Abstract
Our aim was to test the hypothesis that exposure to whole diesel exhaust (WDE) would enhance angiogenesis/vasculogenesis. Male apolipoprotein E-deficient mice, with either scaffold implantation subcutaneously or hindlimb ischemia, were exposed to either WDE (containing diesel exhaust particle [DEP] at a concentration of about 1 mg/m3) or filtered air 6 hours/day, 5 days/week in a whole body exposure chamber for 2, 5, or 8 weeks, respectively. WDE exposure significantly increased total cell counts in the scaffolds, aortic, and perivascular fat tissues. Macrophage infiltration was enhanced and CD31 expression increased in the scaffolds, which was coupled by increased α-smooth muscle actin (α-SMA) expression. WDE exposure led to increased CD31 expression, while decreasing endothelial nitric oxide synthase in the aortic wall. The vessel volume measured by micro-CT was increased in ischemic and non-ischemic hindlimbs in response to WDE exposure. DEP exposure induced capillary-like tube formation in endothelial cells in vitro, and caused capillary sprouting from aortic rings ex vivo. In addition, WDE exposure significantly increased mRNA expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF)-1α, while decreasing prolylhydroxylase (PHD) 2 expression. WDE exposure increases inflammatory cell infiltration, enhances the vessel volume/flow, and increases capillary tube formation and sprouting, thereby inducing angiogenesis and vasculogenesis. The angiogenic effects may occur through increasing HIF-1α and VEGF while decreasing PHD2 expression.
Keywords: particulate matter, diesel exhaust, hypoxia-inducible factor 1, prolylhydroxylase 2
1. Introduction
Epidemiologic studies have shown a strong link between airborne particulate matter (PM) exposure and cancer morbidity and mortality (Cohen, 2000; Liu et al., 2009; Pope et al., 2002; Straif et al., 2009; Vineis et al., 2004; Yang and Omaye, 2009). Although multiple mechanisms have been proposed, few studies have mechanistically evaluated the impact of diesel exhaust, primarily generated from motor vehicle sources, on the angiogenesis that cancers are dependent upon for their growth.
Angiogenesis constitutes physiological and pathological responses to ischemia. For multicellular organisms to grow beyond their sizes, they must recruit new blood vessels by vasculogenesis and angiogenesis (Carmeliet and Jain, 2000). Therefore, vasculogenesis and angiogenesis play a key role in the development of tumors. Recently, a growing body of epidemiological and clinical evidence has led to a heightened concern about the potential deleterious effects of ambient air pollution on health and its relation to systemic diseases, such as cardiovascular diseases (Brook et al., 2004; Miller et al., 2007; Nemmar et al., 2003). These associations are strongest for fine and ultrafine particulate air pollutants, of which the combustion-derived PM in diesel exhaust (DEP) is an important component (Laden et al., 2000). However, to our knowledge, there has been no report about the effect of diesel exhaust exposure on angiogenesis and vasculogenesis, especially at an environmentally relevant exposure level.
Hypoxia-inducible factor (HIF) 1 is composed of an inducible α-subunit (HIF-1α) and a constitutive β-subunit (HIF-1β). HIF-1α contains an oxygen-dependent degradation domain that, when hydroxylated by specific prolyl hydroxylases, binds the von Hippel–Lindau protein (VHL), leading to HIF-1α ubiquitinylation and degradation by the 26S proteasome. VHL binding depends on the hydroxylation of proline residue 402, 564, or both by the prolylhydroxylase 2 (PHD2). PHD1 and PHD3 also hydroxylate HIF-1α when overexpressed, but their physiological functions have not been established. PHD2 activity is reduced under hypoxic conditions as a result of either substrate limitation or inhibition of the catalytic center, resulting in stabilization of HIF-1α (Schofield and Ratcliffe, 2004; Semenza, 2007). At low oxygen levels, the prolyl hydroxylases lose their activity, which prevents VHL protein binding. This results in HIF-1α stabilization, nuclear translocation, dimerization with HIF-1β, recruitment of coactivators, and binding to hypoxia-response elements in the promoter of target genes. Vascular endothelial growth factor (VEGF), which is a well-known pro-angiogenic agent, is induced by the transcription factor HIF-1α under hypoxia (Forsythe et al., 1996; Yancopoulos et al., 2000).
To investigate if the vasculogenic/angiogenic effect of exposure to WDE in the initiation of the angiogenesis/vasculogenesis (other than progression of tumor) to diesel exhaust was via hypoxia, two ischemic models were designed for our study. One was created by the ligation of femoral artery and vein, which is a clinically extreme hypoxia situation. Another model was based on implantation of scaffold into the dorsum of the mouse, which was intended to mimic the very low-grade inflammation and hypoxia that human body/tissues may encounter to traffic corridor exposure where high WDE levels occur. Through these two clinically relevant models, we examined the effects of WDE exposure on angiogenesis and vasculogenesis under both extreme hypoxia and very mild hypoxic circumstances. In this study, we intended to investigate that diesel exhaust-induced angiogenesis and vasculogenesis in vivo in a mouse model of apolipoprotein E-deficient (ApoE−/−) mice and vasculogenesis in vitro. This murine model, along with in vitro vasculogenesis assay, provides insights into the mechanisms responsible for air pollution-induced angiogenesis and vasculogenesis.
2. Materials and Methods
2.1. Animals
Twelve-week-old male ApoE−/− mice from The Jackson Laboratory (Bar Harbor, ME) were housed at constant temperature (22 ± 2°C) on a 12-h light/dark cycle. They were fed ad libitum on standard laboratory mouse chow and had free access to water. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and the study protocols were approved by the Institutional Animal Care and Use Committee of New York University and the Ohio State University.
2.2. Scaffold Preparation and Implantation
To provide a biologically-compatible platform for cell growth, a system of scaffold with matrigel was used. The scaffolds consisted of a blend of NaCl and poly-L-lactic acid sponge (PLLA, Boehringer Ingelheim Inc., Winchester, VA). The sponges were leached out of salt by water, and cut into rectangular pieces of 6 × 6 × 6 mm. The physical characterization of the scaffold has been documented previously (Sun et al., 2005). The scaffolds were sterilized overnight in 70% (vol/vol) ethanol and washed three times in PBS. Then, 1×106 murine endothelial cells (ATCC # CRL-2586) were seeded into each piece of the scaffold with matrigel (growth factor-reduced, Becton Dickinson, MA). After the mice were anesthetized by a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) intraperitoneally, an incision on the dorsal region (between the scapulae) was performed, and one scaffold containing the cells was implanted subcutaneously into the dorsum of each mouse (near the scapula).
2.3. Hindlimb Ischemic Model
After anesthesia as above, another set of the mice were subjected to unilateral femoral artery and vein ligation, as described previously (Sun et al., 2005). Briefly, severe unilateral hind limb ischemia was surgically created via ligation and division of the left superficial femora artery and vein, left external iliac artery and vein, and left deep femoral and circumflex arteries and veins.
2.4. Mouse Diesel Exhaust Exposure
Mice, with either scaffold implantation or hindlimb ischemia, were exposed to either diluted whole diesel exhaust (WDE, containing diesel exhaust particles [DEP] at a concentration of about 1 mg/m3, as well as all of the gaseous pollutants in the exhaust) based on previous studies (McDonald et al., 2004a; Reed et al., 2004), or to filtered outdoor air (FA) for 6 hours/day, 5 days/week for 2, 5, or 8 weeks while within a whole body exposure chamber (n = 8) in a diesel exhaust exposure facility at New York University School of Medicine’s A.J. Lanza Laboratory in Sterling Forest at Tuxedo, NY. The diluted air was sufficient to maintain the carbon monoxide (CO) concentration in the exposure chamber at 5.0 ± 1.5 ppm.
The WDE was produced by a 5500-watt single-cylinder diesel engine generator (Model YDG 5500EE-6EI, Yanmar, Osaka, Japan) that contains a 418cc displacement air-cooled engine (Model LE100EE-DEGY6). Engine oil (SAE, 15W/40, Delo400, Chevron Products Company, San Ramon, CA) was changed every two weeks during the series of daily exposures. The diesel engine used #2 on-road diesel fuel from a local gas station (SOS fuels, Tuxedo, NY) and the diesel engine was run at a maximum engine load condition. The characterization and exposure concentrations of the WDE, including both the gaseous components and the DEP components when using #2 diesel fuel have been described previously (McDonald et al., 2004a; McDonald et al., 2004b). The FA control mice in the experiment were subjected to an identical protocol, with the exception that a High Efficiency Particulate Air filter (HEPA, Pall Life Sciences, East Hills, NY) was positioned in the inlet valve to the exposure system (a separate line from the diesel exposure system) to remove essentially all of the ambient air PM from the fresh air stream.
2.5. Mouse Sacrifice
Mice were euthanized immediately after each exposure period. The implanted scaffolds were retrieved and aortas dissected out. The scaffolds were fixed in 10% formalin, and embedded in paraffin, while the aorta and skeletal muscles distal to the ischemic surgery site were removed, snap-frozen in OCT, and stored at −80 °C.
2.6. Micro-CT Scanning
To investigate the functional vascular blood flow, micro-CT scanning was used. In brief, after exposure to WDE or FA for 8 weeks, the mice were euthanized and perfused with radiopaque polymer contrast Microfil® MV-122 (Flowtech, Carver, MA). The images were acquired on an Invion™ micro-CT scanner (Siemens Medical Solution, Knoxville, TN) with 4K × 4K detector using the following parameters: 80 kV voltage, 500 mA current, effective pixel size 20.30 mM, exposure time of 515 ms with high system magnification and binning of 2 giving image resolution of 37.20 mM with slice thickness of 0.010 mm. The images were analyzed with IRW software from Siemens.
2.7. Histology and Immunohistochemical Staining
Hematoxylin and eosin (H&E) staining was performed on the sections of thoracic aorta and retrieved scaffolds. The images were digitized with a digital camera, and analyzed under a research microscope (Zeiss 510 META, Jena, Germany) with Metamorph V.7.1.2 software (Universal Imaging, West Chester, PA). The percentage of total cells was calculated by the area of positive hematoxylin staining over the area of entire analyzed tissue.
For immunohistochemical staining, after quenching the endogenous peroxidase activity with 0.3% H2O2, the sections were incubated in 1% BSA/PBS for 10 minutes, followed by overnight incubation with primary antibodies (1:200 diluted in 1% BSA/PBS) at 4°C. After incubation with appropriate peroxidase-conjugated secondary antibodies, the stain was developed using Fast 3, 3′-diaminobenzidine tablet sets (D4293; Sigma). The sections were then counterstained with hematoxylin and examined by light microscopy. The primary antibodies were rat anti-CD31 (BD/pharmingen, San Diego, CA), mouse monoclonal anti-α-smooth muscle actin (α-SMA, 1:200; Sigma), rabbit anti-endothelial nitric oxide synthase (eNOS, Cell Signaling Technology, Danvers, MA), and rabbit anti-CD68 and rabbit anti-inducible NOS (iNOS, Santa Cruz Biotechnology Inc., Santa Cruz, CA).
2.8. DEP Extraction
The particles used in in vitro assays were collected from the filters of the diesel exhaust exposure system where the mice were exposed. The particles were extracted from the filters in 95% ethanol, and ultrasonicated for 20 min. The supernatant liquid was then decanted into evaporative concentrator tubes and concentrated under a stream of nitrogen gas in a Dry Bath Incubator (Fisher Scientific, Pittsburgh, PA). Thereafter, PBS was added to re-suspend the DEP to 0.5 mg/ml.
2.9. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from ATCC (# CRL-1730) and cultured at 37 °C in a 5% CO2 atmosphere in M199 culture medium (Lonza, Williamsport, PA) supplemented with 10% fetal bovine serum (FBS; Lonza), 5 units/ml heparin, 2mM L-glutamine(Sigma, St. Louis, MO), 10 ng/ml β-Endothelial Cell Growth Factor (β-ECGF; Sigma), 100 U/ml penicillin, and 100μg/ml streptomycin (Invitrogen, Carlsbad, CA). The HUVECs at 6th to 8th passage were used in this study.
2.10. Cytotoxicity Evaluation
Cell viability was evaluated under the microscope with the trypan blue dye exclusion method. The HUVECs (1.5 ×105/well) were cultured in a 24-well plate coated with 0.2% gelatin (Sigma) followed the incubation with DEP for 24 hours. Live cell number was counted after 1, 3, 5, 7 and 9 days by trypan blue dye exclusion. In addition, the cells (1.5 ×105/well) were incubated with DEP for 10, 20, 30 and 40 hours, and then the numbers of live cells were counted. The total number of viable cells in the FA control group (DEP-free) was considered as 100% viability, while DEP-treated cells were compared with the FA control group for the determination of percentage viability.
2.11. Endothelial Capillary Tube Formation Assay in Vitro
Confluent HUVECs were treated with different concentration of DEP (0, 5, 15, and 30 μg/ml) for 4 or 24 hours, then resuspended in a density of 3 ×104 cells/ml with M199 supplemented with 10% FBS but without β-ECGF and seeded onto extracellular matrix (ECM) gel (Sigma). The cells were incubated at 37 °C for 24 hours in a humidified 5% CO2 incubator. Three random fields of view in three replicate wells in triplicate were visualized under 100×magnifications.
2.12. Capillary Sprouting From Aortic Rings ex Vivo
The aortas were harvested and 1–2 mm thick aortic rings were transversally cut after the dissection of all connective tissue. The rings were treated with different concentration of DEP (0, 5, 15, and 30 μg/ml) for 24 h and then seeded in a 24-well ECM gel-coated plate. DMEM supplemented with 10% FBS, 20 U/ml of heparin (Sigma), and penicillin/streptomycin was added to each well of gelled ECM. After 5 days of culture, the number and length of capillary sprouting from each aortic ring were imaged using an inverted microscope and assessed.
2.13. Quantitative Real-time Polymerase Chain Reaction
RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA were converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The quantification of gene expression was determined by real-time polymerase chain reaction (RT-PCR). The primers for human and mouse HIF-1α, PHD2, VEGF and β–actin are showed in Table E1 in the online data supplement.
2.14. Statistical Analysis
Data are expressed as mean ± SEM unless otherwise stated. All of the values were analyzed using one-way ANOVA and the Newman-Keuls-Student t test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. WDE Exposure
There were no significant differences in the WDE concentrations among the 3 exposure time periods. The details of WDE exposure data are listed in Table E2 in the online data supplement.
3.2. WDE Exposure – Effect on Functional Vascular Blood Flow
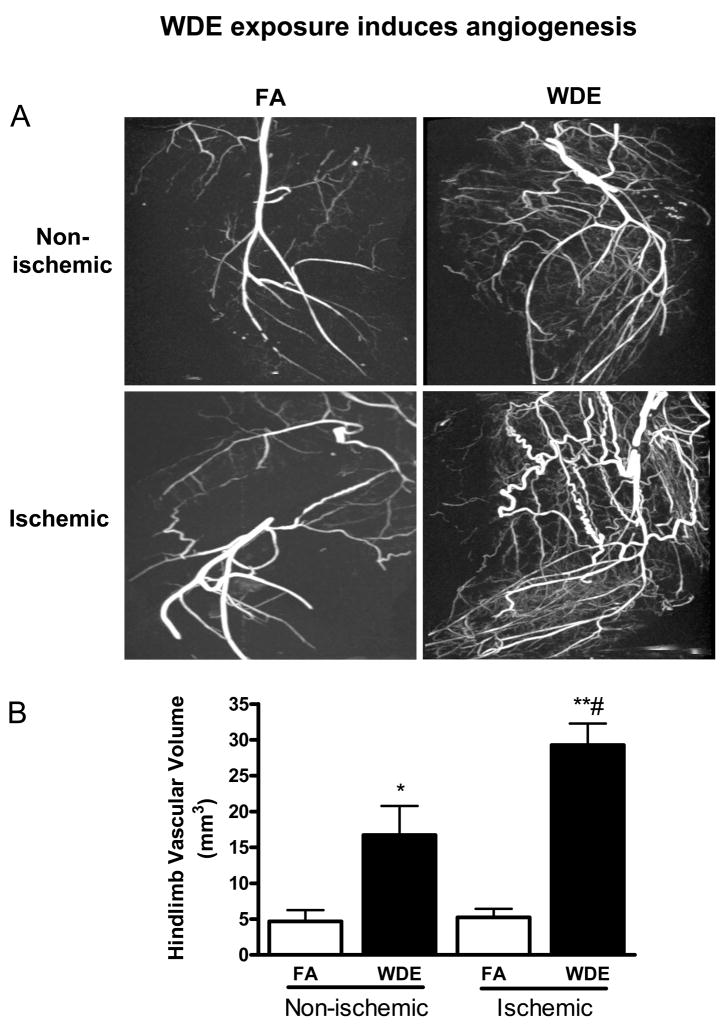
Micro-CT imaging (Figure 1) showed a significant increase in vessel volume in both ischemic and non-ischemic hindlimbs in response to WDE exposure. In hindlimb ischemia, WDE exposure induced 6-fold increase in vascular volume compared to the FA group (29.3 ± 2.9 mm3 and 5.2 ± 1.2 mm3, respectively, p = 0.0007). In contrast, vessel volume increased 4-fold in non-ischemic hindlimb (16.7 ± 4.1 mm3 and 4.7 ± 1.6 mm3, respectively, p = 0.046).
Figure 1.
WDE exposure induces angiogenesis in ischemic hindlimb. (A) Representative micro-CT scanning images of ischemic and non-ischemic hindlimbs in FA and WDE groups. (B) Graph shows 6- and 4-fold increase in vascular volume in ischemic and non-ischemic hindlimbs in the WDE group comparing to the FA group, respectively. *p<0.05 vs. FA in ischemic or non-ischemic hindlimbs; **p<0.001 vs. FA in ischemic or non-ischemic hindlimbs; #p<0.05 vs. WDE non-ischemic hindlimb. N = 3 –4.
3.3. WDE Exposure – Effect on Inflammation
The total cells in the scaffolds, aortic wall, and perivascular fat tissue were counted based on H & E staining (see Figure E1 and E2 in the online data supplement). The total cells were increased significantly in the WDE group compared to the FA group at week 2. The total cells were also increased significantly in both WDE and FA groups at week 2 compared to week 5 and week 8. There was no significant difference between the WDE and the FA groups at either week 5 or week 8. In addition, the total cell infiltration was significantly enhanced in the WDE group at week 5 in both perivascular fat tissue and the aortic wall, but was only enhanced in the aortic wall at week 8. There was no significant difference of total cell number between the 2 groups at week 2.
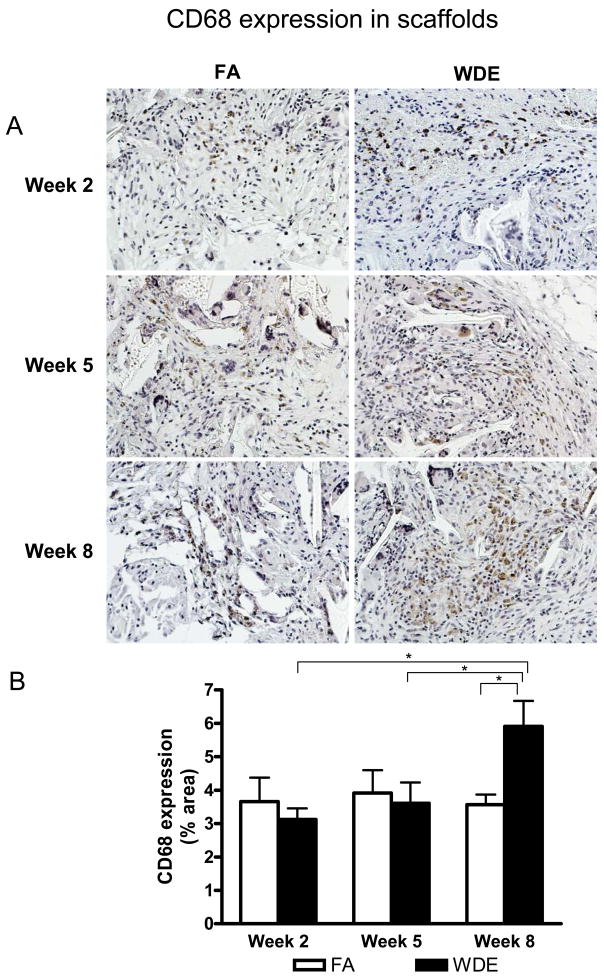
Figure 2 shows the representative images and statistical analyses for CD68 staining in the scaffolds. There was no significant difference in CD68 staining between the WDE group and the FA group at weeks 2 and 5. However, the expression of CD68 was significantly increased in the WDE group in the scaffolds compared to the FA group at week 8. Yet, in the aortic vessels (including the wall and perivascular fat tissue), there was no significant difference between the 2 groups throughout the entire experimental time period (see Figure E3 in the online data supplement).
Figure 2.
WDE exposure induces macrophage infiltration. Representative immunohistochemical staining images (A) and statistical analysis (B) of CD68 in the scaffolds at exposure week 2, week 5 and week 8. Original magnification ×200. *p<0.05. N = 8.
3.4. WDE Exposure – Effect on Angiogenesis
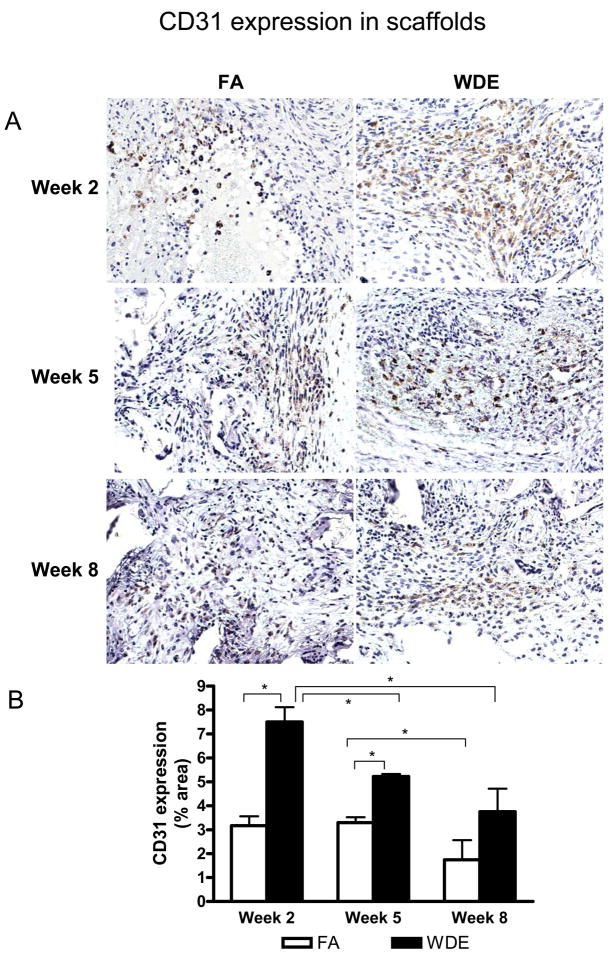
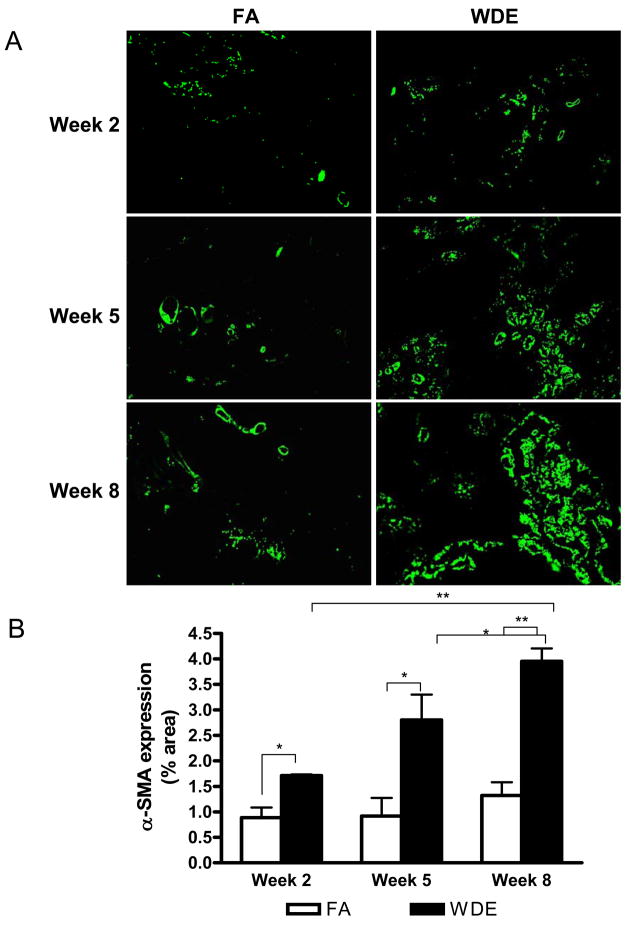
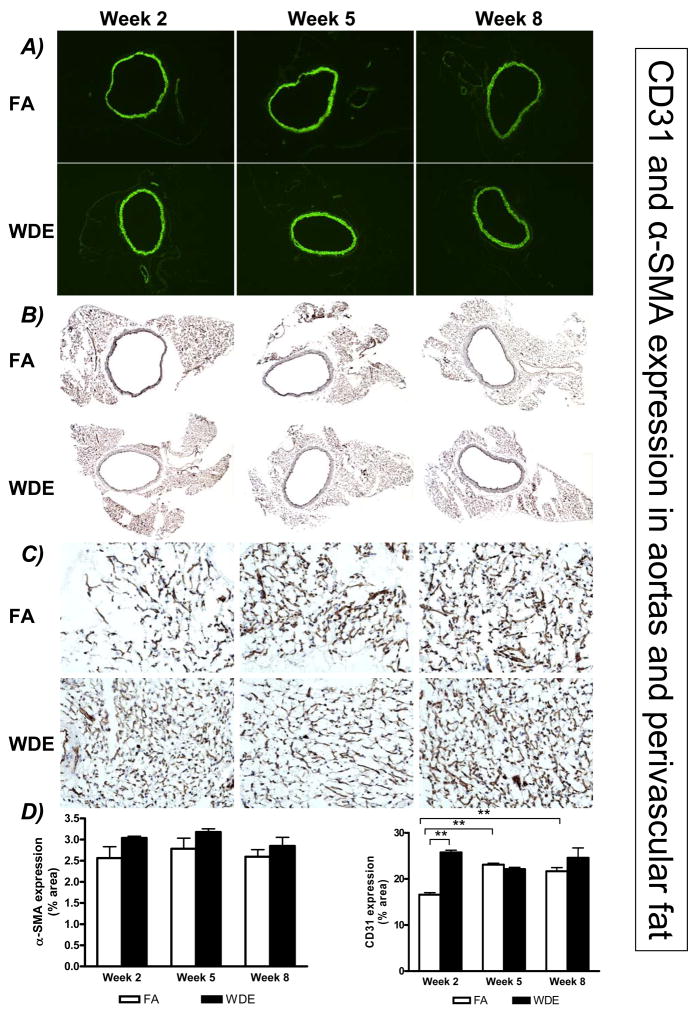
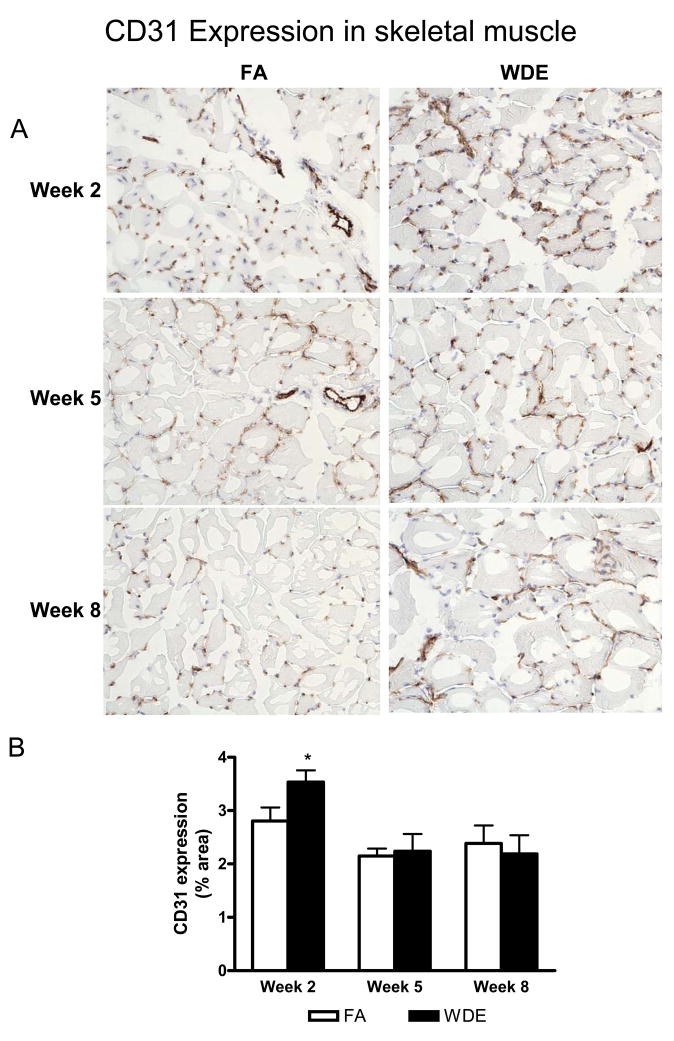
Figure 3 shows representative immunohistochemical staining images of CD31 in the scaffolds at weeks 2, 5 and 8. The CD31 expression in the scaffolds showed an increase in the WDE group, being especially significant at weeks 2 and 5, compared to the FA group. Figure 4 shows representative immunofluorescent staining images of α-SMA in the scaffolds at weeks 2, 5 and 8. It shows clearly that WDE exposure significantly increased the expression of α-SMA in the scaffolds compared to the FA group from week 2 through week 8. As shown in Figure 5, in the aortic vessel, CD31 expression was increased in the WDE group only at week 2, and there was no significant difference in the expression of CD31 between the 2 groups at weeks 5 and 8, neither was there a difference in α-SMA expression in the vessel from week 2 through week 8. Likewise, as shown in Figure 6, the expression of CD31 in skeletal muscle was significantly higher at week 2 in the WDE group than in the FA group, whereas there was no significant difference between these 2 groups at weeks 5 or 8.
Figure 3.
WDE exposure induces angiogenesis in the scaffolds. Representative immunohistochemical staining images (A) and statistical analysis (B) of CD31 in the scaffolds at exposure week 2, week 5 and week 8. Original magnification ×200. *p<0.05. N = 8.
Figure 4.
WDE exposure induces vasculogenesis in the scaffolds. Representative immunofluorescent staining images (A) and statistical analysis (B) of α-SMA in the scaffolds at exposure week 2, week 5 and week 8. Original magnification ×200. *p<0.05; **p<0.001. N = 8.
Figure 5.
WDE exposure on angiogenesis in aortic and perivascular fat tissues. Representative immunofluorescent staining images of α-SMA (A), immunohistochemical staining images of CD31 in the aorta (B) and perivascular fat tissue (C), and statistical analysis in the aorta and perivascular fat tissue (D) at exposure week 2, week 5 and week 8. A–B) Original magnification ×40; C) Original magnification ×200. **p<0.001. N = 8.
Figure 6.
WDE exposure induces angiogenesis in ischemic skeletal muscle tissue. Representative immunohistochemical staining images (A) and statistical analysis (B) of CD31 in ischemic skeletal muscle tissue at week 2, week 5 and week 8. Original magnification ×200. *p<0.05. N = 8.
3.5. WDE Exposure and NOS Expression
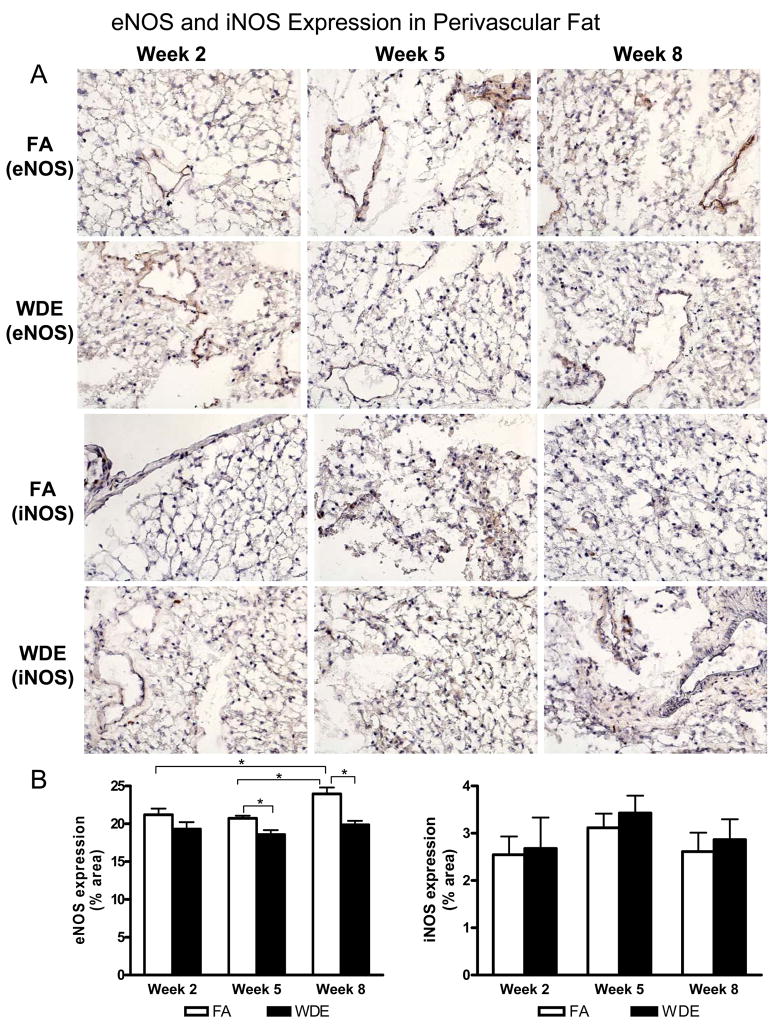
Representative immunohistochemical staining images of eNOS and iNOS in the perivascular fat tissue are shown in Figure 7. The expression of eNOS was decreased significantly in the perivascular fat tissue by WDE exposure at weeks 5 and 8, but there were no significant changes in iNOS expression seen between the 2 groups. In addition, there was no significant difference for the expression of either eNOS or iNOS in the aorta wall (data not shown).
Figure 7.
WDE exposure on eNOS and iNOS expression in perivascular fat tissues. Representative immunohistochemical staining images (A) and statistical analysis (B) of eNOS and iNOS in perivascular fat at week 2, week 5 and week 8. Original magnification ×200. *p<0.05; N = 8.
3.6. Cytotoxic Effects of DEP on Endothelial Cells
As shown in Figure E4 in the online data supplement, 5 μg/ml of DEP had moderate cell growth inhibition with 24 and 72 hours of incubation (about 41.9% and 43.1%), while 15 μg/ml of DEP showed significant cell growth inhibition with 24 and 72 hours of incubation (about 71.0% and 82.0%). Nevertheless, 30 μg/ml of DEP seriously damaged the cells; the results showed more than 95% cell growth inhibition with 24 hours of incubation. Also, after 20 hours of incubation, 5, 15 and 30 μg/ml of DEP affected cell viability slightly (about 4.7%, 8.4% and 16.5%) compared to the FA control groups for the same incubation time; but with an incubation time of 40 hours at 37 °C, all three of these concentrations of DEP showed significant cytotoxicity to the HUVECs, especially 30 μg/ml of DEP (about 38.8%, 56.9% and 78.5%, respectively). Accordingly, we investigated the effects of DEP on angiogenesis of endothelial cells in less than 24 hours in the in vitro study, and there were no significant morphological or functional damages to the cells during this period.
3.7. Role of DEP on Sprouting and Capillary Tube Formation
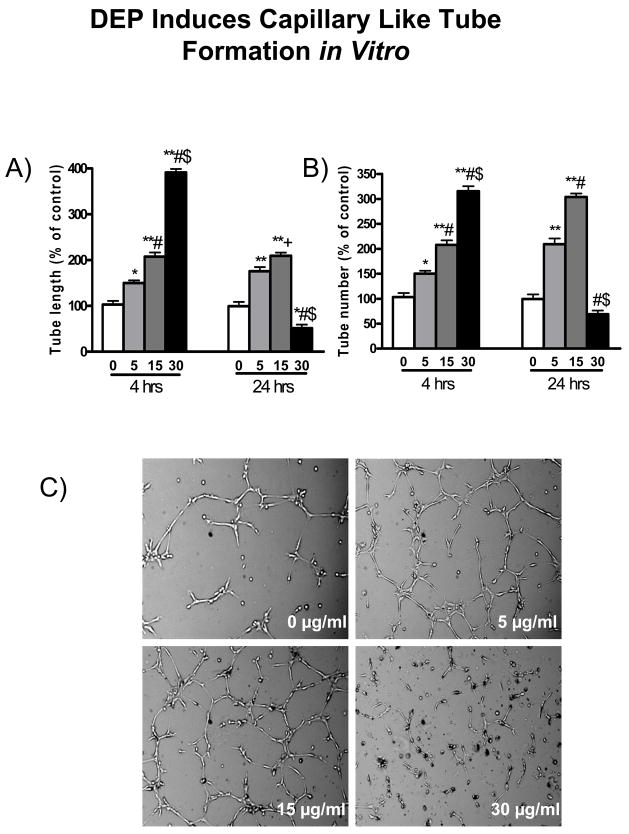
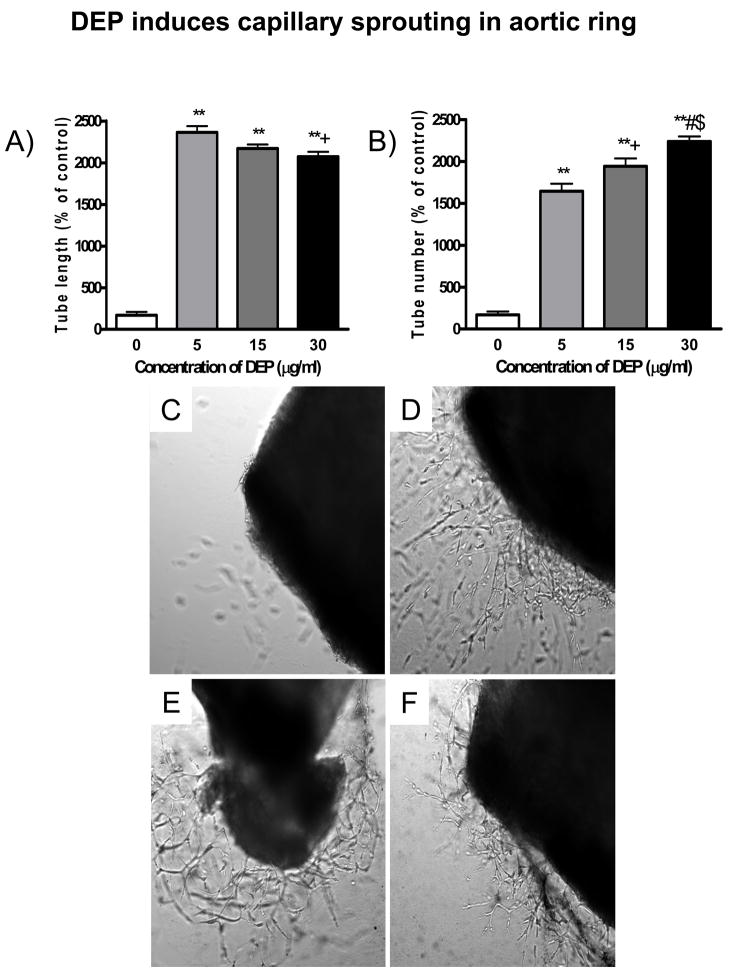
To investigate the role of DEP on vasculogenesis, we measured endothelial sprouting and network formation after exposure to DEP. As shown in Figure 8, DEP led to the formation of capillary-like structures after a short exposure time. The maximum capillary formation occurred in response to 4 hours of exposure to 30 μg/ml concentration of DEP. Thus, DEP induced the formation of capillary-like structures in a dose-dependent manner in 4 hours of exposure. Similarly, in response to 24 hours of exposure, the maximum capillary formation occurred at 15 μg/ml concentration of DEP, whereas it was significantly decreased at 30 μg/ml concentration of DEP. On the other hand, DEP induced capillary sprouting from mice aortic ring exposed ex vivo. As shown in Figure 9, treatment of aortic rings from mice with DEP for 5 days markedly induced capillary sprouting, and resulted in a significant increase in the number and length of newly sprouted vessels.
Figure 8.
DEP exposure induces capillary tube formation in endothelial cells. A) indicates the analysis of capillary tube length, B) shows the analysis of capillary tube number, and C) depicts representative images of cultured HUVECs exposed to DEP for 24 hours. Original magnification ×100. *p<0.05 vs. 0 μg/ml; **p<0.001 vs. 0 μg/ml (control); +p<0.05 vs. 5 μg/ml; #p<0.001 vs. 5 μg/ml; $p<0.001 vs. 15 μg/ml at the same time point.
Figure 9.
DEP induces capillary sprouting in mouse aortic ring. A and B depict semi-quantitative analysis of capillary sprouting length and number in response to DEP exposure at different concentrations. C–F depict representative images of capillary sproutings in DEP-treated aortic rings of C57BL/6 mice after 5 day incubation with DEP. C) 0 μg/ml; D) 5 μg/ml; E) 15 μg/ml; F) 30 μg/ml. **p<0.001 vs. 0 μg/ml of DEP; +p<0.05 vs. 5 μg/ml of DEP; #p<0.001 vs. 5 μg/ml of DEP; $p<0.05 vs. 15 μg/ml of DEP. N = 8.
3.8. Gene Expression Analyses of HIF-1α, PHD2 and VEGF
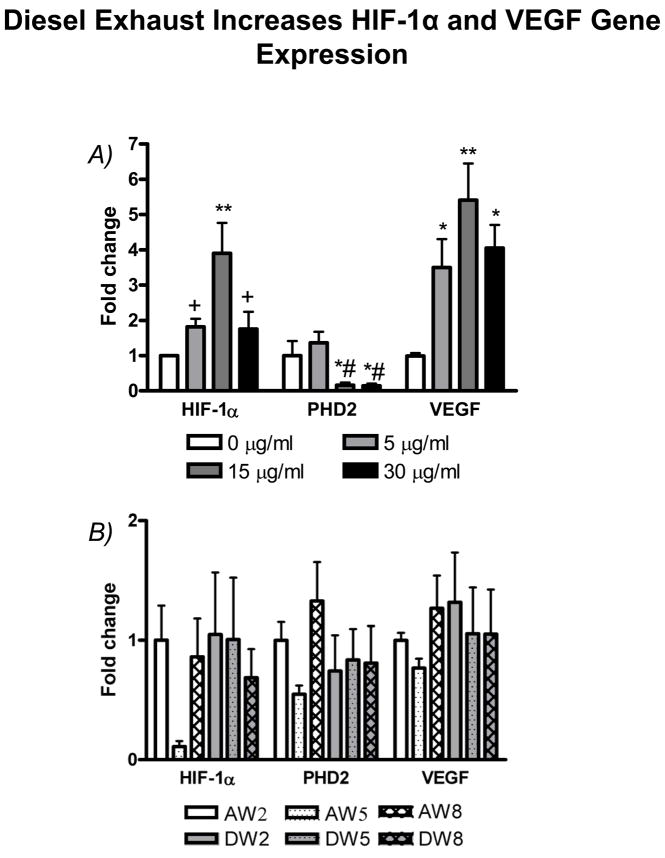
Figure 10A shows the data for gene expression from HUVECs in vitro. The mRNA level of HIF-1α was significantly higher in DEP exposed groups compared to the control group, with the highest level at 15 μg/ml of DEP exposure. PHD2 mRNA level was significantly lower in the concentration of 15 or 30 μg/ml of DEP than in the concentration of 0 or 5 μg/ml of DEP exposure. VEGF is the major angiogenic mediator generated by different cell types, including vascular endothelial cells. Similar to the change in HIF-1α, VEGF was significantly increased in response to DEP exposure, while VEGF expression was at the highest level with 15 μg/ml of DEP exposure. Yet, as shown in Figure 10B, we did not find any significant difference for these gene expressions in the ischemic skeletal muscles among the groups exposed to either WDE or FA in vivo.
Figure 10.
Diesel exhaust exposure on gene expression of HIF-1α, PHD2, and VEGF. A) Gene expression in confluent HUVECs treated with different concentrations of DEP (0, 5, 15, 30 μg/ml) for 4 hours in vitro. B) Gene expression in the ischemic hindlimb skeletal muscles exposed to WDE or FA for 2, 5 and 8 weeks. Data are expressed as fold change over the group exposed to FA for 2 weeks. AW2, AW5, or AW8, groups exposed to FA for 2, 5 or 8 weeks; DW2, DW5, or DW8, groups exposed to WDE for 2, 5 or 8 weeks. *p<0.05 vs. control (0 μg/ml); **p<0.001 vs. 0 μg/ml; +p<0.05 vs. 15 μg/ml; #p<0.05 vs. 5 μg/ml. N = 8.
4. Discussion
In the current study, by using two in vivo models, i.e. scaffold implantation with endothelial cells and hindlimb ischemia, were designed to provide two extremely hypoxic conditions that occur in human bodies in real world. The major findings in a murine model of hindlimb ischemia exposed by inhalation to WDE for up to 8 weeks, along with ex vivo aortic ring and in vitro endothelial cell culture experiments are the following: 1) WDE exposure significantly increased inflammatory cell infiltration in the tissues and scaffolds; 2) WDE exposure induced vasculogenesis, which was manifested by increased CD31 and α-SMA expression in the scaffolds; 3) WDE exposure resulted in decreased eNOS expression, which may lead to hypoxia by decreasing the vascular production of nitric oxide (NO); 4) WDE exposure enhanced functional vascular blood flow, and induced capillary tube formation and capillary sprouting. Inflammation provides a potential mechanistic link between PM air pollution exposure and adverse health effects observed in epidemiologic studies, yet considerable uncertainty remains as to whether inflammation is mediating these effects, and where that inflammation is occurring (Frampton, 2006). In our current study, H&E staining was performed to measure the total amount of cells in the scaffolds. Since we seeded the same amount of endothelial cells in each scaffold (1×106 cells), the difference between WDE and FA groups should be due to either the increase in inflammatory cell infiltration, or the vasculogenic effect (endothelial proliferation). The transient increase of total cells in WDE group at week 2 in our study, which was coupled with significant increase of CD31 staining, but no increase of macrophages, indicates that there was no significant increase of inflammatory cell infiltration at week 2 induced by WDE exposure. At week 8, we did see a significant increase in macrophages in the scaffolds in the WDE group, indicating that PM exposure-induced inflammation has “low-grade” characteristics, and is not an acute event, which is consistent with other reports (Al-Humadi et al., 2002; McCreanor et al., 2007). PM air pollution has been shown to be associated with several adverse cardiovascular health outcomes, and patients with diabetes may be especially vulnerable (O’Neill et al., 2005). One potential pathway is via inflammation and endothelial dysfunction. In one previous study, PM2.5 exposure in subjects with type 2 diabetes mellitus showed consistent association with increased inflammatory markers, suggesting that inflammatory mechanism may explain the increased risk of air pollution-induced cardiovascular events (O’Neill et al., 2007). To study the effects of DEP on pulmonary and systemic inflammatory responses, another study in mice using LPS challenge found that DEP exacerbated pulmonary inflammation and vascular permeability, and increased fibrinogen and E-selectin levels, suggesting that adverse health effects of PM air pollution occur in sensitive populations with predisposing vascular and/or pulmonary disorders, including ischemic vascular diseases and respiratory infection (Inoue et al., 2006).
As currently understood, neovascularization is the result of several processes, including angiogenesis, arteriogenesis, and vasculogenesis. The term angiogenesis describes the sprouting of new capillaries from postcapillary venules (Carmeliet, 2003), and in adults, it is stimulated mainly by tissue hypoxia (Pugh and Ratcliffe, 2003). Angiogenesis leads predominantly to the development of capillaries, although the formation of larger-size vessels has also been noted in certain animal models. In contrast, arteriogenesis refers to the process of maturation or perhaps de novo growth of collateral conduits that are frequently of a sufficient diameter to be visualized angiographically (Buschmann and Schaper, 2000; de Muinck and Simons, 2004; Helisch and Schaper, 2003). Vasculogenesis is the process of an in situ formation of blood vessels from circulating endothelial progenitor cells and vascular progenitor cells (Asahara et al., 1999; Luttun and Carmeliet, 2003). The functional significance of vasculogenesis has not been conclusively established. To our knowledge, there has been no report specifically about ambient air PM affecting angiogenesis, let alone on an effect of WDE on angiogenesis and vasculogenesis. In our current study, we clearly demonstrated in-depth effects of WDE exposure on angiogenesis (in the aortic vessels), arteriogenesis (in the aortic vessels and scaffolds), and vasculogenesis (in the scaffolds). Furthermore, through micro-CT scanning, our data demonstrate that WDE enhances the vessel volume in both of the ischemic and non-ischemic hindlimb, increases capillary tube formation, as well as capillary sprouting, representing new vascular formation at a clinically functional level. The formation of a vasculature by vasculogenesis and angiogenesis is essential for embryonic development, tissue remodeling in adults, and the unrestrained growth of tumors. Pathological angiogenesis is a requisite for solid tumor growth. In a Lewis lung cancer mice model, sidestream cigarette smoke significantly increased tumor size, weight, capillary density, VEGF and monocyte chemoattractant protein (MCP)-1 levels, and circulating endothelial progenitor cells, revealing that sidestream smoke promotes tumor angiogenesis and growth (Zhu et al., 2003). One of the mechanisms involving in angiogenesis is in the HIF pathway. HIF is a transcription factor that regulates a master genetic program that controls many forms of energy homeostasis at the cellular and systemic levels including glycolysis (local energy production), erythropoiesis (blood oxygen delivery), and angiogenesis (blood flow regulation) (Bruegge et al., 2007). Under hypoxic condition, HIF-1α heterodimerizes with HIF-1β, and translocates to the nucleus where it activates transcription from a number of hypoxia-responsive genes such as VEGF. The current study confirmed that DEP exposure promoted the expressions of HIF-1α and VEGF while decreasing PHD2 expression in vitro, suggesting that WDE exposure may induce angiogenesis through a hypoxia/HIF pathway, which needs further investigation. Additionally, exposure to WDE significantly decreased eNOS in our study, which may reduce NO formation, thereby potentiating cytotoxicity.
Clearly, the data have shown that WDE exposure has an ability to increase inflammatory cell infiltration, enhance the vessel volume and blood flow, and induce angiogenesis and vasculogenesis. By using ApoE−/− mouse model, Quan et al (Quan et al., 2009) compared the effects of WDE with diesel exhaust gases (DEG) on cardiovascular system. Their data indicate that the particles in the WDE, rather than gaseous components, are responsible for atherosclerosis exacerbation, which is consistent with what we have found in our current study that the different angiogenic effects are from the PM components in the diesel exhaust since both groups were exposed to the same gaseous components.
5. Conclusion
This study demonstrates that WDE exposure increases inflammatory cell infiltration in the tissues, enhances vessel volume and blood flow, and increases capillary tube formation and capillary sprouting, thereby inducing angiogenesis and vasculogenesis via increasing HIF-1 and VEGF expression and decreasing PHD2 expression. These findings may have significant impact on our understanding of vehicular traffic-related air pollution, especially PM, on long-term human health effects, especially tumor initiation and development.
Supplementary Material
Acknowledgments
This study was supported in part by Health Effects Institute (HEI) award #4747-RFPA05-3/06–8 to Dr. Sun, who is also supported by National Institutes of Health (NIH) grant K01ES016588. The whole-body diesel exhaust particle exposure was performed in facilities at New York University School of Medicine that were supported by NIH grants (Center Grant ES00260 and ES015495 to Dr, Chen), and a Health Effects Institute grant (4750-RFA05-1A/06–11 to Drs. Lippmann and Chen). The authors wish to thank Ximei Jin and Mianhua Zhong for their technical support.
Footnotes
Conflict of interest statement
Authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Humadi NH, Siegel PD, Lewis DM, Barger MW, Ma JY, Weissman DN, Ma JK. Alteration of intracellular cysteine and glutathione levels in alveolar macrophages and lymphocytes by diesel exhaust particle exposure. Environ Health Perspect. 2002;110:349–353. doi: 10.1289/ehp.02110349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Bruegge K, Jelkmann W, Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr Med Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis) J Pathol. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Cohen AJ. Outdoor air pollution and lung cancer. Environ Health Perspect. 2000;108(Suppl 4):743–750. doi: 10.1289/ehp.00108s4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Muinck ED, Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol. 2004;36:25–32. doi: 10.1016/j.yjmcc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW. Inflammation and airborne particles. Clin Occup Environ Med. 2006;5:797–815. doi: 10.1016/j.coem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Sakurai M, Oda T, Tamura H, Yanagisawa R, Shimada A, Yoshikawa T. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp Biol Med (Maywood) 2006;231:1626–1632. doi: 10.1177/153537020623101007. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Tsai SS, Chiu HF, Wu TN, Chen CC, Yang CY. Ambient exposure to criteria air pollutants and risk of death from bladder cancer in Taiwan. Inhal Toxicol. 2009;21:48–54. doi: 10.1080/08958370802207326. [DOI] [PubMed] [Google Scholar]
- Luttun A, Carmeliet P. De novo vasculogenesis in the heart. Cardiovasc Res. 2003;58:378–389. doi: 10.1016/s0008-6363(03)00258-x. [DOI] [PubMed] [Google Scholar]
- McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, Chung KF, Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Barr EB, White RK, Chow JC, Schauer JJ, Zielinska B, Grosjean E. Generation and characterization of four dilutions of diesel engine exhaust for a subchronic inhalation study. Environ Sci Technol. 2004a;38:2513–2522. doi: 10.1021/es035024v. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Harrod KS, Seagrave J, Seilkop SK, Mauderly JL. Effects of low sulfur fuel and a catalyzed particle trap on the composition and toxicity of diesel emissions. Environ Health Perspect. 2004b;112:1307–1312. doi: 10.1289/ehp.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003;107:1202–1208. doi: 10.1161/01.cir.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Quan C, Sun Q, Lippmann M, Chen L. Comparative effects of diesel exhaust and ambient particles on cardiovascular system [abstract] Toxicological Sciences (The Toxicologist) 2009;108:924. [Google Scholar]
- Reed MD, Gigliotti AP, McDonald JD, Seagrave JC, Seilkop SK, Mauderly JL. Health effects of subchronic exposure to environmental levels of diesel exhaust. Inhal Toxicol. 2004;16:177–193. doi: 10.1080/08958370490277146. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110–1116. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- Vineis P, Forastiere F, Hoek G, Lipsett M. Outdoor air pollution and lung cancer: recent epidemiologic evidence. Int J Cancer. 2004;111:647–652. doi: 10.1002/ijc.20292. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Heeschen C, Sievers RE, Karliner JS, Parmley WW, Glantz SA, Cooke JP. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell. 2003;4:191–196. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.