Abstract
Proteome microarrays hold great promise for various biotechnological and biomedical applications including mapping protein-protein interactions, drug discovery and biomarker discovery. However, the need to express, purify and print thousands of functional proteins at high density on a microarray substrate presents challenges which limit their wide-spread availability and use. We report the development of new methods, based on photocleavage, for the purification and printing of nascent proteins. Photocleavable biotin (PC-biotin) is incorporated into nascent proteins by misaminoacylated tRNAs used in a coupled transcription/translation rabbit reticulocyte cell-free expression system. Proteins were affinity isolated onto (strept)avidin coated beads and then photo-released (PC-SNAG). Compared to polyhistidine tag based affinity purification, PC-SNAG provided a higher purity yet comparable yield using a GST test protein. Antibody mediated PC-SNAG is also demonstrated. PC-SNAG proteins were found to exhibit native enzymatic activity and were suitable for the printing of ordered protein microarrays used in protein-protein interaction assays. Alternatively, when beads carrying photocleavably tethered proteins were placed in close proximity to an activated planar surface and then illuminated, proteins were transferred directly to the surface (PC-PRINT) to form discrete spots whose dimensions match that of the beads. PC-PRINT can provide an inexpensive method to fabricate very large scale, high density proteome microarrays. Moreover, transferring the proteins off the beads significantly reduces background auto-fluorescence observed with common bead types. In order to decode nascent proteins which are deposited by PC-PRINT from individual beads, the feasibility of using photocleavable quantum dot codes is demonstrated.
Keywords: Cell-free protein expression, photocleavage, proteomics, photocleavable biotin, Protein microarrays, proteome microarrays, tRNA mediated protein engineering, protein interaction, protein isolation, microarray printing, misaminoacylated tRNA
Introduction
Protein microarrays (1–3) can facilitate a variety of proteome-wide screening applications such as mapping protein-protein interactions in cellular pathways (4–6), detecting protein-drug interactions (6), determining kinase substrate preferences (6, 7), evaluating antibody specificity (8) and discovering biomarkers (9) such as novel autoantigens (10, 11). However, significant challenges throughout the entire microarray fabrication process limit their wide spread availability and use (4). For example, the fabrication of large-scale proteome microarrays requires the low-cost expression of thousands of human proteins followed by their rapid purification and printing to a surface at high densities and in functional form.
Conventional methods of protein microarray fabrication typically involve gene cloning, cellular transfection, protein expression in cell cultures, tag-mediated affinity purification and mechanical protein printing to microarray surfaces (5–8). While some of this can be done in parallel and is partially automatable, the process is still tedious and expensive, especially for proteins expressed in mammalian cells. Moreover, because of these limitations, proteins are not easily produced on-demand, but instead in bulk quantities, leading to storage and stability issues (4). This strategy also makes the process less amenable to fabricating custom arrays of smaller subsets of proteins. Finally, despite the automation, such approaches to microarray fabrication are not truly multiplexed and hence have not achieved optimal throughput.
Cell-free protein expression is currently being explored as an attractive means to produce proteins for microarrays (12–14) and has the potential to overcome many of the aforementioned limitations. Key advantages of cell-free expression include speed of production (e.g. 1 hr reaction time), elimination of the need for transfection and cell culture, ease of manipulation and protein recovery as well as the ability to express proteins that cellular systems cannot, such as those that are toxic to or degraded by the host cell or form insoluble inclusion bodies. Furthermore, unlike cellular expression methods which use DNA cloned into plasmid vectors, many cell-free synthesis systems can directly accept linear PCR DNA, avoiding gene cloning procedures in initial screening applications. Importantly, eukaryotic, especially mammalian cell-free protein synthesis systems such as rabbit reticulocyte lysate are capable of producing soluble, properly folded, post-translationally modified and functional proteins, including multi-pass integral membrane proteins that can be inserted into phospholipid/membrane vesicles (15–22). Finally, cell-free expression is compatible with tRNA mediated protein engineering (TRAMPE), whereby misaminoacylated tRNAs are used to co-translationally incorporate non-native amino acids into the nascent proteins (23–33). These non-native amino acids can include detection and affinity tags which are useful for microarray fabrication and read-out.
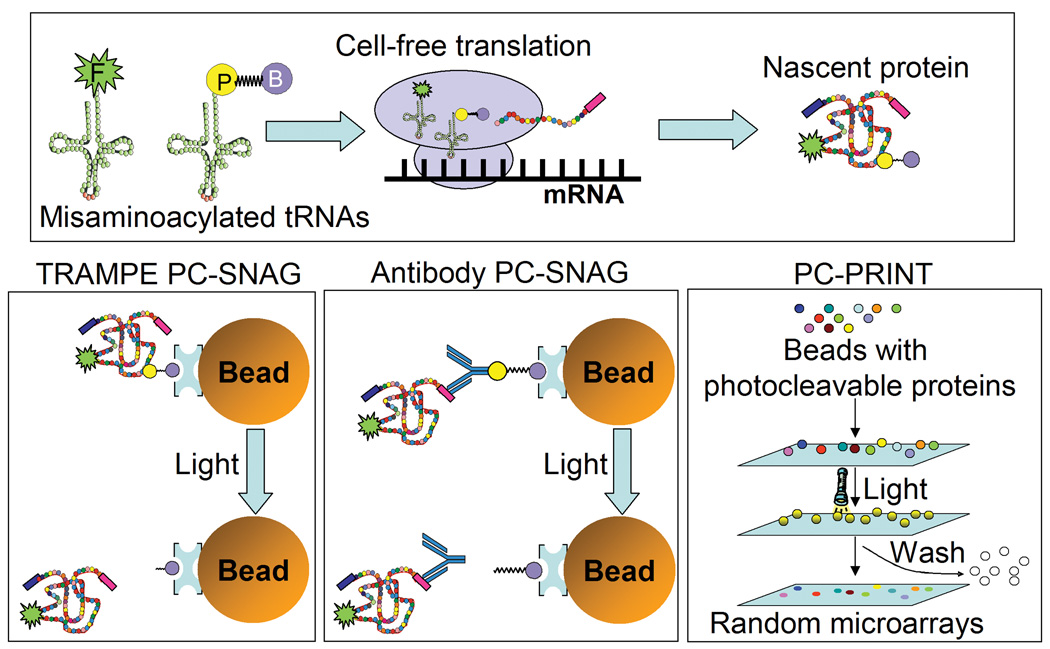
We report here the development of new methods, based on photochemical cleavage, for the purification and surface printing of cell-free expressed nascent proteins. Previously, we reported the TRAMPE incorporation and subsequent detection of either fluorescein, BODIPY-FL, biotin, photocleavable biotin (PC-biotin) or a dual biotin/BODIPY-FL marker at the N-terminus of proteins using formyl methionine initiator or initiator-suppressor tRNAs in a prokaryotic cell-free protein synthesis system (29, 31). We have also reported the incorporation of either the BODIPY-FL detection tag alone or both BODIPY-FL and biotin, each at random lysine positions, using a eukaryotic cell-free expression system for a molecular diagnostic ELISA assay (33). Here, for the first time, TRAMPE incorporation of PC-biotin was achieved in a eukaryotic (mammalian) cell-free protein synthesis system (Figure 1, top panel) and used for capture of the nascent protein onto (strept)avidin coated beads followed by photo-release of the functional nascent protein in pure form (PC-SNAG) (Figure 1, lower left panel). In addition, along with PC-biotin, simultaneous TRAMPE labeling with a BODIPY-FL fluorophore was performed (Figure 1, top panel) which allows protein quantification and tracking. Here, all TRAMPE labeling was done with total tRNA preparations for truly random incorporation at any amino acid position.
Figure 1. Photocleavage based isolation of cell-free expressed proteins and bead mediated photo-printing of microarrays.
The target protein can be cell-free produced and simultaneously labeled using tRNA mediated protein engineering (TRAMPE). For TRAMPE, the cell-free expression lysate is supplemented with fluorescent (F) and photocleavable biotin (PC-biotin; P-B) misaminoacylated tRNAs. PC-biotin labeled nascent proteins are isolated on (strept)avidin coated affinity beads and photo-released in pure form, in a process termed PC-SNAG. “TRAMPE PC-SNAG” is based on directly incorporated PC-biotin labels while the “Antibody PC-SNAG” mechanism does not use PC-biotin tRNAs for labeling, but instead isolation is via photocleavable antibodies (PC-antibodies) bound to beads through PC-biotin. Photo-release can be into solution and microarrays printed using traditional mechanical arrayers. Alternatively, rather than photo-release into solution, proteins can also be directly photo-transferred from individual beads to activated microarray substrates, and the beads washed away following light treatment. This photo-transfer process, termed “PC-PRINT”, creates high density random microarray spots that mirror the position and dimensions of the original beads. Decoding agents can also be applied by PC-PRINT, to identify the protein spots. In all cases, photo-release of the protein is achieved with 5 min of near-UV light illumination.
In a second example of PC-SNAG (antibody based), nascent proteins TRAMPE labeled with fluorescence only, were purified from the cell-free reaction mixture using PC-biotin conjugated antibodies (PC-antibodies) tethered to (strept)avidin coated beads (Figure 1, lower middle panel). In this case, the antibody, which is directed at a common C-terminal epitope on the nascent proteins, remains attached to the nascent protein after photo-release, while the PC-biotin moieties are completely removed.
Although broadly applicable, we demonstrate a novel use of these TRAMPE labeled and PC-SNAG purified proteins in the fabrication of ordered protein microarrays. Alternatively, using a newly developed and related method termed PC-PRINT, beads carrying photocleavably tethered proteins were placed in close proximity to an activated planar surface and then illuminated, causing the proteins to be transferred directly to the surface to form discrete spots whose dimensions match that of the beads. Feasibility studies show that PC-PRINT may provide an inexpensive method to fabricate very large scale, high density proteome microarrays.
Materials and Methods
Materials
Purified recombinant firefly luciferase, the cell-free expressible firefly luciferase T7 plasmid and the TNT® T7 Quick for PCR DNA cell-free expression lysate were from Promega Corp. (Madison, WI). Sulfo-NHS-LC-biotin, 10 kDa MWCO Slide-A-Lyzer MINI Dialysis Units, NeutrAvidin agarose beads and NeutrAvidin Biotin Binding Protein were from Pierce Biotechnology, Inc. (Rockford, IL). The BODIPY-FL SSE labeling reagent and Alexa Fluor® 488 labeled goat anti-mouse secondary antibody were from Invitrogen Corp. (Carlsbad, CA). All oligonucleotides, both modified and unmodified were from Sigma-Genosys (The Woodlands, TX). Fluorescent, 705 nm emissions, anti-FITC antibody coated quantum dot nanocrystals were from Quantum Dot Corp. (Hayward, CA). The streptavidin-alkaline phosphatase conjugate was from Bio-Rad Laboratories (Hercules, CA). Aldehyde and epoxy activated glass microarray substrates, SuperEpoxy and SuperAldehyde, were from TeleChem International, Inc. (ArrayIt™ Division, Sunnyvale, CA). The Cy5-NHS mono-reactive ester labeling reagent was from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ). The mouse monoclonal anti-HSV tag antibody was from EMD Biosciences, Inc. (San Diego, CA). The recombinant human p53-GST fusion protein was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Source DNA for human gene cloning was either human mRNA from Clontech Laboratories, Inc. (Mountain View, CA) or cDNAs from the I.M.A.G.E. Consortium (http://image.llnl.gov/). The Ultrafree-MC, Durapore PVDF membrane, 500 µL capacity, micro-centrifuge filtration devices were from Millipore (Billerica, MA). The Luc-1 mouse monoclonal anti-luciferase antibody, cycloheximide, mammalian protease inhibitor cocktail, calcineurin from bovine brain, casein, β-casein and all other reagents were from Sigma-Aldrich (St. Louis, MO).
DNA for Cell-Free Expression
All cell-free expression plasmids containing human gene inserts were constructed in-house using the pETBblue-2 Perfectly Blunt® cloning kits according to the manufacturer’s instructions (EMD Biosciences, Inc., San Diego, CA). Human gene inserts for cloning were obtained by standard PCR using either an mRNA library or individual cDNAs as the template. All genes inserted in the pETBlue-2 plasmid contain both a C-terminal HSV epitope tag followed by a polyhistidine (His6) tag. The clones were verified by DNA sequencing, mass of the expressed proteins by SDS-PAGE and the presence of epitope tags by Western blotting.
Misaminoacylated tRNAs for TRAMPE
tRNAs were prepared by chemical aminoacylation (23, 34) using modifications of published procedures (31, 35). The overall strategy was as follows: a total mixture of yeast tRNAs (tRNACOMPLETE; codons for all amino acids) were hydrolyzed of their amino acid, truncated at the acceptor stem using snake venom phosphodiesterase I, ligated to either a PC-biotin or BODIPY-FL labeled lysine-dinucleotide conjugate (labels on ε-amine group of lysine) and purified (Olejnik, J., et al. Manuscript in preparation). The resultant PC-biotin and BODIPY-FL labeled misaminoacylated tRNAs are referred to as PC-biotin tRNACOMPLETE and BODIPY-FL tRNACOMPLETE, respectively.
PC-Antibody Affinity Resin
A monoclonal anti-HSV tag antibody at 1 µg/µL was dialyzed extensively against 200 mM sodium bicarbonate, 200 mM NaCl using 10 kDa MWCO dialysis units. The resultant recovered antibody (0.3–0.4 µg/µL) was labeled for 1 hr with mixing using 20 molar equivalents of a PC-biotin-NHS ester amine-reactive reagent (36) which was synthesized as previously described (37) (added from 5 mM stock in DMF). The reaction was quenched for 15 min by adding one-fifth volume of a 1M glycine stock. Without additional purification, the resultant antibody conjugate solution was mixed 1:1 with 0.1% BSA (w/v), TBS (50 mM Tris, pH 7.5, 200 mM NaCl) and captured on NeutrAvidin agarose beads for 30 min at a ratio of 0.25 µg of antibody conjugate per µL of packed beads. Beads were washed 4X 5 min with 10 bead volumes each wash using 0.1% BSA (w/v), TBS and re-suspended to a 50% slurry (v/v) in the same buffer. The beads were stored at +4°C with a 1.5 mM sodium azide supplement.
Cell-free Expression
Reactions were performed using a commercial transcription/translation coupled rabbit reticulocyte lysate system according to the manufacturer’s instructions (TNT® T7 Quick for PCR DNA; Promega, Madison, WI) with the following modifications: Plasmid DNA was used at ~25 ng/µL and a complete amino acid mixture was added to 50 µM of each. For tRNA mediated dual-labeling, PC-biotin tRNACOMPLETE was used at 1.0 µM along with the BODIPY-FL tRNACOMPLETE at 0.6 µM. For fluorescence labeling only, 1 to 4 µM BODIPY-FL tRNACOMPLETE was used. The reaction was carried out for 30 min at 30°C and stopped by chilling on an ice bath and the addition of equal volume of Translation Dilution Buffer (TDB) [2X PBS (1X = 50 mM sodium phosphate, pH 7.5, 100 mM NaCl), 4 mM cycloheximide, 20 mM EDTA added from a 500 mM pH 8 stock, 2 to 10 mM DTT was used with comparable results, 0.4% (v/v) of a mammalian protease inhibitor cocktail]. In some cases (see below), a detergent (0.02% v/v Triton X-100) or Analytical protein carrier (0.2% w/v BSA or β-casein used interchangeably) was included in the TDB to prevent non-specific target protein losses. The stopped reactions were equilibrated at +4°C for 15 minutes with gentle mixing and clarified by micro-centrifugation prior to further processing.
PC-SNAG
TRAMPE labeled proteins from a 200 µL cell-free expression reaction (400 µL total volume of stopped reaction after mixing with TDB as described above) were captured on 10 µL bead volume of either NeutrAvidin agarose beads or the prepared PC-antibody agarose bead affinity resin (see above). Bead volumes were scaled accordingly for smaller expression reactions (down to 10-fold smaller was tested). The isolation procedure was performed in batch mode by micro-centrifugation in polypropylene tubes or in 0.45 micron pore size, PVDF membrane, micro-centrifuge filtration devices to manipulate the affinity resin and exchange the buffers. The buffer system used for PC-SNAG washing and photo-release steps (PC-SNAG Buffer) was PBS, 1 to 5 mM DTT (used with comparable results). In some cases (see below), detergent (0.01% v/v Triton X-100) or protein carriers (0.1% w/v BSA or β-casein used interchangeably) were included in the PC-SNAG Buffer to prevent non-specific target protein losses. All steps were performed at +4°C with ice cold buffers. After capture on the beads for 30 min, the beads were washed 2X briefly and 2X for 5 min with 400–450 µL each. Photo-release of the captured nascent protein was achieved via illumination of the bead suspension, with mixing, for 5 min using near-UV light (365 nm peak UV lamp, Blak-Ray Lamp, Model XX-15, UVP, Upland, CA) at a 5 cm distance. Light illumination was performed directly in uncovered/uncapped polypropylene micro-centrifuge tubes (or microtiter plates), such that there was no solid barrier between the bead suspension and the light source. The power output under these conditions was 2.6 mW/cm2 at 360 nm, 1.0 mW/cm2 at 310 nm and 0.16 mW/cm2 at 250 nm. Alternatively, illumination could be performed through the side-walls of the closed polypropylene micro-centrifuge tubes with similar results. Depending on the sample concentration needs, photo-release was performed on bead suspensions ranging from 2.5–50% by volume. After photo-release, when needed, remaining bound material was eluted by boiling the beads for 5 min in SDS-PAGE sample loading buffer (62.5 mM Tris, pH 6.8, 350 mM DTT, 2% SDS, 10% glycerol, 0.005% bromphenol blue) with intermittent re-suspension of the beads every 1 min. When the target nascent protein content in the various fractions pertaining to the various steps of PC-SNAG was to be assayed and compared, fluid volumes of the various PC-SNAG steps were kept constant (i.e. wash and elution volumes kept equal to the volume following stopping of the crude cell-free expression reaction).
SDS-PAGE and Functional Activity Assays
Relative nascent protein amounts in the different fractions of the PC-SNAG isolation process were determined by standard SDS-PAGE (38) using NuSep 8–16% pre-cast gradient polyacrylamide Tris-Gycine mini iGels (NuSep, Inc., Austell, GA) (sample loading buffer 62.5 mM Tris, pH 6.8, 350 mM DTT, 2% SDS, 10% glycerol, 0.005% bromphenol blue) and subsequent fluorescence imaging of the incorporated BODIPY-FL label using a FluorImager SI argon laser based scanner (Molecular Dynamics/GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Image quantification was performed using the manufacturer supplied software (ImageQuant; Molecular Dynamics/GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Standard Western blotting was also performed in some cases (Supplementary Methods). To assay the functional activity of firefly luciferase, a commercially available chemiluminescent kit was used according to the manufacturer’s instructions (luciferin substrate based assay; Promega, Madison, WI). Except where noted, all experiments involving SDS-PAGE or functional activity analysis of the PC-SNAG procedure utilized protein carriers during PC-SNAG, as described above.
Immobilized Metal Chelate Chromatography
PC-SNAG was compared to immobilized metal affinity chromatography (IMAC) isolation of polyhistidine (His6) tagged cell-free expressed glutathione-s-transferase (GST). PC-SNAG was essentially performed as described earlier except that to avoid interference in the purity analysis, a 0.01% (v/v) Triton X-100 carrier was used instead of a protein carrier. For comparison of PC-SNAG to His6 isolation, the same expression reaction was divided into equal portions and the isolations performed in parallel. The His6 based isolation was performed essentially the same as for the PC-SNAG method described earlier (batch mode) with the following exceptions: DTT and EDTA buffer constituents were omitted from all steps in the sample preparation and isolation procedures (for both His6 and PC-SNAG isolation in this experiment) due to their incompatibility with IMAC. For His6 isolation, 10 µL of cobalt metal chelate Sepharose beads was used for a total binding capacity of 50 µg of His6 protein (according to manufacturer’s specifications; Talon beads, Clontech Laboratories, Inc., Mountain View, CA) versus the roughly 0.5–1 µg present in 200 µL translation reaction (39). The wash buffer additionally contained 5 mM imidazole (pH 7.5) and the elution buffer contained 150 mM imidazole (pH 7.0) per the manufacturer’s recommendations. Elution volume was the same as for PC-SNAG and was performed for 5 minutes with vortex mixing. Samples were separated by SDS-PAGE and gels imaged fluorescently as described earlier. Next, the same gel was subjected to high sensitivity silver staining for total protein detection according to published procedures (40) and gels imaged using a CCD based ChemiImager 4400 system (Alpha Innotech Corp., San Leandro, CA).
Conventional Microarray Printing of PC-SNAG Purified Proteins
Experiments in Figure 5A used a 0.01% (v/v) Triton X-100 detergent carrier in all PC-SNAG steps, instead of a protein carrier, to prevent loss of the PC-SNAG purified protein by non-specific absorption. PC-SNAG was via TRAMPE PC-biotin labels. Washed affinity beads bearing the captured proteins were re-suspended in 25 µL of PBS, 1 mM DTT, 0.01% (v/v) Triton X-100. Photo-release was performed as described earlier. Microarrays were spotted using a GMS 417 robotic pin-and-ring arrayer (Genetic Microsystems/AffyMetrix; Santa Clara, CA) set to apply approximately 1 nL total volume per spot (measured with fluorescence standards). After protein printing, dried spots were over-printed with PBS, 40% glycerol, 1mM DTT to maintain hydration (6) and allow extended binding to the microarray substrate for 30 min at 37°C in a humidified chamber. Proteins were printed onto anti-HSV antibody coated microarray substrates (antibody to the common C-terminal epitope tag). Antibody coating was achieved via treatment of epoxy substrates with 0.25 mg/mL of anti-HSV diluted in PBS, followed by extensive washing/quenching in TBS with 0.05% (v/v) Tween-20 (TBS-T) and 100 mM glycine.
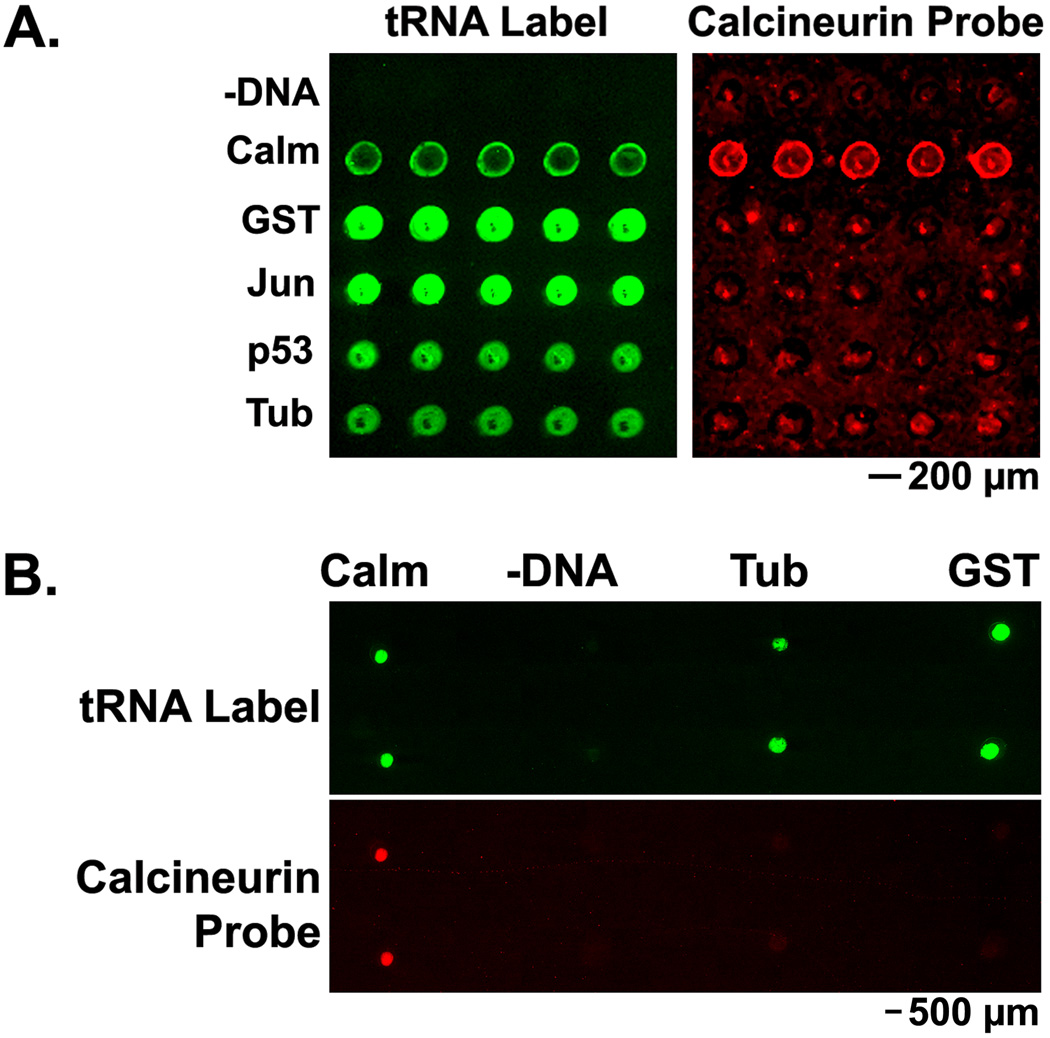
Figure 5. PC-SNAG for fabricating protein microarrays used for protein interaction assays.
Several human proteins were cell-free expressed and TRAMPE labeled. Proteins were then PC-SNAG purified into solution and printed using conventional mechanical pin-type arrayers. Protein interaction assays were performed by treating the microarray with a calcineurin-Cy5 probe (Calcineurin Probe) in the presence of calcium. (A.) TRAMPE based PC-SNAG purification of proteins that were labeled with both PC-biotin and BODIPY-FL using misaminoacylated tRNAs. (B.) Antibody based PC-SNAG purification using a PC-antibody to a common epitope tag in all expressed proteins. In this case, expressed proteins were TRAMPE labeled with BODIPY-FL only. Printed proteins: –DNA = samples differing only by omission of the DNA from the expression reaction; Calm = calmodulin; GST = glutathione-s-transferase; Jun = c-jun; p53 = cellular tumor antigen p53; Tub = α-tubulin. “tRNA Label” indicates fluorescence imaging of internal BODIPY-FL labels.
Experiments in Figure 5B did not use any detergent or protein carriers during the PC-SNAG process. PC-SNAG in this case was via a PC-antibody against the common C-terminal HSV epitope tag in all expressed proteins. Photo-release into solution was performed as described earlier except that after light treatment, the samples were supplemented with glycerol to a 40% final concentration prior to printing. Microarrays were printed to epoxy activated microarray substrates using a MicroCaster manually operated pin-type printing system (Schleicher & Schuell BioScience/Whatman, Inc., Florham Park, NJ).
PC-PRINT of a Chemically Labeled Casein Test Protein from 10 µm Beads
Bovine casein was dissolved to 2 mg/mL in 200 mM sodium bicarbonate and 200 mM NaCl. Any un-dissolved particulate was removed by passing the solution through the aforementioned micro-centrifuge filtration devices. The filtrate was then collected and desalted on a NAP-10 column according to the manufacturer’s instructions (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) against the same buffer. The resultant recovered casein (1.2 mg/mL; 1 mL used) was labeled using 10 molar equivalents of the aforementioned PC-biotin-NHS reagent (added from 50 mM stock in DMF) for 20 min with mixing. Next, this PC-biotin labeled casein was additionally labeled with the Cy5 fluorophore using a 2.7-fold molar excess of the Cy5-NHS monoreactive ester labeling reagent (added from a 27 mM stock prepared in DMSO). The reaction was allowed to proceed for 30 min with gentle mixing and protected from light. This dual-labeled casein was then purified to remove any unreacted or hydrolyzed labeling reagent using a NAP-10 column and TBS for the buffer exchange.
The dual-labeled casein was then loaded onto biotin binding beads for use in PC-PRINT. First however, biotin binding beads were in-house prepared for capture of the dual-labeled casein. For this, 10.2±0.09 µm diameter hydrophobic styrene-divinylbenzene co-polymer beads were used (Bangs Laboratories, Inc., Fishers, IN). The beads were coated with NeutrAvidin by passive absorption. A second set of beads was coated with BSA as a negative control. To do so, 57 mg of beads was washed 3X briefly with 400 µL each of 20 mM sodium phosphate, pH 6.3 and 150 mM NaCl. To wash the beads or exchange the buffers, the aforementioned micro-centrifuge filtration devices were used unless otherwise noted. After washing, the beads were re-suspended in 400 µL of NeutrAvidin or BSA at a 2.5 mg/mL concentration in 20 mM sodium phosphate, pH 6.3 and 150 mM NaCl. Binding was allowed to occur for 2 hr at 37°C with gentle mixing. Beads were then washed for 4X briefly with 400 µL with 5% BSA (w/v) in TBS. Beads were then blocked for 15 min at 37°C in the same buffer. The beads were then washed for 3X briefly with 400 µL with 0.1% sodium azide as a preservative in TBS. Beads were prepared as a 10% (v/v) stock by re-suspension in the same buffer. 100 µL of the 10% (v/v) coated bead stocks was mixed with 100 µL of dual-labeled casein (0.1 µg/µL in 5% BSA (w/v) and TBS). Binding was allowed to occur for 30 min with gentle mixing. Beads were then washed briefly 1X; 400 µL with 5% BSA (w/v) in TBS, 4X 400 µL with PBS and 1X 400 µL with 50% glycerol (v/v), 5 mM DTT in PBS. The washed bead pellets were diluted to a 2% (v/v) suspension with 50% glycerol (v/v), 5 mM DTT in PBS.
The aforementioned bead suspension was applied to epoxy activated microarray substrates in 0.5 µL droplets. The droplets were then each overlaid with 12 mm diameter round microscope cover glasses to disperse the beads and create a thin liquid film. The microarray substrates were then placed on a UV transilluminator light box (TMW-20; UVP, Upland, CA) and prior to light treatment the substrates were allowed to stand undisturbed for 5 min to allow equilibration. The substrates were then illuminated, from the bottom up, through the glass microarray substrate for 5 min. After light treatment, the substrates were then left to stand undisturbed for 10 min. The substrates were rinsed in 1X 1 min with 5% BSA (w/v) in TBS-T (TBS with 0.05% (v/v) Tween-20), 4X briefly with TBS-T and 4X with water on an orbital platform shaker. To confirm that the beads were indeed washed away, the microarray substrates were viewed via visible microscopy (note: when present, the 10 µm diameter beads are clearly visible). Negative controls where only the light treatment was omitted from the PC-PRINT procedure also confirm bead removal (see Results and Discussion).
PC-PRINT of Cell-Free Expressed Proteins from 100 µm Agarose Beads
Cell-free expressions were performed as described earlier using TRAMPE labeling with both BODIPY-FL and PC-biotin. PC-SNAG was performed as described earlier with the following exceptions: The expression reaction was scaled to 20 µL (1 µL NeutrAvidin beads) but the wash volumes were kept the same. No protein or detergent carriers were necessary and hence were omitted. Prior to photo-release (PC-PRINT), the beads were additionally washed 1X briefly in 400 µL of PBS, 40% glycerol, 5 mM DTT (all washes were performed using the aforementioned micro-centrifuge filtration devices). The washed bead pellets were then re-suspended to 1% beads (v/v) in PBS, 40% glycerol, 5 mM DTT. Beads could be stored long term this way at −20°C. In Figure 6B (excluding “Mix” panel), 0.5 µL of the bead suspension was manually applied to aldehyde activated microarray substrates in defined regions and allowed to stand for 5 min undisturbed. To achieve PC-PRINT, the substrate was then top-illuminated, without disturbance, with near-UV light using the same conditions as described for PC-SNAG. Substrates were then incubated for 30 min at 37°C in a humidified chamber, without disturbance, to fully allow transfer and covalent binding of proteins to the substrate. Beads were gently washed away following PC-PRINT (see protein-protein interaction assays), which was confirmed by visible microscopy as well as by negative controls were only the light treatment was omitted (not shown). For the special case of the experiment in Figure 6B, “Mix” panel only, details of the PC-PRINT process are described later.
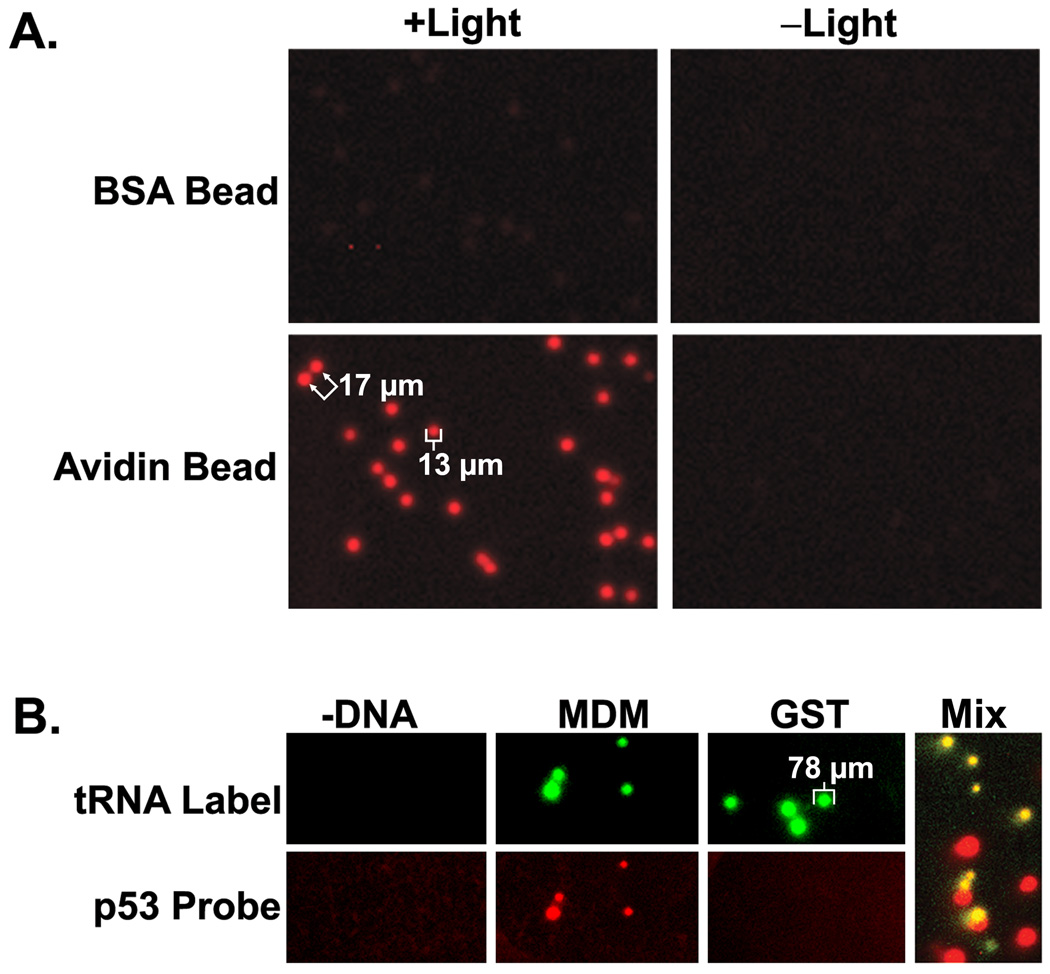
Figure 6. PC-PRINT of proteins in the fabrication of high density random microarrays and demonstration of protein-protein interaction measurements.
(A.) Analytical Biochemistry Research Article – Lim and Rothschild 45 High density PC-PRINT microarrays. NeutrAvidin (“Avidin”) or BSA coated 10 µm beads were used to capture casein that was dual labeled (chemically) with PC-biotin and Cy5 fluorescence. PC-PRINT was then performed in a 0.7 cm circular region, of which a representative portion is shown here to allow sufficient magnification. (B.) (excluding “Mix” panel) PC-PRINT based protein-protein interaction assays. Human proteins were cell-free expressed and TRAMPE labeled with both PC-biotin and BODIPY-FL. Proteins were captured on NeutrAvidin agarose beads (~75–150 µm) followed by PC-PRINT onto activated microarray substrates. Printed proteins: –DNA = samples differing only by omission of the DNA from the expression reaction; MDM = ubiquitin-protein ligase E3 MDM2; GST = glutathione-s-transferase. The microarray was probed with a p53-Cy5 conjugate (“p53 probe”) and “tRNA label” denotes imaging of the directly incorporated BODIPY-FL. (C.) (“Mix” panel only) MDM and GST bait proteins were separately cell-free expressed, tRNA labeled with PC-biotin only and, prior to isolation, each mixed with crude expressed p53 probe that was tRNA labeled with BODIPY-FL only (green). Proteins and complexes were isolated onto beads via the PC-biotin for PC-PRINT (MDM and GST bait beads mixed 1:1 just before PC-PRINT). The printed array was additionally probed with a Cy5 conjugated antibody (red) against a common epitope in all printed proteins. The fluorescence image is a 2-color overlay. Example spot sizes and spacing are indicated in µm.
PC-PRINT of Quantum Dot Nanocrystals
Quantum dot nanocrystals (QDots) with a 705 nm emissions were purchased commercially, coated with an irrelevant antibody (anti-FITC) which served as a medium for chemical attachment of the PC-biotin. QDots were labeled directly as supplied in the manufacturer’s buffer (1 µM QDots in borate buffer, pH 8.3). The aforementioned PC-biotin-NHS ester amine-reactive reagent was added from a 2 mM stock (in DMF) to a final 20 µM concentration. The reaction was carried out for 30 min with mixing. The PC-biotin labeled QDots were then purified on a NAP-10 desalting column according to the manufacturer’s instructions (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) using TBS for the buffer exchange. The resultant QDot solution was mixed with equal volume of 2X PBS, 10 mM DTT, 0.02% (v/v) Triton X-100 yielding a final concentration of 120 nM QDots (as determined in a spectrophotometer based on the manufacturer reported extinction coefficient the QDots). Bead washing and manipulation was performed using the aforementioned micro-centrifuge filtration units. 20 µL bead volume of NeutrAvidin agarose beads was pre-washed 2X 400 µL with PBS, 5 mM DTT, 0.01% (v/v) Triton X-100. 340 µL of the QDot solution (40 pmoles) was then used to re-suspend the washed bead pellet. Binding was allowed to occur for 30 min with mixing. After binding, the beads were washed 2X briefly and 2X 5 min each with PBS, 5 mM DTT, 0.01% (v/v) Triton X-100. Beads were then washed 2X briefly with 400 µL each of 50% glycerol, 5 mM DTT in PBS and re-suspended to 10% beads (v/v) in the same buffer. Beads could be stored long term this way at −20°C. Prior to PC-PR INT, beads were further washed 3X 400 µL and re-suspended to 2% beads (v/v) using 50% glycerol, 5 mM DTT in PBS. PC-PRINT was performed essentially as described above for the cell-free expressed proteins with the following exceptions: PC-PRINT was onto epoxy activated microarray substrates. To more evenly disperse the beads, 15 µL of bead the suspension was deposited onto the microarray substrate then overlaid with a standard 12 mm round microscope cover glass (coverslip) and allowed to stand for 5 min undisturbed prior to light treatment. After PC-PRINT the substrate was washed in a tray 4X 1 min with excess PBS-T (PBS with 0.05% v/v Tween-20) on an orbital shaker. The substrates were then washed 4X in deionized water (dH2O) prior to imaging. Bead removal following washing was confirmed by visible microscopy. Negative controls where only the light treatment was omitted from the PC-PRINT procedure also confirm bead removal (see Results and Discussion).
Protein-Protein Interaction Assays
Bovine calcineurin was chemically labeled using 10 molar equivalents of a Cy5-NHS ester. The reaction was performed for 30 min in 200 mM sodium bicarbonate, 200 mM NaCl (0.4 µg/µL calcineurin). Unreacted Cy5-NHS ester was quenched for 15 min by adding lysine to a final 10 mM concentration. BSA was then added as a carrier to a final 0.05% (w/v) and any free fluorophore removed by desalting on a G-25 MicroSpin column according to the manufacturer’s instructions (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). A p53-Cy5 conjugate was also prepared for interaction assays from a commercially available human recombinant p53-GST fusion protein in the same manner with the following major exceptions: The manufacturer supplied p53 was exchanged into 200 mM sodium bicarbonate, 200 mM NaCl, 5 mM DTT and 10 mM EDTA by dialysis prior to labeling. After labeling and quenching, the sample was mixed with equal volume of 2X PBS, 10 mM DTT, 0.2% (w/v) β-casein prior to processing on a NAP-10 desalting column according to the manufacturer’s instructions (GE Healthcare Bio-Sciences Corp., Piscataway, NJ), against a 1/2X concentration of the same buffer.
For calcineurin-Cy5 probing, printed microarray substrates were rinsed 3X briefly with excess TBS and blocked for 10 min using 1% BSA (w/v) in TBS. The calcineurin-Cy5 conjugate was diluted 1/30 (approximate 5 µg/mL final) in TBS containing 1% BSA (w/v), 2 mM CaCl2 and used to probe the substrates for 45 min. The substrates were then washed 3X 1 min with 2 mM CaCl2 in TBS followed by a brief wash with 2 mM CaCl2 in water. Probing with the p53-Cy5 conjugate was performed in a similar manner with the following exceptions: After microarray printing, PC-PRINT substrates were washed (to remove beads) and blocked using PBS, 5 mM DTT, 100 mM glycine, 6% (w/v) BSA for 15 min followed by rinsing 3X briefly with TBS. The p53-Cy5 probe was used at approximately 20 µg/mL in 25 mM sodium phosphate, pH 7.5, 5% BSA (w/v), 150 mM NaCl, 0.05% β-casein (w/v), 5 mM DTT. Post-probing washes were in PBS followed by water.
For Figure 6B “Mix” panel only, the protein interaction assay was performed prior to PC-PRINT. Cell-free expression reactions were performed as described earlier except that tRNA mediated labeling only with PC-biotin (1 µM tRNA) was done for the MDM2 and GST “bait” proteins and only with BODIPY-FL (2 µM tRNA) for the p53 probe. After stopping the reactions with TDB, equilibration and clarification of the samples was performed as described earlier. Each crude “bait” protein was separately mixed with equal volume of p53 “probe” (equivalent of 20 µL unpurified expression reaction for each) and binding was allowed to occur for 15 min at +4°C. Each PC-biotin labeled “bait” protein and any p53 “probe” bound to it was captured on NeutrAvidin beads, the beads washed and microarray fabrication performed similar to as described earlier for PC-PRINT of cell-free expressed proteins from 100 µm agarose beads, with the following exceptions: The 1 µL volume of NeutrAvidin agarose beads used to capture each sample was pre-washed 2X briefly in 400 µL of 5 mM DTT in PBS. The “bait-probe” sample mixtures were added to the washed bead pellets and captured for 1 hr at +4°C. Beads were washed 2X briefly with 400 µL each of 5 mM DTT in PBS. The washed bead pellets were then re-suspended to 0.5% beads (v/v) in 40% glycerol, 5mM DTT in PBS, then the 2 bead populations (GST and MDM2 “baits” each probed separately with p53) were mixed 1:1 just prior to PC-PRINT. PC-PRINT was performed using a cover glass overlay as done with the aforementioned quantum dot experiments. Following PC-PRINT to epoxy activated substrates, they were washed 1X briefly in water.
Microarray Imaging and Quantification
In all cases, following the final washing, microarray substrates were dried by placing them in 50 mL conical tubes padded at the bottom with lint-free paper towels and spun in a standard swing-bucket clinical centrifuge at 2,500 rpm for 1 min. Substrates were imaged on an ArrayWoRxe BioChip reader (Applied Precision, LLC, Issaquah, WA). Microarrays printed from 10 µm beads were imaged at 3 µm resolution and all others at 9.7 µm. Quantification of the microarrays was performed on the original 16-bit grayscale images using the image analysis software ImageQuant (Molecular Dynamics/GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Results and Discussion
Photocleavage Based Purification of Nascent Proteins
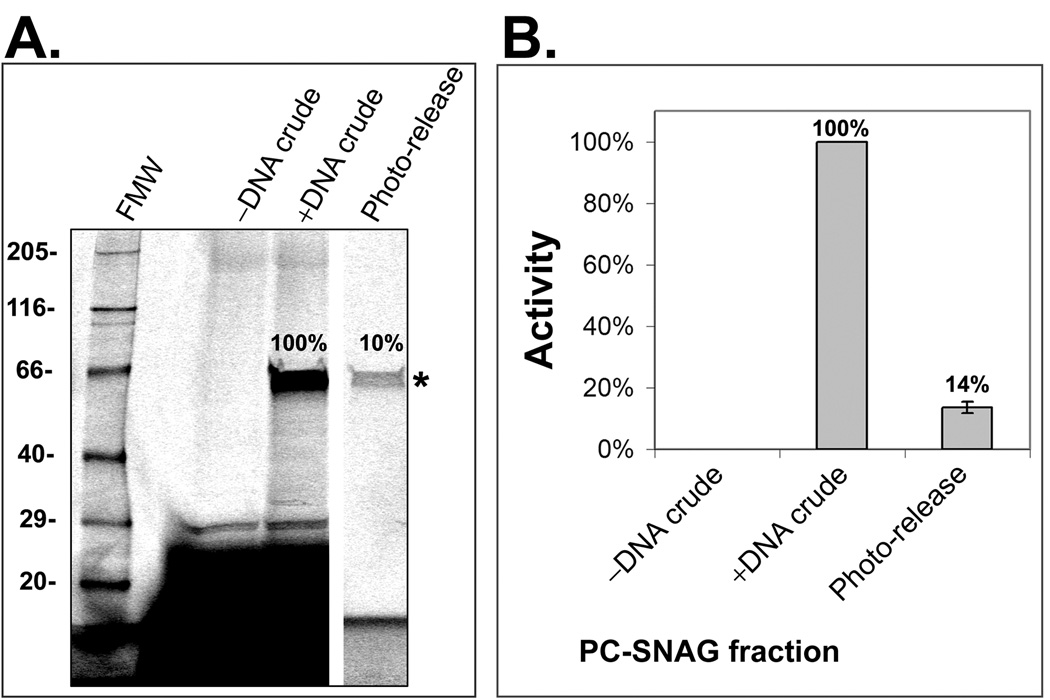
For the first time, trace labeling of cell-free expressed proteins with photocleavable biotin (PC-biotin), at random positions throughout the polypeptide chain, was accomplished using TRAMPE in a mammalian expression system. Additional simultaneous TRAMPE labeling with a BODIPY-FL fluorophore was also performed. Specifically, a yeast total tRNA extract (tRNACOMPLETE) was chemically aminoacylated with a modified lysine to form either BODIPY-FL-lysine tRNACOMPLETE or PC-biotin-lysine tRNACOMPLETE, both of which were used during protein expression in a coupled transcription/translation rabbit reticulocyte cell-free lysate. Furthermore, a unique method of purification of these cell-free expressed proteins was developed by capturing them by their directly incorporated TRAMPE-mediated PC-biotin labels onto a (strept)avidin affinity media and subsequently photo-releasing them into solution in a pure and functional form (PC-SNAG). To control for protein yield and function, PC-SNAG was initially evaluated using luciferase as a test protein. PC-SNAG yield was determined relative to the measured amount of starting crude nascent luciferase. Yield, as determined by three different assays, was 10±1% (n=2) using SDS-PAGE with fluorescence detection of the TRAMPE BODIPY-FL labels (Figure 2A), 14±2% (n=2) using a functional activity assay (Figure 2B) and 9±0.3% (n=2) using Western blot (Supplementary Methods and Supplementary Figure 1). SDS-PAGE and Western blot based quantification compared to the functional assay supports the conclusion that the TRAMPE labeled and PC-SNAG isolated luciferase retains full enzymatic activity. The Western blot assay served to further validate the fluorescence SDS-PAGE measurements and was also used to calculate absolute yields based on known standards. The absolute PC-SNAG yield was determined for 6 distinct test proteins in all (luciferase and human proteins c-jun, MDM2, p53, PKAcα and GST A2), with an average 325±137 pg per µL of cell-free expression reaction (see Supplementary Methods and Supplementary Figure 1 for luciferase example).
Figure 2. Analysis of PC-SNAG based on SDS-PAGE and a luciferase functional activity assay.
Luciferase was cell-free expressed and TRAMPE labeled using PC-biotin and BODIPY-FL misaminoacylated tRNAs together. PC-SNAG was achieved based on the directly incorporated PC-biotins. (A.) Fractions were separated by SDS-PAGE and luciferase (asterisk) imaged via the TRAMPE incorporated BODIPY-FL labels. (B.) Functional activity of luciferase was measured using a chemiluminescent substrate based assay. FMW = fluorescent molecular weight standards; –DNA Crude = Crude unprocessed cell-free expression reaction performed without the expressible luciferase DNA; +DNA Crude = Crude unprocessed cell-free expression reaction performed with the luciferase DNA (.i.e. actual total starting luciferase); Photo-Release = The photo-released luciferase fraction obtained by PC-SNAG. The photo-released luciferase is expressed as a percent of the “+DNA Crude” luciferase.
The relatively low yield of TRAMPE based PC-SNAG is most likely due to the relatively low incorporation of PC-biotin into the nascent proteins. In particular, PC-biotin tRNACOMPLETE competes with native tRNAs, which are charged and rechargeable with natural amino acids and hence more readily accepted by the protein synthesis machinery. In a separate series of experiments, where the PC-biotin labeled nascent luciferase fraction was purified from the unlabeled fraction (without photocleavage) and then analyzed, an average of approximately 1 PC-biotin per protein molecule was estimated (Supplementary Methods and Supplementary Figure 2). Since luciferase is a 550 amino acid protein, an average labeling ratio of 1 per luciferase molecule, in the labeled fraction, is expected if the overall incorporation rate is ≤1/550 amino acids (assuming constant labeling rate). The capture efficiency of luciferase onto the NeutrAvidin beads during PC-SNAG was 25% of the total expressed luciferase, as determined by fluorescence SDS-PAGE (not shown; see Figure 4B for an example determination using a GST test protein). A total of 6 proteins were tested in all with an average capture efficiency of 34± 12% (luciferase and human proteins calmodulin, c-jun, MDM2, GST and p53). These data suggest that <100% of the total nascent protein molecules have an incorporated TRAMPE label, although label inaccessibility for binding may also contribute.
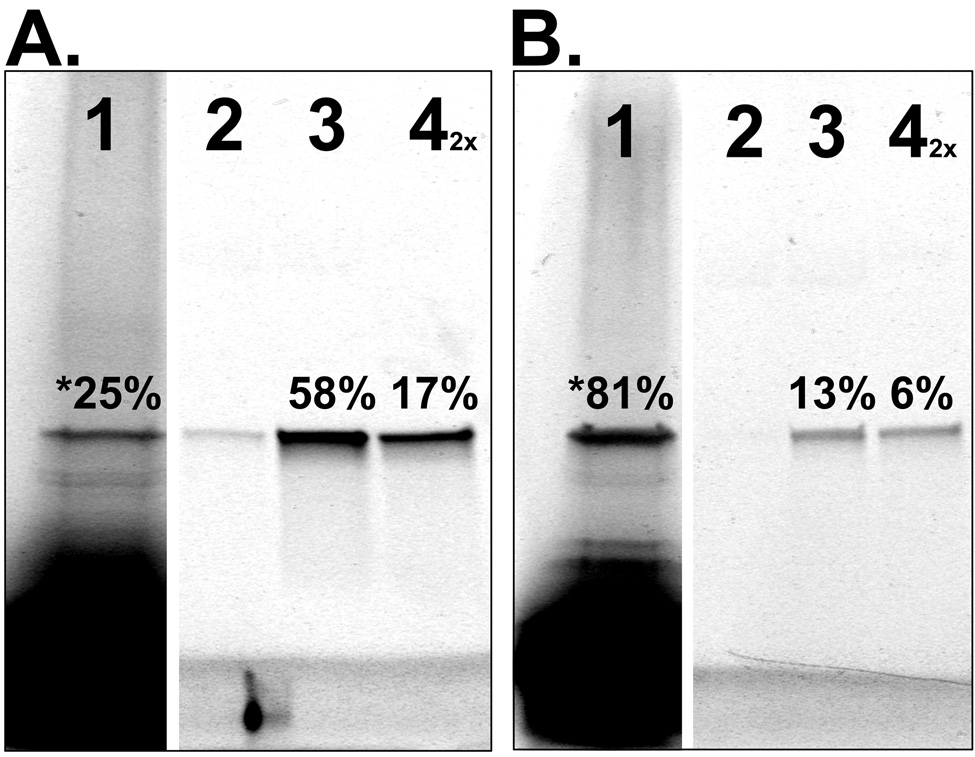
Figure 4. Demonstration and Analysis of PC-SNAG by fluorescence SDS-PAGE.
Human GST was cell-free produced using a coupled transcription/translation rabbit reticulocyte lysate. (A.) GST was TRAMPE labeled only with BODIPY-FL and PC-SNAG was achieved using a PC-antibody against a C-terminal HSV epitope tag. (B.) GST was TRAMPE labeled using PC-biotin and BODIPY-FL misaminoacylated tRNAs together and PC-SNAG was achieved based on the directly incorporated PC-biotins. All fractions from the PC-SNAG process were collected and separated by SDS-PAGE followed by fluorescence imaging of the incorporated BODIPY-FL labels. Wash fractions were collected and quantified but are not shown. Distribution of GST across the various fractions is expressed as a percent of the total GST. Lanes: 1 = Initial unbound fraction. The asterisk denotes that the number represents the total unbound GST, calculated by summing the initial unbound (shown) and wash fractions (not shown); 2 = Minus light elution (negative control); 3 = Plus light elution; 4 = Remaining bound to beads eluted by denaturation (the 2X indicates this fraction was loaded on the gel at twice the amount relative to other fractions).
Purity and yield of this new TRAMPE-based PC-SNAG method were compared to the conventional immobilized metal affinity chromatography (IMAC) method of protein isolation; using a C-terminal polyhistidine (His6) tag in cell-free expressed human GST. PC-SNAG and His6 isolation procedures were performed in parallel and in essentially identical manner with the His6 method differing only by the use of low level imidazole in the buffer for stringent washing, a cobalt chelate agarose bead resin instead of NeutrAvidin agarose beads (same bead volumes; excess target protein binding capacity in both cases) and a high imidazole concentration in the buffer for elution instead of elution by light (same duration).
As shown by fluorescence SDS-PAGE (Figure 3A), the isolated nascent GST containing the TRAMPE BODIPY-FL labels appears as a single band using either PC-SNAG or His6 isolation. Quantification of the fluorescent bands shows that TRAMPE based PC-SNAG had a similar yield (PC-SNAG 82% of His6), despite being impaired by the low PC-biotin incorporation discussed earlier. This, in part, may be related to the extremely strong biotin-(strept)avidin affinity of Ka=1015 M−1 (41), which could provide superior capture efficiency and less loss during washing. It is also possible that the His6 yield is compromised due to the presence of incompatible agents in the mammalian rabbit reticulocyte cell-free lysate (Promega Corp., Madison, WI), such as the DTT reducing agent or the competitive binding of non-His6 protein contaminants (see below).
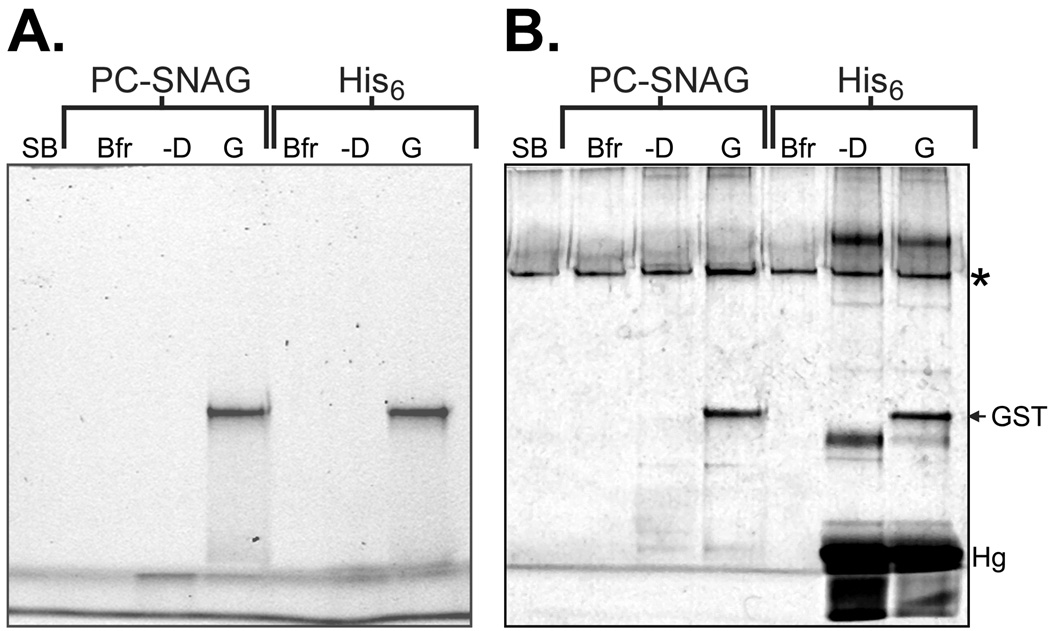
Figure 3. Comparison of PC-SNAG yield and purity to polyhistidine tag mediated isolation.
GST containing a C-terminal polyhistidine (His6) tag was cell-free expressed and TRAMPE labeled using PC-biotin and BODIPY-FL misaminoacylated tRNAs together. PC-SNAG was achieved based on the directly incorporated PC-biotins. The expression reaction mixture was split and GST was isolated either by PC-SNAG or by the His6 tag using a cobalt metal chelate affinity resin using standard procedures. (A.) BODIPY-FL fluorescence SDS-PAGE image. (B.) Silver stain for total protein of the same gel. Lanes: SB = SDS-PAGE gel loading sample buffer; Bfr = Elution buffer used for PC-SNAG or polyhistidine isolation; –D = Isolated material derived from a blank expression reaction performed lacking only the added GST DNA; G = Isolated material derived from an expression reaction performed with the added GST DNA. The asterisk denotes a general contaminant not attributable to the test samples, “GST” shows the position of the GST band on the gel and “Hg” indicates hemoglobin contamination.
While the yields were similar, the purity of PC-SNAG isolation was superior to that of the His6 method. In particular, silver staining reveals 2–3 major contaminant bands of similar or greater intensity than GST, including hemoglobin, as well as several minor contaminants in the His6 tag method (Figure 3B). The superior purity of PC-SNAG could be explained by the high selectivity of the (strept)avidin-biotin interaction as opposed to the relative promiscuity of protein binding to polyvalent metal ions. A few of the many examples include immobilization of antibodies on chelated cobalt affinity resins via the histidine rich region of the Fc domain (42) and purification of phosphoproteins on chelated ferric ion affinity resins (43); various other metal binding proteins could be potential contaminants, especially if present in high concentration relative to the target protein. Furthermore, elution of His6 tagged proteins involves competitively stripping off all that is bound to the chelated metal affinity resin, including specifically or non-specifically bound protein contaminants. Conversely, since only the target protein is attached by a PC-biotin, the photocleavage step of PC-SNAG releases only the nascent target protein, while bound contaminants would not be expected to be eluted by light treatment as there is no photocleavable linkage. In the future, a more comprehensive comparison of a wider variety of test protein species and isolation conditions would be informative.
It should be noted that a universal contaminant was observed with both purification methods (Figure 3B asterisk), and even in lanes loaded only with plain SDS-PAGE sample buffer (all lanes were loaded with either authentic sample or plain SDS-PAGE sample buffer). Due to the extremely high sensitivity of silver staining, this most likely pertains to a reagent contamination with human skin cytokeratins (subunit molecular weight roughly 55–70 kDa). Human skin cytokeratins are a ubiquitous contaminant (e.g. human skin and air-borne dust particles), known to show up in silver stained electrophoretic gels and nearly impossible to completely eliminate with such high sensitivity procedures (40). Low levels of cytokeratin, or some other protein contaminant, were likely present in one of the SDS-PAGE sample buffer component reagents.
PC-Antibody Based Isolation of Nascent Proteins
Photocleavable affinity agents and ligands have been previously reported for various uses such as affinity chromatography (44) and solid-phase radiolabeling of antibodies followed by their photo-release (45). In addition to TRAMPE based PC-SNAG, here we also report the photocleavable antibody (PC-antibody) mediated purification of cell-free expressed proteins (PC-antibody based PC-SNAG; Figure 1, lower middle panel). In contrast to TRAMPE based PC-SNAG, capture efficiency is expected to be significantly improved by using PC-antibodies. In this case, 100% of the protein molecules contain the epitope tag to which the PC-antibody binds, as opposed to the estimated <100% labeling by TRAMPE (discussed earlier). In order to characterize this approach, human GST was TRAMPE labeled only with BODIPY-FL and captured with a PC-antibody directed against its C-terminal HSV epitope tag. Capture efficiency based on fluorescence SDS-PAGE measurements was 75% of the total nascent GST, 77% of the bound material was photo-released with an overall PC-SNAG yield of 58% (Figure 4A). In contrast, for TRAMPE based PC-SNAG, a 19% capture efficiency, 68% release of the bound GST and 13% yield was observed (Figure 4B). In both cases, as expected, <3% of the bound material is released in the absence of the proper light treatment. As anticipated, the 4.5-fold yield increase with the PC-antibody is primarily achieved through a 4-fold increase in capture efficiency.
It should be noted that with the PC-antibody approach, the PC-biotin is photocleaved from the antibody, but the antibody remains bound to the target protein. In general, presence of bound antibody could cause interference with downstream assays, e.g. functional assays, or mediate unwanted background in various protein interaction assays. In the context of fabricating protein microarrays, the PC-antibody approach is not unlike other methods using antibodies to tether the proteins to the array surface (4), or methods using recombinant target proteins bearing a large fusion tag (e.g. GST tag) to facilitate protein purification (5). However, the presence of a bound antibody or large fusion tag could result in background arising from interaction with the labeled probe used to treat the microarray or impair the biological function of the printed target proteins. On the other hand, such methods could be used to uniformly orient the target proteins on the surface (5) as well as act as a buffer layer to reduce the potential for surface induced protein denaturation.
PC-SNAG Application to Microarrays
Because PC-SNAG provides a rapid method to go from gene to purified protein, it is an effective method for producing proteins for a microarray. The process of going from expressible DNA to cell-free synthesized and PC-SNAG purified protein is extremely rapid, and can be completed in less than 2 hours. Furthermore, since only ≤1 pg/spot protein is required for an assayable proteome microarray (5), the lower yield of TRAMPE is not problematic. As such, the aforementioned measured TRAMPE based PC-SNAG yields are adequate for at least 300 spots per µL of cell-free expression reaction input, while the PC-antibody based method can afford a ~5-fold yield improvement. Whether it be by PC-antibodies or direct PC-biotin TRAMPE labels, we report for the first time the application of PC-biotin purified proteins to the fabrication of microarrays. Examples of microarrays produced by conventional mechanical printing of PC-SNAG purified cell-free expressed human proteins are shown in Figure 5. As a control for background, samples differing only by omission of the expressible DNA from the cell-free reaction (“-DNA”) were also applied to the microarrays. The microarrays were treated with a calcineurin-Cy5 fluorescent probe, to assay the established calmodulin-calcineurin binding interaction (46).
In one scenario involving 5 human test proteins, TRAMPE based PC-SNAG was followed by printing onto microarray substrates coated with a common HSV epitope tag antibody (Figure 5A). TRAMPE labeling with BODIPY-FL allowed tracking of all protein spots while the calcineurin-Cy5 probe bound only to calmodulin. Averaged over 5 replicate spots, the BODIPY-FL signal to noise ratios were 6:1, 14:1, 19:1, 9:1 and 7:1 for calmodulin, GST, c-jun, p53 and tubulin respectively. The signal to noise ratio for the calcineurin-Cy5 probe binding was 4:1 for calmodulin while all other proteins were essentially equal to background. As a measure of selectivity of the calcineurin-Cy5 binding, the calcineurin-Cy5 to BODIPY-FL signal ratio was calculated (background mathematically subtracted from data). Using this calculation, calmodulin had a 28, 41, 8 and 5-fold higher ratio versus GST, c-jun, p53 and tubulin respectively.
In another scenario, PC-SNAG was achieved using a PC-antibody directed against a common epitope tag, followed by printing onto epoxy activated microarray substrates (Figure 5B). Averaged over 5 replicate spots (2 shown), the BODIPY-FL signal to noise ratios were 7:1, 7:1 and 9:1 for calmodulin, tubulin and GST respectively. The signal to noise ratio for the calcineurin-Cy5 probe binding was 5:1 for calmodulin while tubulin and GST were essentially equal to background. As a measure of selectivity of the calcineurin-Cy5 binding, the calcineurin-Cy5 to BODIPY-FL signal ratio was calculated (background mathematically subtracted from data). Using this calculation, calmodulin had a 10 and 31-fold higher ratio versus tubulin and GST respectively.
The BODIPY-FL signal readout reveals differences in microarray spot morphology and uniformity as well as signal and background levels between the TRAMPE and PC-antibody based methodologies. Overall, the PC-antibody approach produced more uniform spot morphologies and more uniform signal to noise ratios among different proteins. This could be the result of a variety of parameters including differences in composition and surface tension of the protein printing buffer used, arrayed protein concentration (function of expression level, purification efficacy and protein immobilization efficiency), type of array surface coating used (i.e. method of surface attachment of arrayed proteins) and the mechanical protein printing method used (see materials and methods for details of procedural differences between the TRAMPE and PC-antibody methods). In addition, the significantly larger protein yields (e.g. see Figure 4) with the PC-antibody purification method (potential saturation) may explain the reduced protein to protein variability in signal to noise ratios with this method (albeit with elevated overall background level as well).
Printing Proteins Directly to Surfaces Using Photocleavable Linkers (PC-PRINT)
In a process similar to PC-SNAG, a novel, non-mechanical, parallel microarray photo-printing process (PC-PRINT) was demonstrated. Instead of photo-release into solution, proteins are photo-transferred from the affinity capture beads directly to activated microarray substrates. The beads initially contact or are in close proximity to the microarray substrate and are then washed away following PC-PRINT (light treatment), leaving a random microarray of spots that mirror the original position and dimensions of the beads (Figure 1, bottom right panel). Here, the basic PC-PRINT phenomena is demonstrated along with compatibility of PC-PRINT with cell-free expressed proteins and protein interaction assays. Lastly, preliminary evidence of possible strategies for decoding the random microarrays is shown.
PC-PRINT and High Density Microarrays
To demonstrate PC-PRINT (Figure 6A), 10±0.09 µm diameter polymer beads coated with NeutrAvidin (“Avidin”), or BSA as a negative control, were used to capture a dual-labeled casein test protein that was conjugated to both PC-biotin and Cy5 fluorescence (PC-biotin-casein-Cy5). PC-PRINT was performed in a 0.7 cm diameter circular region (small sub-region shown in Figure 6A) onto epoxy activated microarray substrates (antibody coated substrates are also PC-PRINT compatible). The 10 µm diameter beads, which are easily observed by visible microscopy, are washed away after PC-PRINT (not shown). Bead removal is also confirmed by imaging the microarray in the “fluorescein” channel of the microarray scanner (not shown), since the polymer beads significantly auto-fluoresce in this channel. After Cy5 fluorescence imaging, sharply resolved 13 µm diameter fluorescent microarray features were observed only when PC-PRINT was performed from the NeutrAvidin beads loaded with PC-biotin-casein-Cy5 (Figure 6A lower left). Spots could be distinguished at a center-to-center spacing as low as 17 µm. A negative control performed from the PC-biotin-casein-Cy5 loaded NeutrAvidin beads, but without light treatment, results in no significant signal (Figure 6A lower right), further confirming that beads do not remain non-specifically stuck to the microarray substrate after washing. Using BSA beads for capture of PC-biotin-casein-Cy5 yields no significant PC-PRINT signal, thus showing bead-binding selectivity (Figure 6A top panels). At the bead density used, 4,896 spots were counted in the 0.7 cm diameter circular area, equivalent to a spot density of 13,000 spots/cm2 or ~240,000 spots on an entire 2.5 × 7.5 cm standard microarray substrate. Since the spot size on PC-PRINT microarrays is controlled by the dimensions of the bead, which could be ≤1 µm in diameter, spot densities as high as 4 × 109/cm2 may be possible, for example as already achieved with beads arrayed in etched fiber optic bundles (47). In contrast, conventional printers are limited to printing features of approximately 75–400 µm. State-of-the-art protein microarrays (5, 6) currently use ~100 µm spots and substantially lower densities of approximately 2,000/cm2.
PC-PRINT Arrays and Protein-Protein Interaction Assays
To demonstrate compatibility of this new PC-PRINT method with conventional microarray-based protein interaction assays, the human proteins MDM2 and GST were cell-free expressed using PC-biotin and BODIPY-FL TRAMPE labeling. The proteins where then isolated on agarose beads (~75–150 µm diameter range) in a similar manner as with TRAMPE based PC-SNAG. PC-PRINT was subsequently performed from the agarose beads onto aldehyde activated microarray substrates. The different bead species underwent PC-PRINT in separate defined regions by manual application. Removal of the beads after PC-PRINT was confirmed by visible microscopy. Results of the experiment are shown in Figure 6B (excluding “Mix” panel). As evidenced by the BODIPY-FL signal from the TRAMPE labels (green), PC-PRINT forms discrete protein spots, corresponding to MDM and GST, that approximate the dimensions of the agarose beads. As a negative control, a sample differing only by omission of the expressible DNA from the cell-free reaction showed no significant BODIPY-FL signal above background. The signal to noise ratio for the BODIPY-FL labeling was 11:1 for both MDM2 and GST (11 spots analyzed each). Probing of the microarray with a recombinant human p53-Cy5 fluorescent conjugate was done to allow detection of the established p53-MDM2 interaction (48). As anticipated, only the expected interaction of the p53-Cy5 probe with the MDM2 spots was observed, with a signal to noise ratio of 12:1 (11 spots), while binding to GST was essentially equal to background. As a measure of selective probe binding, the p53-Cy5 to BODIPY-FL signal ratio was 23-fold higher for MDM2 than for GST (background mathematically subtracted from data).
With PC-PRINT, new protein interaction assay formats are possible that can afford specific advantages. For example, prior to PC-PRINT, it is possible to expose the beads to a mixture containing both the “bait” protein (binds selectively to beads) and a probe (“prey”) protein which interacts with the bait. In one scenario, human MDM2 and GST bait proteins were cell-free expressed with TRAMPE PC-biotin labeling but not BODIPY-FL. Each of the crude bait proteins was separately mixed with unpurified cell-free expressed human p53 probe that was TRAMPE labeled with BODIPY-FL only. After possible interactions were allowed to occur, each PC-biotin labeled bait protein and any bound p53 probe was isolated on NeutrAvidin agarose beads which were then pooled prior to PC-PRINT. The printed microarray was probed with a Cy5 labeled antibody directed against the common HSV epitope tag in the bait proteins. The results clearly indicate that p53 interacts with MDM2 but not GST. The image (Figure 6B “Mix” panel) is a 2-color fluorescence overlay, whereby the red spots correspond to GST detected with the epitope tag antibody but with no detectable binding to the p53 probe (green). The yellow spots correspond to MDM2 also detected with the antibody but additionally bound to the p53 probe. Results were confirmed with non-mixed beads and negative controls which show no non-specific signal from the probes (not shown). Alternatively, bait proteins could be separately isolated on the beads first, the beads pooled, and then probed in a single tube to simplify the probing step. An important feature of PC-PRINT in these experiments is the ability to gently and selectively photo-transfer only the bait and hence bound probe from the bead to the microarray surface. Therefore, any non-specifically agarose-bound or NeutrAvidin-bound p53 probe is expected to be left behind on the bead surface, since it is not photocleavably linked. Other bound non-specific contaminates, e.g. from the cell-free expression lysate, that may themselves auto-fluoresce or mediate non-specific probe binding, will also not be photo-transferred to the microarray. In contrast, utilization of conventional affinity elution methods require more harsh or non-specific treatments which can release bound contaminants (e.g. see Figure 3B) that would also bind the microarray surface and increase background.
In comparison to “on-bead” assay platforms, such as flow cytometry based bead technologies or bead arrays, PC-PRINT affords additional advantages with respect to background. Bead surfaces are optimally porous or textured to increase binding capacity of the assayed protein, but this can cause undesirable light scattering (background) effects in fluorescence assays. Furthermore, many bead materials including plastics and magnetic resins also exhibit significant auto-fluorescence background. PC-PRINT of proteins from beads to optically flat, glass microarray substrates essentially eliminates such fluorescence background problems (Supplementary Figure 4). Lastly, the use of a fixed PC-PRINT microarray affords parallel signal readout and extended fluorescence exposure times not possible with flow cytometry based techniques.
The PC-PRINT process is also highly efficient because the isolated proteins are photo-transferred directly from the beads to the neighboring (or contacting) microarray surface, hence dilution of the protein prior to interaction with the microarray surface is minimized (i.e. concentration of applied protein is maximized). We have measured a 9-fold improvement, using identical bead to fluid volume ratios, with PC-PRINT versus off-line photo-release followed by mechanical array spotting (Supplementary Methods and Supplementary Figure 5). Off-line release prior to microarray deposition causes the protein to first become diluted into the solution-phase (a minimum practical fluid volume is required). Furthermore, due to its relatively low concentration and high purity, the now solution-phase protein is susceptible to non-specific adsorption onto the walls of the vial or tube in which it is stored prior to microarray printing. Although detergent or proteinaceous carriers can alleviate this phenomena, such additives may be undesirable and/or incompatible with microarrays. For example, proteinaceous carriers would not be compatible with printing to a non-specific protein-binding surface (e.g. commonly used epoxy, aldehyde or nitrocellulose microarray surfaces). Detergents on the other hand, even non-ionic, can have undesirable effects on protein function and are well known to block deposition of proteins onto nitrocellulose and hydrophobic surfaces, for example.
Feasibility of Decoding PC-PRINT Arrays
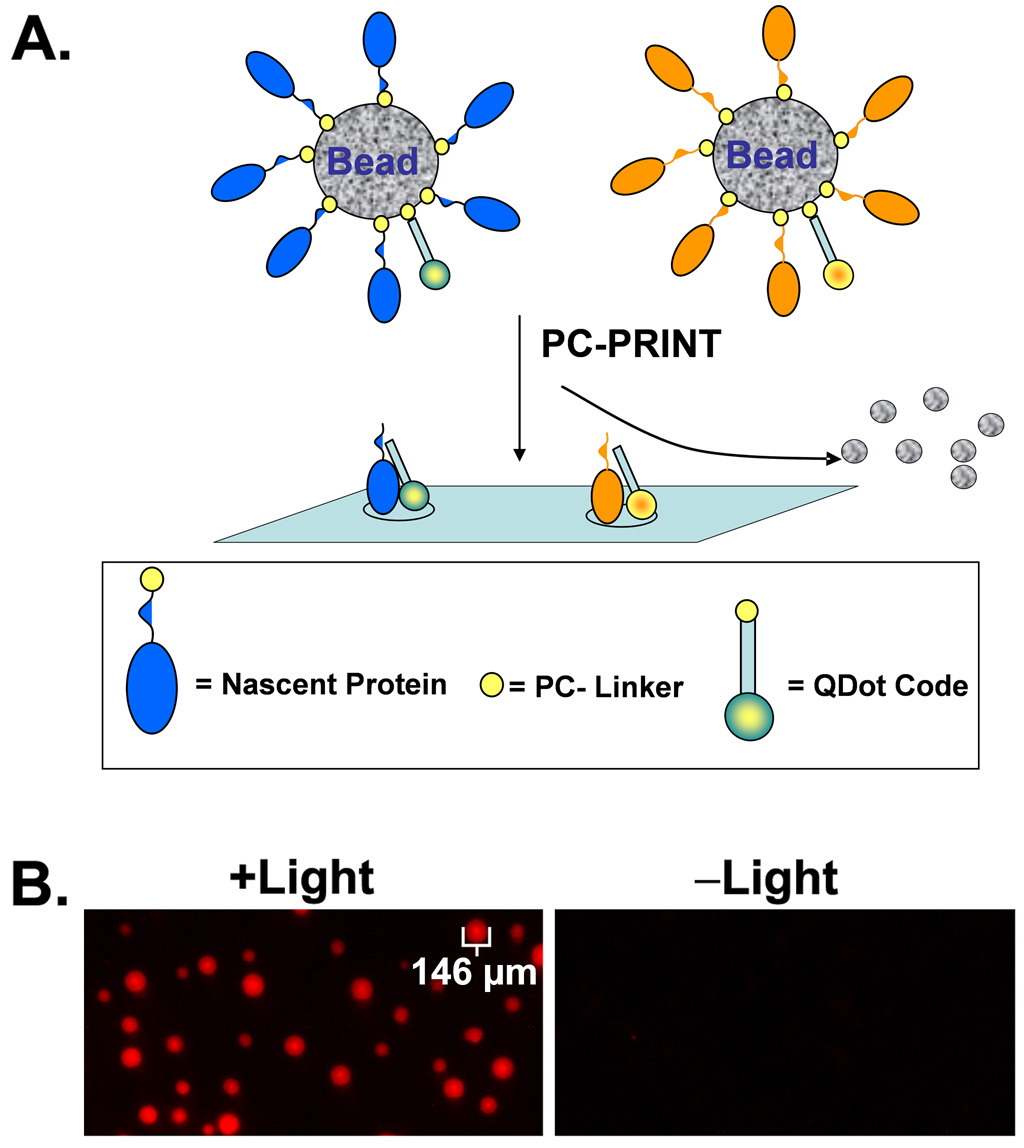
While PC-PRINT would derive its power from the ability to parallel print thousands of different proteins onto a microarray surface at high densities, the identity of each protein in a particular spot usually needs to be determined before useful information can be extracted. This can be achieved using a number of possible methods. For example, one approach is based on mixing different quantum dot nanocrystals which can use the same excitation wavelength yet emit with different wavelengths. More than 40,000 distinct codes should be possible, for example when these quantum dots are permanently embedded in beads at different ratios of color and intensity level, for multiplexed assays (49). In the case of PC-PRINT, the nanocrystals could be instead photo-transferred along with the nascent proteins onto a microarray, thus preserving information about the identity of the protein. Figure 7A is a diagrammatic representation of coding PC-PRINT arrays with fluorescent quantum dot nanocrystals (other possible methods of coding are discussed later).
Figure 7. Feasibility of decoding random PC-PRINT microarrays using fluorescent quantum dot nanocrystals.
(A.) Diagrammatic representation of co-transfer of nascent proteins and quantum dot nanocrystal codes using PC-PRINT. Other coding molecules (not shown) such as DNA or peptide mass tags can be applied by PC-PRINT in the same manner as the quantum dot nanocrystals. (B.) Feasibility of PC-PRINT transfer of fluorescent quantum dot nanocrystals to a microarray substrate. Fluorescent quantum dot (“QDot”) nanocrystals (705 nm emissions) were chemically labeled with PC-biotin, captured on NeutrAvidin agarose beads and applied by PC-PRINT onto activated microarray substrates. +Light = PC-PRINT with proper near-UV light treatment; –Light = PC-PRINT in the absence of the proper light treatment.
In order to demonstrate basic feasibility, we created beads coated with photocleavable quantum dots (PC-quantum dots). Protein coated (immunoglobulin) 705 nm emitting quantum dots were purchased commercially, conjugated to PC-biotin and captured on NeutrAvidin coated agarose beads (~75–150 µm diameter range). The data shows (Figure 7B) that PC-PRINT results in transfer of the PC-quantum dots from the beads to the microarray substrate in a light-dependent manner, and the spot dimensions approximate the dimensions of the beads. In separate experiments, PC-PRINT signal was readily detectible when beads were loaded to ≤10% of the manufacturer reported bead binding capacity (not shown), thus adequate capacity should remain for capturing the expressed proteins as well. Hence, in the future, decoding of random PC-PRINT microarrays might be achieved using fluorescent quantum dot nanocrystals, but other scenarios are also possible. In analogy to a bead-based multiplexed SNP assay, specific DNA sequences attached to the beads can be used for fluorescence hybridization based decoding of more than a thousand beads (50). Coding DNA sequences could be attached to beads through a photocleavable linker for PC-PRINT along with the nascent proteins. One strategy could employ PC-biotin phosphoramidites (51) or photocleavable amine phosphoramidites (52), developed by us, to incorporate the photocleavable affinity linker at the 5’ end. Chemical labeling of amine modified DNA with the PC-biotin amine-reactive reagent could also be employed. In fact, basic proof-of-principle for PC-PRINT of DNA oligonucleotides onto microarray substrates (epoxy activated) is demonstrated here for the first time. DNA applied to microarrays by PC-PRINT was detected by fluorescence hybridization probing (Supplementary Methods and Supplementary Figure 3). Importantly, epoxy activated microarray substrates have already been shown here to also be compatible with protein PC-PRINT. Alternatively, unique peptide identifiers (codes) could be photocleavably attached (53) to the beads for PC-PRINT onto activated (or affinity coated) MALDI-TOF plates, along with the nascent proteins, in cases where the microarray assay is amenable to a mass spectrometry platform. Finally, on a mass spectrometry platform, the expressed nascent proteins themselves might serve as intrinsic codes (unique mass or mass fingerprint or tandem mass spectrometry based sequencing).
Conclusions
In summary, PC-biotin, used in TRAMPE or on capture antibodies, provides a fast and efficient means to isolate cell-free expressed proteins at high purity (PC-SNAG). Proteins are enzymatically functional, can be printed to microarrays and participate in biologically significant protein-protein interactions. TRAMPE based PC-SNAG in particular shows similar yield and superior purity to conventional polyhistidine tag mediated purification when a comparison was made using a GST test protein. PC-biotin also facilitates a novel, non-mechanical, parallel microarray printing process called PC-PRINT, whereby proteins photocleavably tethered to beads are directly photo-transferred to planar microarray substrates. This affords all the benefits of PC-SNAG (e.g. high purity) while maintaining protein sorting in the resultant spots when PC-PRINT is performed from a mixed bead population. In addition, transferring the proteins off the beads onto a planar, highly polished and optically flat microarray substrate greatly reduces background noise from auto-fluorescence and light scattering observed with common polymer or magnetic bead types (Supplementary Figure 4).
Future Advancements
In the future, the decoding of PC-PRINT arrays will need to be developed to maturity by achieving co-transfer of both the code and nascent protein from the bead to a surface. The practical limits of the number of achievable codes will also need to be assessed.
Finally, while the current work utilized separate reactions to prepare beads coated with the different photocleavable nascent protein species, it is possible to envision processes that would allow entire Libraries of in vitro Expressed Proteins Displayed on Beads (LIVE-PDB) to be created in a few fully multiplexed reactions. Recent progress by us (Lim et al. unpublished preliminary data) has demonstrated that beads coated with PCR primers can be used to produce beads coated with different nascent proteins, by first using a single multiplexed solid-phase PCR reaction followed by a single multiplexed cell-free expression reaction that is based on self-assembling (4, 54) cell-free expression techniques.
Supplementary Material
Acknowledgments
This work was partially funded by the SBIR grants AI052525 and GM063369 from the National Institutes of Health to AmberGen, Inc. We thank Edyta Olejnik and Jerzy Olejnik for preparation of the PC-biotin-NHS ester and tRNACOMPLETE and Sadanand Gite for preparation of some of the plasmid constructs used for cell-free protein expression.
Abbreviations
- BODIPY-FL
4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene propionic acid
- TRAMPE
tRNA protein mediated protein engineering
- PC-biotin
photocleavable biotin
- PC-antibody
photocleavable antibody
- PC-SNAG
photocleavage mediated purification of biomolecules
- PC-PRINT
photocleavage based transfer (printing) of biomolecules from beads to a planar surface
- HSV tag
herpes simplex virus epitope tag (QPELAPEDPED)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu H, Snyder M. Curr Opin Chem Biol. 2001;5:40–45. doi: 10.1016/s1367-5931(00)00170-8. [DOI] [PubMed] [Google Scholar]
- 2.Lopez MF, Pluskal MG. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:19–27. doi: 10.1016/s1570-0232(02)00336-7. [DOI] [PubMed] [Google Scholar]
- 3.Melton L. Nature. 2004;429:101–107. doi: 10.1038/429101a. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, Rosen B, Lau AY, Walter JC, LaBaer J. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]
- 6.MacBeath G, Schreiber SL. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Klemic JF, Chang S, Bertone P, Casamayor A, Klemic KG, Smith D, Gerstein M, Reed MA, Snyder M. Nat Genet. 2000;26:283–289. doi: 10.1038/81576. [DOI] [PubMed] [Google Scholar]
- 8.Michaud GA, Salcius M, Zhou F, Bangham R, Bonin J, Guo H, Snyder M, Predki PF, Schweitzer BI. Nat Biotechnol. 2003;21:1509–1512. doi: 10.1038/nbt910. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan C. Nat Biotechnol. 2005;23:3–4. [PubMed] [Google Scholar]
- 10.Robinson WH, Fontoura P, Lee BJ, de Vegvar HE, Tom J, Pedotti R, DiGennaro CD, Mitchell DJ, Fong D, Ho PP, Ruiz PJ, Maverakis E, Stevens DB, Bernard CC, Martin R, Kuchroo VK, van Noort JM, Genain CP, Amor S, Olsson T, Utz PJ, Garren H, Steinman L. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 11.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, de Vegvar HE, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 12.Kawahashi Y, Doi N, Takashima H, Tsuda C, Oishi Y, Oyama R, Yonezawa M, Miyamoto-Sato E, Yanagawa H. Proteomics. 2003;3:1236–1243. doi: 10.1002/pmic.200300444. [DOI] [PubMed] [Google Scholar]
- 13.He M, Taussig MJ. Nucleic Acids Res. 2001;29:E73-3. doi: 10.1093/nar/29.15.e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, Rosen B, Lau AY, Walter JC, LaBaer J. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- 15.Middleton RB, Bulleid NJ. Biochem J. 1993;296(Pt 2):511–517. doi: 10.1042/bj2960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pensiero MN, Dveksler GS, Cardellichio CB, Jiang GS, Elia PE, Dieffenbach CW, Holmes KV. J Virol. 1992;66:4028–4039. doi: 10.1128/jvi.66.7.4028-4039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vorburger K, Kitten GT, Nigg EA. Embo J. 1989;8:4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose S, Kim S, Miyazaki H, Park YS, Murakami K. J Biol Chem. 1985;260:16400–16405. [PubMed] [Google Scholar]
- 19.Gibbs PE, Zouzias DC, Freedberg IM. Biochim Biophys Acta. 1985;824:247–255. doi: 10.1016/0167-4781(85)90055-7. [DOI] [PubMed] [Google Scholar]
- 20.Popov M, Tam LY, Li J, Reithmeier RA. J Biol Chem. 1997;272:18325–18332. doi: 10.1074/jbc.272.29.18325. [DOI] [PubMed] [Google Scholar]
- 21.Lyford LK, Rosenberg RL. J Biol Chem. 1999;274:25675–25681. doi: 10.1074/jbc.274.36.25675. [DOI] [PubMed] [Google Scholar]
- 22.Beckler GS, Thompson D, Van Oosbree T. Methods Mol Biol. 1995;37:215–232. doi: 10.1385/0-89603-288-4:215. [DOI] [PubMed] [Google Scholar]
- 23.Noren CJ, Anthony-Cahill SJ, Griffith MC, Schultz PG. Science. 1989;244:182–188. doi: 10.1126/science.2649980. [DOI] [PubMed] [Google Scholar]
- 24.Rothschild KJ, Gite S. Curr Opin Biotechnol. 1999;10:64–70. doi: 10.1016/s0958-1669(99)80012-3. [DOI] [PubMed] [Google Scholar]
- 25.Rothschild KJ, Gite S, Mamaev S, Olejnik J. In: CRC Handbook of Organic Photochemistry and Photobiology. Lenci F, editor. 2003. pp. 1–21. Chapter 133. [Google Scholar]
- 26.Forster AC, editor. Engineering Translation. 2005. [DOI] [PubMed] [Google Scholar]
- 27.Anthony-Cahill SJ, Griffith MC, Noren CJ, Suich DJ, Schultz PG. TIBS. 1990;14:400–403. doi: 10.1016/0968-0004(89)90287-9. [DOI] [PubMed] [Google Scholar]
- 28.Sonar S, Lee CP, Coleman M, Patel N, Liu X, Marti T, Khorana HG, Rajbhandary UL, Rothschild K. Nature Structural Biology. 1994;1:512–517. doi: 10.1038/nsb0894-512. [DOI] [PubMed] [Google Scholar]
- 29.Gite S, Mamaev S, Olejnik J, Rothschild K. Anal Biochem. 2000;279:218–225. doi: 10.1006/abio.1999.4472. [DOI] [PubMed] [Google Scholar]
- 30.Mamaev S, Olejnik J, Olejnik EK, Rothschild KJ. Anal Biochem. 2004;326:25–32. doi: 10.1016/j.ab.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Olejnik J, Gite S, Mamaev S, Rothschild KJ. Methods. 2005;36:252–260. doi: 10.1016/j.ymeth.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Krieg UC, Johnson AE, Walter P. J Cell Biol. 1989;109:2033–2043. doi: 10.1083/jcb.109.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gite S, Lim M, Carlson R, Olejnik J, Zehnbauer B, Rothschild K. Nat Biotechnol. 2003;21:194–197. doi: 10.1038/nbt779. [DOI] [PubMed] [Google Scholar]
- 34.Hecht SM, Alford BL, Kuroda Y, Kitano S. J Biol Chem. 1978;253:4517–4520. [PubMed] [Google Scholar]
- 35.Lodder M, Wang B, Hecht SM. Methods. 2005;36:245–251. doi: 10.1016/j.ymeth.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Olejnik J, Sonar S, Krzymanska-Olejnik E, Rothschild KJ. Proc. Nat. Acad. Sci. (USA) 1995;92:7590–7594. doi: 10.1073/pnas.92.16.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandori MW, Hobson DA, Olejnik J, Krzymanska-Olejnik E, Rothschild KJ, Palmer AA, Phillips TJ, Sano T. Chem Biol. 2002;9:567–573. doi: 10.1016/s1074-5521(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 38.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Beckler G, Bhatia A, Brisco P, Brondyk W, Butler B, DeMars S, Doers M, Doyle K. In: Protocols and Applications Guide (Promega) Doyle K, editor. Madison, WI: Promega Corporation; 1996. pp. 261–262. [Google Scholar]
- 40.Sinha P, Poland J, Schnolzer M, Rabilloud T. Proteomics. 2001;1:835–840. doi: 10.1002/1615-9861(200107)1:7<835::AID-PROT835>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Wilchek M, Bayer EA. Methods Enzymol. 1990;184:5–13. doi: 10.1016/0076-6879(90)84256-g. [DOI] [PubMed] [Google Scholar]
- 42.Hale JE. Anal Biochem. 1995;231:46–49. doi: 10.1006/abio.1995.1501. [DOI] [PubMed] [Google Scholar]
- 43.Muszynska G, Andersson L, Porath J. Biochemistry. 1986;25:6850–6853. doi: 10.1021/bi00370a018. [DOI] [PubMed] [Google Scholar]
- 44.Thiele C, Fahrenholz F. Anal Biochem. 1994;218:330–337. doi: 10.1006/abio.1994.1187. [DOI] [PubMed] [Google Scholar]
- 45.Ranadive GN, Bloomer WD. Nucl Med Biol. 1995;22:607–612. doi: 10.1016/0969-8051(94)00147-c. [DOI] [PubMed] [Google Scholar]
- 46.Klee CB, Crouch TH, Krinks MH. Proc Natl Acad Sci U S A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michael KL, Taylor LC, Schultz SL, Walt DR. Anal Chem. 1998;70:1242–1248. doi: 10.1021/ac971343r. [DOI] [PubMed] [Google Scholar]
- 48.Bottger A, Bottger V, Garcia-Echeverria C, Chene P, Hochkeppel HK, Sampson W, Ang K, Howard SF, Picksley SM, Lane DP. J Mol Biol. 1997;269:744–756. doi: 10.1006/jmbi.1997.1078. [DOI] [PubMed] [Google Scholar]
- 49.Han M, Gao X, Su JZ, Nie S. Nat Biotechnol. 2001;19:631–635. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 50.Gunderson KL, Kruglyak S, Graige MS, Garcia F, Kermani BG, Zhao C, Che D, Dickinson T, Wickham E, Bierle J, Doucet D, Milewski M, Yang R, Siegmund C, Haas J, Zhou L, Oliphant A, Fan JB, Barnard S, Chee MS. Genome Res. 2004;14:870–877. doi: 10.1101/gr.2255804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olejnik J, Krzymanska-Olejnik E, Rothschild KJ. Nucleic Acids Research. 1996;24:361–366. doi: 10.1093/nar/24.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olejnik J, Krzymanska-Olejnik E, Rothschild KJ. Nucleic Acids Res. 1998;26:3572–3576. doi: 10.1093/nar/26.15.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olejnik J, Ludemann HC, Krzymanska-Olejnik E, Berkenkamp S, Hillenkamp F, Rothschild KJ. Nucleic Acids Res. 1999;27:4626–4631. doi: 10.1093/nar/27.23.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nord O, Uhlen M, Nygren PA. J Biotechnol. 2003;106:1–13. doi: 10.1016/j.jbiotec.2003.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.