Figure 2.
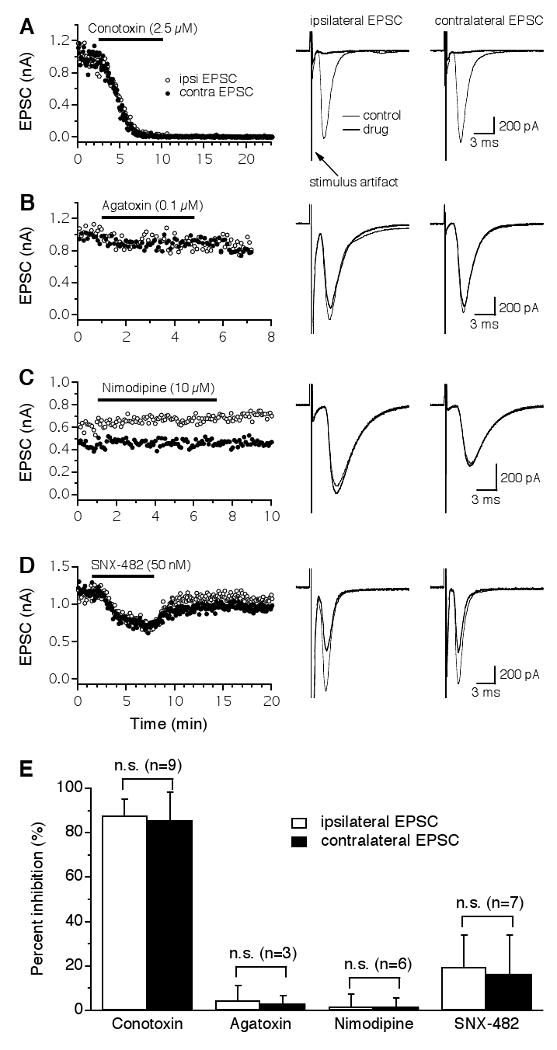
Glutamatergic transmission in the NL is predominantly triggered by Ca2+ entry through N-type VGCCs. A: Both the ipsilateral (open circles) and the contralateral (filled circles) EPSCs recorded from the same NL neuron were largely and irreversibly blocked by the N-type blocker ω-Conotoxin-GVIA (ω-CTx-GVIA, 2.5 μM), with nearly identical time courses. Averaged traces under control (thin traces) and drug (thick traces) conditions are shown on the right. Stimulus artifacts are truncated for clarity. B-C: No inhibition of EPSCs was produced by the P/Q-type blocker ω-Agatoxin-IVA (ω-Aga-IVA, 0.1 μM) or the L-type blocker nimodipine (10 μM). D: A small and reversible inhibition of the EPSCs by the R-type blocker SNX-482 (50 nM) was observed. The different time courses of the EPSCs observed in the sampled neurons may be due to different locations of the neurons along the tonotopic frequency axis. E: Summary of the effects of different VGCC blockers on EPSCs of NL neurons. ω-CTx-GVIA inhibited the ipsilateral and the contralateral EPSCs by 88% and 86%, respectively. Blockers for P/Q- and L-type VGCCs had no effects on EPSCs. SNX-482 produced a small inhibition with a large variation among cells. No differences in the percent inhibition by either drug were detected between the ipsilateral and the contralateral EPSCs. Error bars represent standard deviations. n.s.: non-significant (paired t-test, p > 0.05; the number of cells is indicated in parentheses).
