Abstract
Hypertonic saline (HTS) is an accepted treatment for traumatic brain injury (TBI). However, the behavioral and cognitive consequences following HTS administration have not thoroughly been examined. Recent preclinical evidence has suggested that nicotinamide (NAM) is beneficial for recovery of function following TBI. The current study compared the behavioral and cognitive consequences of HTS and NAM as competitive therapeutic agents for the treatment of TBI. Following controlled cortical impact (CCI), bolus administrations of NAM (500 mg/kg), 7.5% HTS, or 0.9% saline vehicle (1.0 mL/kg) were given at 2, 24, and 48 hrs post-CCI. Behavioral results revealed that animals treated with NAM and HTS showed significant improvements in beam walk and locomotor placing compared to the Vehicle group. The Morris water maze (MWM) retrograde amnesia test was conducted on day 12 post-CCI and showed that all groups had significant retention of memory compared to injured, Vehicle-treated animals. Working memory was also assessed on days 18-20 using the MWM. The NAM and Vehicle groups quickly acquired the task; however, HTS animals showed no acquisition of this task. Histological examinations revealed that the HTS-treated animals lost significantly more cortical tissue than either the NAM or Vehicle-treated animals. HTS-treated animals showed a greater loss of hippocampal tissue compared to the other groups. In general, NAM showed a faster rate of recovery than HTS without this associated tissue loss. The results of this study reiterate the strengths of NAM following injury and show concerns with bolus administrations of HTS due to the differential effects on cognitive performance and apparent tissue loss.
Keywords: Therapy, hippocampus, neurodegeneration, retrograde amnesia, traumatic brain injury, controlled cortical impact
1. Introduction
Traumatic brain injury (TBI) is a major unrecognized public health epidemic. According to the Centers for Disease Control and Prevention, 1.4 million incidences of TBI occur each year in the United States alone with at least 1.1 million patients treated and released from emergency departments and another 235,000 that required hospitalization. Annually 50,000 people die and an additional 80,000 experience long-term disabilities following TBI (Thurman et al., 1999). Currently there are approximately 5.3 million Americans living with disabilities resulting from TBI (Langlois et al., 2006; Rutland-Brown et al., 2006; Thurman et al., 1999; Thurman & Guerrero, 1999). Included in these disabilities are motor, sensory, cognitive, emotional and psychiatric impairments (Thurman et al., 1999a, 1999b. This crisis is currently increasing based on causality reports from current military actions (Bliese et al., 2008).
Patient outcome is greatly influenced by cerebral edema and intracranial hypertension. Osmotherapy has become a common practice in the management of cerebral edema and intracranial hypertension. HTS was first introduced as a possible treatment following TBI in 1919 (Weed and McKibben, 1919). However, it hasn't been until recently that it has reemerged in the clinical setting. In 1988, Worthley showed that the bolus administration of HTS following TBI reduced intracranial pressure (ICP) in two patients (Worthley et al., 1988). Mostly through case studies and retroactive reports, additional adult human studies have shown reductions in ICP and brain edema in TBI patients following the administration of HTS (Qureshi et al., 1998; Suarez et al., 1998; Ware et al., 2005) even when conventional methods (mannitol, glycerol, etc.) have failed (Schatzmann et al., 1998; Horn et al., 1999; Munar et al., 2000). One difficulty in comparing these studies is the difference in HTS concentrations and routes of administration utilized across the studies. Pediatric studies, which more generally use HTS infusions, also indicate a reduction in ICP (Fisher et al., 1992) and increased cerebral perfusion pressure (CPP) (Khanna et al., 2000). However, 6 months after HTS treatment, neurological function was no different than those patients treated with conventional methods (Cooper et al., 2004). A current clinical practice suggests a rapid 250 mL bolus of 7.5% saline, followed by another 250 mL if needed, be administered following hemorrhage due to convenience of administration on the battlefield (Rhee et al., 2003). A current clinical trial has demonstrated that administration of 250 mL of 7.5% HTS in 6% dextran 70 within 4 hrs of severe TBI resulted in reduced serum biomarkers for TBI and correlated with favorable outcome (Baker et al., 2009).
Additionally, HTS therapy has been studied in many different animal models of TBI. HTS solutions reduced brain edema in rabbits (Scheller et al., 1991; Zornow et al., 1990). These studies suggest there is no difference between the reduction in edema between HTS and mannitol, an osmotic diuretic agent used to treat ICP. However, both are better at reducing edema than normal 0.9% saline. Additionally, studies in sheep show a reduction of edema with an intact blood-brain barrier (Battistella and Wisner, 1991) and decreased ICP (Anderson et al., 1997) following HTS administration. The administration of HTS in one month old piglets increased blood flow and oxygenation without increasing ICP (Taylor et al., 1996). However, in cats, HTS failed to restore blood flow following TBI (DeWitt et al., 1996). Furthermore, HTS administration reduced edema following TBI in rats (Wisner et al., 1990). Nevertheless, additional studies have shown that although administration of HTS reduced ICP in rats, only the combination of HTS and dextran 70 reduced neuronal loss and improved neurological outcome (Zausinger et al., 2004). In addition to reducing brain edema, HTS at both 2 mL/kg and 4 mL/kg doses has been shown to reduce secondary injury and increase cell survival in rats (Soustiel et al., 2006; Elliot et al., 2007). Following TBI plus hemorrhage, rats showed a significant improvement in performance over time following HTS treatment in a reference memory paradigm of the MWM. In addition, hippocampal cell counts revealed no significant ipsilateral difference between any of the groups. However, animals treated with HTS showed overall reduction in cell loss in the contralateral hippocampus (Sell et al., 2008). Although human and animal studies have shown the potential of HTS therapy, one in vitro study did not reveal such promising results. Himmelseher and colleagues (Himmelseher et al., 2001) investigated the effects of HTS on healthy and glutamate-injured brain cells and found a reduction of neurons but not astrocytes.
A possible alternative to HTS therapy, Nicotinamide (NAM), an amide of vitamin B3 and one of the precursors for nicotinamide adenine dinucleotide (NAD+), which plays a critical role in oxidative metabolism by increasing neuronal ATP concentrations, may protect against neuronal damage and toxicity (Grover et al, 2003). Not only does the administration of NAM following TBI reduce neuronal death (Holland et al., 2008; Hoane et al., 2006), it also attenuates cerebral edema (Hoane et al., 2006a). Following both CCI and fluid percussion injury (FPI) models of TBI, NAM (500 mg/kg) administered at 15 mins and 24 hrs post-injury improved behavioral impairments and reduced both reference and working memory MWM latencies (Hoane et al., 2006b; 2003). Additionally, lesion size and GFAP expression was also reduced (Hoane et al., 2003). It has also recently been shown that the window of opportunity for 50 mg/kg NAM therapy extends to at least 4-8 hrs post-CCI (Hoane et al., 2008).
Although the complete mechanisms of NAM following brain injury are not completely understood, Yang and colleagues (Yang et al., 2002) suggest that the inhibition of Poly (ADP-ribose) polymerase (PARP) by NAM administered shortly after ischemic stroke in rats is responsible for the observed reduction in apoptosis and necrosis. Doses of NAM (500 mg/kg) administered 2 hrs following ischemic stroke also reduced infarct volumes (Ayoub et al., 1999) and improved sensory and motor behavior (Mokudai et al., 2000) in rats. Additionally, administration of NAM (500 mg/kg) improved rotorod performance and reduced caspase-3 activity (reducing apoptosis) in seven day old rat pups following hypoxic-ischemic injury (Feng et al., 2006).
The purpose of the current study was to compare the behavioral and cognitive effects of NAM and HTS following a unilateral CCI. Additionally, histological examinations were conducted to measure cortical and hippocampal tissue loss. Although, the effects of HTS on cerebral edema and intracranial hypertension following TBI are highly recognized, its effects on cerebral tissue and subsequent behavioral and cognitive consequences have yet to be solidly determined. Furthermore, the positive effects of NAM on neuronal loss and subsequent behavioral and cognitive consequences in the treatment of TBI are well established. Therefore, a comparison of such treatments was warranted in order to establish clinical relevance of these agents.
2. Results
2.1 Subjects
A total of 32 animals underwent CCI surgery or Sham operations. Weight analysis of all animals using a one-way ANOVA showed there were no significant differences between the animals at the time of CCI surgery, [F(3,28) = 2.59, p > 0.05]. The mean weight at the time of CCI surgery was 379 g (SD = 13.72).
2.2 Behavioral Results
Beam Walk Test
Post-CCI performance was scored on all behavioral tests on Days 2, 6, 10, 14, and 18. Difference scores were calculated by subtracting post-CCI scores from pre-injury criterion scores. These difference scores were entered into a GLM repeated measures procedure with group (NAM, HTS, Vehicle, Sham) as the between subjects factor and Day of testing (pre-injury, post-CCI Day 2, 6, 10, 14, 18) as the within subjects factor. A significant main effect was obtained, [F(3,28) = 47.72, p < 0.001], indicating that beam walk performance was different between the groups following CCI (Fig. 1). A significant within subjects effect was also obtained, [F(2.62,73.21) = 106.65, p < 0.001] signifying that over time, there was a change in the performance on the beam walk. Significant differences in the rate of recovery between the groups were also revealed with the interaction effect between group and Day of testing [F(7.84,73.21) = 14.25, p < 0.001]. Tukey's HSD means comparisons outlined these significant differences. A significant injury effect was observed on all testing days (p < 0.05) where Vehicle animals performed significantly worse than Shams. By Day 10 both the NAM and HTS groups had recovered to Sham levels. In addition, on all testing days, a significant treatment effect was evident wherein, the NAM group performed significantly better than the Vehicle group (p < 0.05). On all testing days, with the exception of day 6, a significant treatment effect was also evident between the HTS groups compared to the Vehicle group (p < 0.05) (Fig. 1).
Figure 1.
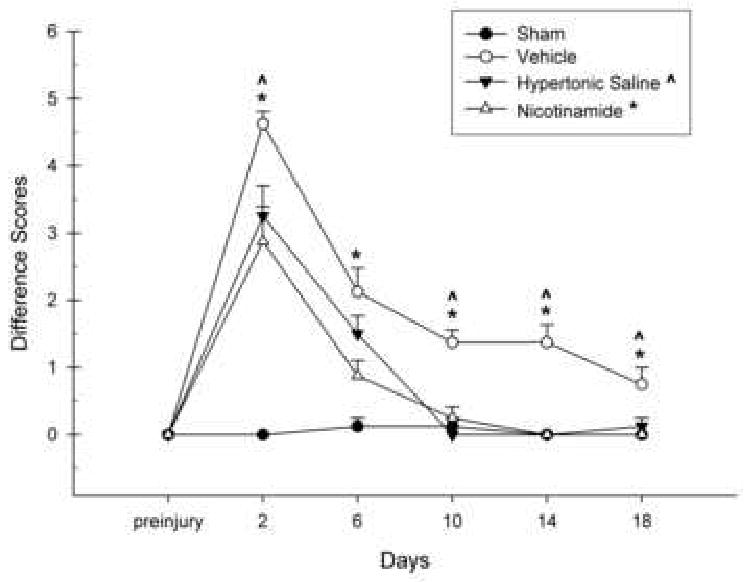
The effects of Nicotinamide (NAM) (500 mg/kg, ip), 7.5% Hypertonic Saline (HTS) (1 mL/kg, ip), or Vehicle (0.9% saline, 1 mL/kg, ip) administration following CCI or Sham surgery on the beam walk task. The graph shows the plotted mean (±SEM) beam walk difference scores. Treatment with NAM (* p = < 0.05) and HTS (ˆ p= < 0.05) significantly improved beam walk performance compared to the Vehicle group.
Forelimb Flexion Test
Difference scores were calculated by subtracting post-CCI scores from pre-injury baseline scores for both the left and right forelimbs. These difference scores were entered into a GLM repeated measures procedure with group (NAM, HTS, Vehicle, Sham) as the between subjects factor and Day of testing (preinjury, post-CCI Days 2, 6, 10, 14, 18) as the within subjects factor. No significant effects were obtained for the left forelimb (p > 0.05) (data not shown). However, a significant main effect was obtained for the right forelimb, [F(3,28) = 6.25, p < 0.01], indicating that right forelimb flexion performance was different between the groups following CCI (Fig. 2). A significant within subjects effect was also obtained, [F(3.72,104.14) = 33.93, p < 0.001], indicating a change in forelimb flexion over time. Significant differences in the rate of recovery between the groups were also revealed with the interaction effect between group and Day of testing, [F(11.16,129.54) = 3.72, p < 0.001]. However, Tukey's HSD means comparisons indicated no statistically significant treatment effects. However, an injury effect was apparent on Days 2, 6, 10, and 14 wherein the Vehicle group was significantly different than Shams (p < 0.05). An injury effect was also noted when comparing all groups to Shams on Day 2 of testing (p < 0.01). A general improvement in performance was observed in both the NAM and HTS-treated groups; however, these effects were no statistically significant (Fig. 2).
Figure 2.
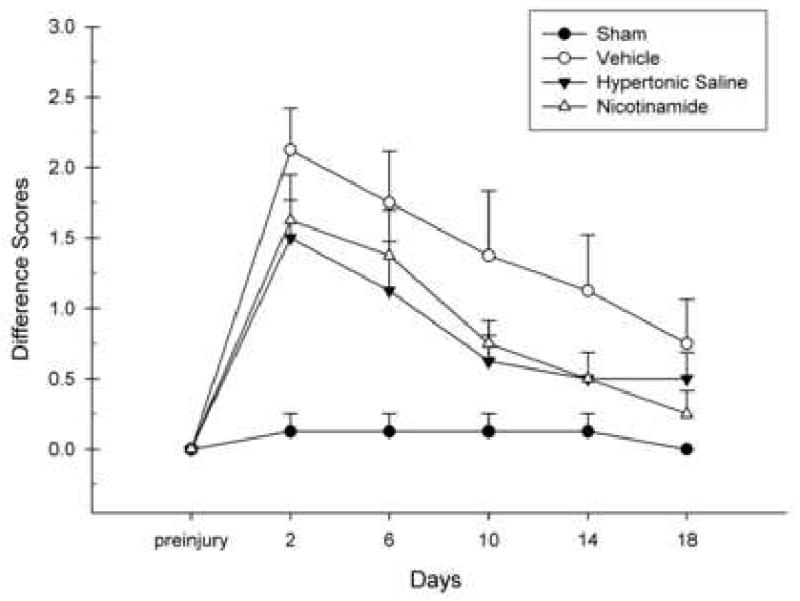
The effects of Nicotinamide (NAM) (500 mg/kg, ip), 7.5% Hypertonic Saline (HTS) (1 mL/kg, ip), or Vehicle (0.9% saline, 1 mL/kg, ip) administration following CCI or Sham surgery on the forelimb flexion task. The graph shows the plotted mean (±SEM) forelimb flexion difference scores. The Vehicle group showed significantly greater flexion following injury on all test days compared to Shams with the exception of Day 18. The NAM and HTS groups had a greater amount of flexion compared to Shams at the beginning of testing. The NAM-treated animals' flexion was not significantly different than Shams beginning on Day 10. The amount of flexion between the HTS and Shams were not significantly different beginning on Day 6.
Locomotor Placing Test
The number of foot-faults per quadrant was calculated for each Day of testing and multiplied by 100 to derive a standardized locomotor placing percentage score. These scores were entered into a GLM repeated measures procedure with group (NAM, HTS, Vehicle, Sham) as the between subjects factor and Day of testing (preinjury, post-CCI Days 2, 6, 10, 14, 18) as the within subjects factor. A significant group main effects was observed, [F(3,28) = 3.58, p < 0.05] (Fig. 3). A significant within groups effect was also obtained, [F(3.43,96.14) = 18.19, p < 0.001], as well as a significant interaction effect, [F(10.30,96.14) = 2.62, p < 0.01], indicating a change among groups across time and an interaction between group and Day of testing, respectively. A significant injury effect was only obtained on Day 10 for the Vehicle group (p < 0.05). Significant treatment effects were obtained starting on Day 6 with the HTS group performing significantly better than Vehicles (p < 0.01) and on Day 10 with the NAM group performing significantly better than Vehicles (p < 0.05) (Fig. 3).
Figure 3.
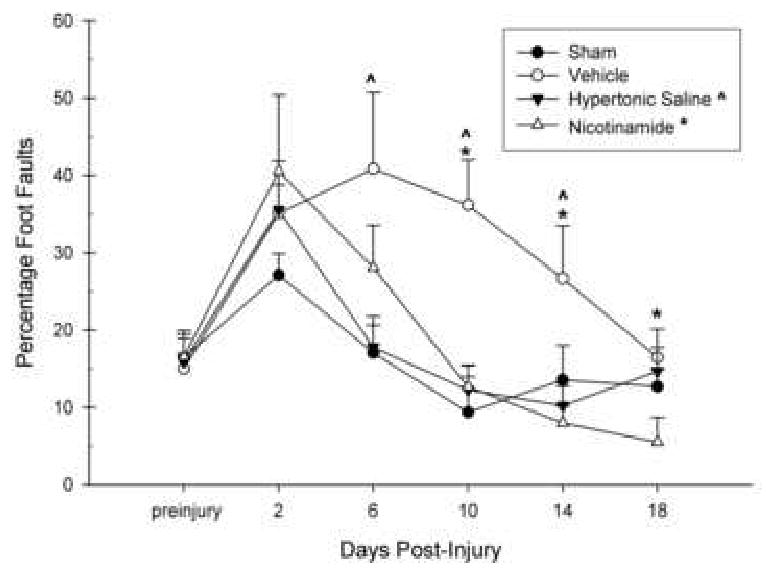
The effects of Nicotinamide (NAM) (500 mg/kg, ip), 7.5% Hypertonic Saline (HTS) (1 mL/kg, ip), or Vehicle (0.9% saline, 1 mL/kg, ip) administration following CCI or Sham surgery on locomotor placing. The graph shows the plotted mean (±SEM) percentage foot faults on the locomotor placing task. NAM (* = p < 0.05) and HTS-treated (ˆ = p < 0.05) animals showed a more rapid rate of recovery than the Vehicle-treated animals. The NAM and HTS -treated animals were not significantly different than Shams on all testing days.
Morris Water Maze
In the retrograde amnesia paradigm of the MWM, the latency to find and mount the submerged platform during each trial was recorded. Daily trials (16 pre-injury, 2 post-injury) were entered into a repeated measures GLM analysis with Group (NAM, HTS, Vehicle, Sham) as the between subjects factor and Trial number as the within subjects factor. The pre-injury (16 trials) and post-injury (2 trials) analyses were ran separately. There were no significant differences between the groups in the latency to locate the platform across all pre-injury training trials prior to CCI, [F(3,28) = .73, p > 0.05] (Fig. 4). There were significant changes in the groups across the trials represented by the within subjects effect, [F(7.18,200.99) = 20.71, p < 0.001], indicating learning over the trials. No interaction effect between group and trial number was obtained, [F(21.54,200.99) = 0.91, p > 0.05].
Figure 4.
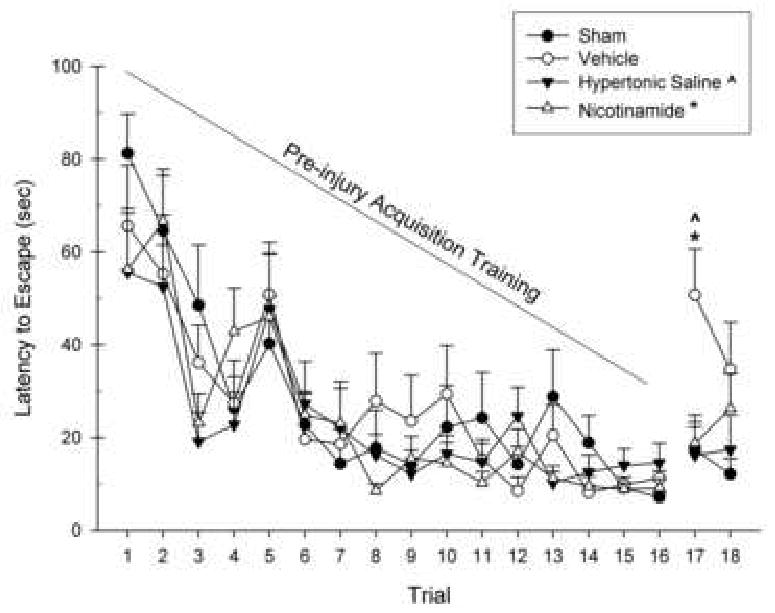
The effects of Nicotinamide (NAM) (500 mg/kg, ip), 7.5% Hypertonic Saline (HTS) (1 mL/kg, ip), or Vehicle (0.9% saline, 1 mL/kg, ip) administration following CCI or Sham surgery on the retrograde amnesia version of the MWM. The graph shows the plotted mean (±SEM) swim latencies to the submerged platform. Trials 1-16 were learning trials that occurred prior to injury and there were no significant differences between any of the groups. The retrograde memory test was assessed on Trial 17 and 18 were performed on Day 12 post-injury. Treatment with both NAM (* = p < 0.05) and HTS (ˆ = p < 0.05) significantly reduced latencies compared to the Vehicle-treated animals on trial 17. The NAM and HTS -treated animals were not significantly different than Shams.
Analysis of the retrograde amnesia test indicated a significant difference between the groups in the latency to locate the platform, [F(3,28) = 5.58, p < 0.01]. No significant changes were found across the trials, [F(1,28) = 0.41, p > 0.05]. No interaction effect between group and Trial number was indicated, [F(3,28) = 1.02, p > 0.05]. Tukey's HSD mean comparisons revealed an injury effect which was observed on trial 17 (p < 0.05) indicated by a significant difference between the Vehicle and Sham groups (Fig. 4). A treatment effect was also obtained for trial 17 for NAM (p < 0.05) and HTS (p < 0.05). No significant differences were observed between the 2 treatment groups and Sham group on either post-injury testing trial.
In the working memory paradigm of the MWM, only the average of the last 3 trials of each day was calculated since the first trial is considered an information trial. Each average was entered into a repeated measures GLM analysis with Group (NAM, HTS, Vehicle, Sham) as the between subjects factor and Day (18, 19, 20) as the within subjects factor. A significant difference between the groups in the latency to locate the platform across all testing days was found, [F(3,28) = 7.05, p = 0.001] (Fig. 5). A significant change across testing days was indicated by the within subjects effect, [F(1.63,45.71) = 4.92, p < 0.05]. However, an interaction effect between group and day was not obtained, [F(4.90,45.71) = 1.29, p > 0.05]. Tukey's HSD means comparisons revealed an injury effect on Day 18 with the latencies of the Vehicle group being significantly longer than those of the Sham group (p < 0.05). On all testing days, the HTS group performed significantly worse than the Sham group (p < 0.05) and significantly worse than the NAM group on Day 20. Furthermore, the NAM-treated group was no different from Shams on all testing days (Fig. 5).
Figure 5.
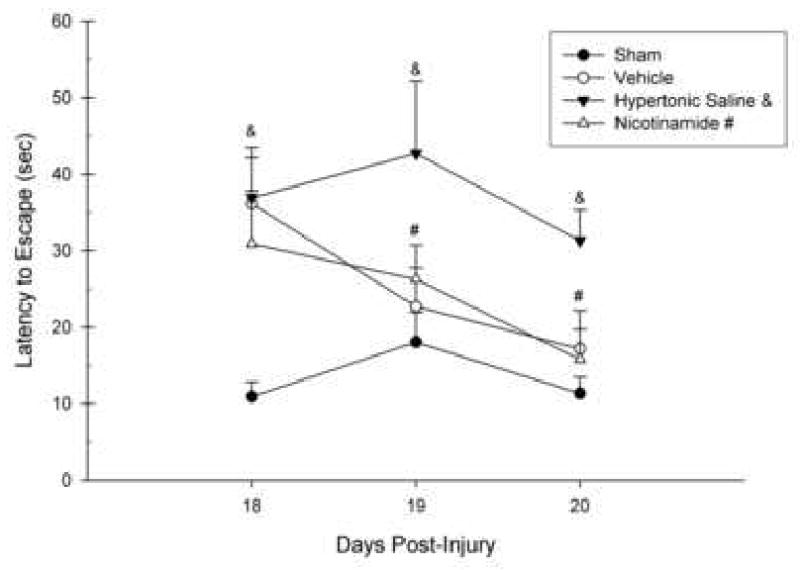
The effects of Nicotinamide (NAM) (500 mg/kg, ip), 7.5% Hypertonic Saline (HTS) (1 mL/kg, ip), or Vehicle (0.9% saline, 1 mL/kg, ip) administration following CCI or Sham surgery on the working memory task in the MWM. The graph shows the plotted mean (±SEM) swim latencies to the submerged platform. The latencies in NAM-treated animals were not significantly different than Shams on all testing days. The Vehicle group was not significantly different from the Sham group after the first day of testing, suggesting quick recovery. The animals treated with HTS had significantly longer latencies than the Sham animals on all testing days (& = p > 0.05) as well as the NAM-treated animals on the final testing day (& = p > 0.05). NAM-treated animals were not significantly different from shams on the final 2 days of testing (# = p > 0.05).
2.3 Histology
Ipsilateral and contralateral volume measurements of both the cerebral cortices and hippocampus were taken by tracing four coronal sections selected throughout the lesion area for each animal. The scores calculated using the Calverlieri method, were analyzed using a one-way ANOVA. A significant difference between cortical loss was identified between groups [F(3,28) = 100.54, p < 0.001]. Tukey's HSD analysis revealed a significant injury effect between all groups compared to Shams (p < 0.001); as well as, volume differences in the HTS group were significantly different than the Sham and NAM groups (p <0.05) (Fig. 6). Representative (average) cortical loss is shown for the groups in Figure 7.
Figure 6.

Cortical lesion analysis. Plotted is the mean (±SEM) percentage cortical volume differences (ipsilateral/contralateral) for each group. Nicotinamide (NAM), Hypertonic Saline (HTS) and Vehicle-treated cortical differences were significantly greater than Sham. The HTS groups cortical difference (ˆ = p < 0.05) was also significantly larger than the Vehicle-treated group and the NAM-treated group (** = p < 0.007).
Figure 7.

Histology plate. Shown are representative cresyl violet images (0.44×) of sections (40 μm) in the Sham, Vehicle, Hypertonic Saline-treated and Nicotinamide-treated brain at coordinates -0.40 mm (top row), -2.12 mm, -3.30 mm, and -4.80 mm (bottom row) relative to bregma. Scale bar = 2.0 mm.
There was also a significant difference in hippocampal loss between groups [F(3,28) = 3.13, p < 0.05]. Post hoc analysis revealed no differences between Sham, Vehicle, and NAM groups. However, a significant injury effect was found between the Sham and HTS group (p < 0.05) (Fig. 8). A histology plate showing representative (average) hippocampal atrophy is provided in Figure 9.
Figure 8.
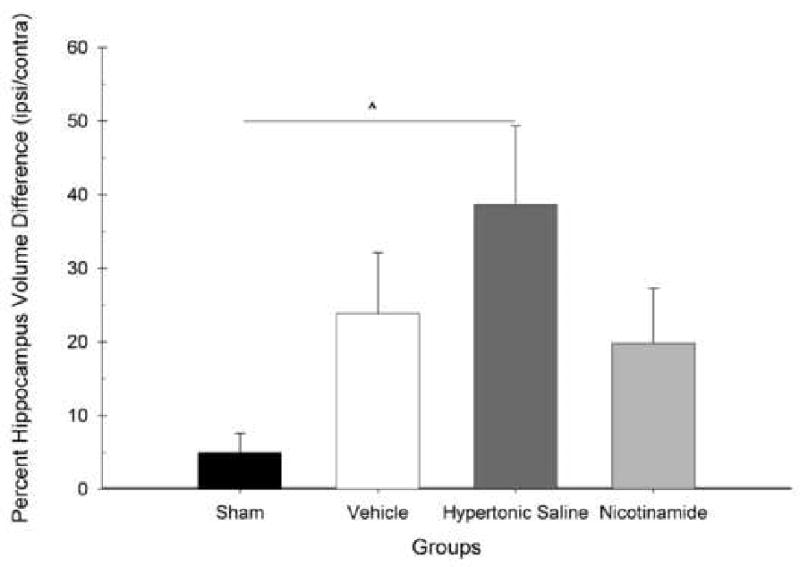
Hippocampal lesion analysis. Plotted is the mean (±SEM) percentage hippocampus volume differences (ipsilateral/contralateral) for each group. No hippocampal volume differences were found between the Nicotinamide and Vehicle-treated group compared to Shams. The Hypertonic Saline-treated group (ˆ = p < 0.05) had significantly more hippocampal loss compared to Shams and the treated groups did not.
Figure 9.
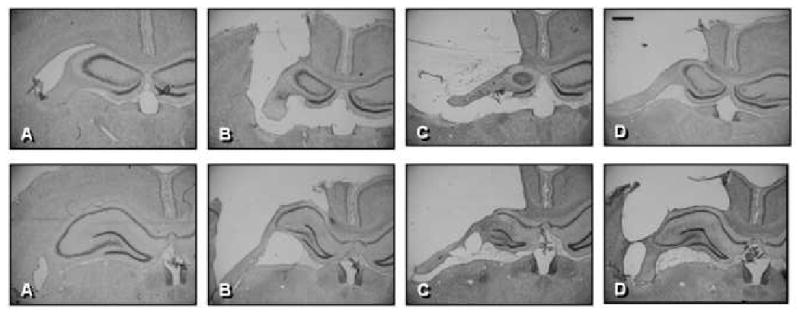
Histology plate. Shown are representative Cresyl Violet images (1.4×) of sections (40 μm) in the Sham (A), Vehicle (B), Hypertonic Saline-treated (C) and Nicotinamide-treated (D) brains at bregma coordinates -2.12 mm (top row), and -3.30 mm (bottom row). Scale bar = 500 μm.
3. Discussion
Currently, HTS is an accepted form of therapy following TBI. It is well established that HTS exerts its benefits by reducing edema and intracranial pressure. Nonetheless, the behavioral and cognitive consequences of such treatment have yet to be solidly identified. Recent evidence has shown that NAM, which also reduces edema, is a beneficial agent in the recovery of function following TBI. The current comparison investigated the behavioral and cognitive consequences of the use of HTS and NAM as competitive therapeutic agents in the treatment of TBI.
Following CCI, animals treated with NAM and HTS recovered to Sham levels in the beam walk, forelimb flexion and locomotor tasks within a short time frame. In addition, animals treated with NAM performed significantly better than the Vehicle-treated animals on all testing days on the beam walk task (Fig. 1). In the same task, the animals treated with HTS also performed better than the Vehicle group on all testing days with the exception of Day 6. Furthermore, locomotor placing scores were significantly better in both the NAM (Day 10 and 14) and HTS (Day 10) treated animals compared to the Vehicle group (Fig. 3). Although recovery was observed between both of these groups on all of the aforementioned tasks, an interesting trend was noticed at Day 18 in the HTS group. Although not significant, it appears that the HTS animals might have begun to rebound from these potential benefits. Therefore, testing beyond 18 days is needed in future studies to see if these rebound effects persist.
For the assessment of cognitive performance, all animals were pre-trained in the MWM for a period of 4 days (16 trials) prior to CCI. Retrograde amnesia for the platform location was assessed by placing the animal back in the MWM 12 days after CCI and measuring the latency to find the previously learned platform location. As expected, all groups displayed a normal learning curve in the four day training period prior to CCI. However, when placed back in the water to test for retrograde amnesia (12 days after CCI), the HTS and NAM-treated animals mounted the hidden platform significantly faster than the Vehicle group (Fig. 4). Thus, it was shown that both HTS and NAM-treated animals retained the memories for a task learned prior to injury and interestingly enough did not show signs of retrograde amnesia. These results are similar to past reference memory performance shown in animals treated with HTS following TBI and hemorrhage (Sell et al., 2008). However, these same results did not hold true for the working memory portion of the MWM that began on Day 18 post-injury. Although an apparent injury effect was observed on the first day of working memory testing, Day 18, the Vehicle animals quickly acquired the task and were no longer different from Sham animals on the next two days of testing. This enhanced recovery may be attributed to the excessive training that occurred before injury. An interesting finding in this task was that although the NAM-treated animals were no different than the Sham animals on any of the testing days, the HTS-treated animals had significant deficits in this task (Fig. 5). Not only did the HTS animals never acquire the task and continued to show significantly longer latencies to mount the platform than the Sham animals, they also showed significantly longer latencies than the NAM animals on the last testing day.
The diminished cognitive improvement in the HTS-treated group on the working memory test may be related to the histological observations. As expected, due to the location and impact of the CCI, a significant injury effect was observed in the cortex between all groups compared to the Sham animals. However, what was unforeseen was the increased tissue damage observed in the HTS group. Not only did the HTS animals have significantly more cortical loss than the Sham animals, more importantly, they also had significantly more cortical loss than the NAM-treated animals (Fig. 6). A similar finding was also observed in the hippocampus. There was no significant hippocampal volume difference between the Sham, NAM and Vehicle-treated animals. However, the HTS-treated animals lost significantly more hippocampal tissue than did the Sham group (Fig. 7). This is in contrast to previous findings that HTS at both 2 mL/kg and 4 mL/kg doses reduced secondary injury and increased cell survival (Soustiel et al., 2006; Elliot et al., 2007). As mentioned before, time course studies may further illustrate the extent and distribution of these specific tissue losses.
It appears that HTS may be effective when it comes to protection against retrograde amnesia. However, there was no statistical evidence for beneficial cognitive effects in the working memory task which was performed almost a week later (Day 18-20). One possible explanation for these differential effects may be the result of the severe ipsilateral hippocampal damage that was observed in the HTS-treated animals. It is believed that the hippocampus may not be responsible for storage and retrieval of old spatial memories which would explain the safeguarding of the retrograde memories (Haijima & Ichitani, 2008). In contrast, NAM was shown to provide significant improvements in recovery without the associated tissue loss seen with HTS therapy. However, in the current study NAM treatment failed to show significant reductions in cortical loss which have been previously shown (Hoane et al., 2003; 2006; 2008). Treatment in the current study was delayed 2 hrs post-injury and was followed with only a 2 day dosing regimen; whereas, in our previous papers the dosing has either started sooner (Hoane et al., 2003; 2006), or with later administrations (eg, 4-8 hrs) and longer treatment durations (Hoane et al., 2008a; 2008b). Thus, these results further support the preclinical efficacy of NAM therapy for TBI and may suggest that there may be some risk, or reduced effectiveness with HTS. Furthermore, the combination of these 2 treatments might be an interesting treatment option in a future preclinical study.
4. Experimental Procedures
4.1 Subjects
Four groups of 4 month old Sprague-Dawley rats (mean weight = 379g) made up of eight animals per group were used in this study. Throughout both pre-training and post-injury testing, animals were housed individually in standard housing cages and maintained at 22° C on a 12:12 hour light:dark cycle, with food and water provided ad lib. All experimental procedures were reviewed and approved by the Southern Illinois University Institutional Animal Care and Use Committee, and the study was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care.
4.2 CCI Surgery
Rats were anesthetized using a mixture of Isoflurane (2-4%), nitrous oxide (0.2 L/min), and oxygen (0.8 L/min). They were kept at a stage III level of anesthesia (Guedel, 1920; Friedberg et al., 1999) throughout the surgical procedure during which time the animal is unresponsive with no hindlimb withdrawal reflex. Body temperature was maintained at 37° C using a heating pad and YSI model 73ATA feedback device attached to a rectal probe. After shaving the top of the head and application of Betadine, the animals were placed in a stereotaxic frame and a midline incision was made in the skin and underlying fascia on the skull. The underlying fascia was retracted. A unilateral 5.0 mm diameter circular craniotomy was performed in the left hemisphere and made using a dental drill keeping the dura intact. The craniotomy was centered at 3.0 mm posterior to bregma and 2.5 mm lateral to the midline. After clearing the craniotomy of any bone fragments, a 4.0 mm impact tip was lowered until it contacted the dura. The impact tip was raised and pre-set to be lowered 2.0 mm below the dura surface upon impact. Injury parameters were set at 3.5 m/s (40 psi) velocity. The impact tip maintained contact for 500 ms before retraction. The animal was then sutured and returned to his home cage. To ensure testing under blind conditions, Sham-operated uninjured animals underwent the same surgical procedures but did not receive a brain injury.
4.3 Drug Administration
Two hrs following injury rats received either NAM, dissolved in 0.9% saline (500 mg/kg, ip; n=8), 7.5% HTS (1 mL/kg, ip; n=8), or Vehicle (0.9% saline, 1 mL/kg, ip; n=8). A second dose was given at 24 hrs post-injury. This was followed by a final dose at 48 hrs post-injury. A group of Sham animals (n=8) which received no injections were also included in the study. The dose of NAM was dissolved in vehicle and was chosen based on previous studies (Hoane et al., 2006b; 2003) as was HTS (Wisner et al., 1990; Zausinger et al., 2004; Soustiel et al., 2006; Elliot et al., 2007). All analyses were conducted without knowledge of drug treatment.
4.4 Behavioral Assessment
Behavioral testing was conducted on Days 2, 6, 10, 14, and 18 post-injury with the exception of cognitive tasks. The retrograde amnesia portion of the MWM was performed on Day 12 post-injury, whereas the working memory (anterograde amnesia) portion of the cognitive testing was performed on Days 18-20.
Beam Walk Test
Motor coordination and vestibulomotor function was assessed using the beam walk test (Feeney et al., 1982; Schmanke et al., 1996; Smith et al., 2005). Animals were trained to walk down a 120 cm long, 2.5 cm wide elevated beam. Post-injury performance was rated on a seven point scale with 1 indicating the inability to transverse the beam and 7 indicating the animal traversed the beam normally with no more than two foot slips (Hoane et al., 2006b; Smith et al., 2005; 2006). Each animal was scored once per testing day.
Forelimb Flexion Test
After injury, neurological function was addressed by assessing flexion and adduction of the forelimbs (Smith et al., 2005, 2006; McIntosh et al., 1989; Hoover et al., 2004). Each animal was placed on a flat surface and swiftly lifted by the base of its tail. The amount of flexion of each forelimb was rated on a four-point scale with 4 indicating normal behavior and 1 indicating complete adduction of the forelimb. Each animal was scored once per testing day.
Locomotor Placing Test
Coordination of limb placing during a locomotion task was assessed (Smith et al., 2005, 2006; Hoane et al., 2004; Barth et al., 1990). The testing apparatus consisted of fifteen test tube racks (Nalge Nunc International, Product # 5970-0330) bound together to create an 86 × 55 cm grid surface with 3 × 3 cm square openings which is divided into 30 sectors. Rats were placed on the apparatus and allowed to freely explore for a single 1.5 minute testing period at every post-injury test day. The total number of sectors crossed and foot-faults were recorded. For each rat, a standardized percentage of foot-faults per sector was calculated.
Morris Water Maze
This test assessed cognitive ability (learning and memory). The effects of both retrograde amnesia and working memory were tested using a large, standard MWM (Hoane et al., 2006b, 2003). The apparatus consisted of a circular (1.5 m diameter, 76 cm deep) blue fiberglass tank filled with water to a depth of 32 cm. The temperature of the water was maintained at 24 °C. A Plexiglas escape platform (10 cm2 diameter) was submerged 1.0 cm below the water surface.
In the retrograde amnesia paradigm, pre-injury training began 4 days prior to injury and consisted of 4 trials a day for 4 days. The platform was placed and remained in the southeast quadrant, 1 cm below the water. Each trial ended when the rat found the platform or 90 seconds elapsed. If the animal did not find the platform by the end of 90 sec, it was guided to it. In either case, it remained on the platform for 10 sec. Inter-trial intervals were 10 mins in length. Starting points varied from four different locations on each trial. On Day 12 following injury, each animal had two trials starting off at different locations. The platform remained in the same place as the pre-injury training (southeast quadrant). The mean latency to find the platform from both trials served as the dependent measure.
In the working memory paradigm, rats were tested post-injury on Days 18-20. Each rat received 4 trials daily in a MWM for 3 consecutive days (Hoane et al., 2006b, 2003). On each training day, the platform was placed in a different random location. Rats were given 4 trials per day, starting from one of four different directions on each trial (north, south, east, west). The order of the starting position was randomized across training days. On each trial, the rat was placed into the water facing the wall of the pool. The animal was allowed to swim around the pool until it found and climbed upon the hidden platform. Once the platform was found, the rat was allowed to stay on the platform for 10 sec. If the platform was not found within 90 sec, the trial was terminated and the rat guided to the platform where it remained for 10 sec. Inter-trial intervals were 10 min in length.
4.5 Histology
On day 21 post-injury, animals were anesthetized with urethane (3.0 g/kg, 0.5 g/mL, ip) and transcardially perfused with 0.9% phosphate-buffered saline (PBS), followed by 10% phosphate-buffered formalin (PBF). Brains were post-fixed in PBF for 48 hr and cryoprotected in 30% sucrose for 2 days prior to frozen sectioning. Coronal sections (40 μm) were sliced using a sliding microtome. Sections were mounted on gelatin-subbed microscope slides, stained with cresyl violet, dehydrated, and cover slipped.
4.6 Lesion analysis
Quantitative measurements of cortical and hippocampal loss were analyzed with an Olympus microscope (BX-51) and DP-70 camera. Images of the sections throughout the extent of the injury were captured using the digital capturing system and area measurements of the cortex and hippocampus were determined using ImageTool software (Hoane et al., 2006b, 2003; Barbre and Hoane, 2006). The Calvalieri method was used to calculate the volumes of the remaining, intact and healthy tissue (Coggeshall, 1992). The number of sections and the section thickness (40 μm) were multiplied by the mean area of the lesion cavity (calculated at four stereotaxic coordinates surrounding the lesion (AP: -2.12, -2.8, -3.8, and -4.8 relative to bregma) (Paxinos and Watson, 2005). The extent of cortical and hippocampal injury was measured by calculating the volume of remaining tissue (Hoane et al., 2003, 2005).
4.7 Statistical Analysis
Analysis of variance (ANOVA) tests were performed using procedures for general linear models (SPSS 13.0 for Windows) with options for repeated measures, where appropriate, for all behavioral measures. The between factor was group (NAM, HTS, Vehicle, Sham). The within group factor was Day of testing (i.e. pre-injury baseline, post-injury Days 2, 6, 10, 14, 18). Greenhouse-Geisser corrections were used to Control for type I error in the repeated measures. For all corrected analyses, the actual corrected degrees of freedom are reported. Planned mean comparisons for each time point were analyzed using Tukey's HSD. Histological data was analyzed using one-way ANOVA procedures and Tukey's HSD. A significance level of p < 0.05 was used for all statistical analyses.
Acknowledgments
We would like to thank Jason Beare for his help in image capturing and analysis. Funded by NIH grant NS045647 to MRH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JT, Wisner DH, Sullivan PE, Matteucci M, Freshman S, Hildreth J, Wagner FC. Initial small-volume hypertonic resuscitation of shock and brain injury: short- and long-term effects. J Trauma. 1997;42:592–600. doi: 10.1097/00005373-199704000-00003. [DOI] [PubMed] [Google Scholar]
- Ayoub IA, Lee EJ, Ogilvy CS, Beal MF, Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal cerebral ischemia in Wistar rats. Neurosci Lett. 1999;259:21–24. doi: 10.1016/s0304-3940(98)00881-7. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Rhind SG, Morrison LJ, Black S, Crnko NT, Shek PN, Rizoli SB. Resuscitation with hypertonic saline-dextran reduces serum biomarker levels and correlates with outcome in severe traumatic brain injury patients. J Neurotrauma. 2009;26:1227–1240. doi: 10.1089/neu.2008.0868. [DOI] [PubMed] [Google Scholar]
- Barbre AB, Hoane MR. Magnesium and riboflavin combination therapy following cortical contusion injury in the rat. Brain Res Bull. 2006;69:639–646. doi: 10.1016/j.brainresbull.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Beh Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Battistella FD, Wisner DH. Combined hemorrhagic shock and head injury: effects of hypertonic saline (7.5%) resuscitation. J Trauma. 1991;31:182–188. [PubMed] [Google Scholar]
- Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA, Hoge CW. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol. 2008;76:272–281. doi: 10.1037/0022-006X.76.2.272. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Traumatic brain injury in the United States. [April 1, 2008]; Available online at: http://www.cdc.gov/ncipc/pub-res/research_agenda/agenda.htm.
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Prough DS, Deal DD, Vines SM, Hoen H. Hypertonic saline does not improve cerebral oxygen delivery after head injury and mild hemorrhage in cats. Crit Care Med. 1996;24:109–117. doi: 10.1097/00003246-199601000-00019. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Jallo JJ, Gaughan JP, Tuma RF. Effects of crystalloid-colloid solutions on traumatic brain injury. J Neurotrauma. 2007;24:195–202. doi: 10.1089/neu.2006.0094. [DOI] [PubMed] [Google Scholar]
- Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69:117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4:4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Burkett DE, Parham CS, Scalese RJ, Sadanaga KK. Protective effect of mitochondrial KATP activation in an isolated gracilis model of ischemia and reperfusion in dogs. J Cardiovasc Pharmacol. 2003;42:790–792. doi: 10.1097/00005344-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Guedel AE. Signs of inhalational anesthesia. A fundamental guide. In: Guedel AE, editor. Inhalation anesthesia. New York: MacMillian; 1920. [Google Scholar]
- Haijima A, Ichitani Y. Anterograde and retrograde amnesia of place discrimination in retrosplenial cortex and hippocampal lesioned rats. Learning & Memory. 2008;15:477–482. doi: 10.1101/lm.862308. [DOI] [PubMed] [Google Scholar]
- Himmelseher S, Pfenninger E, Morin P, Kochs E. Hypertonic-hyperoncotic saline differentially affects healthy and glutamate-injured primary rat hippocampal neurons and cerebral astrocytes. J Neurosurg Anesthesiol. 2001;13:120–130. doi: 10.1097/00008506-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J Neurotrauma. 2003;20:1189–1199. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Becerra GD, Shank JE, Tatko L, Pak ES, Smith M, Murashov AK. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma. 2004;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Gilbert DR, Holland MA, Pierce JL. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci Lett. 2006;408:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to four hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Pierce JL, Kaufman NA, Beare JE. Variation in chronic nicotinamide treatment after traumatic brain injury can later components of functional recovery independent of histological damage. Oxid Med & Cell Long. 2008;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J Neurotrauma. 2006;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Wolyniak JG, Akstulewicz SL. Administration of riboflavin improves behavioral outcome and reduces edema formation and glial fibrillary acidic protein expression after traumatic brain injury. J Neurotrauma. 2005;22:1112–1122. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- Holland MA, Tan AA, Smith DC, Hoane MR. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Hoover RC, Motta M, Davis J, Saatman KE, Fujimoto ST, Thompson HJ, Stover JF, Dichter MA, Twyman R, White HS, McIntosh TK. Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J Neurotrauma. 2004;21:501–512. doi: 10.1089/089771504774129847. [DOI] [PubMed] [Google Scholar]
- Horn P, Münch E, Vajkoczy P, Herrmann P, Quintel M, Schilling L, Schmiedek P, Schürer L. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res. 1999;21:758–764. doi: 10.1080/01616412.1999.11741010. [DOI] [PubMed] [Google Scholar]
- Khanna S, Davis D, Peterson B, Fisher B, Tung H, O'Quigley J, Deutsch R. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (Vitamin (3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke. 2000;31:1679–1685. doi: 10.1161/01.str.31.7.1679. [DOI] [PubMed] [Google Scholar]
- Munar F, Ferrer AM, de Nadal M, Poca MA, Pedraza S, Sahuquillo J, Garnacho A. Cerebral hemodynamic effects of 7.2% hypertonic saline in patients with head injury and raised intracranial pressure. J Neurotrauma. 2000;17:41–51. doi: 10.1089/neu.2000.17.41. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fifth. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, Ulatowski JA. Use of hypertonic (3%) saline/acetate infusion in the treatment of cerebral edema: Effect on intracranial pressure and lateral displacement of the brain. Crit Care Med. 1998;26:440–446. doi: 10.1097/00003246-199803000-00011. [DOI] [PubMed] [Google Scholar]
- Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54:S52–62. doi: 10.1097/01.TA.0000064507.80390.10. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States. J Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Schatzmann C, Heissler HE, König K, Klinge-Xhemajli P, Rickels E, Mühling M, Börschel M, Samii M. Treatment of elevated intracranial pressure by infusions of 10% saline in severely head injured patients. Acta Neurochir Suppl. 1998;71:31–33. doi: 10.1007/978-3-7091-6475-4_9. [DOI] [PubMed] [Google Scholar]
- Scheller MS, Zornow MH, Seok Y. A comparison of the cerebral and hemodynamic effects of mannitol and hypertonic saline in a rabbit model of acute cryogenic brain injury. J Neurosurg Anesthesiol. 1991;3:291–296. doi: 10.1097/00008506-199112000-00009. [DOI] [PubMed] [Google Scholar]
- Schmanke TD, Avery RA, Barth TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma. 1996;13:293–307. doi: 10.1089/neu.1996.13.293. [DOI] [PubMed] [Google Scholar]
- Sell SL, Avila MA, Yu G. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108:873–881. doi: 10.1097/ALN.0b013e31816c8a15. [DOI] [PubMed] [Google Scholar]
- Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Tan AA, Duke A, Neese SL, Clough RW, Browning RA, Jensen RA. Recovery of function following vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J Neurotrauma. 2006;23:1549–1560. doi: 10.1089/neu.2006.23.1549. [DOI] [PubMed] [Google Scholar]
- Soustiel JF, Vlodavsky E, Zaaroor M. Relative effects of mannitol and hypertonic saline on calpain activity, apoptosis and polymorphonuclear infiltration in traumatic focal brain injury. Brain Res. 2006;1101:136–144. doi: 10.1016/j.brainres.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Suarez JI, Qureshi AI, Bhardwaj A, Williams MA, Schnitzer MS, Mirski M, Hanley DF, Ulatowski JA. Treatment of refractory intracranial hypertension with 23.4% saline. Crit Care Med. 1998;26:1118–1122. doi: 10.1097/00003246-199806000-00038. [DOI] [PubMed] [Google Scholar]
- Taylor G, Myers S, Kurth CD, Duhaime A, Yu M, McKernan M, Gallagher P, O'Neill J, Templeton J. Hypertonic saline improves brain resuscitation in a pediatric model of head injury and hemorrhagic shock. J Pediatr Surg. 1996;31:65–70. doi: 10.1016/s0022-3468(96)90321-8. [DOI] [PubMed] [Google Scholar]
- Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–957. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57:727–736. [PubMed] [Google Scholar]
- Weed LH, McKibben PS. Experimental alteration of brain bulk. Am J Physiol. 1919;48:531–558. [Google Scholar]
- Wisner DH, Schuster L, Quinn C. Hypertonic saline resuscitation of head injury: effects on cerebral water content. J Trauma. 1990;30:75–78. doi: 10.1097/00005373-199001000-00011. [DOI] [PubMed] [Google Scholar]
- Worthley L, Cooper D, Jones N. Treatment of resistant intracranial hypertension with hypertonic saline: report of two cases. J Neurosurg. 1988;68:478–481. doi: 10.3171/jns.1988.68.3.0478. [DOI] [PubMed] [Google Scholar]
- Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, Chan P, Adams JD. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav. 2002;73:901–910. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Thal SC, Kreimeier U, Messmer K, Schmid-Elsaesser R. Hypertonic fluid resuscitation from subarachnoid hemorrhage in rats. Neurosurgery. 2004;55:679–686. doi: 10.1227/01.neu.0000134558.28977.ee. [DOI] [PubMed] [Google Scholar]
- Zornow MH, Oh YS, Scheller MS. A comparison of the cerebral and haemodynamic effects of mannitol and hypertonic saline in an animal model of brain injury. Acta Neurochir Suppl (Wien) 1990;51:324–325. doi: 10.1007/978-3-7091-9115-6_109. [DOI] [PubMed] [Google Scholar]


