doi: 10.1016/s0367-326x(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 18.Shoji N, Iwasa A, Takemoto T, Ishida Y, Ohizumi Y. Cardiotonic principles of ginger (Zingiber officinale Roscoe) J Pharm Sci. 1982;71:1174–1175. doi: 10.1002/jps.2600711025. [DOI] [PubMed] [Google Scholar]
- 19.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Coulter JA, McCarthy HO, Xiang J, Roedl W, Wagner E, Robson T, Hirst DG. Nitric oxide--a novel therapeutic for cancer. Nitric Oxide. 2008;19:192–198. doi: 10.1016/j.niox.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. 2009;9:701–715. doi: 10.1016/j.intimp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Sood S, Li N, Yang P, Newman RA, Yang CS, Chen X. Chemoprevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch by topical application of a dual inhibitor of epidermal growth factor receptor (EGFR) and ErbB2 tyrosine kinases. Oral Oncol. 2008;44:652–657. doi: 10.1016/j.oraloncology.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thun MJ, Blackard B. Pharmacologic effects of NSAIDs and implications for the risks and benefits of long-term prophylactic use of aspirin to prevent cancer. Recent Results Cancer Res. 2009;181:215–221. doi: 10.1007/978-3-540-69297-3_20. [DOI] [PubMed] [Google Scholar]
- 25.Ha SK, Lee P, Park JA, Oh HR, Lee SY, Park JH, Lee EH, Ryu JH, Lee KR, Kim SY. Apigenin inhibits the production of NO and PGE2 in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem Int. 2008;52:878–886. doi: 10.1016/j.neuint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Murakami A. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 2009;61:193–203. doi: 10.1159/000212751. [DOI] [PubMed] [Google Scholar]
- 27.Warren CA, Paulhill KJ, Davidson LA, Lupton JR, Taddeo SS, Hong MY, Carroll RJ, Chapkin RS, Turner ND. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J Nutr. 2009;139:101–105. doi: 10.3945/jn.108.096271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang GY, Taboada S, Liao J. Induced nitric oxide synthase as a major player in the oncogenic transformation of inflamed tissue. Methods Mol Biol. 2009;512:119–156. doi: 10.1007/978-1-60327-530-9_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.