Abstract
The 5-HT3 receptor antagonist, ondansetron, has been shown to correct the auditory gating deficit in medicated schizophrenia patients. Inhibition of 5-HT3 receptors releases acetylcholine, the endogenous ligand for nicotinic acetylcholine receptors. The schizophrenia-related auditory gating deficit is modulated, in part, by nicotinic acetylcholine receptors, as is the mouse (DBA/2) model of the deficit. The present study assessed the effects of both acute and chronically administered ondansetron on auditory gating in DBA/2 mice. Auditory gating is defined as a decrease in amplitude of response to the second of a paired identical auditory stimulus presented 0.5 seconds following an initial auditory stimulus. Acute ondansetron administration at the lowest dose (0.1 mg/kg, IP) tested had no effect, while other doses (0.33 and 1 mg/kg, IP) produced improvements in auditory gating. The improvements were produced through both an increase in response to the first auditory stimulus and a decrease in the response to the second auditory stimulus. Co-administration of an α7 nicotinic acetylcholine receptor antagonist, α-bungarotoxin, or the α4β2 nicotinic acetylcholine receptor antagonist dihydro-β-erythroidine, with the 0.33 mg/kg dose of ondansetron blocked the improvement in auditory gating produced by ondansetron alone. There was no difference in response between the chronically injected mice and naïve mice. Both showed improved auditory gating, thus, demonstrating no “carry over” effect of daily injections. These data demonstrate that indirect stimulation of nicotinic acetylcholine receptors by ondansetron can improve auditory gating parameters in DBA/2 mice.
Keywords: nicotinic acetylcholine receptors, ondansetron, 5-HT3 receptor, DBA/2 mice, auditory gating, schizophrenia
1. Introduction
The serotonergic and cholinergic neurotransmitter systems have both been implicated in the pathophysiology of schizophrenia (Keshavan et al., 2008). The serotonergic system has been of interest since the 1950's after observations that the psychotomimetic effects of LSD, a serotonin receptor partial agonist, produced clinical effects resembling those of schizophrenia (Fischman, 1983; Woolley and Shaw, 1954). Later studies determined a decrease in 5-HT2A receptors in the prefrontal cortex of postmortem brain from subjects with schizophrenia (Burnet et al., 1996; Dean and Hayes, 1996; Laruelle et al., 1993) as well as decreased cortical 5-HT2A receptor mRNA (Burnet et al., 1996; Hernandez and Sokolov, 2000). The majority of serotonin receptors belong to a superfamily of G-protein coupled receptors, however, the 5-HT3 serotonin receptor is a ligand gated ion channel.
The 5-HT3 receptors belong to the same superfamily of ligand gated ion channels as the nicotinic acetylcholine receptors. There is a 30% sequence homology between the 5-HT3 receptor and the α7 nicotinic acetylcholine receptor, possessing the greatest sequence similarity within this superfamily of ion channels (Maricq et al., 1991). Like the 5-HT3 receptor, nicotinic acetylcholine receptors are implicated in the pathophysiology of schizophrenia. Postmortem brain tissue from schizophrenia patients exhibit a decreased density of α7 nicotinic acetylcholine receptors in the CA3 region of the hippocampus and the dentate gyrus as compared to tissue from non-schizophrenia subjects (Breese et al., 1997; Freedman et al., 1995). Furthermore, an endophenotype of the illness, an auditory gating deficit, is linked genetically to chromosome 15q14, which is the locus of the α7 nicotinic acetylcholine receptor gene (Chini et al., 1994; Freedman et al., 1997; Leonard et al., 2002; Orr-Urtreger et al., 1995).
The auditory gating deficit in schizophrenia patients manifests as an inability to inhibit, or filter, the responses to repetitive auditory stimuli. In normal subjects, the ability to filter repetitive auditory information allows for focused attention, while schizophrenia patients report “difficulties in maintaining attention and complain of intrusion of unwanted sensory information” (Adler et al., 1999). Auditory gating is modulated by both the cholinergic and serotonergic systems, among others. The interaction of the serotonergic and cholinergic systems occurs via antagonism of serotonergic receptors. Specifically, antagonism of serotonin 5-HT3 receptors facilitates the release of acetylcholine, purportedly through disinhibition of GABAergic interneurons (Fink and Gothert, 2007). The release of GABA may correct a deficient inhibitory circuit in the hippocampus thus improving the auditory gating deficit (Adler et al., 1998). The increased release of acetylcholine following blockade of 5-HT3 receptors should activate nicotinic acetylcholine receptors, thus improving auditory gating. Therefore, the interaction of these neurotransmitter systems can produce various outcomes on auditory gating.
Auditory gating is assessed by recording the electrical responses of the brain to two identical auditory stimuli separated by 500 milliseconds. In control subjects the amplitude of the response to the second stimulus (test) is smaller than the amplitude of the response to the first stimulus (conditioning). However, in schizophrenia patients the response to the test stimulus is of similar magnitude to the response to the conditioning stimulus (Adler et al., 1982).
The equivalent of the human P50 gating measure in rodents is the P20-N40 waveform complex (Bickford-Wimer et al., 1990; Bickford and Wear, 1995; Simpson and Knight, 1993; Stevens et al., 1998). Although it has been proposed that the P20 and N40 waveform responses vary independently according to the pharmacologic or behavioral manipulation (Connolly et al., 2004; Maxwell et al., 2004; Metzger et al., 2007; Umbricht et al., 2004), the entire complex has been shown to have less variability than either the P20 or N40 component alone (Hashimoto et al., 2005). In rodents the P20-N40 complex has been localized to the dorsal hippocampal CA3 region (Bickford-Wimer et al., 1990). The DBA/2 inbred mouse strain is a model for the auditory gating deficit, because 1) it spontaneously fails to inhibit responses to repetitive auditory stimuli (Stevens et al., 1996) 2) the expression level of α7 nicotinic acetylcholine receptors in the hippocampus of DBA/2 mice is decreased as compared to mice with normal auditory gating (Adams et al., 2001) and 3) activation of α7 receptors produce an improvement in the auditory gating deficit of DBA/2 mice (Stevens et al., 1998). Recently, another subtype of nicotinic acetylcholine receptor, α4β2, has also been implicated in the auditory gating deficit in the DBA/2 mouse model (Metzger et al., 2007; Radek et al., 2006; Wildeboer and Stevens, 2008). Therefore, the DBA/2 mouse serves as a relevant model for studying abnormalities in nicotinic receptor function in relation to deficient auditory gating.
Two 5-HT3 antagonists that produce improvements in auditory gating are tropisetron and clozapine. Tropisetron is an antiemetic and anti-nausea drug used by patients receiving chemotherapy. This compound improves auditory gating in both DBA/2 mice and in subjects with schizophrenia (Hashimoto et al., 2005; Koike et al., 2005). Tropisetron, however, is not only a 5-HT3 antagonist, but is also a partial agonist at the α7 nicotinic acetylcholine receptor (Macor et al., 2001; Papke et al., 2004; Simpson et al., 2000). Thus, the improvement in auditory gating by tropisetron is proposed to be primarily through the α7 receptor (Hashimoto et al., 2005). Clozapine has also been shown to improve auditory gating in both schizophrenia patients and DBA/2 mice (Nagamoto et al., 1996; Simosky et al., 2003). While clozapine lacks direct agonist activity at the α7 receptor, the study by Simosky and colleagues (2003) demonstrated that the improvement in auditory gating with clozapine was produced by activation of nicotinic receptors, presumably through the increased release of hippocampal acetylcholine (Shirazi-Southall et al., 2002). However, it should be noted that clozapine also antagonizes other neuronal receptors including dopaminergic receptors (Fjalland and Boeck, 1978). Another 5-HT3 antagonist, ondansetron, improves the auditory gating deficit in medicated schizophrenia patients (Adler et al., 2005). Like clozapine, ondansetron does not have a physiologically relevant binding affinity for α7 or α4β2 nicotinic acetylcholine receptors (Macor et al., 2001) nor does it display direct agonist activity for nicotinic receptors (Papke et al., 2004). Ondansetron has not yet been tested in DBA/2 mice. The purpose of this study was to determine if ondansetron produces an improvement in auditory gating in DBA/2 mice. We hypothesized that ondansetron would improve auditory gating in DBA/2 animals and that the mechanism of improvement would be via nicotinic acetylcholine receptors.
2. Results
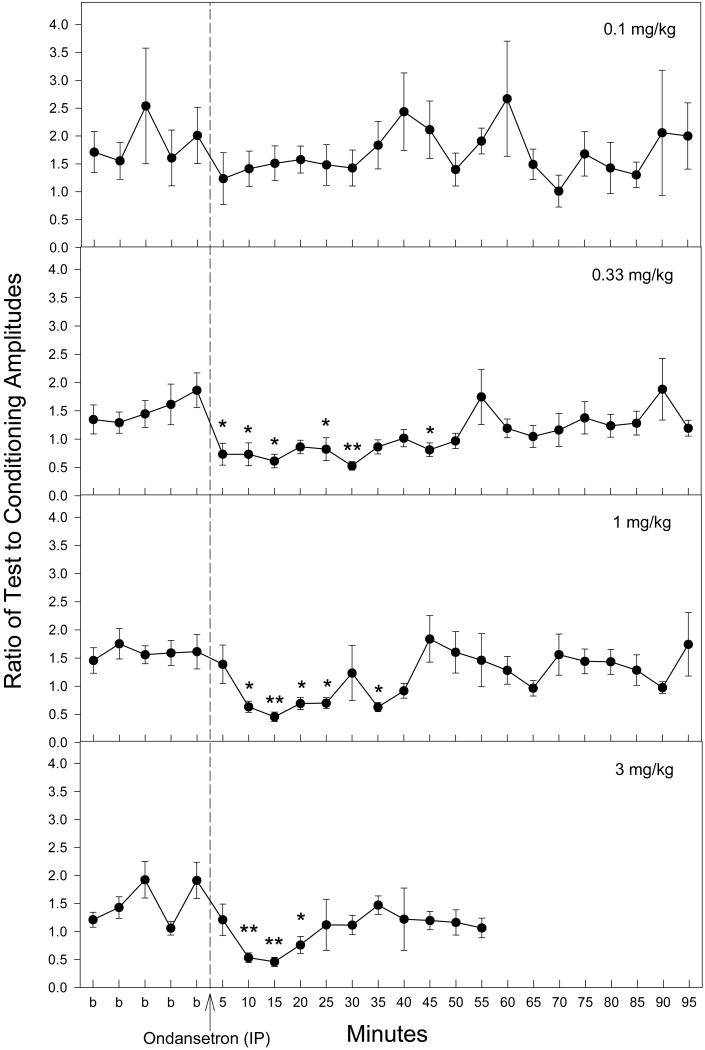
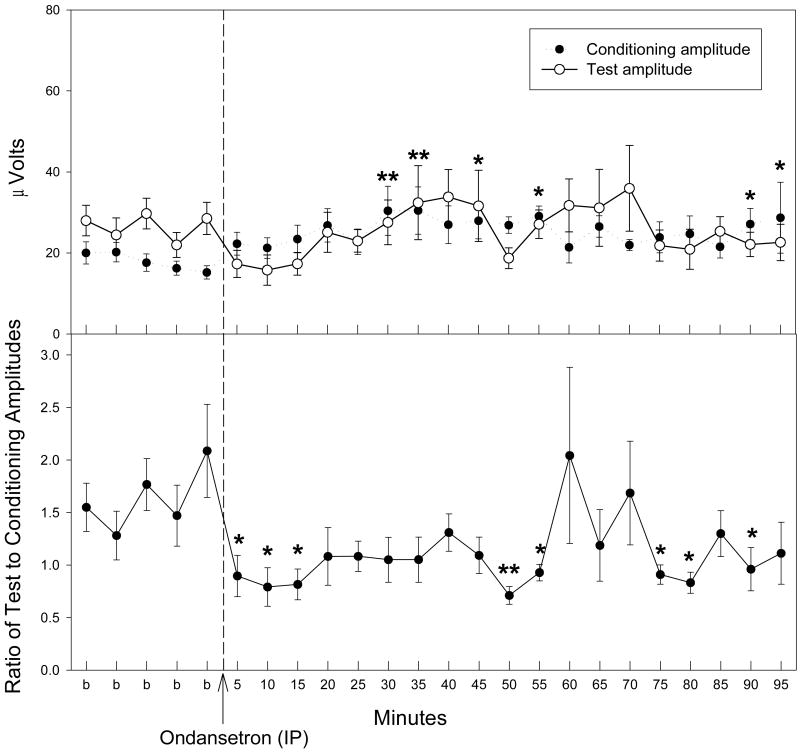
Administration of ondansetron produced improvements in the inhibitory processing of the P20-N40 auditory evoked potential of DBA/2 mice at three of the four doses tested (Fig. 1). The doses selected were based on a previous study in which schizophrenia patients received an acute administration of ondansetron and evoked potentials were measured (Adler et al., 2005). The lowest dose, 0.1 mg/kg IP, produced no significant effects on TC ratios, defined as the ratio of amplitudes of the evoked potential of the test response to that of the conditioning response. The other three doses tested, 0.33, 1, and 3 mg/kg IP produced significant changes on TC ratio as determined by a repeated measures MANOVA [0.03 mg/kg: F(23,161) = 2.50, p < 0.001; 1 mg/kg: F(23,161) = 2.25, p = 0.002; 3 mg/kg: F(15,105) = 2.62, p = 0.002], although the highest dose (3 mg/kg) was actually lethal to several mice at approximately 55 minutes post injection. Fisher's PLSD a posteriori analysis revealed several time points with significant decreases in TC ratio post-ondansetron relative to the average of baseline measurements (Fig. 1).
Figure 1.
TC ratios indicate an improvement in auditory gating following ondansetron administration (IP). Fisher's PLSD determined several time points following ondansetron administration where TC ratios were significantly decreased as compared to the averaged baseline (b) measurement for the corresponding dose. The recording for the 3 mg/kg dose only proceeded for 55 minutes following ondansetron injection due to loss of several animals. Data are mean ± SEM. For 0.1 mg/kg, n = 6; for all other doses, n = 8. *p < 0.05, **p < 0.01.
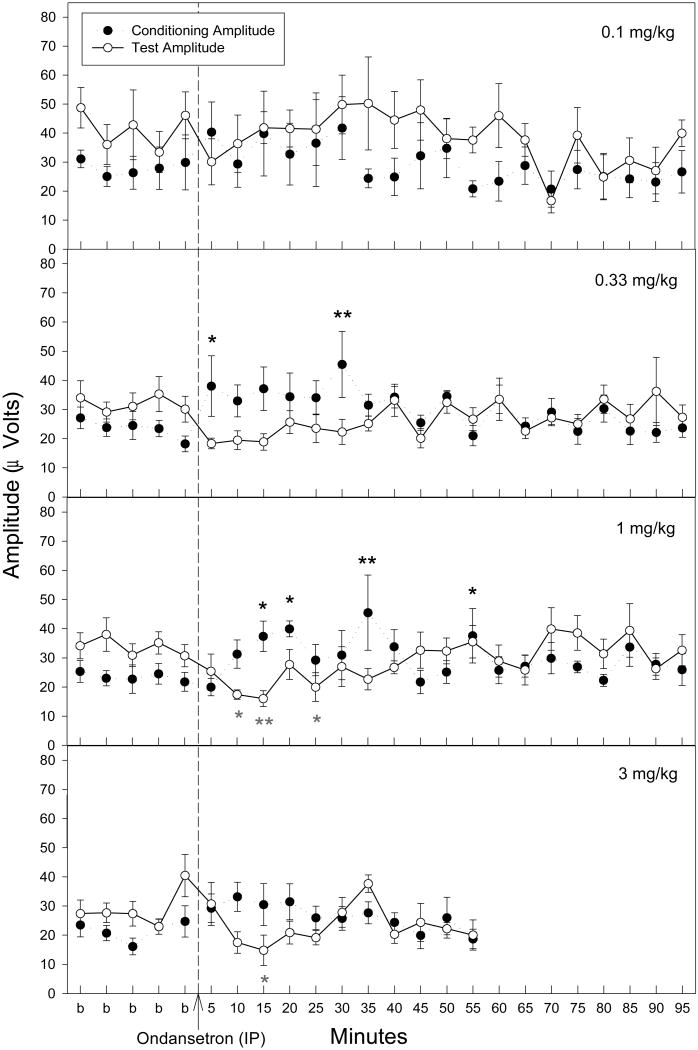
Acute ondansetron administration influenced both the conditioning and test amplitudes. Sample waveforms are displayed in Figure 2. The conditioning amplitude was significantly increased compared to the average of baseline measurements after acute administration of ondansetron at both 0.33 and 1 mg/kg [0.33 mg/kg: F(23,161) = 1.8, p = 0.019; 1 mg/kg: F(23,161) = 1.85, p = 0.015] (Fig. 3). Fisher's PLSD a posteriori analysis indicated that this significant increase in conditioning amplitude occurs at 5 and 30 minutes post-injection for the 0.33 mg/kg dose and at 15, 20, 35 and at 55 minutes post-injection for this 1 mg/kg dose. The 0.1 and 3 mg/kg acute ondansetron doses did not produce significant effects on conditioning amplitude (Fig. 3). The test amplitude was affected following acute administration of ondansetron (Fig. 3). The 1 and 3 mg/kg doses of ondansetron produced a significant effect on test amplitude [1mg/kg: F(23,161) = 1.77, p = 0.022; 3 mg/kg F(15,105) = 2.42, p = 0.005] with significant decreases at several time points following ondansetron injection as determined by Fisher's PLSD a posteriori analysis. Although the 0.33 mg/kg dose of ondansetron did not reach significance with a repeated measures MANOVA (p = 0.077), there was a trend towards a decrease in TAMP for the first three time-points following ondansetron administration (Fig. 3).
Figure 2.
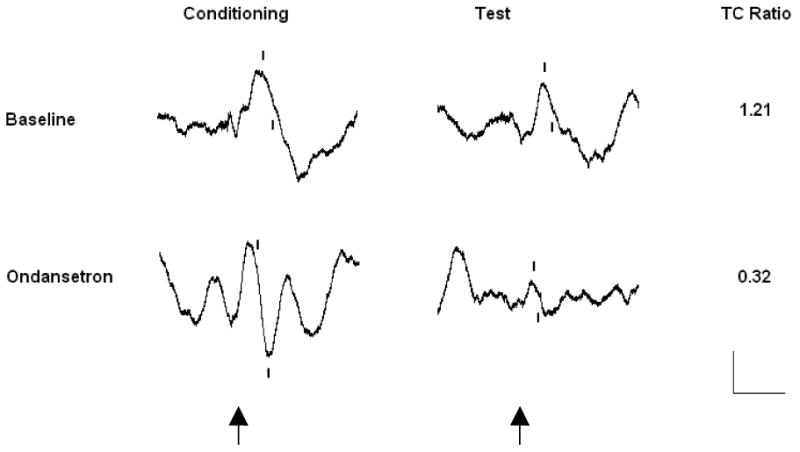
Representative P20-N40 waveforms averaged from an individual DBA/2 mouse. Waveforms obtained prior to drug administration (Baseline) and after 0.33 mg/kg ondansetron administration (Ondansetron). Tick marks denote the P20-N40 wave and stimulus onset is denoted by arrows. Calibration 40 μV/50 ms.
Figure 3.
Conditioning (●) and test (○) amplitudes before and after ondansetron administration. Asterisks indicate time points following ondansetron administration where a significant effect, as determined by Fisher's PLSD, on either the conditioning amplitude (black asterisks) or test amplitude (grey asterisks) were found as compared to an averaged baseline (b) for the corresponding dose. The recording for the 3 mg/kg dose only proceeded for 55 minutes following ondansetron injection. Data are mean ± SEM. For 0.1 mg/kg, n = 6; for all other doses, n = 8. *p < 0.05, **p < 0.01.
Previous experiments included vehicle control administration of saline via IP injection (Hashimoto et al., 2005). The administration of saline failed to produce any change in any auditory gating parameter, therefore, saline control studies were not conducted.
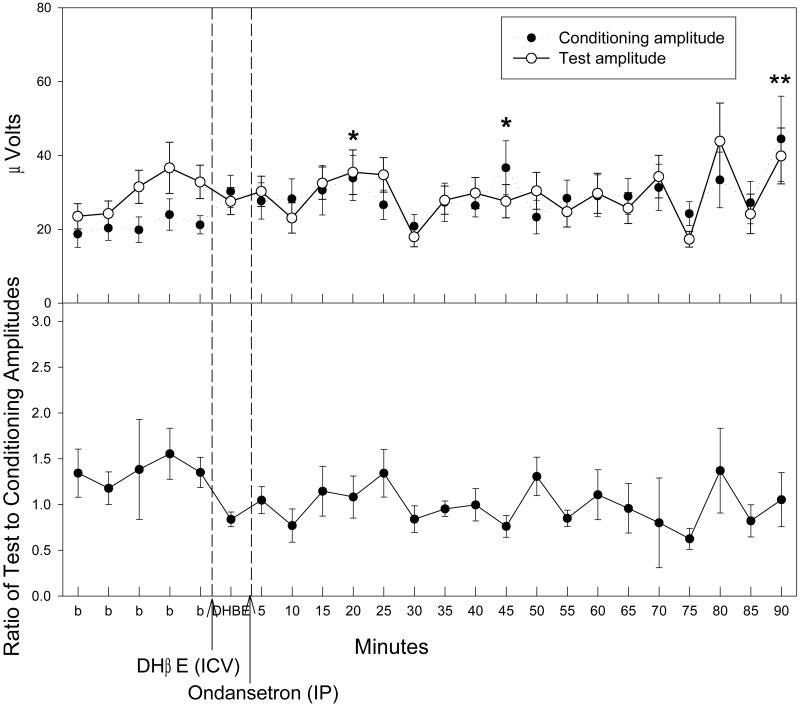
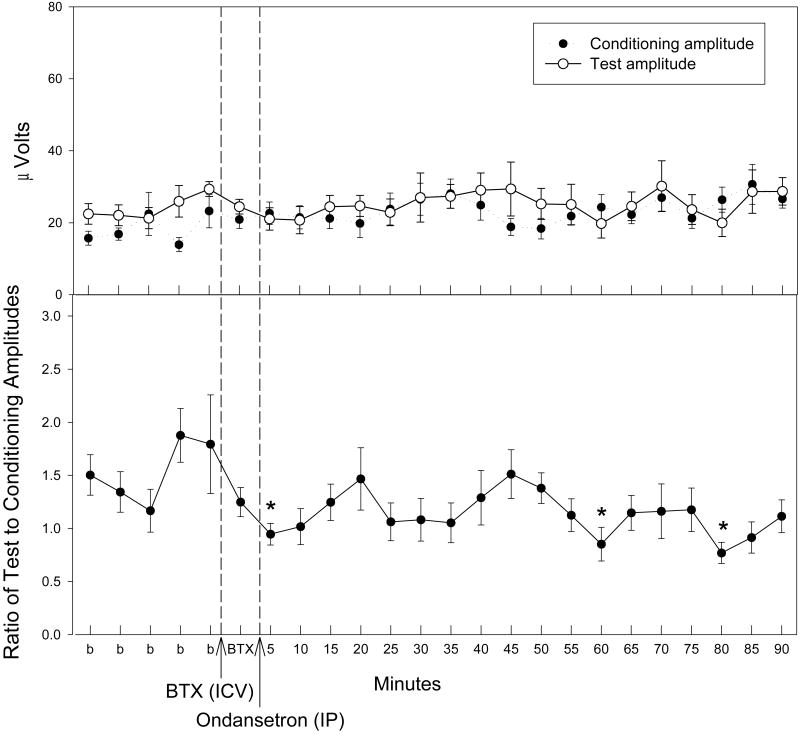
In order to determine the involvement of nicotinic acetylcholine receptors following ondansetron administration, selective antagonists for either the α7 or α4β2 subtype of receptor were administered five minutes prior to the injection of ondansetron following five baseline recordings. Antagonism of the α4β2 subtype by DHβE (30 nM, ICV) prevented significant decreases in TC ratio following ondansetron administration (0.33 mg/kg, IP) (Fig. 4). However, there were significant changes in both test amplitude [F(23,161) = 1.88, p = 0.013] and conditioning amplitude [F(23,161) = 1.88, p = 0.013] following ondansetron administration (Fig. 4). A posteriori analysis revealed several time points in which the conditioning amplitude was significantly increased as compared to the baseline averages post ondansetron injection (Fig. 4). Although the repeated measures MANOVA was significant for the test amplitude, a posteriori analysis revealed no significance at individual time points. Antagonism of the α7 subtype by α-BTX (1.25 nM, ICV) antagonized the effects of both the conditioning and test amplitudes following ondansetron (0.33 mg/kg, IP) administration (Fig. 5). However, there were significant changes in TC ratio following α-BTX and ondansetron administration [F(23,161) = 1.74, p = 0.026]. A Fisher's PLSD a posteriori analysis revealed three time points following antagonism of α7 and administration of ondansetron (0.33 mg/kg, IP) in which the TC ratios were significantly lower than the baseline average (Fig. 5).
Figure 4.
The effect of dihydro-β-erythroidine (DHβE) injection (30 nM, ICV), an α4β2 nicotinic acetylcholine receptor antagonist, on conditioning (●) and test (○) amplitudes (top figure) and TC ratios (bottom figure) prior to and following ondansetron (0.33 mg/kg, IP) administration. Asterisks indicate time points of significantly higher conditioning amplitudes as compared to an averaged baseline (b) determined by Fisher's PLSD. Data are mean ± SEM. n = 8. *p < 0.05, **p < 0.01.
Figure 5.
The effect of α-bungarotoxin (BTX) injection (1.25 nM, ICV), an α7 nicotinic receptor antagonist, on conditioning (●) and test (○) amplitudes (top figure) and TC ratio (bottom figure) prior to and following ondansetron (0.33 mg/kg, IP) administration. Asterisks indicate time points of significantly lower TC ratios as compared to an averaged baseline (b) determined by Fisher's PLSD. Data are mean ± SEM. n = 8. *p < 0.05.
Chronic daily administration of ondansetron also produced significant effects on the conditioning amplitude and TC ratios. The six day daily- injection regimen was based upon a 1-week repeated-dosing pilot study in schizophrenia patients conducted by Dr. Larry Adler at the Veterans Affairs Medical Center in Denver, CO (personal communication Dr. Merilyne Waldo). Ondansetron (0.33 mg/kg, IP) was administered once daily for six consecutive days. On the seventh day auditory gating measurements were performed. Following baseline recordings, a seventh and final injection of ondansetron was administered. A repeated measures MANOVA of the TC ratio for pre- and post-injection analyses was significant [chronic 0.33 mg/kg: F(23,161) = 1.72, p = 0.029] (Fig. 6). Fisher's PLSD a posteriori analysis revealed several time points, both immediately following injection and at later time points in the recording, which were significantly decreased as compared to pre-drug baseline measurements. Although the final injection of 0.33 mg/kg ondansetron had no significant impact on the test amplitude it did significantly impact the conditioning amplitude as determined by a repeated measures MANOVA [chronic 0.33 mg/kg: F(23,161) = 1.71, p = 0.029] (Fig. 6). Fisher's PLSD a posteriori analysis revealed time points of the conditioning amplitude in the middle and end of the recording which were significantly increased as compared to the baseline recording.
Figure 6.
The effect of chronic ondansetron administration (0.33 mg/kg/day, IP) 6 days prior to the auditory gating measurement on the 7th day of ondansetron administration. There was a significant difference in the conditioning amplitude (●) (top figure) with asterisks indicating specific time points at which the conditioning amplitude was significantly higher as compared to an averaged baseline (b) as determined by Fisher's PLSD. The TC ratio also proved to be significantly different following the 7th daily administration of ondansetron (bottom figure). Asterisks mark specific time points following drug administration where TC ratios were significantly decreased as compared to an averaged baseline (b) determined by Fisher's PLSD. Data are mean ± SEM. n = 8. *p < 0.05, **p < 0.01.
In order to verify that there was no carry-over effect of daily ondansetron administration (IP) an ANOVA was performed on the baseline TC ratios between animals that received daily injections (chronic 0.33 mg/kg) and animals that received only one IP injection (acute 0.33 mg/kg). The ANOVA revealed no significant between-subjects effect in baseline recordings of the 2 groups of animals F(1,14) = 0.30, p = 0.594].
3. Discussion
The goal of the present study was to determine if the 5-HT3 antagonist ondansetron produces an improvement in auditory gating in DBA/2 mice and if the improvement is mediated by a nicotinic receptor mechanism. Although ondansetron is not a nicotinic receptor agonist, we hypothesized that indirect activation of nicotinic receptors by acetylcholine, released following inhibition of 5-HT3 receptors, would result in improved auditory gating. Our results indicate that three (0.33, 1, and 3 mg/kg) of the four doses of ondansetron tested produced a significant improvement in auditory gating of DBA/2 mice as measured by TC ratios. The significant effects on TC ratios are reflective of both increases in conditioning amplitude and decreases in test amplitude. Previous studies in our laboratory confirm that vehicle control injections to DBA/2 mice produce no effect upon TC ratio (Hashimoto et al., 2005), indicating that the results observed are produced by administration of ondansetron. The effects on the test amplitude have been proposed to be mediated via α7 nicotinic acetylcholine receptor activation as demonstrated with the α7 selective partial agonist DMXB-A (Stevens et al., 1998). A growing body of literature indicates modulation of the conditioning amplitude, or response, by α4β2 agonists (Metzger et al., 2007; Phillips et al., 2007; Radek et al., 2006; Stevens and Wear, 1997; Wildeboer and Stevens, 2008). A recent study with β2-nicotinic receptor knockout mice on a C57BL/6 background reported a difference in auditory-event-related potentials of the knockout versus wild-type mice following nicotine administration (Rudnick et al., 2009). This study measured effects on P20 and N40 responses independently and determined that β2-knockout animals displayed an increase in P20 with no effect on N40, whereas wild-type mice displayed an increase in P20 and a decrease in N40. This effect was observed during the conditioning response only. The effect on the conditioning response is consistent with our observed results. However, we utilized DBA/2 mice whereas the previous study utilized C57BL/6. Also, we studied an anesthetized preparation whereas the previous study recorded auditory event-related potentials from awake animals. Despite the difference in methodology, both our study and Rudnick et al. (2009) support the notion that β2-containing nicotinic receptors modulate auditory gating. The release of acetylcholine following ondansetron administration would be expected to act on both nicotinic receptor subtypes in the hippocampus, where the auditory gating recording occurs. Thus, as predicted, significant effects on both the test and conditioning amplitudes were observed.
These data parallel the results observed by Adler et al. (2005) in which acute ondansetron administration improved deficient auditory gating of medicated schizophrenia patients. However, in that study, the involvement of nicotinic receptors was not examined. To understand the possible involvement of α7 and α4β2 nicotinic receptors in the present study, antagonists for each subtype were administered separately to animals five minutes prior to ondansetron administration. Any effect of ondansetron, either directly or indirectly, on α7 or α4β2 receptors would be inhibited by the nicotinic receptor antagonists. Our results indicate that the significant improvements in auditory gating following ondansetron administration were prevented by the α4β2 antagonist DHβE, demonstrating involvement of the α4β2 nicotinic acetylcholine receptor. The α7 antagonist, α-BTX, prevented the significant decreases in TC ratio, with the exception of three discrete time points (5, 60 and 80 minutes). The significant improvements in gating at the 60 and 80 may be due to normal variations in animal gating or it is possible that choline, resulting from the degradation of acetylcholine by acetylcholinesterase, was activating α7 receptors thus producing the significant decreases in TC ratio at the later time points. It is also possible that activation of muscarinic receptors by acetylcholine, following ondansetron administration, produced an effect on auditory gating. Although a previous study determined that scopolamine, a non-subtype selective muscarinic receptor antagonist, does not alter the auditory gating of anesthetized rats (Luntz-Leybman et al., 1992) it has yet to be determined if muscarinic agonists can improve the gating deficit. Therefore, in addition to the nicotinic receptor mediated effects on auditory gating, muscarinic receptors may also be involved following ondansetron administration.
The terminal half-life of ondansetron at 1 mg/kg intravenous in rats has been reported as 24.4 ± 4.91 minutes (Yang and Lee, 2008). Assuming the half-life in mice is similar to that of rats, the effects of ondansetron on TC ratio should be observed through the 25 minute time point for the 1 mg/kg dose with effects possibly lasting longer due to the release and degradation of acetylcholine, as well as possible effects upon muscarinic receptors. In this study the significant effects of the 1 mg/kg dose of ondansetron on TC ratio was observed up to the 35 minute time point. At the lower dose of 0.33 mg/kg the significant effects on TC ratio are observed up to 45 minutes post-injection. The highest dose tested, 3 mg/kg, produced significant effects on TC ratio as late as 20 minutes post-injection. The 3 mg/kg dose also proved to be lethal in several of the anesthetized DBA/2 mice. The data for the 3 mg/kg dose is only presented through the 55 minute post-injection time point as to include the average for all 8 animals tested. A previous report cited a dose of 5 mg/kg and higher as highly toxic in DBA/2 mice (Hendrie, 1990). Although a lower dose was utilized for this study toxicity was still observed.
Ondansetron is not an agonist for the α7 or α4β2 nicotinic acetylcholine receptor. However, the current data demonstrate that the α7 and α4β2 receptors are involved in the effect of ondansetron upon auditory gating because inhibition of either receptor subtype prevented the significant decreases in TC ratio following ondansetron administration. The results from this current study conflict with a previous study in which ondansetron co-administered with acetylcholine dose-dependently inhibited the activation of α7 and α4β2 nicotinic receptors expressed in Xenopus oocytes (Papke et al., 2004). The disparity in outcomes is likely due to differences in receptor system expression. The present study utilized an in vivo system whereas Papke et al. (2004) utilized an in vitro method of studying nicotinic receptor function. The Papke study also utilized α7 receptor subunit clones from rat and α4β2 subunit clones from human, whereas the present study utilized mice.
There appear to be no lasting changes on auditory gating following chronic administration of ondansetron; the baseline records following six days of daily ondansetron administration were not different from the baselines of naïve mice. In other words, daily IP injection over the course of six days did not appear to alter the efficacy of the seventh injection, suggesting there was neither desensitization of receptors nor any “carry over” effect of the prior injections. However, the method of chronic administration was daily IP injections. Experiments involving 7-day osmotic mini-pump implants (0.5 μl/hour) were carried out to determine how 24-hour, 7-day a week exposure to ondansetron affected auditory gating (data not shown). Several concentrations, 0.33, 1, 3 and 7.5 mg/ml were tested but no significant differences in TC ratio, or test and conditioning amplitudes were found during evoked potential recordings on the seventh day following pump implantation. Chronic administration of vehicle control via osmotic mini-pump has been previously performed in our laboratory (unpublished data) with no effects upon any auditory gating parameter, therefore, saline control groups were not included in this study. A possible explanation for the difference may be that the peak circulating levels of ondansetron via IP injections is greater than that of osmotic mini-pump administration. It is also possible that a desensitization of serotonergic or nicotinic receptors occurs due to a consistent presence of ondansetron in vivo. Overall, the metabolism and clearance of ondansetron during the 24 hours between IP injections appears to be necessary for observed improvement in auditory gating of DBA/2 mice.
As previously published, a 5-HT3 antagonist, tropisetron, has been demonstrated to improve auditory gating in DBA/2 mice, a model for the auditory gating deficit in schizophrenia patients (Hashimoto et al., 2005). However, tropisetron functions not only as a 5-HT3 antagonist but also as a partial agonist for α7 nicotinic acetylcholine receptors. The ability of ondansetron to improve auditory gating indicates the possibility of improved gating via a pathway other than administration of direct nicotinic receptor agonists (e.g. nicotine). It also further supports the involvement and interaction of several neurotransmitter systems in the pathophysiology of schizophrenia. Thus, the cholinergic system can mediate the ability to gate auditory stimuli but the manipulation of other transmitter systems may have similar effects on gating by affecting processes upstream in the pathway.
4. Experimental Procedure
2.1 Animals
Male DBA/2 mice (20-25 g) were obtained from Harlan Sprague Dawley (Indianapolis, IN) and housed five to a cage at the Center for Comparative Medicine (CCM) at the University of Colorado Denver, School of Medicine (UCD-SOM) or the Veterans Affairs Medical Center (VAMC), Denver, CO. All experiments were carried out at the facility where the mice were housed. The mice were provided water and food (Harlan Teklad, Indianapolis, IN) ad libitum. Lighting was cycled at 12 hour intervals (lights on at 6:00 AM). Approximately one quarter of the procedures were carried out at UCD-SOM with the remaining performed at VAMC. Animals in CCM were housed in ventilated cage rack while animals in VAMC were housed in static microisolater cages with filtration covers. All animal procedures were approved by the Institutional Animal Care and Use Committee of the VAMC and UCD-SOM and conform to the Principles of Laboratory Animal Care (Institute of Laboratory Animal Resources, 1996).
2.2 Surgery
For electrophysiological recording of auditory gating, mice were anesthetized by intraperitoneal (IP) injection of the anesthetic chloral hydrate (400 mg/kg) followed by an injection (IP) of pyrazole (400 mg/kg) to retard the metabolism of chloral hydrate. Once surgical plane of anesthesia was attained, mice were placed in a Kopf stereotaxic instrument (Kopf Instruments, Tujunga, CA) on a heating pad (35° C) to maintain a stable core temperature. Hollow earbars, attached to miniature earphones connected to an audio amplifier, were placed adjacent to the externalization of the aural canal. During recording, chloral hydrate and pyrazole were supplemented as necessary (5 mg/kg, IP) to maintain a surgical plane of anesthesia as evidenced by lack of reflexive limb withdrawal in response to toe pinch.
The scalp was incised and burr holes drilled over the over the dorsal CA3 region of the hippocampus [-1.8 mm posterior from bregma, +2.7 mm lateral from midline (Paxinos and Franklin, 2001) and the contralateral anterior cortex. The recording electrode, a Teflon-coated stainless-steel cut wire (0.127 mm diameter), was inserted into the CA3 pyramidal cell layer of the hippocampus (1.5 to 1.7 mm ventral from the dorsal brain surface). Final placement of the recording electrode was determined by the presence of complex action potentials typical of hippocampal pyramidal neurons (Miller et al., 1992). The reference electrode, identical to the recording electrode, was placed on dura through the burr hole over the cortex. Electrical responses were amplified 1000 times with analog to digital conversion (SciWorks, DataWave, Berthoud, CO) and averaged by computer.
2.3 Experimental Protocol
Tones (3000 Hz, 10 ms, 70 dB), as auditory stimuli, were presented in pairs separated by a 500 millisecond interval and 10 seconds between pairs of stimuli. Responses to 16 pairs of tones were filtered with a bandpass between 10 and 5000 Hz. The N40 wave was defined as the maximum negativity between 20 and 60 milliseconds after stimulus onset and measured relative to the proceeding positivity, the P20 wave. The measure of the animals' auditory inhibition is reported as a TC ratio. The TC ratio is defined as the ratio of the amplitudes of the response to the second tone, the test amplitude (TAMP), to the response to the first tone, the conditioning amplitude (CAMP). A decrease in TC ratio after drug administration, as compared to pre-drug baseline indicates improved inhibition of auditory processing. Five baseline records, at 5-minute intervals, were obtained prior to compound administration.
Ondansetron was dissolved in 0.9% NaCl and administered IP at four doses (0.1 mg/kg, n = 6; 0.33 mg/kg, n = 8; 1 mg/kg, n = 8; 3 mg/kg, n = 8). After injection, recordings continued for up to 95 minutes, at 5 minute intervals. For chronic administration studies, daily IP injections of 0.33 mg/kg were administered for 6 continuous days. On the 7th day mice were tested for effects on auditory gating. Following five baseline recordings each animal received a final (7th) IP injection of 0.33 mg/kg ondansetron (n = 8).
Antagonist experiments involving dihydro-β-erythroidine (DHβE) or α-bungarotoxin (α-BTX) required a third burr hole to be drilled over the anterior lateral ventricle (+0.8 mm anterior from bregma, -0.5 mm lateral from midline (Paxinos and Franklin, 2001)) ipsilateral to the recording electrode for placement of an injection cannula. A 26-gauge needle attached to a 10 μl Hamilton syringe (Hamilton, Reno, NV) was inserted into the anterior lateral ventricle (2.0 mm below the dura) for intracerebroventricular (ICV) administration of antagonist. Either 30 nM DHβE or 1.25 nM α-BTX was administered (1μl) following baseline recordings (Simosky et al., 2003). After injection of either antagonist, one record at 5 minutes was obtained then followed by an injection of 0.33 mg/kg IP ondansetron. Records at 5-minute intervals were obtained for 90 minutes of records, thereafter. Following each antagonist experiment, the mice were decapitated under anesthesia and the brains were removed to verify cannula placement.
2.4 Compounds
Ondansetron hydrochloride dihydrate was obtained from Sigma (St. Louis, MO), α-BTX and DHβE hydrobromide were obtained from Tocris (Ellisville, MO). Both ondansetron and DHβE dosing were based on salt weights. α-BTX dosing was according to the free base weight. All compounds were dissolved in 0.9% NaCl.
2.5 Statistical analysis
The time course of ondansetron alone or in conjunction with antagonist was analyzed, for each dose, using repeated measures MANOVA. A significant p-value obtained from a MANOVA was followed by Fisher's protected least-significant difference (PLSD) a posteriori analysis to compare individual post injection time points to collapsed average baseline values.
Acknowledgments
This study was supported by NIH R01 MH 73725 (K.E.S.), a T32 MH15442 institutional postdoctoral research training grant (K.M.W.), and research funds from the Developmental Psychobiology Endowment Fund at the University of Colorado Denver (K.M.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Literature References
- Adams CE, Stitzel JA, Collins AC, Freedman R. Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–90. doi: 10.1016/s0006-8993(01)03115-8. [DOI] [PubMed] [Google Scholar]
- Adler LE, Cawthra EM, Donovan KA, Harris JG, Nagamoto HT, Olincy A, Waldo MC. Improved p50 auditory gating with ondansetron in medicated schizophrenia patients. Am J Psychiatry. 2005;162:386–8. doi: 10.1176/appi.ajp.162.2.386. [DOI] [PubMed] [Google Scholar]
- Adler LE, Freedman R, Ross RG, Olincy A, Waldo MC. Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry. 1999;46:8–18. doi: 10.1016/s0006-3223(99)00085-2. [DOI] [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–54. [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol Psychiatry. 1990;27:183–92. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Wear KD. Restoration of sensory gating of auditory evoked response by nicotine in fimbria-fornix lesioned rats. Brain Res. 1995;705:235–40. doi: 10.1016/0006-8993(95)01157-9. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, Demasters BK, Freedman R, Leonard S. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–98. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15:442–55. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S. Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHRNA7) Genomics. 1994;19:379–81. doi: 10.1006/geno.1994.1075. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–88. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Dean B, Hayes W. Decreased frontal cortical serotonin2A receptors in schizophrenia. Schizophr Res. 1996;21:133–9. doi: 10.1016/0920-9964(96)00034-5. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- Fischman LG. Dreams, hallucinogenic drug states, and schizophrenia: a psychological and biological comparison. Schizophr Bull. 1983;9:73–94. doi: 10.1093/schbul/9.1.73. [DOI] [PubMed] [Google Scholar]
- Fjalland B, Boeck V. Neuroleptic blockade of the effect of various neurotransmitter substances. Acta Pharmacol Toxicol (Copenh) 1978;42:206–11. doi: 10.1111/j.1600-0773.1978.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–92. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology (Berl) 2005;183:13–9. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Hendrie CA. The 5HT3 antagonist GR38032F is highly toxic in DBA/2 mice. Psychopharmacology (Berl) 1990;101:429–30. doi: 10.1007/BF02244065. [DOI] [PubMed] [Google Scholar]
- Hernandez I, Sokolov BP. Abnormalities in 5-HT2A receptor mRNA expression in frontal cortex of chronic elderly schizophrenics with varying histories of neuroleptic treatment. J Neurosci Res. 2000;59:218–25. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, C. o. L. S., National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: What we know in 2008 Part 3: Neurobiology. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Koike K, Hashimoto K, Takai N, Shimizu E, Komatsu N, Watanabe H, Nakazato M, Okamura N, Stevens KE, Freedman R, Iyo M. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1993;50:810–8. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–96. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–6. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001;11:319–21. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–7. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004;29:739–46. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of nicotine vary across two auditory evoked potentials in the mouse. Biol Psychiatry. 2007;61:23–30. doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Miller CL, Bickford PC, Luntz-Leybman V, Adler LE, Gerhardt GA, Freedman R. Phencyclidine and auditory sensory gating in the hippocampus of the rat. Neuropharmacology. 1992;31:1041–8. doi: 10.1016/0028-3908(92)90106-y. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R. Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry. 1996;40:181–8. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Seldin MF, Baldini A, Beaudet AL. Cloning and mapping of the mouse alpha 7-neuronal nicotinic acetylcholine receptor. Genomics. 1995;26:399–402. doi: 10.1016/0888-7543(95)80228-e. [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK, Rose GM. Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett. 2004;14:1849–53. doi: 10.1016/j.bmcl.2003.09.104. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego: 2001. [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–23. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacology (Berl) 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Rudnick ND, Koehler C, Picciotto MR, Siegel SJ. Role of beta2-containing nicotinic acetylcholine receptors in auditory event-related potentials. Psychopharmacology (Berl) 2009;202:745–51. doi: 10.1007/s00213-008-1358-6. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–94. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–96. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Knight RT. Multiple brain systems generating the rat auditory evoked potential. II. Dissociation of auditory cortex and non-lemniscal generator systems. Brain Res. 1993;602:251–63. doi: 10.1016/0006-8993(93)90690-o. [DOI] [PubMed] [Google Scholar]
- Simpson K, Spencer CM, McClellan KJ. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2000;59:1297–315. doi: 10.2165/00003495-200059060-00008. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–62. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–7. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57:869–74. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D'Adamo P, Lipp HP. Midlatency auditory event-related potentials in mice: comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Wildeboer KM, Stevens KE. Stimulation of the alpha4beta2 nicotinic receptor by 5-I A-85380 improves auditory gating in DBA/2 mice. Brain Res. 2008;1224:29–36. doi: 10.1016/j.brainres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. A Biochemical and Pharmacological Suggestion About Certain Mental Disorders. Proc Natl Acad Sci U S A. 1954;40:228–31. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos. 2008;29:414–26. doi: 10.1002/bdd.628. [DOI] [PubMed] [Google Scholar]