Abstract
Rationale
Fatty acid nitroalkenes are endogenously-generated electrophilic byproducts of nitric oxide and nitrite-dependent oxidative inflammatory reactions. Current evidence indicates nitroalkenes support post-translational protein modifications and transcriptional activation that promote the resolution of inflammation.
Objective
The aim of this study was to assess whether in vivo administration of a synthetic nitroalkene could elicit anti-inflammatory actions in vivo using a murine model of vascular injury.
Methods and Results
The in vivo administration (21 days) of nitro-oleic acid (OA-NO2) inhibited neointimal hyperplasia after wire injury of the femoral artery in a murine model (OA-NO2 treatment resulted in reduced intimal area and intima to media ratio vs. vehicle (V) or oleic acid (OA) treated animals, P<0.0001). Increased heme oxygenase-1 (HO-1) expression accounted for much of the vascular protection induced by OA-NO2 in both cultured aortic smooth muscle cells and in vivo. Inhibition of heme oxygenase (HO) by Sn(IV)-protoporphyrin (SnPP) or HO-1 siRNA reversed OA-NO2 -induced inhibition of platelet-derived growth factor-stimulated rat aortic smooth muscle cell migration. The up-regulation of HO-1 expression also accounted for the anti-stenotic actions of OA-NO2 in vivo, since inhibition of neointimal hyperplasia following femoral artery injury was abolished in HO-1 mice (OA-NO2-treated WT vs. HO-1−/− mice, P=0.016).
Conclusions
In summary, electrophilic nitro-fatty acids induce salutary gene expression and cell functional responses that are manifested by a clinically significant outcome, inhibition of neointimal hyperplasia induced by arterial injury.
Keywords: fatty acids, arteries, stenosis, nitric oxide
Basal and inflammatory redox signaling reactions are broadly regulated by nitric oxide (NO). For example, secondary reactions of NO, promoted by a pro-oxidative inflammatory milieu, yield oxidizing, nitrosating and nitrating species that transduce NO signaling via cGMP-independent and -dependent mechanisms. Nitro-fatty acid derivatives (NO2-FA) are one class of lipid oxidation byproducts generated by NO-mediated inflammatory reactions1. Current insight indicates that the robust and reversible electrophilic reactivity of NO2-FA supports post-translational protein modifications and transcriptional activation reactions that promote the resolution of inflammation2, 3. In this regard, in vitro studies reveal NO2-FA inhibit platelet aggregation, neutrophil activation, nuclear factor kappa B (NFκB)-mediated cytokine release and stimulate heme oxygenase-1 (HO-1) expression, all via cGMP-independent mechanisms4, 5. NO2-FA also serve as ligands for peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear lipid receptor that regulates the expression of cell differentiation, development and inflammatory-related genes5, 6.
In the context of vascular responses to inflammation, NO2-FA in part inhibit vascular smooth muscle cell proliferation via activation of the Nrf2 (nuclear factor erythroid 2-related factor 2)/Keap 1 (Kelch-like ECH-associating protein) pathway7. Under basal conditions, Keap1 represses nuclear translocation of Nrf2 and Nrf2-dependent transcription. When cells are exposed to reactive species, including thiol-reactive electrophiles such as NO2-FA, Nrf2 escapes Keap1-mediated repression to activate antioxidant responsive element 8-regulated gene expression. Expression of ARE-dependent gene products, including HO-19, 10, attenuates inflammatory responses and maintains cellular redox homeostasis.
HO-1 is the rate-limiting enzyme in the degradation of heme, yielding biliverdin, iron, and carbon monoxide (CO). HO-1, especially when up-regulated, limits vascular inflammatory injury via metabolic, vasodilatory, and immune-modulatory actions11, 12. Nitro-linoleic acid has recently been reported to transcriptionally activate cultured vascular endothelial HO-1 expression via PPARγ- and NO-independent mechanisms13, 14 but no phenotypic responses to elevated HO-1 expression via this mechanism have been observed either in vitro or in vivo. Herein, we reveal that the extended in vivo administration of the nitroalkene derivative of oleic acid at nM concentrations potently inhibits neointimal hyperplasia after arterial injury via HO-1-dependent mechanisms, revealing the ability of endogenously-produced inflammatory byproducts to limit the progression of vascular inflammatory injury.
Methods
OA-NO2 Synthesis
Nitro-oleic acid (OA-NO2) used in this study was synthesized via nitroselenation as previously described 15.
Wire-Mediated Vascular Injury
All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Approval 0702181). Vehicle (V), OA (2 mg/kg/d), or OA-NO2 (2 mg/kg/d) were delivered by osmotic mini-pumps (21d delivery, ALZET®, Durect Corporation, CA, USA). Sn(IV) protoporphyrin (SnPP) was administered to mice (i.p., 50 μmol/kg) one time immediately prior to mini-pump implantation and femoral wire injury and then every 3d for 21d. Unilateral femoral artery injury was achieved by 3 passes of a 0.36 mm angioplasty guide wire.
Detection and Quantitation of OA-NO2 in Serum
Serum OA-NO2 levels in treated mice were quantitated using 13C isotope dilution by reverse-phase HPLC with electrospray ionization triple quadrupole mass spectrometry (ESI MS/MS) detection in the negative ion mode. Multiple reaction monitoring (MRM), following the transitions m/z=326/46 (OA-NO2) and m/z = 344/46 ([13C]OA-NO2), was utilized to quantify serum OA-NO2 levels following [13C]OA-NO2 internal standard addition prior to serum lipid extraction.
Vessel Morphometry
Intimal and medial cross-sectional areas of injured and non injured femoral arteries were measured in three sets of three serial 6 μm thick cross-sections of each artery, spaced at 300 μm intervals. Endothelial cells and smooth muscle cells were visualized by immunofluorescent staining against CD31 and smooth muscle α-actin respectively. Elastic lamina were visualized by autofluorescence.
Immunofluorescence
6 μm thick cross-sections of injured femoral arteries were stained with antibodies against HO-1 or Ki67 followed by incubation with fluorescently labeled secondary antibodies. Images were obtained using a Zeiss confocal microscope. Nuclei were stained using Hoechst stain (10 mg/ml, Sigma-Aldrich, Inc, St Louis, MO). Quantitation of proliferating cells was achieved by dividing the number of Ki67 positive nuclei by the total number of nuclei.
Cells and Cell Culture
Rat aortic smooth muscle cells (RASMC) were isolated via explant and cultured in DMEM containing 10% FBS in 5% CO2 at 37 °C. All experiments were performed using RASMC between passage 3 and 8. Cell proliferation was assessed using the Cyquant NF proliferation assay as described by the manufacturer (Invitrogen, Carlsbad, CA). Migration studies were performed using the wound assay as described in supplemental materials. For some experiments, RASMC were transfected with 50 μM siRNA against HO-1 or non-targeting control siRNA (Dharmacon Lafayette, CO) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA).
Real-Time Quantitative PCR
Total RNA from RASMC and femoral artery tissue was isolated with TRIzol® and further purified using the RNeasy Mini kit (Qiagen, Valencia, CA). Complimentary DNA was obtained using iScript reagents (Bio-Rad Laboratories, Hercules, CA) or SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Quantitative mRNA expression was assessed using real-time PCR with TaqMan Fast Universal PCR Master Mix or Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) using primers specific for HO-1, actin or GAPDH. Samples were run in triplicate on the StepOne or Prism 7000 detection systems (Applied Biosystems, Foster City, CA).
Western Blot analysis
Protein preparation, SDS-PAGE, and Western analysis were performed as previously.9 Equal amounts of protein were loaded and both HO-1 (1:5,000) and HO-2 (1:1000) were detected using Stressgen antibodies (Stressgen Biotechnologies, Ann Arbor, MI).
Heme Oxygenase Enzyme Activity
Heme oxygenase activity was measured by bilirubin generation in microsomal preparations from mouse liver as described previously16.
Statistical Analysis
Results are expressed as mean ± SD or SEM. Statistical analysis was performed using one-way ANOVA or unpaired students t-test as appropriate. Differences between groups were assessed by Bonferroni post hoc test. A value of p < 0.05 was considered statistically significant. SPSS 15.0 was used for all calculations.
Results
In Vivo Delivery of Nitro-Oleic Acid (OA-NO2)
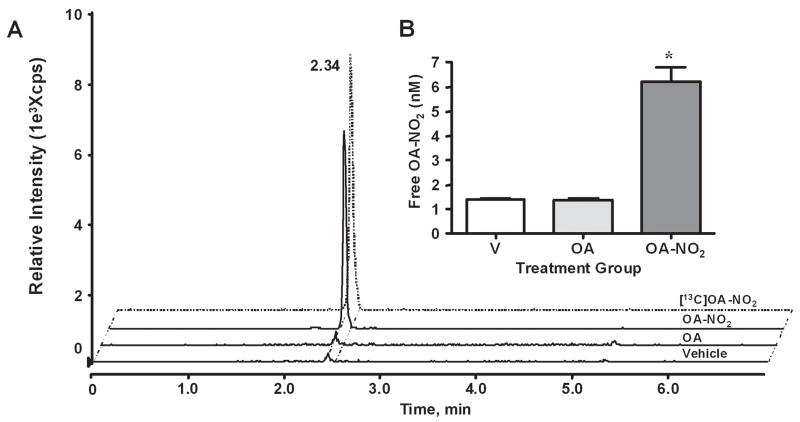
To test the effect of NO2-FA on intimal hyperplasia, C57BL/6 mice were administered vehicle (V), oleic acid (OA), or OA-NO2 via osmotic mini-pump implantation immediately prior to unilateral femoral artery injury. Serum OA-NO2 levels in treated mice were quantified using HPLC-mass spectrometry. Representative chromatographs of serum lipid extracts reveal identical retention times for both OA-NO2 administered in vivo and internal standard (Figure 1A). Serum OA-NO2 levels were significantly greater in OA-NO2 treated mice (6.21±0.60 nmol/L), compared to V- and OA-treated mice (1.43±0.02 nmol/L and 1.36±0.08 nmol/L respectively, p ≤ 0.0001, Fig. 1B).
Fig. 1.
Quantitation of OA-NO2 in serum following administration of V, OA, or OA-NO2. (A) Serum from treatment groups was analyzed by HPLC ESI MS/MS in the negative ion mode using [13C]OA-NO2 as an internal standard (dashed tracing) and by acquiring MRM transitions consistent with the loss of the nitro functional group: m/z 326/46 and m/z 344/46 for OA-NO2 and [13C]OA-NO2 respectively. (B) Free OA-NO2 levels (nM) were quantitated using ANALYST 1.4 quantitation software. Data are expressed as mean ± SD of 6–7 mice per group where *p ≤ 0.001.
Inhibition of Neointimal Proliferation by OA-NO2
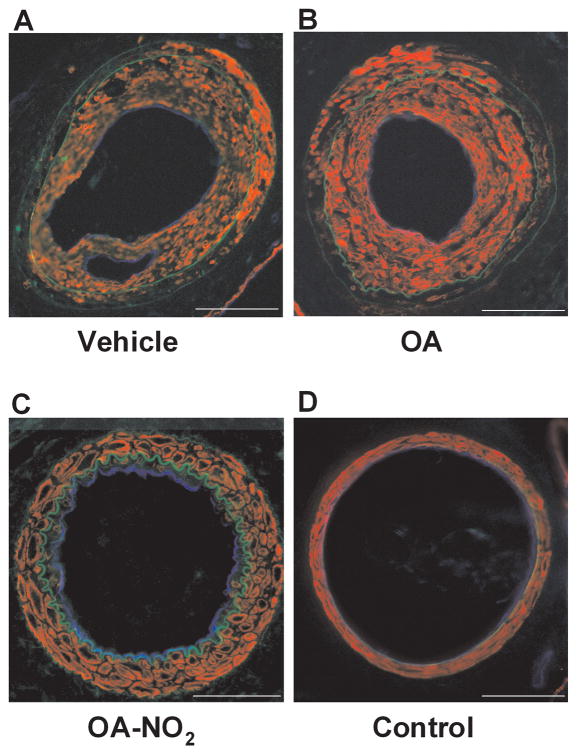
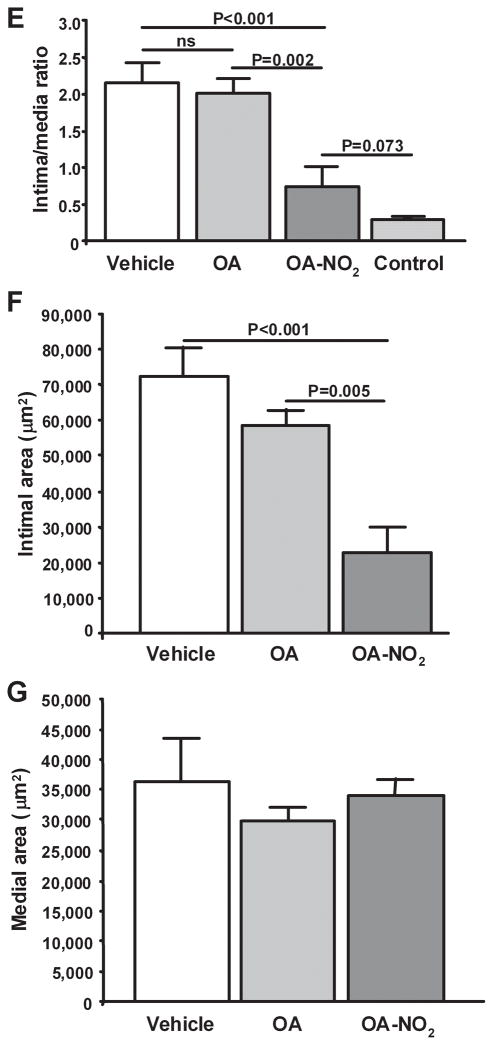
The influence of OA-NO2 on neointimal formation was investigated in a murine model where endoluminal injury to the common left femoral artery was induced by an angioplasty guide wire. This injury induces a highly reproducible neointima that can be quantified after three to four weeks17, 18. Fig. 2A–D shows representative micrographs of injured vessels from V, OA, or OA-NO2 treated animals and the contra-lateral uninjured femoral artery from V-treated mice. Vessels were isolated and stained for smooth muscle α-actin (red) and endothelial CD31 (blue). Green fluorescence represents autofluorescence of the elastic lamina. Morphometric analysis of injured vessels from V or OA treated animals revealed an intima to medial area ratio of >2, reflecting considerable neointimal hyperplasia. In contrast, injured vessels from OA-NO2-treated mice displayed a significantly reduced intimal area and intima to media ratio compared to V or OA treated animals (n=6–7 per group, P<0.0001; Figures 2E and 2F). Medial areas in all groups were not significantly different (Fig. 2G).
Fig. 2.
Nitro-oleic acid decreases neointimal formation. Femoral artery tissue sections from (A) V, (B) OA, (C) OA-NO2 treatment groups, as well as (D) control mice were labeled with anti-smooth muscle actin (red) and anti-CD31 (blue), with autofluorescence used to visualize the inner and outer elastic membrane (green) 21d after wire-induced endoluminal injury (Magnification 20X, Olympus Provis I fluorescence microscope; bar indicates 100 μm). (E–G) Quantitative morphometric analysis of artery remodeling 21d after injury. Data are expressed as mean ± SEM of 6–7 mice per group.
Induction of HO-1 Expression by OA-NO2
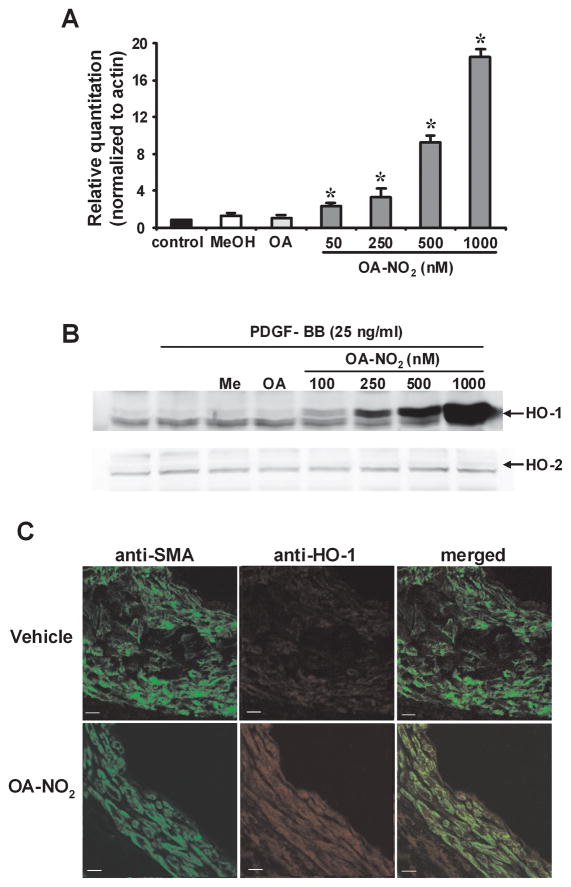
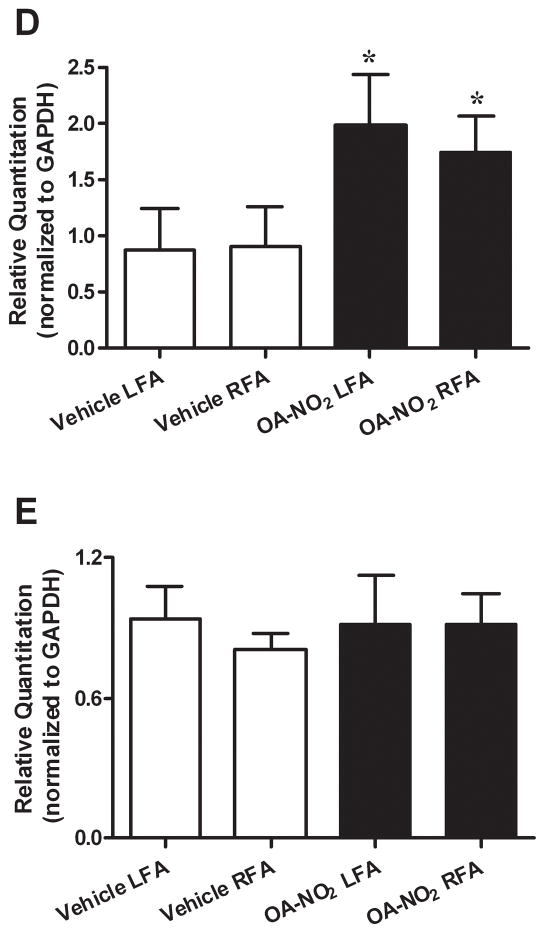
To investigate whether OA-NO2 induces HO-1 expression in vascular smooth muscle cells in vitro, rat aortic smooth muscle cells (RASMC) were grown to 100% confluence and maintained in serum-free media. Two hours after incubation with OA-NO2 (50–1000 nmol/L) HO-1 mRNA levels increased in a dose-dependent fashion (P<0.01, Fig. 3A). Western blot analysis also revealed increased expression of HO-1 protein, with no alterations in HO-2 occurring in response to OA-NO2 (100–1000 nmol/L; Fig. 3B) after 24h. Administration of OA-NO2 in vivo increased HO enzyme activity by two-fold in liver tissue (V: 1.04±0.18 vs. OA-NO2: 2.05±0.28 nmol bilirubin/mg protein/h, P = 0.02; n=6 animals per group). Furthermore, HO-1 expression was induced in vivo in the vasculature by OA-NO2 treatment. Following wire-induced injury (21d), arterial segments immunostained for HO-1 reveal that HO-1 is abundantly expressed throughout the vascular wall in OA-NO2 treated mice. In contrast, there was a significantly lower extent of vessel wall HO-1 expression in OA- or V-treated mice (Fig. 3C). In addition, quantitative real-time PCR revealed that HO-1 mRNA expression was increased in both injured and the contralateral uninjured femoral artery artery tissue 3d after OA-NO2 treatment, compared with V-treated mice following femoral artery injury (Fig. 3D). This supports that OA-NO2 is a potent inducer of HO-1 expression both in vitro and in vivo. Levels of HO-2 mRNA expression did not change in all femoral artery treatment groups (Fig. 3E).
Fig. 3.
Nitro-oleic acid induces HO-1 expression in vitro and in vivo. (A) HO-1 and HO-2 (B) expression in vitro. RASMC were treated with OA-NO2, OA, or vehicle (MeOH) at the indicated concentrations for 2h [real-time PCR analysis, *P<0.01 (ANOVA)] or 18h (immunoblot analysis). Real time PCR data are expressed as mean ± SD of 3–4 independent experiments. (C) HO-1 expression in vivo. Injured femoral arteries were labeled with anti-smooth muscle actin (green) and anti HO-1 (red), 21d after injury. Representative images are shown for V (top 3 panels) and OA-NO2 (bottom 3 panels) treated mice (Magnification 40X; Zeiss confocal microscope; bar indicates 10 μm). (D) HO-1 mRNA levels are increased in isolated femoral arteries 3d following OA-NO2 administration [compared to vehicle, mean ± SD, *P<0.01 (ANOVA)], where HO-2 mRNA levels remain unchanged (E).
Effects of OA-NO2 on Vascular Smooth Muscle Cell Proliferation and Migration
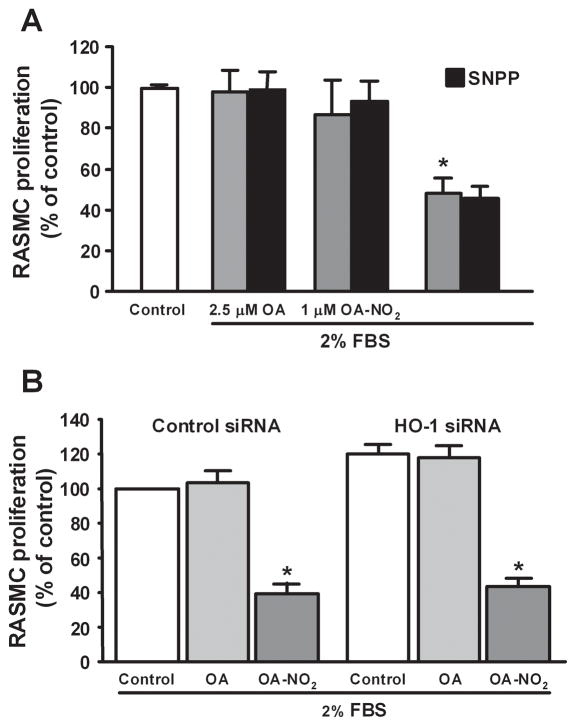
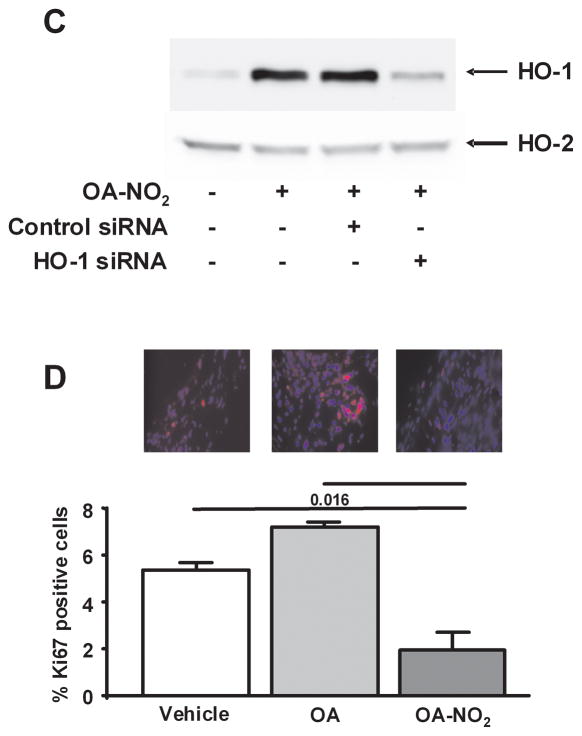
Treatment of RASMC with OA-NO2 significantly inhibited cell proliferation at a concentration of 2.5 μM (P<0.001; Fig. 4A). This anti-proliferative effect of OA-NO2 in vitro was not attenuated by either addition of the HO-1 inhibitor Sn(IV) protoporphyrin (SnPP, 50 μmol/L) or suppression of HO-1 expression by siRNA (50 μM) treatment (Fig. 4A and 4B). Effective inhibition of HO-1 expression by siRNA treatment was confirmed by western blotting, where HO-2 expression remained unaffected (Fig. 4C). Of note, immunostaining of femoral artery sections with a Ki67 antibody revealed that OA-NO2 significantly inhibited smooth muscle cell proliferation in vivo (P=0.001; Fig. 4D).
Fig. 4.
Nitro-oleic acid inhibits vascular smooth muscle cell proliferation in vitro and in vivo. (A, B) RASMC were serum starved for 24h, stimulated to proliferate with DMEM (2% serum), and treated with OA (2.5 μM) or OA-NO2 (1, 2.5 μM), and with SnPP (50 μM) or HO-1 siRNA (50μM). Concentrations for HO-1 siRNA experiments were 2.5 μm for OA and OA-NO2. After 24h cell proliferation was assessed using Cyquant NF proliferation assay. Data are presented as mean ± SEM of 3 independent experiments (*P<0.001). (C) Western blot analysis confirmed OA-NO2 (2.5 μM)-induced HO-1 gene expression is knocked-down in cells transfected with siRNA for HO-1 (50 μM), where HO-2 expression is unaffected. Control siRNA (50 μM) did not change OA-NO2 (2.5 μM) -induced HO-1 gene expression. (D) OA-NO2 significantly inhibited smooth muscle cell proliferation in vivo. Proliferative cells were visualized by staining femoral arterial sections with Ki67. Color threshold was used for quantification of Ki67 positive cells in 6 different fields of view per section (3 sections per animal, 6–7 mice per group). Magnification 40X; Olympus Provis I fluorescence microscope; Bar indicates 10 μm.
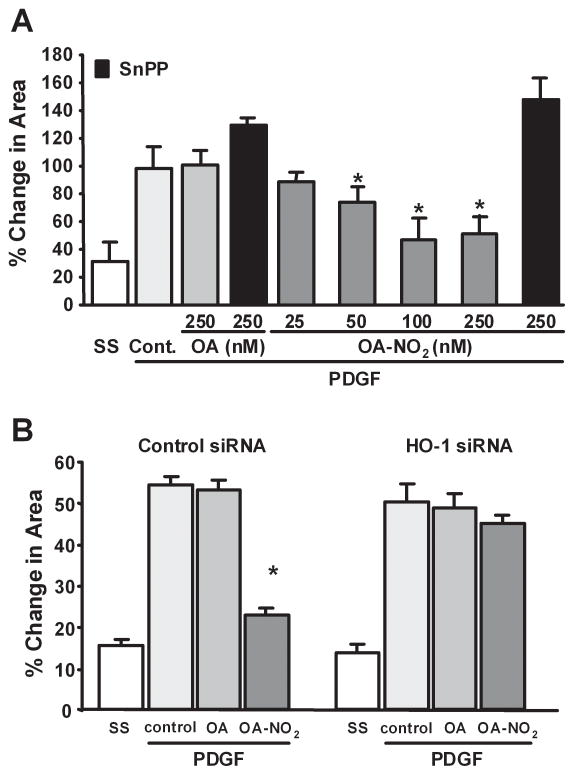
In addition to proliferation, the migration of vascular smooth muscle cells from the media to the intima after arterial injury is a key step in the development of neointimal hyperplasia. To define whether OA-NO2 influences neointimal formation by limiting vascular smooth muscle cell migration, RASMC monolayers were wounded by scratching. Images taken immediately after wounding and 18h later revealed that OA-NO2 (50–250 nmol/L) significantly inhibited RASMC migration in a dose-dependent manner, whereas OA had no effect (P<0.01; Fig. 5A). These in vitro responses reveal that OA-NO2 preferentially inhibits RASMC migration rather than limiting cell proliferation, since OA-NO2 is at least a 20-fold more potent inhibitor of vascular smooth muscle cell migration.
Fig. 5.
Nitro-oleic acid inhibits vascular smooth muscle cell migration and neointima formation via HO-1-dependent mechanisms. (A, B) OA-NO2 inhibition of RASMC migration. After inducing monolayer wounding, cells maintained in serum free media were treated with PDGF (20 ng/ml) and OA (250 nM) or OA-NO2 (25, 50, 100 and 250 nM), with SnPP (50 μM) or HO-1 siRNA (50 μM). Concentrations for HO-1 siRNA transfected experiments were as follows: OA (250 nM), OA-NO2 (250 nM) and PDGF (20 ng/ml). Quantitative image analysis was conducted 18h later to reveal extents of migration of RASMC into the denuded area. Data are presented as mean ± SEM of 6 independent experiments (*P<0.01).
To test the hypothesis that OA-NO2 inhibits vascular smooth muscle cell migration via induction of HO-1 expression and activity, RASMC were co-incubated with 50μmol/L SnPP or HO-1 siRNA and OA-NO2 (250 nmol/L). Both SnPP and HO-1 siRNA reversed the inhibitory effect of OA-NO2 on smooth muscle cell migration (Fig. 5A and 5B), indicating a significant role for HO-1 in the inhibition of vascular smooth muscle cell migration by OA-NO2.
HO-1 Mediated Anti-Stenotic Actions of OA-NO2 In Vivo
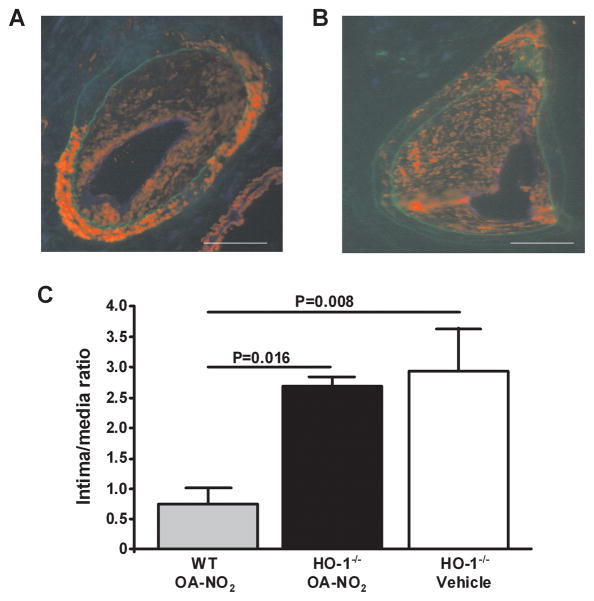
Two lines of evidence support that HO-1 expression and activity transduces OA-NO2 inhibition of wire-induced neointimal formation: (1) Administration of the HO-1 inhibitor SnPP, from the time of wire-induced injury until pathology evaluation at 21d, significantly attenuated the anti-stenotic actions of OA-NO2 in OA-NO2 treated mice (SnPP: 2.20±0.32%, OA-NO2+SnPP: 1.75±0.27%, P=0.023 vs. OA-NO2 treated animals; n=6–7 per group). (2) OA-NO2-induced inhibition of neointimal hyperplasia was abolished in OA-NO2-treated HO-1−/− mice (P=0.016; Fig. 6). In both SnPP-treated and in HO-1−/− mice, neointima formation was even more pronounced than in wild type mice. These in vivo findings confirm that induction of HO-1 by NO2-FA predominantly mediates the protection of vessels from neointimal hyperplasia.
Fig. 6.
Protective actions of OA-NO2 are inhibited in HO-1−/− mice. Femoral artery tissue sections (HO-1−/− mice) from (A) V and (B) OA-NO2 treatment groups were labeled with anti-smooth muscle actin (red) and anti-CD31 (blue), with autofluorescence used to visualize the inner and outer elastic membrane (green) 21d after wire-induced endoluminal injury (Magnification 20X; Olympus Provis I fluorescence microscope; bar indicates 100 μm). (C) Quantitative morphometric analysis of the intima to media ratio wild-type and HO-1−/− mice treated with or without OA-NO2 for 21d. Data are expressed as mean ± SEM of 4–6 mice per group.
Discussion
This is the first report demonstrating that in vivo supplementation of nM concentrations of an endogenous byproduct of nitro-oxidative inflammatory conditions induces tissue-protective actions. Electrophilic nitro-fatty acids are generated by NO and nitrite (NO2−)-dependent reactions that yield nitrogen dioxide (·NO2) as the proximal instigator of fatty acid olefin nitration. Recent reports support that a) these reactions are accelerated in the hydrophobic milieu of membrane and lipoprotein compartments and b) occur at accelerated rates in cells and organs exposed to inflammatory conditions8, 19, 20. The addition of ·NO2 to the double bond of unsaturated fatty acids yields an array of regio- and stereoisomers detectable in vivo that display kinetically rapid and reversible Michael addition to proteins1. Due to the unique physical characteristics of these derivatives, complex metabolic profiles, tissue and subcellular distribution and signaling actions are expected. In vitro studies indicate that NO2-FA will gain access to both the cytosol and nucleus to stimulate redox-dependent transcription factor and nuclear lipid receptor-dependent gene expression4–6. Current data support that NO2-FA a) covalently adduct macromolecules containing nucleophilic centers (e.g., thiol and histidine residues of proteins and glutathione)3, b) reversibly react with water to form nitro-hydroxy derivatives, c) react with coenzyme A and undergo β-oxidation15 and d) become esterified to complex lipids in membranes and lipoproteins1. Due to these reactivities, the HPLC-MS-based detection of ~10 nM “free” serum OA-NO2 upon continuous osmotic mini-pump infusion over weeks in mice will underestimate the net pool of potentially bioactive OA-NO2-derived species that could manifest adaptive and anti-inflammatory signaling actions1.
Nitro-fatty acid treatment in vitro induced HO-1 expression in cultured vascular endothelial cells and rat aortic segments13. The increased gene expression of HO-1 is stimulated by a broad array of reactive inflammatory mediators and cytokines, leading to protection against vascular injury via multiple mechanisms including heme catabolism and the signaling actions of heme metabolites such as carbon monoxide21. In this regard, increased expression of HO-1 attenuates intimal hyperplasia after arterial injury22 and reduces atherosclerotic lesion formation in LDL-receptor23 and ApoE-deficient mice24. HO-1 and CO can also inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia25–27. Herein, we reveal that induction of HO-1 expression by OA-NO2 potently inhibits vascular smooth muscle cell migration. This occurs at much lower expression levels of HO-1 than required for the inhibition of vascular smooth muscle cell proliferation, since the concentrations of OA-NO2 that induced HO-1 and inhibited vascular smooth muscle cell migration had no effect on cell proliferation in vitro. This supports that the protective actions of HO-1 after endoluminal injury in vivo are mainly a consequence of the inhibition of cell migration, a key step in neointimal formation that is proximal to proliferation of vascular smooth muscle cells. In this context, it is noted that the serum OA-NO2 levels measured upon chronic in vivo administration are not fully representative of the bioactive species that can accumulate. Electrophilic nitro-fatty acids undergo protein adduction and partial β-oxidation to shorter chain metabolites that can retain signaling capabilities28. Thus the higher concentrations of OA-NO2 required for inhibition of smooth muscle cell proliferation in vitro may be a reflection of differences in model systems. The HO-1-mediated inhibition of smooth muscle cell migration is a significant mechanism underlying the anti-stenotic benefits of OA-NO2. This property is further affirmed by the observations that siRNA inhibition of HO-1 expression or SNPP, a competitive inhibitor of HO, reversed the inhibition of RASMC migration but not the anti-proliferative actions of OA-NO2.
The induction of HO-1 expression by NO2-FA is regulated by multiple signaling mechanisms. Initial HO-1 promoter activation analyses revealed a synergy between the cAMP-dependent response element CRE and AP-1 sequences in the -4.5Kb HO-1 promoter region in response to NO2-FA exposure13. More recently, chromatin structure analysis revealed that regulation of human HO-1 expression by NO2-FA requires synergy between CRE, AP-1 and E-box sequences and involves the participation of CREB-114. Finally, NO2-FA activate Nrf2/Keap1-dependent gene expression by electrophilic adduction of critical Keap-1 thiol residues7. In turn, activation of the Nrf2/Keap1 pathway mediates the induction of phase II genes, including HO-129–31.
The loss of OA-NO2-dependent inhibition of wire-induced neointimal hyperplasia in both SnPP treated and HO-1(−/−) mice further supports the hypothesis that increased HO-1 expression in the vascular compartment accounts for a significant component of protection against intimal hyperplasia. This beneficial cardiovascular response to extended administration of fatty acid nitroalkene derivatives is likely to include additional signaling events, however. Mass spectrometric and gene expression analysis of cells exposed to electrophilic species reveals that >300 cellular proteins can be reproducibly post-translationally modified, and the expression of a similar number of genes significantly affected (unpublished data). Characteristic cell and tissue responses are also expected for different electrophiles, with these events a consequence of the charge, size and both the rate and reversibility of reaction with nucleophilic targets32. Recent evidence indicates that reversibly-reactive electrophiles may manifest little or no apparent cytotoxicity when administered at low concentrations32. Moreover, current data support that multiple transcription factors possess electrophile-reactive amino acids critical for the regulation of stress-related adaptive signaling reactions. These highly-conserved genes and their allied signaling pathways promote adaptation to the myriad of electrophilic species present in the diet and those that are endogenously generated by toxin exposure, nitro-oxidative inflammatory conditions and metabolic stress. In this regard, fatty acid nitroalkene derivatives are generated by inflammatory conditions, reversibly react via S-alkylation of protein thiols at kinetically rapid second order rate constants (~300 M−1 sec −1) and activate multiple thiol-dependent transcriptional events1.
In summary, when administered in vivo for extended periods in low concentrations, a prototypic fatty acid nitration product induces anti-inflammatory responses in both cell and animal models of vascular injury. This class of redox-derived electrophilic signaling mediators induces rapid adaptive signaling reactions, in response to changes in tissue metabolic, redox and immune status, by modulating protein function and patterns of gene expression.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by the NIH (HL58115 and HL64937 to B. Freeman; 5T32DK007052-34 to M. Cole; HL085134 to P. Bauer), the Deutsche Forschungsgemeinschaft (Ru 1472/1-1 to T. Rudolph), an American Heart Association Southeast affiliate Pre-doctoral fellowship grant (0815026E to S. Bolisetty) and the University of Pittsburgh School of Medicine (to P. Bauer, B. Freeman).
Non-Standard abbreviations
- CO
carbon monoxide
- ESI MS/MS
electrospray ionization triple quadrupole mass spectrometry
- HO
heme ogygenase
- HPLC
high performance liquid chromatography
- Keap1
Kelch-like ECH-associating protein
- MRM
multiple reaction monitoring
- NFκB
nuclear factor kappa B
- NO2-FA
nitro-fatty acids
- Nrf2
nuclear factor erythroid 2-related factor 2
- OA
oleic acid
- OA-NO2
nitro-oleic acid
- PDGF
platelet derived growth factor
- PPAR
peroxisome proliferator-activated receptor
- RASMC
rat aortic smooth muscle cells
- SnPP
Sn(IV) protoporphyrin
- V
vehicle
- WT
wild type
Footnotes
Disclosures
Dr. Freeman has financial interest in Complexa, Inc.
References
- 1.Freeman BA, Baker PR, Schopfer FJ, et al. Nitro-fatty acid formation and signaling. J Biol Chem. 2008 Jun 6;283(23):15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batthyany C, Schopfer FJ, Baker PR, et al. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006 Jul 21;281(29):20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker LM, Baker PR, Golin-Bisello F, et al. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007 Oct 19;282(42):31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui T, Schopfer FJ, Zhang J, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006 Nov 24;281(47):35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schopfer FJ, Lin Y, Baker PR, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005 Feb 15;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker PR, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005 Dec 23;280(51):42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villacorta L, Zhang J, Garcia-Barrio MT, et al. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H770–776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira AM, Ferrari MI, Trostchansky A, et al. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2009 Jan 1;417(1):223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong P, Stewart D, Hu B, et al. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antioxid Redox Signal. 2002 Apr;4(2):249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 10.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004 Mar;26(3):270–280. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- 12.Ryter SW, Otterbein LE, Morse D, et al. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002 May-Jun;234–235(1–2):249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright MM, Schopfer FJ, Baker PR, et al. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright MM, Kim J, Hock TD, et al. Human heme oxygenase-1 induction by nitro-linoleic acid is mediated by cyclic AMP, AP-1, and E-box response element interactions. Biochem J. 2009 Jun 18; doi: 10.1042/BJ20090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph V, Schopfer FJ, Khoo NK, et al. Nitro-fatty Acid Metabolome: Saturation, Desaturation, β-Oxidation, and Protein Adduction. J Biol Chem. 2009 Jan 16;284(3):1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal A, Balla J, Alam J, et al. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995 Oct;48(4):1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 17.Roque M, Fallon JT, Badimon JJ, et al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000 Feb;20(2):335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Basi DL, Adhikari N, Mariash A, et al. Femoral artery neointimal hyperplasia is reduced after wire injury in Ref-1+/− mice. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H516–521. doi: 10.1152/ajpheart.00246.2006. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell VB, Taylor KB, Parthasarathy S, et al. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. J Biol Chem. 1999 Jul 16;274(29):20083–20091. doi: 10.1074/jbc.274.29.20083. [DOI] [PubMed] [Google Scholar]
- 20.Nadtochiy SM, Baker PR, Freeman BA, et al. Mitochondrial nitroalkene formation and mild uncoupling in ischemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2008 Dec 2; doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulak J, Loboda A, Jozkowicz A. Effect of heme oxygenase-1 on vascular function and disease. Curr Opin Lipidol. 2008 Oct;19(5):505–512. doi: 10.1097/MOL.0b013e32830d81e9. [DOI] [PubMed] [Google Scholar]
- 22.Duckers HJ, Boehm M, True AL, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001 Jun;7(6):693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa K, Sugawara D, Wang X, et al. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001 Mar 16;88(5):506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 24.Juan SH, Lee TS, Tseng KW, et al. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001 Sep 25;104(13):1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 25.Kim HP, Wang X, Nakao A, et al. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tulis DA, Durante W, Liu X, et al. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001 Nov 27;104(22):2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 27.Tulis DA, Keswani AN, Peyton KJ, et al. Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol (Noisy-le-grand) 2005 Oct 3;51(5):441–446. [PMC free article] [PubMed] [Google Scholar]
- 28.Gorczynski MJ, Smitherman PK, Akiyama TE, et al. Activation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) by Nitroalkene Fatty Acids: Importance of Nitration Position and Degree of Unsaturation. J of Med Chem. 2009 doi: 10.1021/jm900326c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motohashi H, Katsuoka F, Engel JD, et al. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen YE, Fu M, Zhang J, et al. Peroxisome proliferator-activated receptors and the cardiovascular system. Vitam Horm. 2003;66:157–188. doi: 10.1016/s0083-6729(03)01005-7. [DOI] [PubMed] [Google Scholar]
- 32.Liebler DC. Protein damage by reactive electrophiles: targets and consequences. Chem Res Toxicol. 2008 Jan;21(1):117–128. doi: 10.1021/tx700235t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.