Abstract
Although activation of spinal glia has been implicated in the development of pathological pain, the mechanisms underlying glial activation are not fully understood. One such mechanism may be triggered by reaction to neuroactive substances released from central axons of sensory afferents. The vanilloid receptor TRPV1, a nonselective cation channel in nociceptive sensory afferents, mediates the release of neurotransmitters, such as glutamate and CGRP in the dorsal horn, which can subsequently activate glia. To test the hypothesis that activation of spinal glia is mediated, at least in part, by TRPV1, we studied the expression of markers for microglia (Ionized calcium-binding adapter molecule 1, Iba1) and astrocytes (Glial Fibrillary Acidic Protein, GFAP) in the spinal cord of TRPV1 knockout mice (KO) vs. wild-type mice (WT) in models of acute (intraplantar capsaicin), inflammatory (Adjuvant-Induced Arthritis, AIA), and neuropathic pain (Partial Sciatic Nerve Ligation, PSNL). We found that i) naïve KO mice had denser immunostaining for both Iba1 and GFAP than naïve WT mice, ii) the immunostaining for Iba1 increased significantly in treated mice, compared to naïve mice, 3 days after capsaicin and 7–14 days after AIA or PSNL, and was significantly greater in WT than in KO mice 3 days after capsaicin, 7–14 days after AIA, and 7 days after PSNL, iii) the immunostaining for GFAP increased significantly in treated mice, compared to naïve mice, 3 days after capsaicin and 14–21 days after AIA or PSNL, and was significantly greater in WT than in KO mice 14 days after AIA or PSNL. Our results suggest that TRPV1 plays a role in the activation of spinal glia in mice with nociceptive, inflammatory, and neuropathic pain.
Introduction
Two major neuronal mechanisms are responsible for the pain hypersensitivity observed after inflammatory tissue damage (inflammatory pain) and after peripheral nerve lesions or spinal cord injury (neuropathic pain): peripheral sensitization, caused by the action of inflammatory mediators on the peripheral terminals of sensory neurons, and central sensitization, caused by an increase in synaptic efficacy at the level of the spinal cord, triggered by chemical, structural, and functional plasticity in dorsal horn neurons (Sandkuhler, 2009). Recent data are beginning to challenge the dominant “neuronal” view of central sensitization. Considerable evidence now points to a role for glia. Indeed, neural plasticity leading to sustained hypersensitivity of nociceptors can be triggered by signaling molecules released by activated glia (Milligan and Watkins, 2009). During the past decade, increasing attention has been paid to neuron-glia interaction as a driving force for the development and maintenance of pathological pain (Scholz and Woolf, 2007).
An important step in our understanding of the mechanisms of nociception was the cloning of TRPV1, a non-selective cation channel that serves as a receptor for noxious heat and vanilloids like capsaicin (Caterina et al., 1997). TRPV1 is synthesized in neurons in dorsal root ganglia (DRG), and is transported along their peripheral axons to skin and internal organs and tissues, including joints (Cho and Valtschanoff, 2008), and centrally to the spinal dorsal horn (Tominaga et al., 1998). TRPV1 in sensory neurons is important for acute thermal nociception and inflammatory hyperalgesia, and is also implicated in neuropathic pain, offering the possibility of novel treatments of both acute pain and chronic pain disorders (Kissin, 2008; Levine and Alessandri-Haber, 2007). Thus, silencing of TRPV1 (Christoph et al., 2006; Christoph et al., 2007) or selective TRPV1 antagonists (Honore et al., 2005; Kanai et al., 2005) reduced the acute pain caused by capsaicin and attenuated the hyperalgesia in models of inflammatory and neuropathic pain (Cui et al., 2006; Szallasi et al., 2007). Providing a link between TRPV1 and glial activation, TRPV1-mediated thermal nociception was enhanced by inflammatory mediators that are released from glia, such as bradykinin, nerve growth factor (NGF), and prostaglandins (Katanosaka et al., 2008; Schnizler et al., 2008).
Recently, KO mice have been used to investigate the role of TRPV1 in acute and chronic nociception (Bolcskei et al., 2005). We applied this experimental strategy to address the influence that the genetic deletion of TRPV1 may have on the nociceptive functions of glia. Specifically, the goal of the present study was to ascertain whether TRPV1 may contribute to glial activation in models of nociceptive, inflammatory, and neuropathic pain. For this, we employed intraarticular injections of capsaicin, known to cause acute pain through specific activation of TRPV1 on nociceptive neurons, ankle injections of complete Freund’s adjuvant (CFA), known to cause arthralgia and arthritis (adjuvant-induced arthritis, AIA), or partial sciatic nerve ligation (PSNL), known to cause neuropathic pain due to peripheral nerve damage. We then investigated the changes in expression of a specific marker of microglial activation, Iba1 (Ionized calcium-binding adapter molecule 1, Imai et al., 1996) and a specific marker for astrocytic activation, GFAP (Glial Fibrillary Acidic Protein, Eng et al., 2000) in the dorsal horn of TRPV1-KO, compared to wild-type mice of the same genetic background.
We hypothesized that TRPV1, expressed by dorsal horn glia and/or neurons mediates, at least in part, the activation of glia, which in turn contributes to the induction and maintenance of pathological pain. If confirmed, this would provide a novel mechanism for involvement of glia in central sensitization, which underlies the hyperalgesia that often develops in the course of inflammatory disease and peripheral nerve damage.
Materials and methods
Animals and treatments
A total of 56 male mice, ages 4–6 months and weighing 25–30 g, were used in this study. These included 28 each of TRPV1-KO and wild-type C57BL/6 mice (Jackson Labs, Bar Harbor, ME). All experimental procedures involving mice were carried out in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, and according to a protocol approved by the Institutional Animal Care and Use Committee at University of North Carolina. Matching numbers of KO and WT mice divided into 4 groups. In group 1, mice without treatment (Naïve, n=6) were sacrificed for collecting data in naïve animals. Mice in the treatment groups were anesthetized with a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, i.p.); group 2 received injections of capsaicin (Cap, n=12), to model acute nociceptive pain; in group 3, we used a model of adjuvant-induced arthritis (AIA, n=20), to study the effects of inflammatory pain; and group 4 received partial sciatic nerve ligature (PSNL, n=18), to create a model of neuropathic pain.
In the Cap group, mice were injected with 20 µl of 1.5% capsaicin (Sigma, St. Louis, MO) in a 7.5% solution of Tween 80 in saline (vehicle) into the footpad of one hind paw; the contralateral paw was injected with the same amount of vehicle. Mice were sacrificed at 1d (n=6) and 3d (n=6) after treatment.
For AIA, mice were injected with 10 µl of complete Freund’s adjuvant (CFA, 20 mg/ml suspension of heat-killed Mycobacterium tuberculosis in vehicle, Difco Lab, Detroit, MI) in each of two sites, front and back of one ankle, using a 30-gauge needle attached to a 10 µl Hamilton syringe. The contralateral ankle was injected with the same volume of vehicle (paraffin oil containing mannide monooleate; Freund’s incomplete adjuvant, IFA). Mice were sacrificed at 7d (n=6), 14d (n=8), and 21d (n=6) after induction of arthritis.
For PSNL, the sciatic nerve on one side was exposed at the level of the upper thigh and chromic collagen suture (6–0 metric) was used to create a tight ligature around approximately 40% of the dorsal nerve fibers in the sciatic nerve (Seltzer et al., 1990). The contralateral sciatic nerve was exposed but left intact (sham). The skin incision was repaired with Vicryl silk (5–0 metric) suture. Mice were sacrificed at 7d (n=6), 14d (n=6), and 21d (n=6) after surgery.
Pain behavior
For assessment of pain behavior, mice were acclimated unrestrained in testing chambers, 2–3 times for 30–60 min prior to testing and nociceptive sensitivity was assessed 2 days and 1 day before treatment (baseline), and then on days 1 and 3 after injections of capsaicin or on days 7, 14, and 21 after induction of AIA or PSNL. The latency to withdrawal from a thermal stimulus was measured using radiant heat test (Hargreaves et al., 1988) with a Model 336 Paw/Tail Stimulator Analgesia Meter (IITC) set at 2% idle light intensity and 40% working light intensity. The stimulus was turned off manually upon the hindpaw withdrawal or automatically if the 20 sec cut-off time was reached. Each mouse received 2 trials of each hindpaw with 10 min between trials and the results were averaged for analysis. Data were expressed as the latency to withdrawal in seconds. An overall mean of post-baseline behavioral data at each time point was calculated for each of the WT and KO groups. The behavioral testing was done by an investigator blinded to the treatment and genotype of the mice.
Tissue processing
For tissue collection, mice under deep anesthesia with sodium pentobarbital (80 mg/kg, i.p.) were perfused intracardially with 30 ml freshly prepared solution of 1% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), followed by 100 ml solution of 4% paraformaldehyde in PB. Blocks including the L4 - L5 spinal cord segments were dissected out, postfixed in 4% paraformaldehyde for 4 hours, cryoprotected in 30% sucrose in PB for 24–48 hours and sectioned on a cryostat at 30 µm. Sections were collected in PB.
Immunohistochemistry
For immunohistochemistry, floating sections were permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) for 15 minutes, blocked with 5% normal donkey serum in 0.1% Triton X-100 in PBS (NDS; Jackson Immunoresearch, West Grove, PA) for 30 minutes, and incubated overnight with the primary antibody in NDS (goat anti-GFAP, Santa Cruz #SC-6170, lot F2306, 1:500 or rabbit anti-Iba1, Wako #19-19741, lot JNG4131, 1:2,000). After several rinses with PBS and incubation with 5% NDS for 30 minutes, sections were incubated for 2 hours with appropriate secondary antibodies raised in donkey and conjugated to Cy3 (for Iba1) or FITC (for GFAP, 1:200; Jackson Immunoresearch). Finally, sections were rinsed in PBS, mounted on slides, and coverslipped with Vectashield (Vector, Burlingame, CA). Digital images were obtained with a Retiga EX cooled CCD camera (Q-Imaging, Surrey, CA) attached to a Leitz DMR fluorescent microscope (Leitz, Wetzlar, Germany) and saved as TIFF files. All images were captured with the camera settings held constant; contrast and brightness were adjusted with Photoshop CS2 (Adobe Systems, San Jose, CA); all enhancements were made uniformly across all images. As a matter of routine control, we processed sections of spinal cord according to the above protocol, except that primary or secondary antibodies were omitted; omission of primary or secondary antibodies completely abolished specific staining. We also immunostained sections of all mice for TRPV1 (goat anti-TRPV1, Santa Cruz #SC-12498, lot L1406, 1:250) to verify the lack of immunostaining in KO mice.
Data collection and statistics
Digital images were analyzed using Image J 1.38x software (NIH, Bethesda, MD). All measurements were performed by an investigator blinded to the source material, including the genotype of the mouse and the side (i.e., the notch identifying the right side in spinal cord sections was blocked out of the image and images of the right dorsal horns were flipped horizontally). For every condition and time point, images of each of the dorsal horns in each of 4–5 sections from each animal spaced 300 µm apart were analyzed from each mouse. In every image, the dorsal horn was manually outlined using the freehand tool, the threshold was set just above the level of background within the outlined region, and the labeling density was measured using the integrated density algorithm of Image J (integrated density is defined as the sum of the values of the pixels in the selection and is equivalent to the product of area of the selection and mean gray value within the selection, which in turn is the sum of the gray values of all the pixels in the selection divided by the number of pixels, and is reported in calibrated units). Data were analyzed with SPSS 11.5x (SPSS, Chicago, IL) and graphed using Kaleidagraph (Synergy Software, Reading, PA). Differences between animal groups were studied for significance with one-way analysis of variance (ANOVA), which assessed the overall influence of genotype, side (left, treated or right, control), and time after each treatment as main factors. In select cases, this was followed by a post hoc general contrast comparison using least significant difference (LSD) test. Significance was set at p<0.05.
Results
Pain behavior
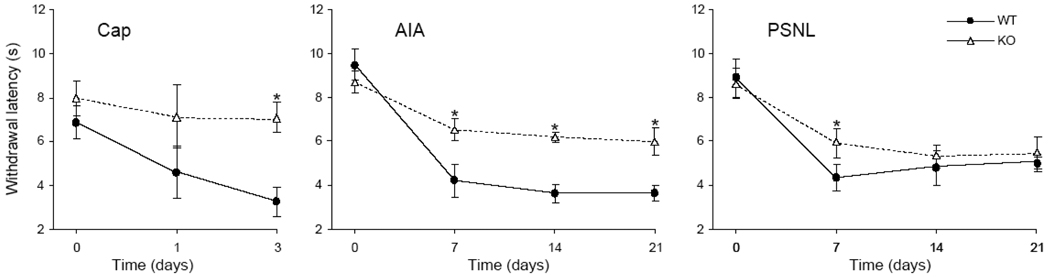
Baseline withdrawal latencies from a thermal stimulus were not significantly different between left vs. right and between WT vs. KO mice. Mice in all treatment groups developed hypersensitivity to thermal stimulation on the treatment side that varied according to pain model, elapsed time after treatment, and genotype (Fig. 1). In the Cap group, WT mice had reduced withdrawal latencies at day 1, compared to baseline, and this tendency became significant by day 3. In contrast, KO mice did not develop hypersensitivity after capsaicin. Surprisingly, KO mice appeared to be slightly more sensitive on the capsaicin-injected side than on the vehicle-injected side, however, this difference was not statistically significant.
Fig. 1.
Sensitivity to noxious heat of wild type mice (WT) vs. TRPV1-KO mice (KO) at baseline (time point 0) and 1–3 days after intraplantar capsaicin (Cap), and 7–21 days after intraarticular CFA (AIA) or partial sciatic nerve ligature (PSNL). Sensitivity on the control side was not significantly different from baseline for all treatments (not shown for clarity). Data are plotted as the mean ± standard error of mean, *p<0.05 for WT vs. KO.
In the AIA model, CFA induced significant thermal hypersensitivity in both WT and KO mice; withdrawal latency decreased to its lowest as early as 7 days post-injection and remained significant until day 21. On the IFA-injected side, the withdrawal latency was not significantly different from baseline, but was significantly longer than that on the CFA-injected side for both genotypes. Importantly, the withdrawal latency on the CFA-injected side in KO mice was significantly longer than that of the WT mice at day 7 and for the rest of the experiment.
Mice in the PSNL group developed significant thermal hypersensitivity on the side of the ligated nerve, compared to baseline, 7 days after the treatment that continued until the end of the experiment. On the contralateral side, the withdrawal latency was not significantly different from baseline for both genotypes. The withdrawal latency on the ligated side in KO mice was significantly longer than that on the ligated side in WT mice at 7 days.
Immunostaining for glial markers
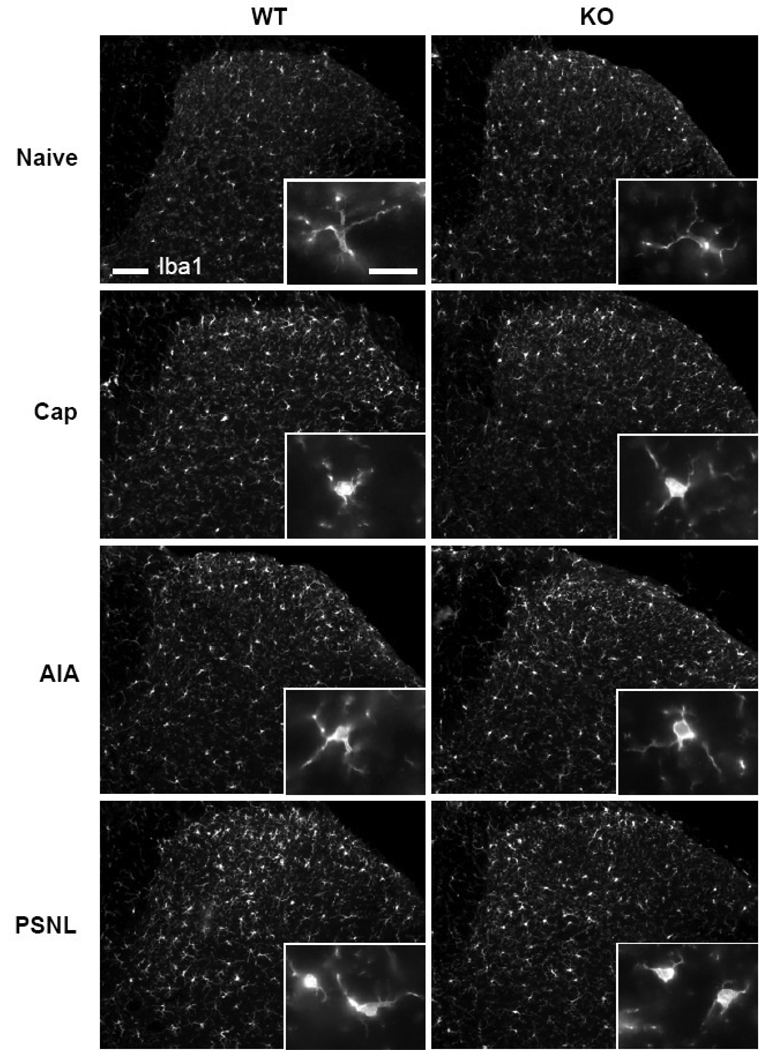
Immunohistochemistry for Iba1 revealed that in both WT and KO mice, the apparent intensity of staining in the dorsal horn increased after all treatments compared to naïve mice (Fig. 2). This increase appeared greater in WT than in KO mice and on the treated side than on the control side, particularly at 3 days after Cap and 7 days after AIA or PSNL. The increased overall staining seemed to reflect the increased size of labeled microglia rather than an increased number of labeled cells: the morphology of labeled cells appeared to change with all treatments, from small cell bodies with thin processes, characteristic of quiescent phenotype in naïve mice, to the enlarged cell bodies with hypertrophied processes, characteristic of the activated phenotype. The changes in cell morphology were noticeable over the entire dorsal horn but were more pronounced in the medial half of the superficial dorsal horn, consistent with the somatotopic representation of the paw.
Fig. 2.
Immunostaining for Iba1 in naïve wild type (WT) and TRPV1-KO (KO) mice and in pain models (the sections are from the dorsal horn of spinal segment L4 ipsilateral to the treatment: Cap, 3 days after intraplantar capsaicin; AIA, 14 days after intraarticular CFA; PSNL, 14 days after partial sciatic nerve ligature). Enlargements in the insets demonstrate the changes in cell shape of microglia between naïve mice and various treatments. Scale bars, 100 µm and 20 µm in the inset.
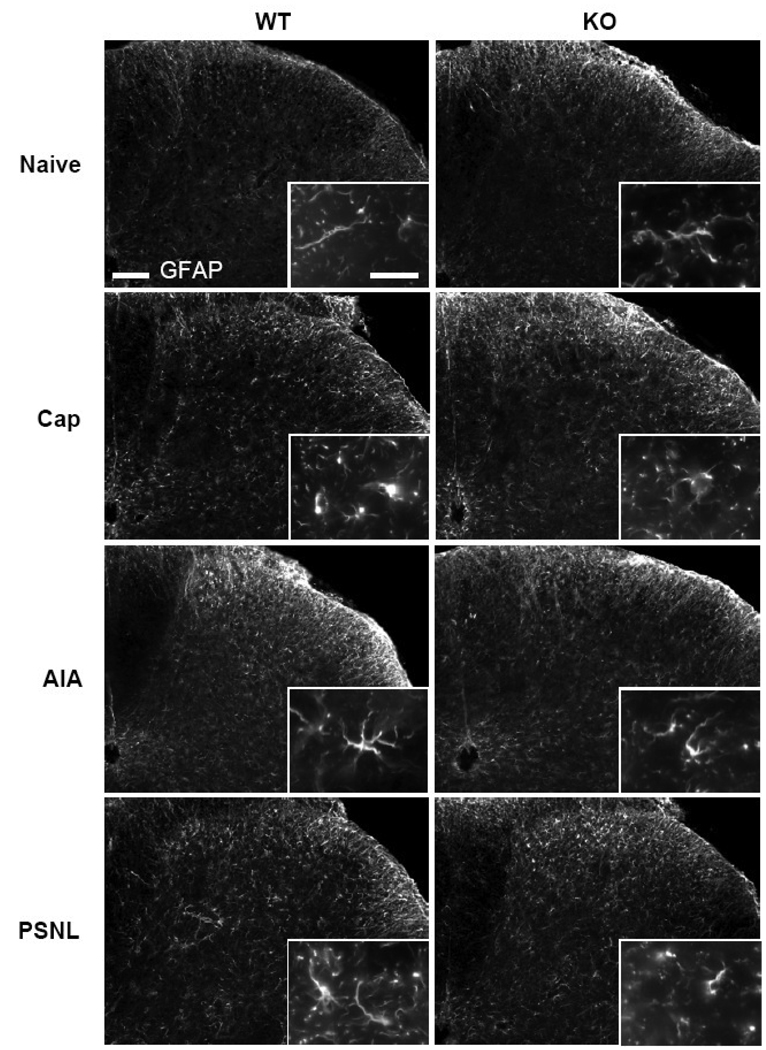
A similar overall increase in staining intensity and changes in cellular morphology with treatment was revealed by immunohistochemistry for GFAP (Fig. 3). The increased intensity of staining, compared to levels in naïve mice, was most pronounced 3 days after capsaicin and 14 days after AIA or PSNL; the left-right difference was particularly apparent on the CFA-injected side of the mice in the AIA group at days 14 and 21. Activated astrocyte morphology, including enlarged cell bodies and thickened processes, was also observed with all treatments and in the areas of the dorsal horn analogous of that for microglia.
Fig. 3.
Immunostaining for GFAP in naïve wild type (WT) and TRPV1-KO (KO) mice and in pain models (the sections are from the dorsal horn of spinal segment L4 ipsilateral to the treatment: Cap, 3 days after intraplantar capsaicin; AIA, 14 days after intraarticular CFA; PSNL, 14 days after partial sciatic nerve ligature). Enlargements in the insets demonstrate the changes in the cell shape of astrocytes between naïve mice and various treatments. Scale bars, 100 µm and 20 µm in the inset.
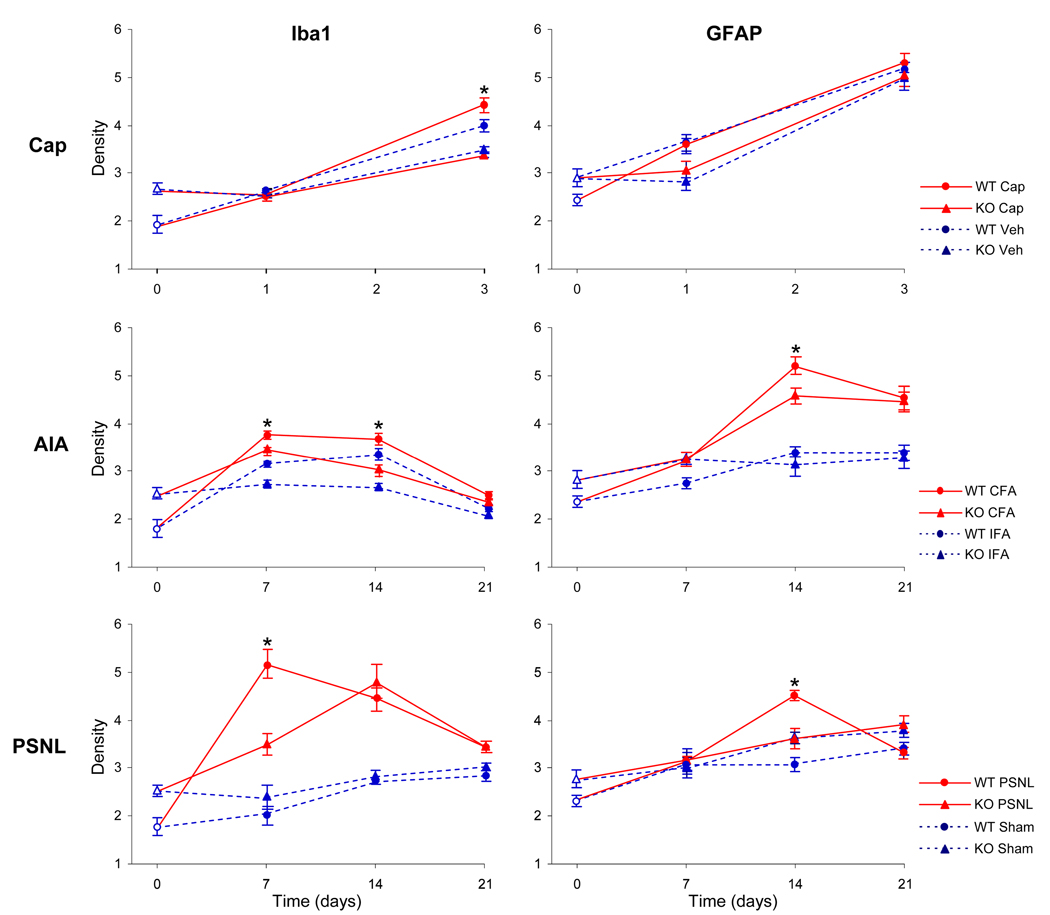
Quantitative densitometry revealed that naïve KO mice had significantly higher overall density of immunostaining for both Iba1 and GFAP than naïve WT mice (Fig. 4). Compared to naïve mice, the immunostaining for Iba1 increased significantly 3 days after capsaicin and 7–14 days after AIA or PSNL; this increase was significantly greater in WT than in KO mice at 3 days after capsaicin, 7–14 days after AIA, and 7 days after PSNL. The immunostaining for GFAP increased significantly 3 days after capsaicin (for both WT and KO mice) and 14–21 days after AIA or PSNL; this increase was significantly greater in WT than in KO mice at 14 days after AIA or PSNL.
Fig. 4.
Density (mean ± standard error of mean) of immunostaining for Iba1 and GFAP in the dorsal horn in naïve wild-type (WT) and TRPV1-KO (KO) mice (open symbols at time point “0”) and after intraplantar capsaicin (Cap) or vehicle (Veh), intraarticular CFA or IFA (AIA), and partial sciatic nerve ligature (PSNL) or sham-operated mice. *p<0.05 for WT vs. KO.
Discussion
The main findings of this study are that i) naïve TRPV1-KO mice had greater immunostaining for both Iba1 and GFAP than naïve WT mice, ii) the immunostaining for Iba1 increased significantly 3 days after capsaicin and 7–14 days after AIA or PSNL and was significantly greater in WT than in KO mice at 3 days after capsaicin, 7–14 days after AIA, and 7 days after PSNL, and iii) the immunostaining for GFAP increased significantly 3 days after capsaicin and 14–21 days after AIA or PSNL and was significantly greater in WT than in KO mice at 14 days after AIA or PSNL. We speculate that TRPV1 plays a role in the activation of spinal glia in mice with acute, inflammatory, and neuropathic pain.
After surveying a variety of markers, we chose Iba1 and GFAP to quantify activated spinal microglia and astrocytes, respectively. Iba1 is highly selectively expressed by microglia and is specifically upregulated with microglial activation (Imai and Kohsaka, 2002; Ito et al., 2001). It showed better correlation to pain hypersensitivity compared to the more popular microglial marker OX-42, which makes it a better indicator of microglial reactivity in pain models (Obata et al., 2006). In turn, GFAP is reportedly the best marker of activated astrocytes in adult animal preparations (Pekny and Pekna, 2004).
The observed changes in morphology of glia, from the “quiescent” phenotype in naïve mice to the “reactive” phenotype in treated mice, were consistent with the activated state of glia (Shapiro et al., 2009). The majority of the Iba1-positive cells in the dorsal horn that displayed this changed morphology also expressed the activated form of p38 MAP kinase and the GFAP-positive cells expressed the activated form of c-Jun-Activated Kinase (JNK), as revealed by a double immunofluorescence using phospho-specific antibodies (Han, Willcockson and Valtschanoff, unpublished observations), providing independent evidence for activation of microglia (Ji and Suter, 2007; Tsuda et al., 2005). However, at least one recent study found no correlation between activation and morphological changes of microglia in the adjuvant-induced inflammatory pain model in rats using OX-42 immunohistochemistry (Lin et al., 2007).
Our results support the notion that glial activation can be induced by C-fiber nociceptive input from the sciatic nerve (Hathway et al., 2009) and are consistent with earlier reports implicating glial activation in rodent models of acute and inflammatory pain (Fu et al., 2000; Sun et al., 2007). Specifically, they corroborate recent studies suggesting that astrocytes play a role in the initiation of acute pain and the maintenance of chronic pain, while microglial activation is important in the early phase of chronic pain (Romero-Sandoval et al., 2008). In a rat model of neuropathic pain, expression of mRNA for several markers of activated microglia was upregulated 4 hours after surgery, continued to increase until day 14, and returned to normal levels by day 28. In contrast, mRNA for GFAP did not significantly increase until day 4 and continued to increase over the duration of the study, demonstrating that peripheral nerve injury induces an early spinal microglial activation that precedes astrocytic activation and implicates astrocytes in the maintenance phase of chronic pain (Tanga et al., 2004). This time course fits the sequential activation of neurons, microglia, and astrocytes, as confirmed by the temporal pattern of activation of MAPK (Scholz and Woolf, 2007; Zhuang et al., 2005).
Intriguingly, capsaicin induced glial activation in KO mice, albeit to a lesser degree than in WT mice. Since this effect was not accompanied by thermal hyperalgesia, it may have been due to a systemic effect of capsaicin that is unrelated to pain. Alternatively, TRPV1 may not be the only sensor for capsaicin in glia. The bilateral activation of glia that we observed in WT mice with AIA may reflect the arthritogenic potential of IFA and/or the ability of CFA, when injected in one joint, to evoke systemic inflammation and arthritis in the contralateral joint (Cannon et al., 1993; Szabo et al., 2005). The effect may also be central and not peripheral, i.e. mediated by spinal cord neurons via chemical signals, possibly growth factors (Koltzenburg et al., 1999) that may be released from activated glia.
It has been reported that TRPV1 is expressed in rat astrocytes and microglia (Doly et al., 2004; Kim et al., 2006); depending on whether or not it is expressed by astrocytes and/or microglia in the mouse, the effects of genetic deletion of TRPV1 on glial activation may be direct or mediated by neurons. For example, it has been shown that TRPV1 expressed by microglia in the rat substantia nigra contributes to microglial cell death via Ca2+ signaling and mitochondrial damage (Kim et al., 2006) and that TRPV1 in astrocytoma cells can contribute to apoptosis via Ca2+ influx and activation of p38 (Amantini et al., 2007). If the same mechanisms were at play in the mouse dorsal horn, this could explain our observation that TRPV1-KO mice have a higher density of astrocytes and microglia under normal conditions than their wild-type littermates.
Several lines of evidence suggest that the pro-nociceptive action of cytokines is mediated, at least in part, by TRPV1. First, long-term exposure of primary afferent neurons to the cytokine Tumor Necrosis Factor-alpha (TNFα) significantly increased the proportion of TRPV1-immunoreactive neurons involved in generation of inflammatory hyperalgesia (Hensellek et al., 2007). Moreover, both thermal hyperalgesia and the increased expression of mRNA and protein for TRPV1 in DRG neurons were reversed by intrathecal application of neurotrophin-3, which blocks the expression of the NGF receptor, in a model of neuropathic pain (Wilson-Gerwing et al., 2005). Second, TRPV1-positive neurons express the β-chemokine-receptor-2 (CCR2) on their processes in the spinal dorsal horn, and intrathecal administration of the cognate chemokine CCL2 produced thermal hyperalgesia, which was completely prevented by an antagonist of the CCR2 (Dansereau et al., 2008), and upregulation of CCR2 may contribute to sustained excitability of TRPV1-positive nociceptors (Jung et al., 2008). Third, the production and release of the proinflammatory cytokine interleukin IL-6 by retinal microglia is partially dependent on the activation of TRPV1 (Sappington and Calkins, 2008), and the proinflammatory cytokine interleukin IL-1β can induce pain hypersensitivity by activating TRPV1-positive nociceptors via a p38-dependent mechanism (Binshtok et al., 2008). Providing a link to the mechanisms of arthritis, joint pathology and pain in a mouse model of osteoarthritis were shown to be dependent on spinal IL-1β (Fiorentino et al., 2008). Fourth, TRPV1 may also be involved in releasing inflammatory signals from immune cells (Basu and Srivastava, 2005; Scholz and Woolf, 2007).
One mediator of neuron-glia interplay in the dorsal horn that may be critically influenced by TRPV1 is glutamate (D'Mello and Dickenson, 2008). This is consistent with evidence that TRPV1-expressing primary afferents are glutamatergic and release glutamate in the dorsal horn (Hwang et al., 2004; Zhou et al., 2009). Conversely, several laboratories have reported that increases in Ca2+ in astrocytes triggers release of glutamate that activates postsynaptic glutamate receptors in neurons (Fiacco et al., 2009). The selective TRPV1 antagonist SB-366791 was found to inhibit glutamatergic transmission of C-fiber input in the rat dorsal horn following peripheral inflammation, suggesting that during inflammation, activation of spinal TRPV1 leads to tonic synaptic release of glutamate (Lappin et al., 2006). Moreover, capsaicin caused glutamate release from axons of small-diameter afferent fibers and significantly decreased withdrawal latencies to thermal stimuli; these effects were inhibited by pretreatment with capsazepine, a competitive antagonist of TRPV1 (Jin et al., 2009). We conclude that TRPV1 is involved in activating spinal glia in mice with nociceptive and pathological pain and that this involvement may have different underlying mechanism at different stages during the progression of the pain state.
Acknowledgements
This work was supported by NIH grant AR053721. We wish to thank Ms. Jieun Han for help with multiple immunofluorescent staining for glial markers, p-p38, and p-JNK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, Giangaspero F, Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- Basu S, Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1 beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Cannon GW, Woods ML, Clayton F, Griffiths MM. Induction of arthritis in DA rats by incomplete Freund's adjuvant. J Rheumatol. 1993;20:7–11. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. doi: 10.1016/j.brainres.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Grunweller A, Mika J, Schafer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Christoph T, Gillen C, Mika J, Grunweller A, Schafer MK, Schiene K, Frank R, Jostock R, Bahrenberg G, Weihe E, Erdmann VA, Kurreck J. Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int. 2007;50:281–290. doi: 10.1016/j.neuint.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008;106:757–769. doi: 10.1111/j.1471-4159.2008.05429.x. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Salio C, Conrath M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci Lett. 2004;357:123–126. doi: 10.1016/j.neulet.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, McCarthy KD. Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol. 2009;49:151–174. doi: 10.1146/annurev.pharmtox.011008.145602. [DOI] [PubMed] [Google Scholar]
- Fiorentino PM, Tallents RH, Miller JN, Brouxhon SM, O'Banion MK, Puzas JE, Kyrkanides S. Spinal interleukin-1 beta in a mouse model of arthritis and joint pain. Arthritis Rheum. 2008;58:3100–3109. doi: 10.1002/art.23866. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Burette A, Rustioni A, Valtschanoff JG. Vanilloid receptor VR1-positive primary afferents are glutamatergic and contact spinal neurons that co-express neurokinin receptor NK1 and glutamate receptors. J Neurocytol. 2004;33:321–329. doi: 10.1023/B:NEUR.0000044193.31523.a1. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci. 2009;109:233–241. doi: 10.1254/jphs.08262fp. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A. Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology. 2005;49:977–984. doi: 10.1016/j.neuropharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Katanosaka K, Banik RK, Giron R, Higashi T, Tominaga M, Mizumura K. Contribution of TRPV1 to the bradykinin-evoked nociceptive behavior and excitation of cutaneous sensory neurons. Neurosci Res. 2008;62:168–175. doi: 10.1016/j.neures.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Kim SR, Kim SU, Oh U, Jin BK. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol. 2006;177:4322–4329. doi: 10.4049/jimmunol.177.7.4322. [DOI] [PubMed] [Google Scholar]
- Kissin I. Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth Analg. 2008;107:271–281. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Lappin SC, Randall AD, Gunthorpe MJ, Morisset V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol. 2006;540:73–81. doi: 10.1016/j.ejphar.2006.04.046. [DOI] [PubMed] [Google Scholar]
- Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007;1772:989–1003. doi: 10.1016/j.bbadis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lin T, Li K, Zhang FY, Zhang ZK, Light AR, Fu KY. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J Neuroimmunol. 2007;192:40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–822. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204:428–437. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Calkins DJ. Contribution of TRPV1 to microglia-derived IL-6 and NFkappaB translocation with elevated hydrostatic pressure. Invest Ophthalmol Vis Sci. 2008;49:3004–3017. doi: 10.1167/iovs.07-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Perez ZD, Foresti ML, Arisi GM, Ribak CE. Morphological and ultrastructural features of Iba1-immunolabeled microglial cells in the hippocampal dentate gyrus. Brain Res. 2009;1266:29–36. doi: 10.1016/j.brainres.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Szabo A, Helyes Z, Sandor K, Bite A, Pinter E, Nemeth J, Banvolgyi A, Bolcskei K, Elekes K, Szolcsanyi J. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314:111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. The glutamatergic nature of TRPV1-expressing neurons in the spinal dorsal horn. J Neurochem. 2009;108:305–318. doi: 10.1111/j.1471-4159.2008.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]