Abstract
Manganese superoxide dismutase (MnSOD) is vital to the protection of mitochondria and cells against oxidative stress. Earlier, we demonstrated that catalytically active homotetramer of MnSOD can be stabilized by oxidative cross-linking. Here we report that this effect may be translated into increased radioresistance of mouse embryonic cells (MECs) by pre-exposure to oxidative stress. Pre-treatment of MECs with antimycin A, rotenone or H2O2 increased their survival after irradiation. Using MnSOD siRNA, we show that MECs with decreased MnSOD levels displayed a lowered ability to preconditioning. Thus oxidative preconditioning may be used for targeted regulation of MnSOD.
Keywords: apoptosis, MnSOD, irradiation, preconditioning, ROS
Introduction
Mitochondrial damage realized through superoxide dependent pathways plays a critical role in radiation injury to cells and tissues [1]. These effects of superoxide and other superoxide-derived reactive oxygen species on mitochondria may be realized immediately due to irradiation (IR) induced radiolysis of water, as well as at later time during the mitochondrial stage of apoptotic response [2]. Manganese superoxide dismutase (MnSOD) provides the first line of defense against superoxide overproduced in the mitochondria [3]. Post-irradiation generation of reactive oxygen and nitrogen species, likely associated with the formation of peroxynitrite, may lead to inactivation of MnSOD [4]. Notably, MnSOD confined to mitochondria - but not MnSOD genetically manipulated to be in the cytosol - is radioprotective [5]. Moreover, treatment with MnSOD plasmid has been shown to improve survival of mice exposed to total body irradiation (9.5Gy) [6].
Adaptive response, or preconditioning, allowing to avoid lethal effects of radiation exposures, has been observed in cell-survival studies from yeast to mammalian and human cells in vitro as well as animal models in vivo [7]. The precise mechanisms by which preconditioning occurs are unknown. Recently, we have shown that treatment of recombinant human (rh)MnSOD with oxidants (hydrogen peroxide (H2O2) plus horseradish peroxidase or Cu2+) induced formation of di-tyrosine cross-linked high molecular weight oligomers with intact activity and increased resistance to unfolding, degradation, and peroxynitrite mediated inactivation [8]. Based on this, we hypothesized that oxidative modification of MnSOD represents a novel radioprotective strategy that may be realized in a preconditioning paradigm. Here we show that short-term pretreatment of cells with pro-oxidant inhibitors of mitochondrial complexes I and III – rotenone or antimycin A (AA) – caused protection against IR-induced cell death. We further demonstrate the involvement of MnSOD oxidative modification in the protective effect.
Materials and methods
Reagents
Annexin-V apoptosis detection kit was purchased from BioVision (Mountain View, CA). Caspase-3/7 activity kit was obtained from Promega (Madison, WI). siRNA and transfection reagents were from Invitrogen (Carlsbad, CA). Other reagents were purchased from Sigma (St.Louis, MO).
Cell culture
Mouse embryonic cells (MECs, courtesy of Dr.X.Wang, University of Texas, Dallas) were cultured in DMEM media supplemented with 15% fetal bovine serum, 25mM of HEPES, 50mg/L of uridine, 110mg/L of pyruvate, 2mM of glutamine, 1×nonessential amino acids, 0.05mM of β-mercaptoethanol, 0.5×106U/L of mouse leukemia inhibitory factor, 100U/L of penicillin, and 100µg/L of streptomycin.
Treatment and irradiation
Cells (0.5–2×105) were seeded in 35 or 60mm cell culture dishes and left to attach overnight. Cells were incubated with AA, rotenone or H2O2 in complete culture medium before IR (30min) and were γ-irradiated using a Shepherd model 143-45A irradiator (J.L. Shepherd & Associates, San Fernando, CA) at a dose-rate of 4Gy/min. Then cells were washed twice and medium was replaced with fresh one.
Caspase activity in cell lysates was measured with a Caspase-Glo-3/7 assay kit. Chemiluminescence was determined at 25°C with ML1000 plate reader (Dynatech Laboratories, Chantilly, VA) and activity was expressed as luminescence intensity per milligram protein produced within 1h incubation.
Cell viability was determined with propidium iodide (PI). For assessments of phosphatidylserine (PS) externalization Annexin V–FITC (plus PI) was employed. Both measurements were performed on FACScan flow cytometer (Beckton-Dickinson, Franklin Lakes, NJ). PS externalization was assessed as the sum of Annexin V(+) cells with PI(−) and PI(+) cells.
Cell morphology
MECs were grown on collagen-coated glass cover slips prior to AA treatment and IR. At 48h after irradiation, cells were fixed with paraformaldehyde (4%), stained with Hoechst3343 (2µg/ml) for 15min followed by fluorescence and visible light microscopy to visualize the morphological characteristics associated with apoptotic cells, including blebs, nuclear breakdown and heterochromatin aggregation [9].
MnSOD activity
Prior to measurements, cells were ruptured by sonication in PBS containing a protease inhibitor cocktail. MnSOD activity was measured using cytochrome c reduction method of McCord and Fridovich [10]. Samples were preincubated with 5mM KCN for 45min at RT to deactivate Cu,Zn-SOD.
MnSOD zymography and Western blotting
Cells were collected and lysed in RIPA buffer with protease inhibitors on ice for 45min, centrifuged at 10,000g for 5min and the supernatants were collected for electrophoresis. For zymography, samples were incubated in Laemmli buffer containing 1.5% SDS and 100mM DTT for 15min, and 30µg of protein was loaded onto a 10% native gel and a modified method of Beauchamp and Fridovich [11] was used to visualize SOD activities. Briefly, the gel was thoroughly washed in PBS then incubated with 0.23mM nitro-blue tetrazolium for 15min at RT. The gel was again washed 4×5min in PBS. Finally, the gel was soaked in the dark in a solution containing 28mM TEMED and 2.8×10−2mM riboflavin for another 15min. Then gel was washed again as previously, and exposed to light. For Western blot analysis samples (20µg protein per lane) were heated for 5min at 100°C, subjected to 12% SDS-PAGE and transferred to a nitrocellulose membrane, which was probed with antibodies against MnSOD or actin, followed by horseradish peroxidase–coupled detection. Typical gels representative of 3 experiments are shown.
siRNA transfection
One day before transfection, MECs were plated in growth medium without antibiotics such that they were 30–50% confluent at the time of transfection. Transfection was carried for 6 h. After that, the medium was removed and replaced with normal culture medium.
Statistical analysis
Data are expressed as means±SD (SE when indicated) of at least three experiments. Changes in variables were analyzed by paired t-test or ANOVA. Differences were considered significant at p<0.05.
Results
Irradiation-induced cell death and oxidative preconditioning of MECs
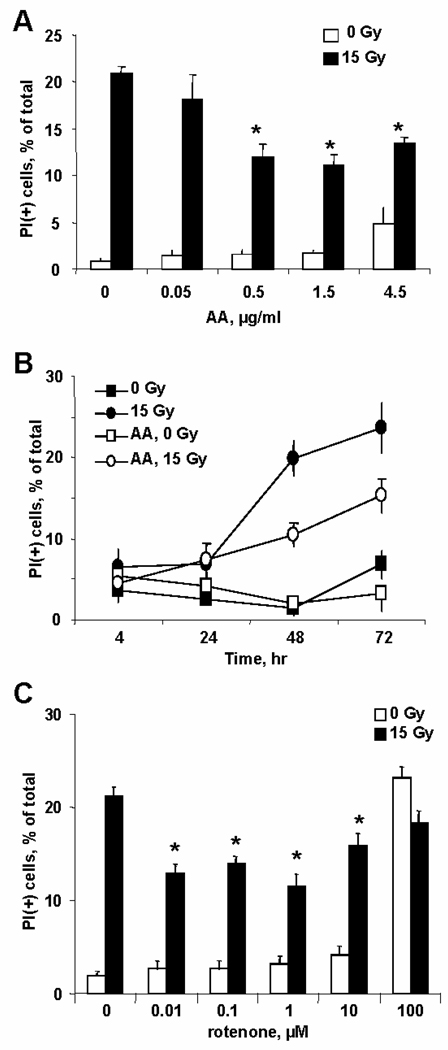
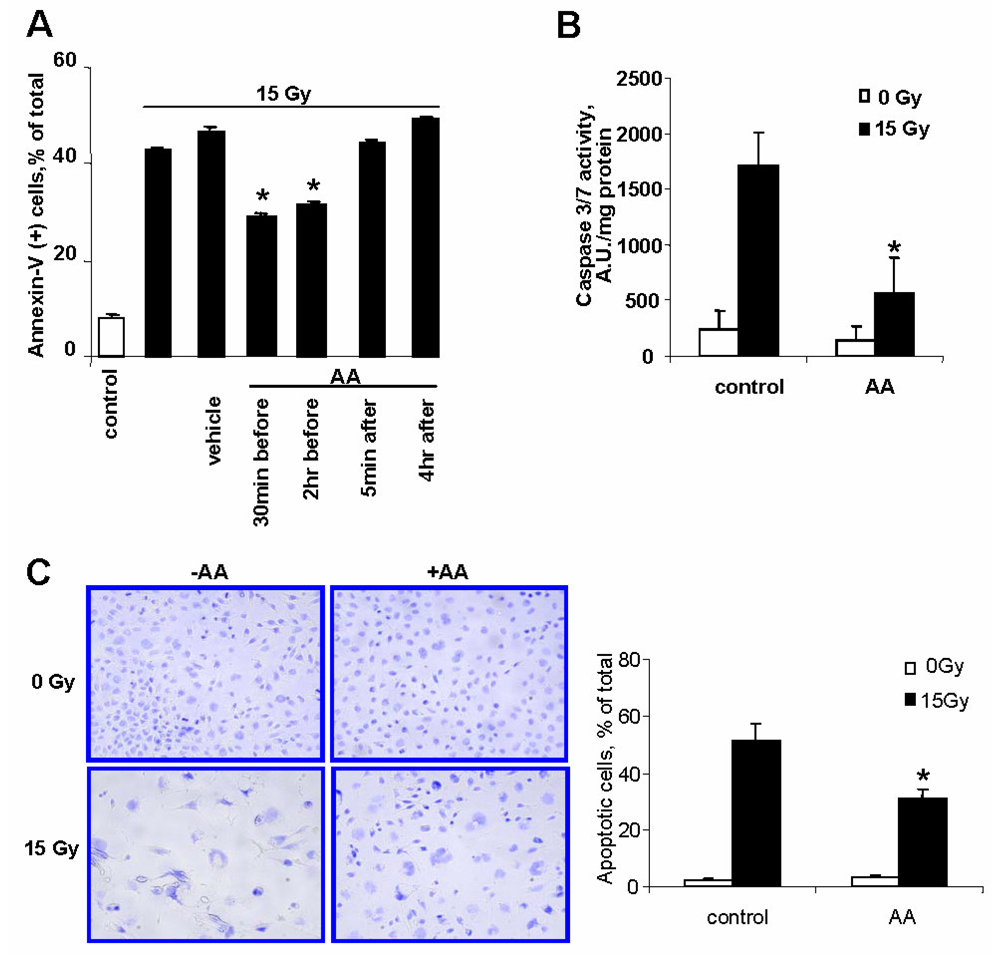
Evaluation of MECs survival 48h after exposure to 15Gy irradiation (IR) by PI positivity showed that IR resulted in ~20% cell death (Fig. 1A). Pretreatment of cells with AA, a mitochondrial complex III inhibitor [12], 30min before IR significantly decreased the number of PI(+) cells. This effect was dependent on AA concentration with saturation at 0.5µg/ml. The protective effect of AA was more pronounced 72h after IR (Fig. 1B). AA alone did not cause any significant cytotoxicity in the dose range 0.05–1.5µg/ml; however, AA was toxic to MECs at doses exceeding 4.5µg/ml. Along with PI-positivity as a marker of late cell death, we evaluated IR-induced phosphatidylserine (PS) externalization by Annexin-V positivity. To this end, we assessed the amounts of Annexin(+)/PI(−) and AnnexinV(+)/PI(+) cells as indicators of early and late apoptosis, respectively. We found that ~45–50% of MECs displayed apoptotic phenotype 48h after IR (Fig. 2A).
Fig. 1.
Protection of MECs against IR-induced death by pretreatment (30min prior to IR) with inhibitors of mitochondrial respiratory complexes – AA (A, B) and rotenone (C). A – Dependence of preconditioning effect on AA concentration (0.05- 4.5µg/ml). The number of PI(+)-cells was measured at 48h after IR. B – Timecourse of IR induced cell death (AA, 1.5µg/ml). C – Protective effect of preconditioning with rotenone (0.01–100µM, other conditions as in A).
Fig. 2.
Biomarkers of IR-induced apoptotic cell death (evaluated at 48h after IR) and protection with AA-pretreatment (AA, 1.5µg/ml). A – PS externalization assessed by Annexin V positivity in PI(+) and PI(−) cells. B – Caspase-3/7 activity. C – Apoptotic morphology.
Pretreatment of cells with AA (1.5µg/ml, 30min) significantly decreased the number of apoptotic cells. This protective effect was also observed when AA pretreatment was performed for 2h. However post-treatment with AA (5min or 4h after IR) did not confer radioprotection.
In line with the high percentage of apoptotic cells, assessments of caspase-3/7 demonstrated a ~7-fold increase of enzymatic activity 48h after IR – the effect that was significantly decreased by AA pre-treatment (1.5µg/ml, 30min) (Fig. 2B).
Typical apoptotic morphology – nuclear fragmentation, chromatin condensation, blebbing - was observed in ~50% of irradiated cells (Fig. 2C). AA pretreatment (1.5µg/ml, 30min) attenuated the apoptotic response as evidenced by a significantly decreased number of cells with characteristic apoptotic morphology (~30%).
We further utilized rotenone, a mitochondrial complex I inhibitor [13]. Pretreatment with rotenone (0.01–10µM, 30min before IR) was as radioprotective as AA. Based on PI assessments of cell viability, rotenone suppressed IR induced cell death by ~30% (Fig. 1C). To support the association of the protective action of mitochondrial respiratory inhibitors with oxidative stress, we additionally evaluated the effects of short-term exposure to H2O2 on preconditioning of MECs to irradiation in the range from 50 µM to 1,000 µM concentrations of the oxidant. At concentrations 500–1,000 µM, the preconditioning effect to 15 Gy irradiation was similar (~30% as evidenced by PS externalization). At lower concentrations (<500 µM), slight but insignificant protective effects were observed (data not shown).
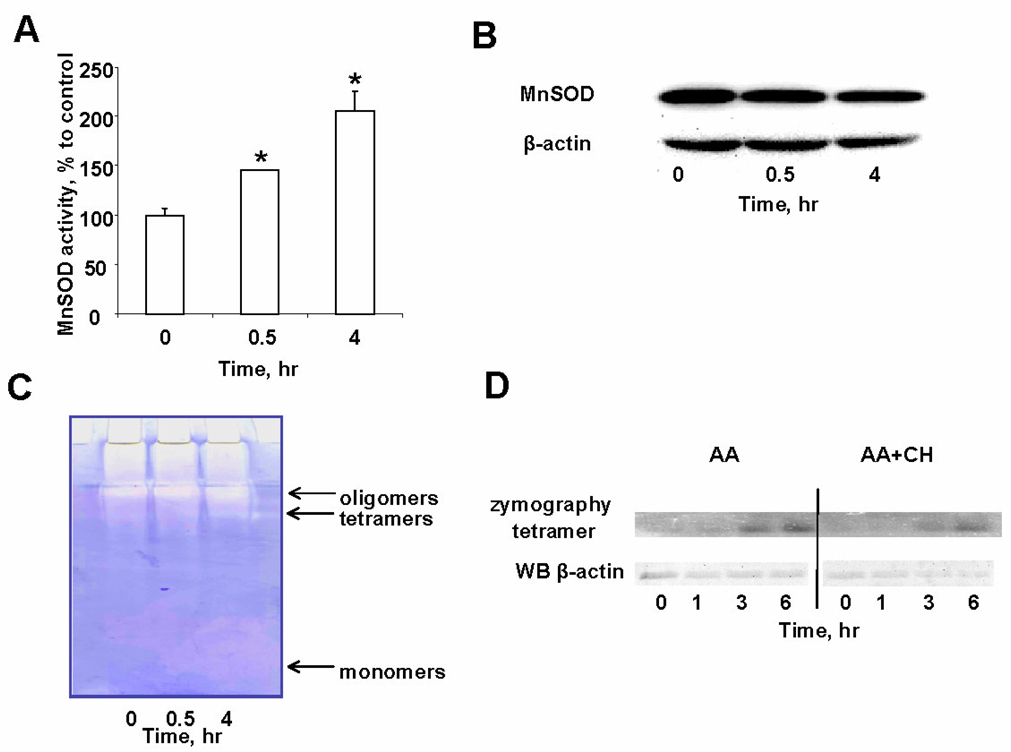
Antimycin A induced increase of MnSOD activity
To investigate possible association of oxidative preconditioning with MnSOD, we evaluated the activity of the enzyme before and after treatment of MECs with AA. The MnSOD activity of control (non-AA-treated) MECs was ~6U/mg protein (Fig. 3A). After 30min of AA treatment (1.5µg/ml), MnSOD activity increased to ~9U/mg protein and after 4h reached ~13U/mg protein [8]. Accordingly, Western blot analysis showed that the expression of MnSOD protein was not increased after AA treatment (Fig. 3B). In-gel zymography was used to examine association of the MnSOD activity with the monomeric or oligomeric forms of the enzyme (Fig. 3C). AA treatment resulted in increased dismutase activity in tetramers and higher molecular weight oligomers (Fig. 3C). There was no detectable MnSOD activity corresponding to monomeric forms of the enzyme. In separate experiments, we confirmed the positioning of monomers and oligomers by Western blotting with anti- MnSOD antibody (data not shown). Notably, MnSOD oligomers were not dissociable by SDS (1.5%) plus DTT (100mM). When AA was used along with an inhibitor of protein synthesis, cycloheximide (CH, 50µM, Fig. 3D), time-dependent accumulation of enzymatically active tetramers was also observed albeit at a slightly lower level. Under these conditions, the content of actin was decreased – an evidence of the inhibition of protein synthesis.
Fig. 3.
AA induced increase in MnSOD activity. A – Time-course of MnSOD activity in MECs after AA pretreatment (1.5µg/ml at indicated time points). Data are expressed as means±SE. B –Western blot analysis with anti-MnSOD antibody. C – In-gel zymography of MECs lysates for MnSOD activity before and after AAtreatment. D – Effect of cycloheximide (CH, 50µM) on MnSOD enzymatic activity. Note that AA (1.5µg/ml) caused time-dependent accumulation of catalytically active MnSOD tetramers (zymography, upper panel) compared to the amount of actin (Western blot, lower panel).
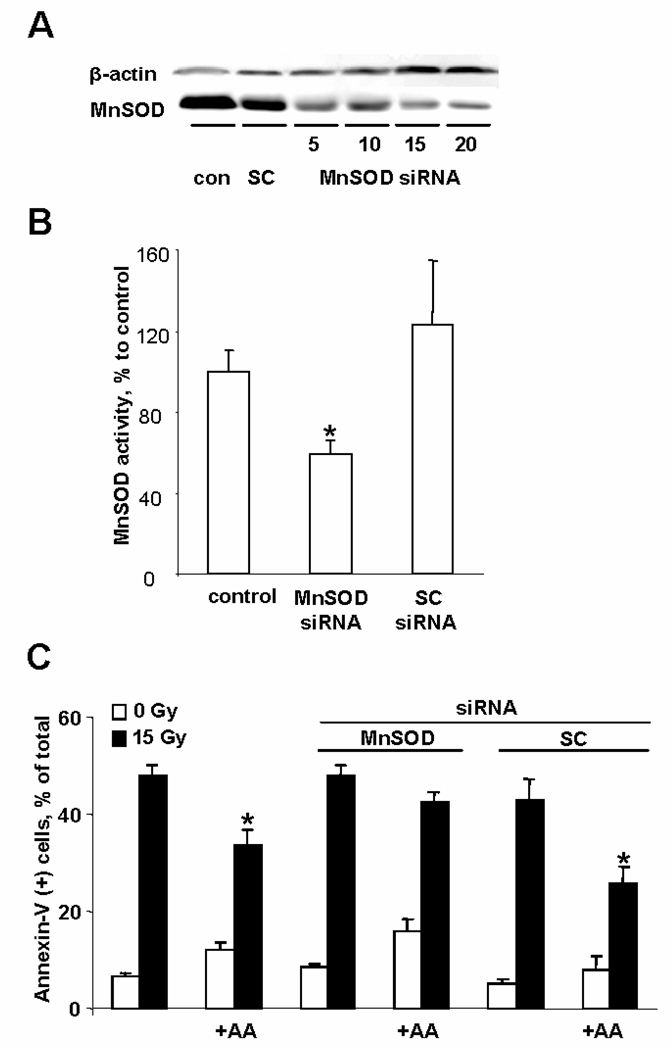
Role of MnSOD in irradiation preconditioning
To determine the extent to which MnSOD participates in AA-mediated preconditioning, we employed MnSOD deficient MECs using siRNA approach. At 48h, siRNA treatment (5–20nM) caused a concentration dependent decrease of MnSOD expression (Fig. 4A). At siRNA concentration 15nM, this was accompanied by a decrease of MnSOD activity to ~60% of control values (Fig. 4B). siRNA treatment was not toxic to cells. MnSOD-depleted MECs were then exposed to AA and irradiated. Two days after IR (day 4 after siRNA treatment), the cells still retained their low levels of MnSOD (data not shown). Importantly, the AA-dependent preconditioning effect against IR was almost completely abolished in MnSOD-depleted cells as evidenced by assessments of PS externalization (Fig. 4C).
Fig. 4.
Effect of MnSOD siRNA on IR-induced preconditioning. A –Western blot with anti-MnSOD antibody at 48h after siRNA treatment (5–20nM; SC – scrambled siRNA). B – MnSOD activity after 48h of either MnSOD-siRNA or SC-siRNA treatment. C – Preconditioning of MnSOD depleted MECs. At 48h after siRNA treatment, cells were exposed to AA (1.5µg/ml, 30min) and irradiated. PSexternalization was measured at 48h after IR. Data are expressed as means ±SE.
Discussion
Adaptive response or preconditioning is an evolutionarily conserved process in which a low dose of a stressful stimulus activates reactions that increase the resistance of the cell or organism to a moderate to severe level of stress [14]. In oxidative stress paradigm, sublethal or slightly toxic doses of oxidative stress induces an adaptive response that protects cells against further insult by a second more cytotoxic challenge with the same or similar damaging agent [15,16].
Preconditioning to radiation in mammalian cells was first described by Olivieri et al. [17], who reported that peripheral blood lymphocytes cultured in [H-3]-thymidine showed a ~50% reduced frequency of chromosome aberrations following a challenge with an acute, higher dose of X-ray exposure. Other studies showed that small doses of H2O2 induced protection against subsequent ionizing radiation [18]. The presence of cycloheximide or benzamide, during this pretreatment, inhibited the preconditioning effects suggesting that protein synthesis was involved in their realization.
MnSOD is one of the essential enzymes engaged in protection against oxidative stress and radiation damage [3]. Presence of sufficient amounts of catalytically active MnSOD is critical for protection. This is because the reaction rate between superoxide and NO is very high (1.6×1010M−1s−1) [19] and it is comparable to the dismutation rate of superoxide catalyzed by MnSOD, which is on the order of 109M−1s−1 [20]. In the past, this has been achieved by increasing the amount of MnSOD via its enhanced expression resulting in IR protection [1,21,22]. Using siRNA against mouse MnSOD, Fan et al. elucidated that MnSOD contributed to low-dose IR-induced adaptive radioresistance [23]. Increased gene expression of MnSOD and catalase as well as enhanced enzymatic activity were also reported in mice 23 days after low dose-rate irradiation [24].
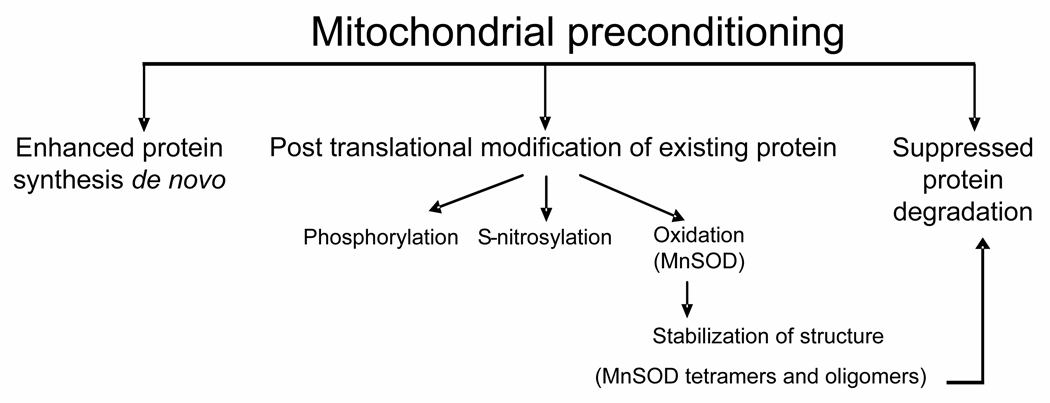
Recently, the role of mitochondria in preconditioning has been emphasized in several publications [25,26] (Schema 1). Among the proposed mitochondrial mechanisms of preconditioning are protein biosynthesis, regulation of ion channels and permeability transition pore stabilization. In the current study, we present a novel concept of preconditioning that is realized via post-translational modification of the existing protein. Our work demonstrates that pre-treatment of cells with oxidants resulted in modification and associated structural stabilization of already expressed MnSOD. Covalent cross-linking has been widely used in biotechnology for protein stabilization against denaturing [27,28]. Our previous work has established that oxidative crosslinking of catalytically active MnSOD homo-tetramer results in the formation of a stabilized structure which retains its catalytic potency [8]. MnSOD forms a locked tetraand higher molecular weight oligomers via Tyr-Tyr cross-links (as evidenced by dityrosine fluorescence, Western blot analysis with anti-dityrosine antibody and massspectrometry). The tetrameric protein contains 9 tyrosine residues per monomer. Two tyrosines – 45 and 166 are positioned within close distance. Tyr45 is located in the in the protein domain responsible for the formation of MnSOD tetramers from two dimers – the tetrameric interface [29]. On the other hand, Tyr166 is located on the dimeric interface responsible for the assembly of MnSOD dimers from its monomers. Except for Tyr45 and Tyr166, there are no other tyrosines that are in close proximity to either the dimeric or tetrameric interface. Structural proximity of tyrosine residues favors their oxidation rather than oxidation of other aminoacid residues resulting in dityrosine formation and cross-linking of subunits. Notably, this covalent oligomerization is associated with increased structural stability of the enzyme and resistance to inactivation by peroxynitrite [8]. Thus, covalent cross-linking of MnSOD leads to overall enhancement of the antioxidant defense. Using siRNA against MnSOD we showed that this antioxidant enzyme contributed to the adaptive protection but was not accountable for the entire AAinduced preconditioning effect. It is likely that other mechanisms such as oxidative stress-induced activation of NF-kB, uncoupling proteins, DNA repair enzymes, glycolytic enzymes and subsequent enhancement of antioxidant and anti-apoptotic protection (eg, catalase, SOD, glutathione peroxidase, glutathione reductase and Bcl-2) also play a role in the protective effects of preconditioning [25,26,30,31].
Schema 1.
Mitochondrial preconditioning pathways.
In our experiments, inhibitors of mitochondrial respiratory complexes I and III – rotenone and AA – were used as inducers of oxidative stress. These inhibitors are known inducers of superoxide production [12,32]. We also employed H2O2 – the product of superoxide radical dismutation – as an alternative way to induce oxidative modification of MnSOD. All these approaches were associated with significant enhancement of resistance to IR. Notably, oxidative stress-dependent chemical modification and stabilization of MnSOD occurred within short interval of time after the pre-treatments (30min). This offers an opportunity for development of a rapid preconditioning protocol. Optimization of radiation preconditioning treatments may include employment of agents with relatively low toxicity. For example, mitochondria-targeted coenzyme Q and its homologues have been shown to induce the production of superoxide radicals in cells [33,34].
Acknowledgements
This work was supported by grants from NIH U19-A1068021-01, NS061817, HL70755.
List of Abbreviations
- H2O2
hydrogen peroxide
- PI
propidium iodide
- PS
phosphatidylserine
- AA
antimycin A
- MnSOD
manganese superoxide dismutase
- IR
ionizing radiation
- MECs
mouse embryonic cells
- SC
scrambled control
Footnotes
References
- 1.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (mnsod)-deficient mice is corrected by human manganese superoxide dismutase-plasmid/liposome (sod2-pl) intratracheal gene therapy. Radiat Res. 2000;154:365–374. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radioprotective gene therapy. Curr Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 3.Macmillan-Crow LA, Cruthirds DL. Invited review: Manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 4.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 5.Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, Greenberger JS. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 6.Epperly MW, Dixon T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of radiation-induced life shortening by systemic intravenous mnsod-plasmid liposome gene therapy. Radiat Res. 2008;170:437–443. doi: 10.1667/rr1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joiner MC. Induced radioresistance: An overview and historical perspective. Int J Radiat Biol. 1994;65:79–84. doi: 10.1080/09553009414550111. [DOI] [PubMed] [Google Scholar]
- 8.Bayir H, Amoscato AA, Rafikov R, Glumac A, Belikova NA, Kagan VE. A novel mechanism of manganese superoxide dismutase (mnsod) structural adaptation to oxidative and nitrative stress. Critical Care Medicine. 2007;35:A31–A31. [Google Scholar]
- 9.Hendzel MJ, Nishioka WK, Raymond Y, Allis CD, Bazett-Jones DP, Th'ng JP. Chromatin condensation is not associated with apoptosis. J Biol Chem. 1998;273:24470–24478. doi: 10.1074/jbc.273.38.24470. [DOI] [PubMed] [Google Scholar]
- 10.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 11.Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 12.Alexandre A, Lehninger AL. Bypasses of the antimycin a block of mitochondrial electron transport in relation to ubisemiquinone function. Biochim Biophys Acta. 1984;767:120–129. doi: 10.1016/0005-2728(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex i inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese EJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Shadley JD, Afzal V, Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of x rays to human lymphocytes. Radiat Res. 1987;111:511–517. [PubMed] [Google Scholar]
- 16.Spitz DR, Dewey WC, Li GC. Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in chinese hamster fibroblasts. J Cell Physiol. 1987;131:364–373. doi: 10.1002/jcp.1041310308. [DOI] [PubMed] [Google Scholar]
- 17.Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:39–47. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- 18.Sen Gupta S, Bhattacharjee SB. Induction of repair functions by hydrogen peroxide in chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:935–942. doi: 10.1080/09553008814551301. [DOI] [PubMed] [Google Scholar]
- 19.Kissner R, Nauser T, Kurz C, Koppenol WH. Peroxynitrous acid--where is the hydroxyl radical? IUBMB Life. 2003;55:567–572. doi: 10.1080/15216540310001628690. [DOI] [PubMed] [Google Scholar]
- 20.Gray B, Carmichael AJ. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electron spin resonance. Biochem J. 1992;281(Pt 3):795–802. doi: 10.1042/bj2810795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger J. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 22.Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, BarSagi D, Greenberger JS. Manganese superoxide dismutase-plasmid liposome (mnsod-pl) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer. 2001;96:221–231. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 23.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-kappab and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 24.Otsuka K, Koana T, Tauchi H, Sakai K. Activation of antioxidative enzymes induced by low-dose-rate whole-body gamma irradiation: Adaptive response in terms of initial DNA damage. Radiat Res. 2006;166:474–478. doi: 10.1667/RR0561.1. [DOI] [PubMed] [Google Scholar]
- 25.Dirnagl U, Meisel A. Endogenous neuroprotection: Mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Murphy E, Steenbergen C. Preconditioning: The mitochondrial connection. Annu Rev Physiol. 2007;69:51–57. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 27.Klibanov AM. Stabilization of enzymes against thermal inactivation. Adv Appl Microbiol. 1983;29:1–28. doi: 10.1016/s0065-2164(08)70352-6. [DOI] [PubMed] [Google Scholar]
- 28.Trehan K. New Age International (P) Ltd. New Delhi: 1990. [Google Scholar]
- 29.Quint P, Ayala I, Busby SA, Chalmers MJ, Griffin PR, Rocca J, Nick HS, Silverman DN. Structural mobility in human manganese superoxide dismutase. Biochemistry. 2006;45:8209–8215. doi: 10.1021/bi0606288. [DOI] [PubMed] [Google Scholar]
- 30.Andoh T, Lee SY, Chiueh CC. Preconditioning regulation of bcl-2 and p66shc by human nos1 enhances tolerance to oxidative stress. FASEB J. 2000;14:2144–2146. doi: 10.1096/fj.00-0151fje. [DOI] [PubMed] [Google Scholar]
- 31.Das DK, Engelman RM, Kimura Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischaemia. Cardiovasc Res. 1993;27:578–584. doi: 10.1093/cvr/27.4.578. [DOI] [PubMed] [Google Scholar]
- 32.Pham NA, Robinson BH, Hedley DW. Simultaneous detection of mitochondrial respiratory chain activity and reactive oxygen in digitonin-permeabilized cells using flow cytometry. Cytometry. 2000;41:245–251. doi: 10.1002/1097-0320(20001201)41:4<245::aid-cyto2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 34.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]