Abstract
We previously demonstrated that there is significantly greater transfer of intravenously-injected Evan’s blue dye into the forebrain of acyclic (reproductive senescent) females compared to young adult females, indicating that blood brain barrier permeability is compromised in the reproductive senescent forebrain. The present study examined brain IgG expression and microvessel tight junction proteins to assess ovarian age-related changes in microvascular permeability, and further compared young and senescent females with age-matched males to distinguish changes attributable to age and reproductive senescence. Blood brain barrier breakdown are often associated with increased extravasation of plasma proteins and high levels of immunoglobulin G (IgG) in brain. In the present study, IgG expression was dramatically increased in the hippocampus and thalamus, but not the hypothalamus of reproductive senescent females compared to young adult females. In males, IgG expression was increased in all these regions in middle aged animals (aged-matched to senescent females) as compared to young males (age-matched to the young adult females). Furthermore, the proportion of hippocampal microvessels with perivascular IgG immunoreactivity was significantly greater in reproductive senescent females as compared to young adult females, while middle aged males and young adult males did not differ. The tight junctions between adjacent microvascular endothelial cells regulated by transmembrane proteins such as claudin-5 and occludin play a critical role in maintaining the blood brain barrier integrity. Increased hippocampal IgG expression in senescent females was paralleled by poor junctional localization of the tight junction protein claudin-5 in hippocampal microvessels. However, there was no difference in hippocampal claudin-5 localization between young adult and middle aged males, indicating that dysregulation of this junctional protein was associated with ovarian aging. Parallel studies in human brain microvessels also revealed age-dependent disruption in claudin-5 distribution in post-menopausal women compared to premenopausal women. Collectively, these data support the hypothesis that constitutive loss of barrier integrity in the forebrain during reproductive senescence may be due, in part, to the selective loss of tight junction proteins in endothelial junctions.
Keywords: Blood brain barrier, IgG, Microvessels, Occludin, Claudin-5, Estrogen, Menopause, Hippocampus
Introduction
The blood brain barrier, composed of endothelial cells, astrocytes and pericytes, plays a central role in maintaining the vascular homeostasis of the central nervous system (Ballabh et al., 2004). In the aging population, common cardiovascular disorders such as hypertension (Hajjar et al., 2005), seizure (Janigro, 1999) and stroke (Mikulis, 2005) all contribute to blood brain barrier dysfunction. Blood brain barrier permeability is altered by a number of factors including increased levels of inflammatory cytokines (Banks et al., 1995) and free radicals (Chan et al., 1991; Greenwood, 1991) which in turn leads to increased influx of cytokines and immune cells into the brain. Moreover, dysfunction of the endothelial barrier facilitates extravasation of plasma proteins into the brain and subsequently triggers a variety of neuro-inflammatory responses within the brain. Aging is associated with degeneration of blood brain barrier/blood cerebrospinal fluid barrier and abnormal accumulation of albumin (Pakulski et al., 2000), and fibrinogen and IgG have been reported in the brains of patients diagnosed with Alzheimer’s Disease (Ryu and McLarnon, 2008).
A principal mechanism of transcapillary passage of large proteins is via openings in the tight junctions between endothelial cells. The intercellular clefts between adjacent endothelial cells of microvessels are sealed by means of specialized protein strands and are referred to as “tight junctions” (Risau and Wolburg, 1990). Three different families of transmembrane proteins are located within endothelial tight junctions: occludin, which was the first tight junction protein identified (Furuse et al., 1993), claudins and junctional adhesion molecules or JAMs (Harhaj and Antonetti, 2004; Sonoda et al., 1999). Occludin and claudin aggregate together to form intramembranous strands with fluctuating channels to promote diffusion of ions and solutes (Bazzoni and Dejana, 2004; Matter and Balda, 2003).
Since the cerebral vasculature is an important target of estrogen (Stirone et al., 2003), age-associated decreases in circulating estrogen in females may adversely affect the structural composition of tight junctions and compromise the integrity of the barrier. Previous work from this laboratory has shown that the blood brain barrier is more permissive in older, acyclic (reproductive senescent) female rats as compared to their younger counterparts (Bake and Sohrabji, 2004). Significantly greater extravasation of Evan’s blue dye (injected into the jugular vein) was observed in both the hippocampus and olfactory bulb of senescent females. Moreover, estrogen replacement to young adults reduced dye extravasation in both regions, however, a similar hormone regimen, paradoxically, increased dye transfer in the senescent hippocampus. The present study extends these findings and further tested the hypothesis that ovarian aging, but not chronological aging affects paracellular transport across the blood brain barrier. To further understand age and ovarian-aging effects on blood brain barrier permeability, the present study employed two sets of analysis. IgG immunoreactivity in the forebrain was assessed to estimate the progressive changes in the barrier as a function of reproductive age in young adult and reproductive senescent females as well age-matched males. Additionally, microvessels from the hippocampus were harvested and analyzed for expression of occludin and claudin-5, two of the principal junction proteins of the blood brain barrier.
METHODS
Animals
Female Sprague Dawley rats of two different age groups, young adult (virgin females, 3 months, average wt. 225–250 g) and reproductive senescent (constant diestrus, 9–11 months, average wt. 300–325 g) were purchased (Harlan Laboratories, IN) as retired breeders. The senescent females met our previously established criteria for reproductive senescence (Bake and Sohrabji, 2004; Jezierski and Sohrabji, 2001; Nordell et al., 2003) which included 4–5 prior successful pregnancies, and were currently acyclic as determined by daily vaginal smears for 3 wks. All senescent females were in constant diestrus for at least 2 weeks prior to the start of the experiment. Male Sprague Dawley rats were also purchased as young adults (3 months, average wt. 300g) and middle-aged (9–11 months, average wt. 450–475 g) from Harlan laboratories (IN). (Males were age-matched to the young adult and reproductive senescent females). All male rats (n=20) were intact animals (not gonadectomized) and used in this study five days after arrival to the animal housing facility. All animals were housed in an AALAC-approved facility, were maintained on a constant photoperiod (12 h light: dark cycles) and were fed ad libitum with laboratory chow (Harlan Telkad 8604) and water. All animal procedures were in accordance with NIH guidelines for human care of laboratory animals and approved by the Institutional Animal Care Committee.
Surgical Procedures
Young adult (n=40) and reproductive senescent females (n=40) were anesthetized with xylazine (200mg/Kg)/ ketamine (10mg/Kg) and bilateral ovariectomies were performed using a dorsal midline incision inferior to palpated rib cage and kidneys. Ovaries were removed and a control or estrogen pellet (Innovative research, Sarasota, FL) was inserted subcutaneously before closing the incision. These are designed for 60 days constant release of 17β-estradiol. However, some studies indicate an initial burst in hormone release during the first week of pellet implantation, followed by slow and steady release in the subsequent weeks (Singh et al., 2008). Twenty-one days after ovariectomy, all animals were anesthetized with xylazine/ketamine and transcardially perfused with saline for 15 min at a flow rate of 8ml/min (~120 mls). For IgG immunohistochemistry, animals were subsequently perfused with fixative containing 4% paraformaldehyde in a 0.1M phosphate buffer (pH 7.4) for another 5 min. Young adult and middle-aged male brains were also processed for IgG immunohistochemistry following the same procedure described above.
Histological procedures for IgG staining
Brains were removed from the cranium, post fixed in perfusion buffer for 2 h and immersed in 0.1M phosphate buffer (pH 7.4). Brains were shipped to Neuroscience Associates (Knoxville, TN) for multibrain embedding and sectioning where they were treated overnight with 20% glycerol and 2% dimethyl sulfoxide to prevent freeze-artifacts followed by multiple embedding (total of 16 brains, 4 animals per each treatment condition) in a gelatin matrix. After curing, the block was rapidly frozen by immersion in isopentane prechilled to −70° C. Coronal freeze cut sections (40 µm) were obtained with an AO 860 sliding microtome and were collected in 4 × 6 array of containers filled with 10% phosphate-buffered formaldehyde. After 24 h, sections were rinsed and stored in Antigen Preserve solution (50% PBS pH 7.0, 50% ethylene glycol, 1% polyvinyl pyrrolidone). Male and female brains were blocked and embedded in separate runs.
IgG immunohistochemistry
IgG staining was performed by Neuroscience Associates (Knoxville, TN) by a modification of a previously reported method (Richmon et al., 1998). Briefly, free-floating sections were treated with hydrogen peroxide for 30 min. Sections were blocked in serum-containing blocking buffer for another 30 min followed by 1 hr incubation with goat antimouse biotinylated antibody at room temperature. Following washes, sections were incubated with avidin-biotin-HRP complex for 1hr and were subsequently treated with diaminobenzidine tetrahydrochloride (DAB) and hydrogen peroxide. Sections were rinsed, mounted on to gelatin-coated glass slides, air-dried and coverslipped with VectaStain ABC kit (Vector, Burlingame, CA).
Overall IgG intensity measurements
Stained slides were digitized and the optical density for IgG staining was determined for specific brain regions (dorsal hippocampus, hypothalamus, thalamus) using Quantity One (Bio-Rad, CA). An average of 10 sections were analyzed for IgG staining for each brain region studied per animal within a treatment group (n=3–4).
Perivascular IgG labeling of microvessels
In the hippocampus, 3 sections (spanning the dorsal hippocampus) were selected per animal and within each section, each microvessel with a diameter of less than (<)30 microns was counted as well as the number of such vessels that were surrounded by a halo of IgG immunoreactivity. This diameter was specifically selected so as to include capillaries and post-capillary venules, where the majority of vascular transfer is likely to occur.
Microvessel tight junction proteins
In order to study extended lengths of vessels immunohistochemistry was performed on brain microvessel preparations, instead of tissue sections. In sections, vessels are likely to go in and out of the plane of focus, precluding accurate estimates of junction protein expression over the length of the vessel.
Rat brain microvessel extraction
Animals were deeply anesthetized and transcardially perfused with saline prior to removal of the hippocampii. Tissues were homogenized in ice-cold 1X phosphate buffered saline (dPBS, Invitrogen,CA) with 6–7 gentle strokes of a loose-fitting pestle of a Dounce homogenizer. The homogenate was spun at 720 g for 5 min at 4°C and the pellet was suspended in PBS and layered over an equal volume of 18% dextran. This mixture was spun at 3300 rpm for 1h at 4°C in a swinging bucket rotor centrifuge. The resulting pellet was suspended again in PBS and was filtered through a 70 micron filter. The microvessels were recovered by gently squirting the filter with ice-cold PBS. The vessel suspension was spun again at 1000 rpm for 5 minutes and the final pellet was suspended in PBS. Fifty microliters of this suspension was dropped onto gelatin-coated (175 bloom, Invitrogen, CA) slides. In order to reduce group differences due to technical artifacts, we ensured that microvessels from each of the treatment groups were represented on a single slide. Therefore a 50 × 75 mm slide was scored into four divisions and microvessel suspension from one animal of each of the four groups (young adult, reproductive senescent, with and without estrogen replacement) was dropped onto a separate quadrant on the slide. Slides were then air-dried and processed for immunohistochemistry.
Human brain tissue collection and microvessel extraction
Human brain tissue was obtained from female patients undergoing craniotomy for traditional indications at St. Joseph Hospital between 2006 and 2008. If any normal brain tissue was resected in the course of the surgical procedure, it was preserved for study rather than discarded. The study was approved by the St. Joseph Hospital Institutional Review Board, and informed consent was obtained from all study subjects. Brain tissue was obtained from 10 adult women, who ranged from 36–84 years of age. The indications for craniotomy in the 10 patients were brain tumors or arteriovenous malformation (AVM). Brain tissue collected during surgery was suspended in ice-cold Dulbecco’s minimum essential media without phenol red and processed immediately upon arrival at the laboratory. Tissue was rinsed in ice-cold PBS and microvessels were extracted using the same procedure described above. Only samples containing 6 or more microvessels were analyzed further. Three subjects were excluded due to inadequate vessel number in the sampled tissue.
For participants between the ages of 35–55, a follow-up interview was conducted to confirm pre or post-menopausal status. Based on age and menstrual history, the sample size was n=3 (mean age 43±3.6 yr) for premenopausal women and n=4 (mean age 71 ± 9.3 yr) for post-menopausal women.
Immunohistochemistry for endothelial markers and tight junction proteins
Vessels were fixed in methanol for 5 min and blocked in blocking buffer (2% normal goat serum or rabbit serum and 0.2% triton X-100 in dPBS) for one hour at room temperature. Slides, with microvessels adhered to them, were then incubated with primary antibodies for PECAM-1 (Santacruz biotechnologies, CA, 1:200) and GFAP (Chemicon, CA, 1:500) for characterization of cellular components of microvessels. Microvessels were also probed with primary antibodies specific for occludin or claudin-5 (Zymed,CA,1:200 dilution) for 2h at room temperature, followed by a 1h incubation with fluorescent-labeled secondary antibodies (Oregon green 488 goat anti-rabbit, 1:500 dilution and Alexafluor 594 goat anti-mouse, 1:2000 dilution, Molecular probes, CA, respectively). Slides were subsequently washed in 1X dPBS (Invitrogen, CA), counterstained with the nuclear dye, Hoechst 33258 for 1 hour at room temperature and cover-slipped with Prolong Antifade mounting media (Molecular Probes, CA). Immunostaining was visualized by fluorescent microscopy at 20× magnification and images were digitized using a digital camera and the Q Capture software package,Q-imaging corporation,Canada. A minimum of 6 separate microvessels were digitized for each subject, for each junction protein.
Analysis of tight junction proteins
Since junctional localization of tight junction proteins is critical for the function of the blood brain barrier, a rating scale was used to judge the localization of these proteins on microvessels. Images of microvessels stained for claudin-5 or occludin were coded and scored independently by 2 raters blind to the treatment condition. Images were scored for (a) continuity and (b) junctional localization (at the interface of adjacent endothelial cells) of the fluorescent signal were rated on a 7-point rating scale, where 0 represented the lowest aspect of the criteria and 7 the highest. Scores were summed as follows C+J, where C=continuity and J=junctional localization. After scoring, the mean rating for all 6 images obtained from one subject were averaged and represented as a single data point. Inter-rater reliability was assessed by correlation analysis and ranged from +0.69 to +0.74 (p<0.01). For data presented here, only scores by one rater (SB) are shown.
Statistical Analysis
For IgG studies and microvessels studies in female animals, group differences were estimated by a two- way ANOVA for age and hormone treatment. For IgG studies and microvessels in male rats as well as human microvessels analysis, group differences were analyzed by t-test. All analyses were performed using the SPSS statistical package (SPSS Inc., IL) and, in all cases, group differences were considered significant at p<0.05.
For human microvessels, 2 correlations were performed. First, the claudin-5 rating score was correlated with the type of surgery (coded 1: tumor, 2: AVM.). Next, the claudin-5 score was correlated with reproductive age (coded 1: premenopausal, 2: postmenopausal).
RESULTS
IgG expression in young adult and reproductive senescent female rats
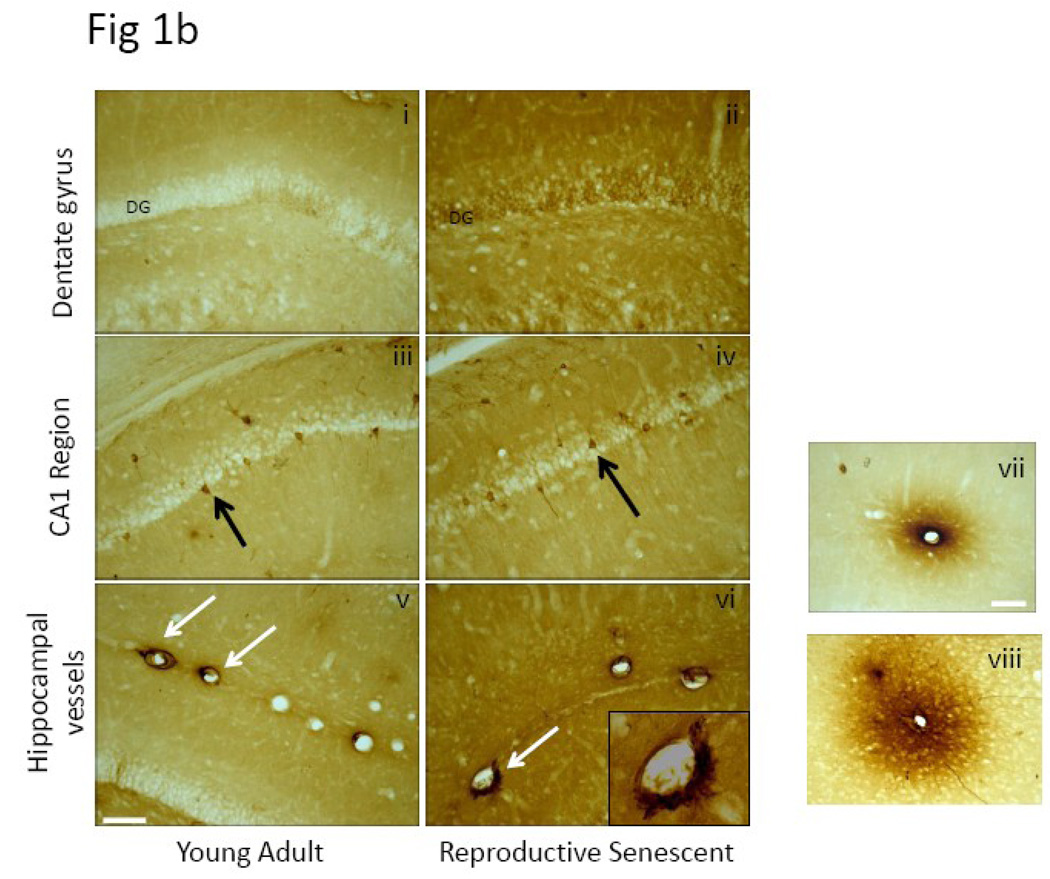
IgG expression in the brain parenchyma is usually indicative of disruption of the blood brain barrier. As shown in Fig 1a-top panel, coronal brain sections probed for IgG indicated an overall greater staining intensity in the forebrain regions of the reproductive senescent females as compared to their young counterparts. Staining intensity was analyzed in the hippocampus, thalamus and hypothalamus by densitometry. IgG immunoreactive staining was seen mainly in the neuropil, as shown here in a higher magnification image from the vicinity of the dentate gyrus of the hippocampal formation (Fig 1b). However, IgG-positive staining was also seen occasionally in neurons. Shown in Fig1b (iii) and (iv) are IgG-positive neurons located in the CA1 region of the hippocampus, indicative of intrathecal IgG synthesis. IgG-positive staining was also seen surrounding the lumen of blood vessels (Fig 1b (v) and (vi)). In the case of microvessels, some lumens were surrounded by a halo of IgG-immunoreactive product (Fig 1b vii, viii), supporting the hypothesis that IgG likely enters the brain parenchyma via the vasculature.
Fig.1. IgG expression in female and male brain.
(a) Coronal brain sections from young adult and reproductive senescent females as well as age-matched males were processed for IgG immunoreactivity. Visual inspection indicated overall greater IgG immunoreactive product in the forebrain of older males and females compared to their younger counterparts. Regional intensity of IgG staining was quantitated by densitometry from digitized images. (b) IgG expression was seen in the parenchyma (i and ii), in neurons (iii and iv) and blood vessels (v and vi) throughout the brain and images shown here are from the hippocampal formation. In microvessels, IgG-positive immunoreactivity was seen as a halo surrounding the lumen of blood vessels (vii and viii) Bars 1b i–vi: 50µm, vii–viii: 20µm.
IgG expression in the hippocampus
Consecutive sections (200 microns apart) through the dorsal hippocampus were analyzed for staining intensity in young and reproductive senescent animals. As shown in Fig 2a and b, there was a prominent difference in the staining intensity for IgG in reproductive senescent animals as compared to young adult females, with significantly greater staining intensity seen in the former (main effect of age, F(1,12): 24.03, p<0.05). There was no main effect of estrogen in this region (F(1,12): 0.573, p>0.05).
Fig.2. IgG immunoreactivity in the hippocampus of the young adult and reproductive senescent brain.
Coronal sections of the young adult and reproductive senescent brain containing the dorsal hippocampus were scanned and the intensity of IgG staining in this region was quantified from the digitized images using Quantity One® software (Bio-Rad, CA). (a) IgG immunoreactivity was stronger in the hippocampus of senescent females as compared to young adults and mean (±SEM) intensity of IgG immunoreactivity is shown in the histogram in (b). Histogram indicates mean (±SEM) optical density of IgG immunoreactivity in hippocampus of young and senescent animals. No significant estrogen effect was observed in either age group. (N=4 per group, * p<0.05) Bar =50 µm. Key: YA: Young adult, RS: reproductive senescent, O: ovariectomized and replaced with control pellet, E: ovariectomized and replaced with estrogen pellet.
IgG expression in the thalamus
As shown in Fig 3a and the histogram in 3b, IgG labeling was significantly greater in the thalamus region of reproductive senescent females as compared to the same region in young females (main effect of age, F(1,12): 25.115, p<0.05). However, estradiol had no effect on IgG-staining in the thalamus (main effect of hormone F(1,12): 1.59, p>0.05).
Fig.3. IgG immunoreactivity in the thalamus of the young adult and reproductive senescent brain.
(a) Stronger IgG immunoreactivity was seen in the thalamus of reproductive senescent females as compared to those of young adult females. (3b) Histogram indicates mean (±SEM) optical density of IgG immunoreactivity in thalamus of young and senescent animals. No significant estrogen effect was observed in either age group. N=4 per group, * p<0.05. Bar=50 µm. Key: YA: Young adult, RS: reproductive senescent, O: ovariectomized and replaced with control pellet, E: ovariectomized and replaced with estrogen pellet.
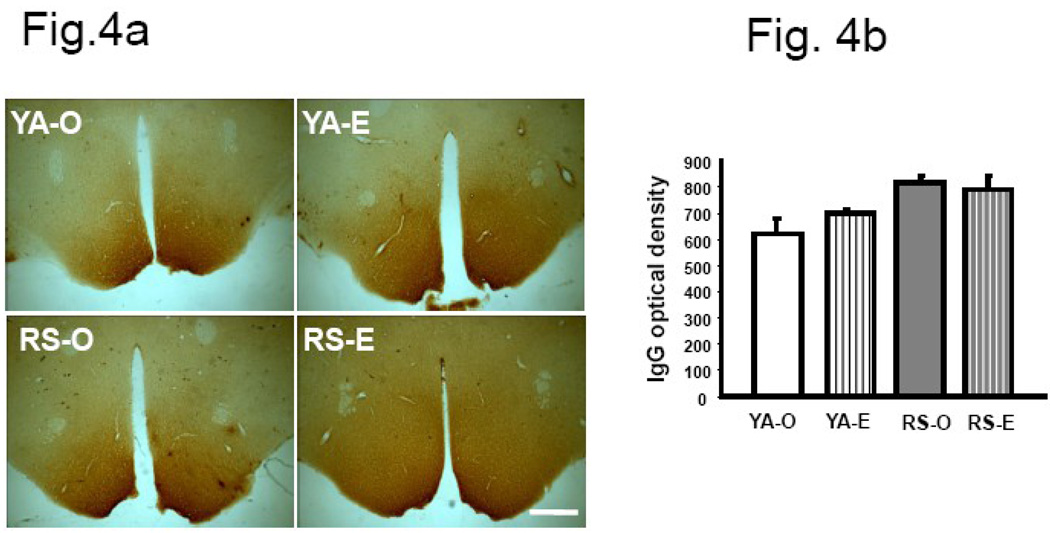
IgG expression in the hypothalamus
Very intense IgG immunoreactivity was seen in the hypothalamus of both young adult and reproductive senescent females. As shown in Fig 4a and the histogram in 4b, there was no difference in the intensity of IgG labeling between young and senescent females or due to hormone treatment (F(3,12): 0.367, p>0.05). Uniform, dark IgG label in this region is not unexpected since the hypothalamus is only poorly protected by the specialized endothelium of the blood brain barrier.
Fig.4. IgG immunoreactivity in the hypothalamus of the young adult and reproductive senescent brain.
(a) Although strong IgG immunoreactivity was visible in the hypothalamus of young and senescent females, there was no difference in staining intensity due to age or estrogen treatment. (b) Histogram shows mean (±SEM) IgG optical density. N=4 per group. Bar=50 µm. Key: YA: young adult, RS: reproductive senescent, O: ovariectomized and replaced with control pellet, E: ovariectomized and replaced with estrogen pellet.
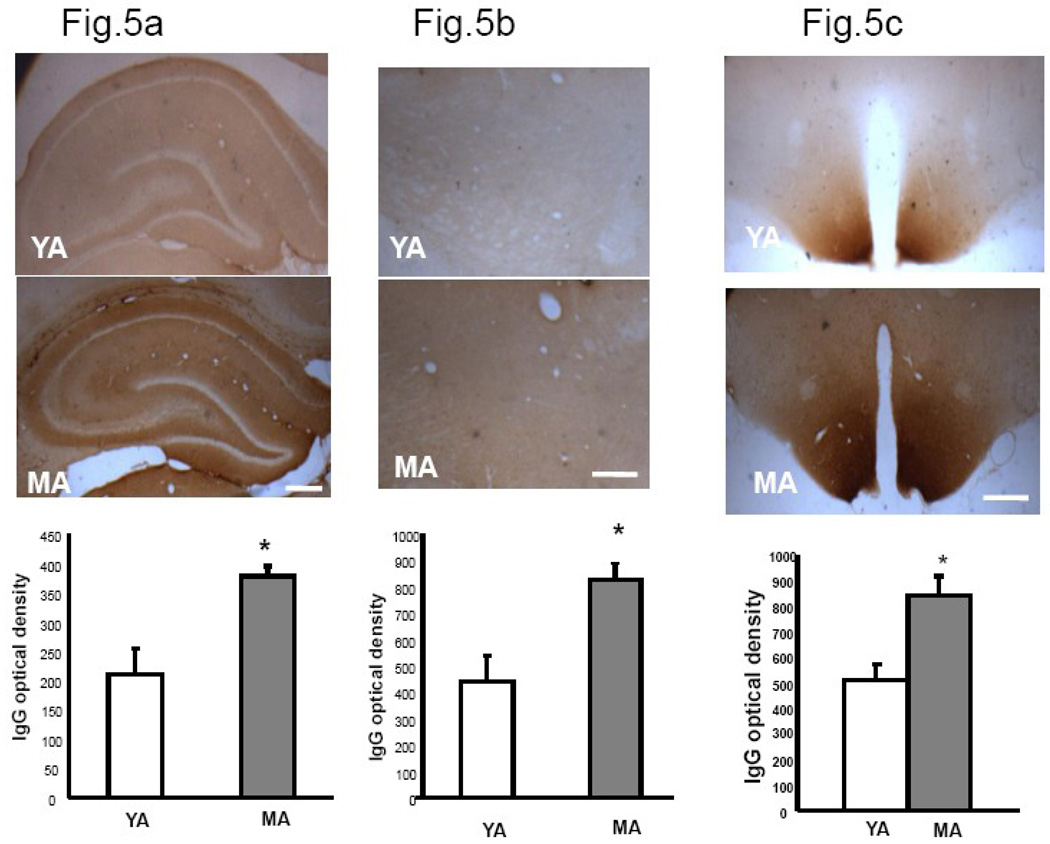
IgG expression in young and middle-aged male rats
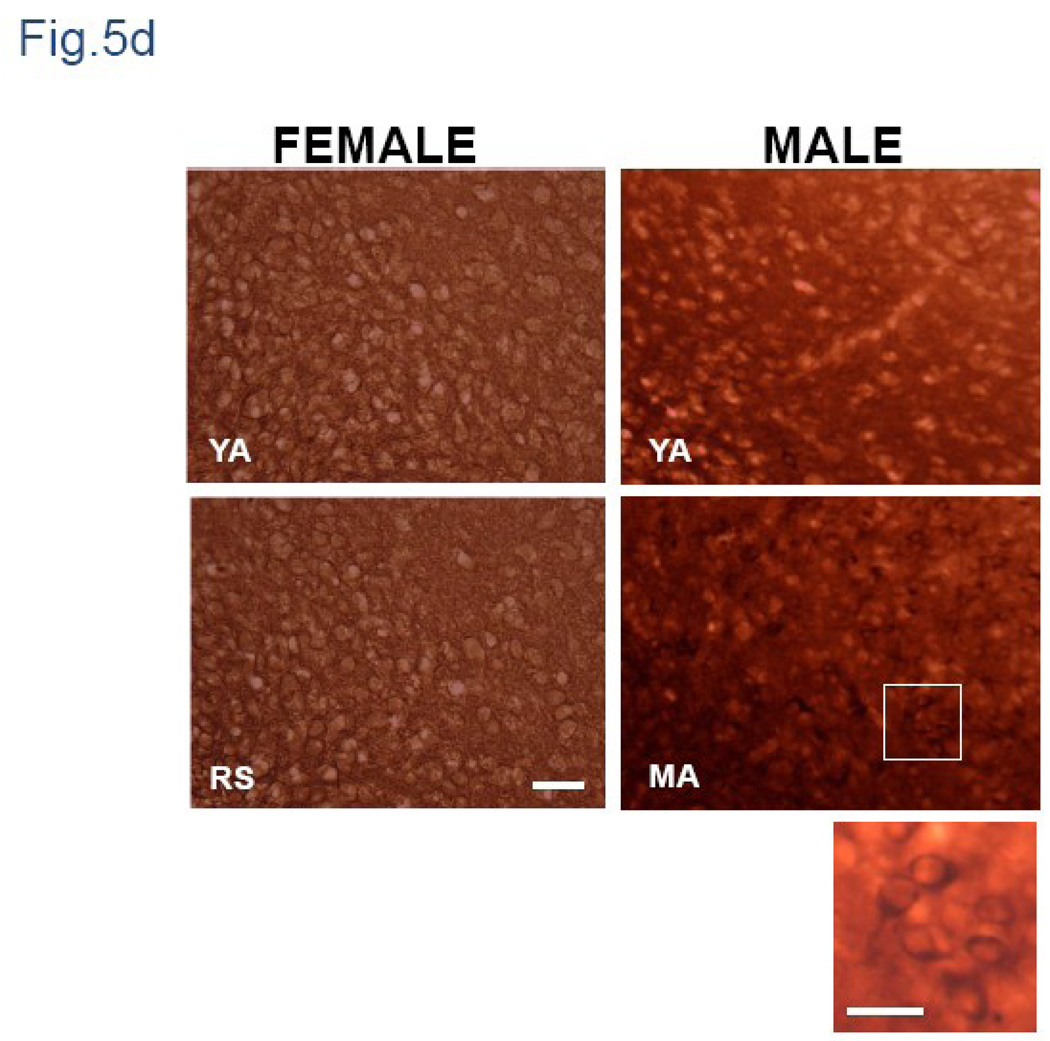
Coronal sections of male brain stained for IgG showed greater staining in middle-aged males than young males (Fig.1a-bottom panel). Similar to the females, there was significantly greater IgG staining in middle-aged males as compared to young adult males in both the hippocampus and thalamus (Fig 5a and 5b; p<0.05). Unlike the female, the intensity of IgG labeling in the hypothalamus was significantly greater in middle aged males compared to young adults (p<0.05). While staining in the female hypothalamus was mainly seen in the neuropil (Fig 5d), the middle aged male hypothalamus had clearly labeled cells and processes (Fig 5d).
Fig.5. IgG immunoreactivity in hippocampus, thalamus and hypothalamus of young adult and middle-aged males.
Coronal sections of young adult and middle-aged males were scanned and optical density was quantified from digitized images using Quantity One® software (Bio-Rad, CA). Histograms represent mean (±SEM) IgG optical density. Stronger immunoreactivity was seen in hippocampus (6a) thalamus (6b) and hypothalamus (6c) of middle-aged males compared to young adults. (6d) Higher magnification view of the hypothalamus shows IgG labeling was largely restricted to the neuropil of females, while in males, intensely stained neuropil as well as prominent cellular labeling was visible. Inset is a magnified image of the hypothalamus of the middle aged male indicating cellular IgG label. YA: Young adult, RS: reproductive senescent (female) MA: Middle-Aged male (age matched to RS female). Bar=50 µm; Inset Bar= 25 µm
Analysis of microvessels with perivascular IgG-immunoreactivity
Approximately 35–50 microvessels were counted per animal. Animals where fewer than 10 vessels were detected were not included since they were not considered a representative sample. The final sample size for this analysis was n=3–4 for the female groups and n=5 for the male groups. The proportion of microvessels with perivascular IgG-immunoreactivity was significantly greater in the senescent females as compared to young adult females (F(1,10): 7.149, p<0.05, but not in middle aged males as compared to young adult males (see Table 1). These data indicate that perivascular leakage of IgG in putative capillaries and post capillary venules is associated with ovarian senescence rather than chronological aging.
Table 1.
Proportion of microvessels with perivascular IgG labeling in the female and male hippocampus.
| Group | Microvessels (<30 µm) *a % (Mean±SD) |
|---|---|
| YA-O females | 16.39 ±5 |
| YA-E females | 22.99 ±4.57 |
| RS-O females | 26.22 ±4.15 |
| RS-E females | 25.54 ±2.79 |
| YA males | 14.42 ±7.34 |
| MA males | 22.85 ±8.97 |
Abbreviation: YA-O, young adult ovariectomized. YA-E, young adult estrogen-treated. RS-O, Reproductive senescent ovariectomized; RS-E, Reproductive senescent estrogen-treated; YA- Young adult, MA-Middle aged.
Key: main effect of age (females)
p<0.05.
Analysis of tight junction proteins in microvessels
We next analyzed the expression of tight junction proteins (occludin and claudin-5) in microvessels extracted from the hippocampus of young adult and reproductive senescent females and age matched males. Extracted microvessels were positive for immunostaining for the endothelial marker, PECAM-1. GFAP positive labeling was seen at the edges of the vessel consistent with localization of astrocytes on microvessels, confirming the structural integrity of the harvested microvessels (Fig. 6).
Fig.6. Cellular characterization of microvessels.
Microvessels were stained with antibodies specific for the endothelial marker, Pecam-1 (in red) and the glial marker, GFAP (in green). The vessels were also counterstained with nuclear dye, Hoeschst 33342 (in blue). Co-localization of pecam-1 and GFAP was observed at several regions of the individual vessel (indicated in white arrows).
Immunohistochemistry and Western blot Analysis
Tight junction protein expression was analyzed by immunohistochemistry using protein-specific antibodies for claudin-5 and occludin. In Western blot analysis, these antibodies detected a size appropriate band for claudin-5 (22kD; Fig 8a) and occludin (66 kD; Fig 8b). The microvessel in Fig 7c was probed with claudin-5 and the arrows indicate continuous, junctional localization of the protein along its length. This vessel would receive a high score (7) for both the scoring parameters (continuity and junctionality) described in the Methods section.
Fig.8. Claudin-5 immunofluorescence in hippocampal microvessels.
(a) Claudin-5 was expressed in hippocampal microvessels derived from both young adult and reproductive senescent females, however claudin-5 expression was brighter and more junctional in microvessels from young adults as compared to those from reproductive senescent females. (b) Histogram represents the average (±SEM) composite rating scores for continuity and junctional localization for claudin-5. Estrogen treatment (closed bars) did not affect the ratings scores in either young adult or senescent females. (c) Western blot analysis showed no change in claudin-5 expression between young adults and reproductive senescent females. N=4–5 per group, 6 vessels were rated per animal, * P<0.05. Key: YA: young adult, RS: reproductive senescent, O: ovariectomized and replaced with control pellet, E: ovariectomized and replaced with estrogen pellet. Bar=50 µm.
Fig.7. Immunostaining for tight junction proteins.
Antibodies specific for (a) occludin and (b) claudin-5 were used for immunohistochemical detection of tight junction proteins in microvessels. These antibodies recognized size-appropriate bands in Western blots. (c) Shown here is an isolated hippocampal microvessel from an estrogen-treated young adult female probed with the claudin-5 antibody. Arrows indicate bright, continuous junctional localization of this protein. Bar=50 µm.
Claudin-5 and Occludin expression in hippocampal microvessels from female and male rats
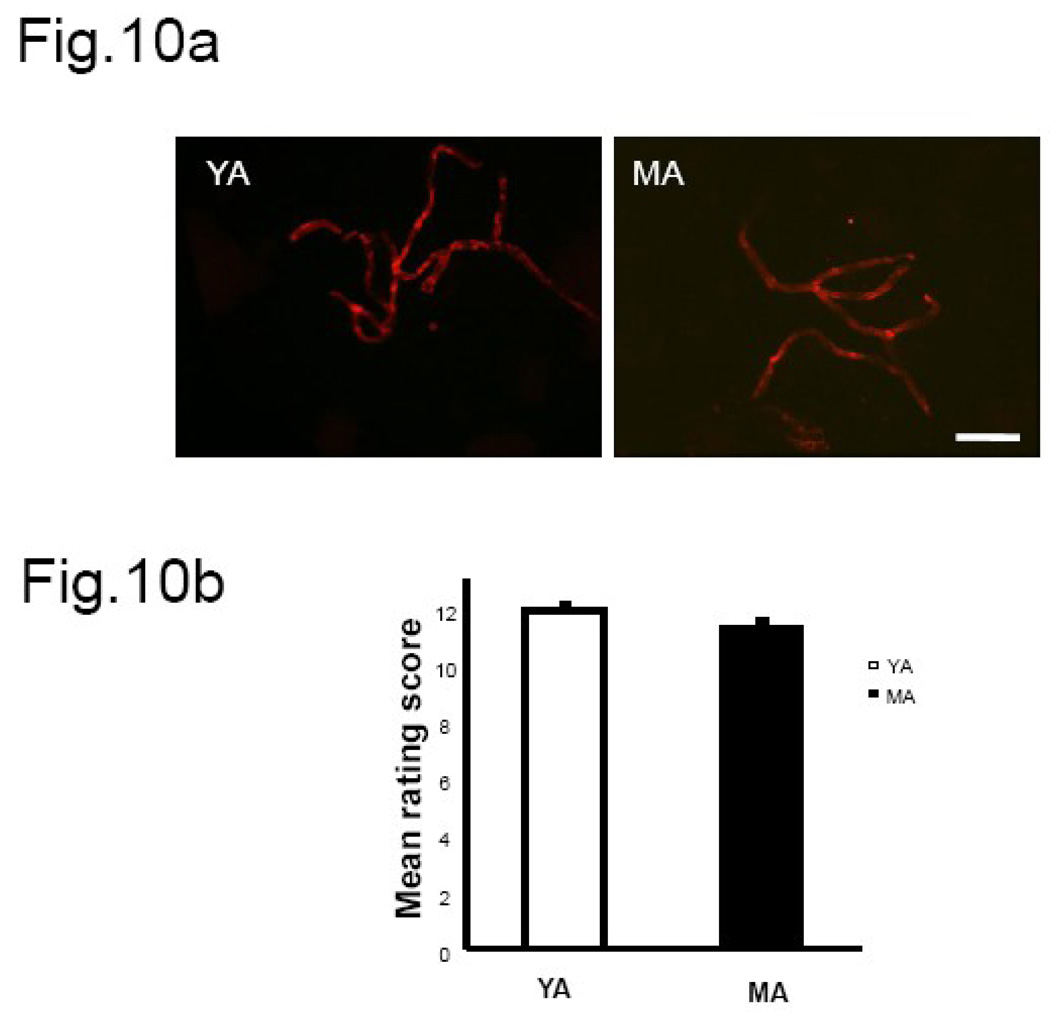
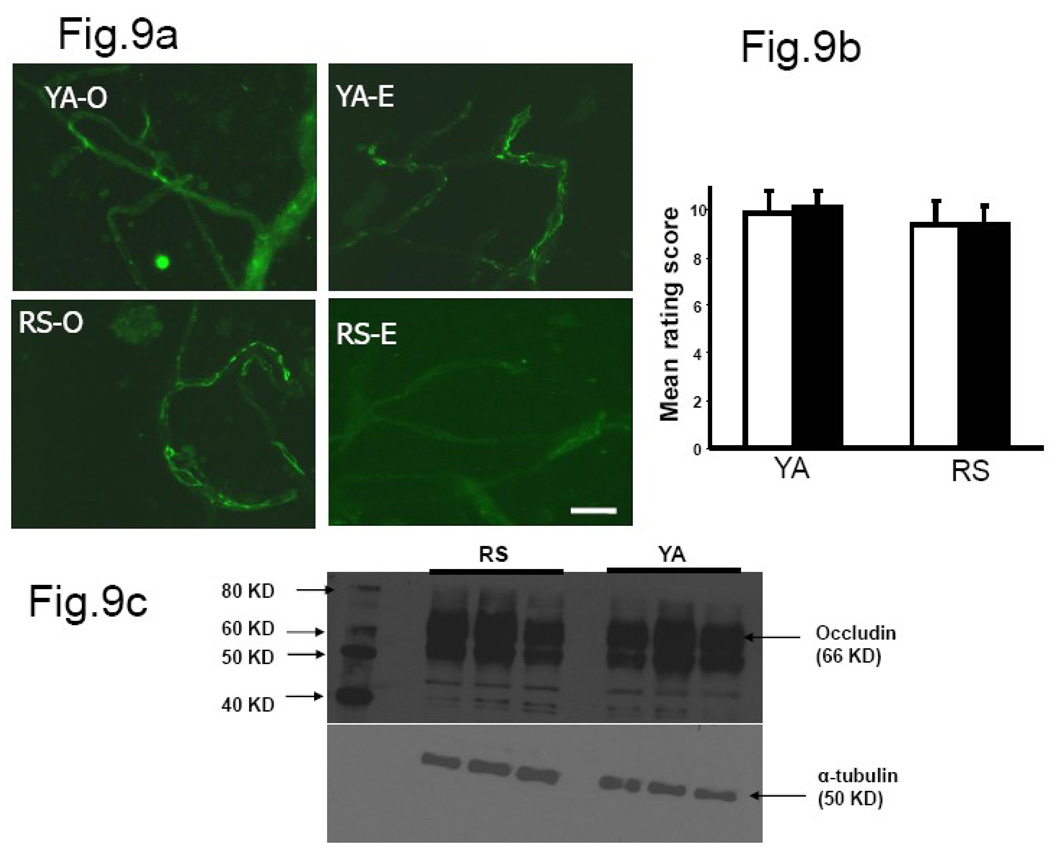
Claudin-5 immunoreactivity was present in both young and senescent microvessels (8a). The mean rating scores (Fig 8b) indicate a significant difference in the distribution of the fluorescent signal between the two age groups (main effect of age, F(1,15): 5.39, p<0.05). Claudin-5 was well localized to the endothelial junctions in vessels from young adult females as compared to the senescent group, However, no estrogen effects (F(1,15): 4.04, p>0.05) were noted in the distribution pattern of claudin-5 in microvessels from young adult and senescent females. Microvessels extracted from young adult and middle-aged males showed uniformly bright claudin-5 immunosignal (Fig 10a). The composite rating scores indicate no difference in continuous distribution and junctional location of claudin-5 immunosignal in young adults and middle-aged males (Fig. 10b). Occludin staining was also present in the hippocampal microvessels of young and senescent females (Fig. 9a). However, the pattern of staining was not different between young and senescent females when subject to blind rating analysis. The composite rating score for continuity and junctional localization of the fluorescent signal was not altered by estrogen (F(1,15): 0.012, p>0.05) in vessels derived from young adults as compared to those derived from reproductive senescent females (Fig.9b). Western blot analysis for claudin-5 and occludin in ovariectomized females indicates that protein expression was not regulated by age (Fig. 8c& Fig. 9c, quantitation not shown).
Fig.10. Claudin-5 immunofluorescence in hippocampal microvessels from male rats.
(a) Claudin-5 staining was bright and well-localized to the cell junctions in microvessels derived from both 3 month old and middle-aged males. (b) Histogram represents the average (±SEM) composite rating score for continuity and junctional localization for claudin-5. There was no difference in claudin-5 expression pattern between young and middle-aged males. N=4 per group, 6 vessels were rated per animal, * P<0.05. Key: YA: young adult, MA: middle aged. Bar=50 µm.
Fig.9. Occludin immunofluorescence in hippocampal microvessels.
(a) Occludin staining was brighter and well-localized to the cell junctions in microvessels derived from both young adult females and reproductive senescent animals. (b) Histogram represents the average (±SEM) composite rating score for continuity and junctional localization for occludin. There was no difference in occludin immunostaining in microvessel tight junctions of young adults and reproductive senescent animals. (c) No change in occludin expression was seen in Western blots from young adult and reproductive senescent microvessels. N=4–5 per group, 6 vessels were rated per animal. Key: YA: young adult, RS: reproductive senescent, O: ovariectomized and replaced with control pellet, E: ovariectomized and replaced with estrogen pellet. Bar=50 µm.
Claudin-5 expression in human brain microvessels
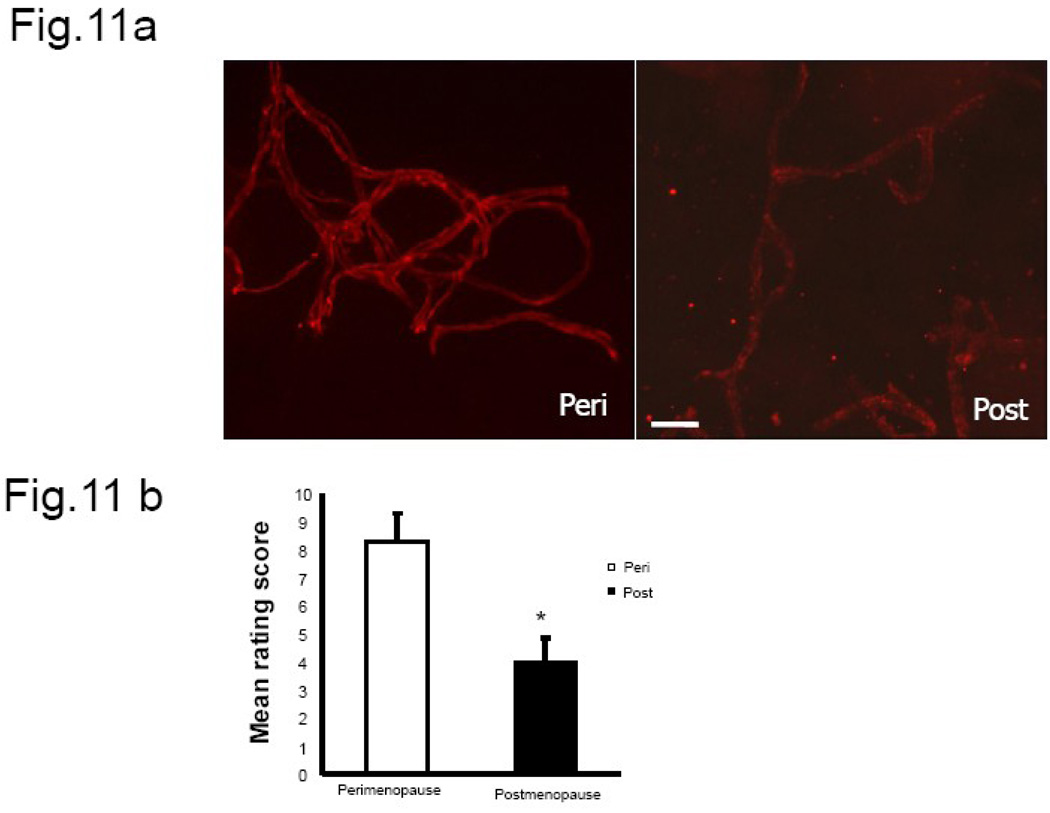
Claudin-5 immunoreactivity in human microvessels also showed an age-dependent dysregulation of claudin-5 localization and distribution. Claudin-5 immunoreactivity in microvessels from premenopausal women showed greater junctional localization with continuous distribution of the protein on the vessels analyzed (Fig.11a). However, in post-menopausal women claudin-5 distribution was frequently disrupted in the long segments of vessels and also showed poor junctional localization. The composite rating scores indicate a significant difference in staining pattern between perimenopausal and post-menopausal women (Fig. 11b). To estimate the involvement of disease/surgery type on claudin-5 expression, a correlation analysis was performed between claudin-5 score and type of surgery and this correlation was not significant (r=+0.348, df(5), p>0.05). However, there was a significant inverse correlation between junctional expression of claudin-5 and advancing age (r=−0.815, df (5), p<0.05).
Fig.11. Claudin-5 immunofluorescence in microvessels from human brain.
(a) Claudin-5 staining in human brain microvessels indicates a change in expression pattern between the pre-menopausal and post-menopausal group. Microvessels collected from younger women showed continuous junctional distribution of claudin-5 compared to older subjects. (b) Histogram represents the average (±SEM) composite rating score for continuity and junctional localization for claudin-5. N= 3–4 per group, 6–8 vessels were rated per sample, * P<0.05. Key: Bar=50 µm.
DISCUSSION
The present study demonstrates that reproductive senescent females and age-matched males have significantly greater IgG immunoreactivity in the hippocampus, a region primarily involved in cognition and memory (Gould et al., 1991), indicating that chronological age may increase IgG accumulation in the brain. However, an age-dependent alteration in the tight junction protein claudin-5 and perivascular IgG immunoreactivity in putative capillaries was seen only in the hippocampus of senescent females but not in males, indicating that hippocampal microvasculature may be uniquely vulnerable to the cumulative decline in ovarian hormones that occur with reproductive senescence. This latter data also confirm the age related difference noted in our previous study showing increased extravasation of Evan’s blue dye into the hippocampus of senescent females, (Bake and Sohrabji, 2004). Collectively, these data indicate that aging is accompanied by constitutive alterations in the blood brain barrier and that these alterations in females may be associated with changes in the microvascular endothelium.
Infiltration of serum proteins into the brain parenchyma is associated with several neurodegenerative disorders such as Alzheimer’s disease (Mori et al., 1991) and multiple sclerosis (Minagar and Alexander, 2003). The presence of perivascular and parenchymal IgG is widely used as a marker for blood brain barrier breakdown in neurovascular injury (Aihara et al., 1994; Kuang et al., 2004; Loftspring et al., 2006). In AD brains, IgG is seen in the brain parenchyma, near vessels and in neurons. In fact, all degenerating neurons expressed IgG, although IgG expression was also seen in non-degenerating cells (D'Andrea, 2003). Aberrant brain localization of the liver enzyme, prothrombin, has also been reported in the pre-frontal cortex of Alzheimer’s disease patients and the extent of prothrombin accumulation is positively correlated with the severity of Alzheimer’s disease (Zipser et al., 2007), suggesting that there may be progressive deterioration of the barrier in the course of this disease.
In the present study, we demonstrate for the first time that, even in the absence of neural injury, middle aged male and female rats have increased IgG accumulation in the hippocampus and thalamus compared to their young counterparts. Furthermore, the proportion of perivascular IgG-immunoreactivity is significantly increased with age, indicating a possible vascular source of brain IgG. However, IgG expression in the brain may not be an unambiguous marker for blood brain barrier deterioration. Originally thought to be expressed only in mature B-lymphocytes, IgG has also been shown to be expressed in epithelial tumor cells (Babbage et al., 2006; Qui et al., 2003). IgG is also seen in neurons, in many cases these are early-generated populations such as the subplate neurons, leading to the proposal that that neuronal IgG resulted from the uptake of peripherally-derived immunoglobulin across an immature blood brain barrier. More recent studies, however, definitely indicate that IgG is synthesized in neurons (Huang et al., 2008). Although the role of neuronal IgG is not known, its expression in early born neurons suggests that it may be implicated in neuronal development. In tumor cells, blockage of IgG increased apoptosis of tumor cells, indicating that IgG may play a role in cell survival (Qui et al., 2003). IgG application to sensory neurons elicits calcium dependent transmitter release (Andoh and Kuraishi, 2004) and reduces infarct size in an ischemic stroke injury (Arumugam et al., 2007). Our data also cautions about the use of IgG as an indicator of blood brain barrier changes. For example, older males had significantly greater expression of IgG in all regions measured when compared to young adult males. However, the proportion of microvessels with perivascular IgG was no different between the two groups, suggesting that the increased density of IgG staining may be due to local synthesis of IgG in older males. In fact, significantly greater IgG expression is seen in the hypothalamus of older males, in a region that is not guarded by the blood brain barrier, as well as cellular staining, that is not readily apparent in the young males or the females. If IgG expression corresponds to neuronal survival, increased local expression of IgG in the older male brain may be indicative of a compensatory, trophic response.
The tight junction family of proteins, on the other hand, is more directly related to the maintenance of the barrier. Studies of the claudin-5 knock-out mouse demonstrate that the absence of this protein resulted in size-dependent permeability of the blood brain barrier (Nitta et al., 2003). In contrast, overexpression of claudin-5 caused reduced paracellular permeability to different size tracers 40 KDa and 342 KDa (Fontijn et al., 2006). Tight junction protein expression is also altered by neuro-vascular injury or inflammation. Inflammation-induced changes in blood brain barrier permeability are accompanied by loss of claudin-3 expression in experimental autoimmune encephalitis in mice (Wolburg et al., 2003). Lambda-carageenan induced inflammatory pain significantly reduced occludin expression in young female rats (Huber et al., 2001) and both occludin and claudin-5 are reduced following injections of the astrocytic toxicant 3-chloropropanediol (Willis et al., 2004) and acute exposure to reactive oxygen species (Schreibelt et al., 2007). However, mouse embryonic stem cells with disrupted alleles of occludin showed no difference in epithelial tight junction morphology and barrier function compared to wild type ES cells (Saitou et al., 1998), suggesting that mere expression of the junction proteins may not be sufficient to ensure tight junction function.
Junctional localization of these proteins is also thought to be critical for its role in maintaining the blood brain barrier. Hypoxia, which increases permeability of the blood brain barrier, causes a relocalization of occludin from cell membrane to cytoplasm in cultured bovine endothelial cells (Brown and Davis, 2005). Post-translational modification such as phosphorylation can also alter the localization of occludin on the cell (Andreeva et al., 2001; Sakakibara et al., 1997), and phosphorylation of occludin on tyrosine residues, but not serine and threonine, resulted in decreased transepithelial/endothelial electrical resistance (TEER) in both MDCK epithelial cells and in bovine brain endothelial cells (Staddon et al., 1995). In view of the importance of junctional localization of these proteins, the present study specifically included this feature as a key aspect of the rating scale instead of using Western blot analysis, which would only reveal differences in total expression of this protein. In order to ensure that sufficient lengths of vessels could be analyzed, microvessel preparations were studied instead of brain sections. This present study shows that there is poor junctional localization of claudin-5 in hippocampal microvessels in reproductive senescent rats. In order to confirm that claudin-5 was altered by ovarian aging and not simply by chronological age, we examined claudin-5 expression in males age-matched to young and senescent females. Interestingly, there was no difference in microvascular distribution of claudin-5 in young and middle aged males, supporting the hypothesis that claudin-5 distribution in tight junction assemblies is not altered as a function of chronological age, but is highly sensitive to persistent changes in cyclicity of ovarian hormones. Parallel studies in human microvessels also indicate a comparable phenomenon, where claudin-5 distribution was severely disrupted in microvessels from post-menopausal women. Due to the limited availability of human tissue, we restricted human microvessel analysis, based on the rat data, to claudin-5 and compared only pre and post-menopausal groups (without segregating for estrogen use). To our knowledge this is the first report of age-related changes in tight junction protein localization in isolated human brain microvessels.
Increased permeability of the blood brain barrier and high levels of circulating cytokines can have serious repercussions on hippocampal function (see review, (Banks et al., 2002) and can impair performance on a spatial-learning task (Morris water maze), by increasing swim times at low doses and reducing the number of correct choices of platform location (Arai et al., 2001). In humans, a strong positive correlation has been noted between elevated levels of circulating inflammatory cytokines and severe cognitive impairment in older women (Yaffe et al., 2004) as well as severe depressive disorders in elderly humans (Miller et al., 2002). In senescent females reduced junction localization of tight junction protein in hippocampal vessels was associated with increased proportion of microvessels with perivascular IgG leakage, indicating that the microvasculature is more permeable in the senescent female forebrain, and may therefore be more vulnerable to behavioral impairment resulting from exposure to circulating cytokines. While studies have shown that the response to peripheral (LPS-induced) activation of central cytokine levels and behavior is modulated by aging, there has been no systematic study of sex differences into the inflammatory response in aging. Our data would suggest that this response is likely to be exacerbated in females.
Our previous studies on the aging blood brain barrier using Evan’s blue dye extravasation indicate that estrogen increases the permeability of the barrier in the older (acyclic) females. Although age-related changes in the barrier properties are partially reflected in the localization of claudin-5 in senescent females, estrogen mediated effects are not regulated via this tight junction protein or occludin. However, this does not preclude the possibility that estrogen may regulate other tight junction family members such as the junction associated molecules (JAMs). Estrogen has been shown to increase occludin mRNA in whole brain homogenates (Kang et al., 2006) and in the colon and a human colon cell line (Braniste et al., 2009). However, estrogen also increases permeability of endometrial tight junctions (Aberdeen et al., 2008) and alters occludin levels in a biphasic manner in human umbilical cord derived endothelial cells (Wolburg et al., 2003) indicating that the hormone may have pleiotrophic effects. Although seemingly contradictory, there are significant differences between the design of these experiments in terms of animal model, route and dose of 17beta-estradiol, regional analysis (whole brain vs hippocampus) that precludes straightforward comparisons and underscores the need for further studies of estrogens effects on paracellular permeability. Furthermore, estrogen’s effects on transcellular passage are also poorly understood. Since estrogen does affect Evan’s dye extravasation in the hippocampus, one possibility is that estrogen’s actions are targeted to transcellular passage, possibly through receptor mediated endocytosis. The estrogen receptor is a ligand-activated transcription factor and could promote transcellular passage by increasing the expression or synthesis of specific binding proteins on endothelial cells. Thus one mechanism by which estrogen may promote Evan’s dye extravasation is via regulation of endothelial albumin- binding protein which can internalize albumin-bound Evan’s dye.
In conclusion, the present study reveals an age-related change in IgG expression in reproductive senescent females and age-matched (middle-aged) males. In older females, but not males, increased IgG expression is correlated with increased perivascular IgG expression as well as changes in the structural composition in the vascular endothelium, indicating that loss of cyclic exposure to gonadal steroids due to reproductive senescence results in distinct cellular and molecular changes in the female brain that is not seen in the male brain (at this age) where gonadal hormonal titers are maintained at a relatively even level. The fact that tight junction protein dysregulation is not altered by estrogen treatment suggests the presence or absence of other regulatory molecules that co-vary with reproductive aging.
Acknowledgements
The authors would like to thank Dr. Danielle Lewis (TAMHSC) for her help with blind rating analysis of microvessels, Dr. Wei-Jung Chen (TAMHSC) for expert statistical advice, as well as Najma Ahmed, Dustin Tyler and Joshua Walters for their excellent technical assistance. We also thank Dr. Rudy Briner for his assistance in recruiting patients (St. Joseph’s Hospital) and Debbie Hieden, RN/CNOR (St. Joseph’s Hospital) for her help in tissue collection and preservation of human samples for this study. The authors are also grateful to two anonymous reviewers for their comments. This work was supported by grants from NIH AG027684 and AG028303 to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberdeen GW, et al. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology. 2008;149:6076–6083. doi: 10.1210/en.2008-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara N, et al. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. doi: 10.1002/cne.903420402. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. doi: 10.1096/fj.02-1169fje. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, et al. Protein Kinase C Regulates the Phosphorylation and Cellular Localization of Occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Arai K, et al. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. J Pharmacol. 2001;87:195–221. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbage G, et al. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 2006;66:3996–4000. doi: 10.1158/0008-5472.CAN-05-3704. [DOI] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17 beta-Estradiol Differentially Regulates 0 Blood-Brain Barrier Permeability in Young and Aging Female Rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Ballabh P, et al. The blood-brain barrier: an overview: Structure, regulation, and clinical implications. Neurobiol Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol Review. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Braniste V, et al. Oestradiol decreases colonic permeability through oestrogen receptor{beta}-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009 doi: 10.1113/jphysiol.2009.169300. (Epub-ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochemical and Biophysical Research Communications. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- Chan PH, et al. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991;29:482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Willis CL, et al. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48:1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR. Evidence linking neuronal cell death to autoimmunity in Alzheimer's disease. Brain Research. 2003;982:19–30. doi: 10.1016/s0006-8993(03)02881-6. [DOI] [PubMed] [Google Scholar]
- Fontijn RD, et al. Limited contribution of claudin-5-dependent tight junction strands to endothelial barrier function. European Journal of Cell Biology. 2006;85:1131–1144. doi: 10.1016/j.ejcb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Biol Chem. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, et al. The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology. 1991;16:67–84. doi: 10.1016/0306-4530(91)90071-z. [DOI] [PubMed] [Google Scholar]
- Greenwood J. Mechanisms of blood-brain barrier breakdown. Neuroradiology. 1991;33:95–100. doi: 10.1007/BF00588242. [DOI] [PubMed] [Google Scholar]
- Hajjar I, et al. Antihypertensive agents for aging patients who are at risk for cognitive dysfunction. Curr Hypertens Rep. 2005;7:466–473. doi: 10.1007/s11906-005-0043-y. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. The Int J Biochem & Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Huber JD, et al. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:1241–1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Huang J, et al. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 2008;40:1604–1615. doi: 10.1016/j.biocel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Janigro D. Blood-brain barrier, ion homeostasis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Research. 1999;37:223–232. doi: 10.1016/s0920-1211(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:311–321. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Kang HS, et al. Effect of estrogen on the expression of occludin in ovariectomized mouse brain. Neurosci Lett. 2006;402:30–34. doi: 10.1016/j.neulet.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Kuang F, et al. Extravasation of blood-borne immunoglobulin G through blood-brain barrier during adrenaline-induced transient hypertension in the rat. Int J Neurosci. 2004;114:575–591. doi: 10.1080/00207450490422731. [DOI] [PubMed] [Google Scholar]
- Loftspring MC, et al. Plasma proteins in edematous white matter after intracerebral hemorrhage confound immunoblots: an ELISA to quantify contamination. J Neurotrauma. 2006;23:1904–1911. doi: 10.1089/neu.2006.23.1904. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Functional analysis of tight junctions. Methods. 2003;30:228–234. doi: 10.1016/s1046-2023(03)00029-x. [DOI] [PubMed] [Google Scholar]
- Mikulis DJ. Functional Cerebrovascular Imaging in Brain Ischemia: Permeability, Reactivity, and Functional MR Imaging. Neuroimaging Clin N Am. 2005;15:667–680. doi: 10.1016/j.nic.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Miller GE, et al. Clinical depression and inflammatory risk markers for coronary heart disease. The Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Mori S, et al. Leakage and neuronal uptake of serum protein in aged and Alzheimer brains. A postmortem phenomenon with antemortem etiology. Lab Invest. 1991;64:345–351. [PubMed] [Google Scholar]
- Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Biol Chem. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell VL, et al. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Pakulski C, et al. High subarachnoid block for severe bronchospasm. Eur J Anaesthesiol. 2000;17:594–595. doi: 10.1046/j.1365-2346.2000.00748.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, et al. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- Richmon JD, et al. Induction of heme oxygenase-1 after hyperosmotic opening of the blood-brain barrier. Brain Res. 1998;780:108–118. doi: 10.1016/s0006-8993(97)01314-0. [DOI] [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends in Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00434.x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, et al. Occludin-deficient Embryonic Stem Cells Can Differentiate into Polarized Epithelial Cells Bearing Tight Junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, et al. Possible Involvement of Phosphorylation of Occludin in Tight Junction Formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;13:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Singh M, et al. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, et al. Clostridium perfringens Enterotoxin Fragment Removes Specific Claudins from Tight Junction Strands: Evidence for Direct Involvement of Claudins in Tight Junction Barrier. J Biol Chem. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JM, et al. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Stirone C, et al. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:184–192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Wolburg H, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathologica. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Yaffe K, et al. The Metabolic Syndrome, Inflammation, and Risk of Cognitive Decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Zipser BD, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]