Abstract
Accumulating evidence indicates that the storage and processing capabilities of the human working memory system co-vary with individuals’ performance on a wide range of cognitive tasks. The ubiquitous nature of this relationship suggests that variations in these processes may underlie individual differences in intelligence. Here we briefly review relevant data which supports this view. Furthermore, we emphasize an emerging literature describing a trait in genetically heterogeneous mice that is quantitatively and qualitatively analogous to general intelligence (g) in humans. As in humans, this animal analog of g co-varies with individual differences in both storage and processing components of the working memory system. Absent some of the complications associated with work with human subjects (e.g., phonological processing), this work with laboratory animals has provided an opportunity to assess otherwise intractable hypotheses. For instance, it has been possible in animals to manipulate individual aspects of the working memory system (e.g., selective attention), and to observe causal relationships between these variables and the expression of general cognitive abilities. This work with laboratory animals has coincided with human imaging studies (briefly reviewed here) which suggest that common brain structures (e.g., prefrontal cortex) mediate the efficacy of selective attention and the performance of individuals on intelligence test batteries. In total, this evidence suggests an evolutionary conservation of the processes that co-vary with and/or regulate “intelligence” and provides a framework for promoting these abilities in both young and old animals.
Keywords: General Intelligence, Fluid Intelligence, Learning, Selective Attention, Working Memory, Short-Term Memory, Aging, Transgenics, Prefrontal Cortex, Mice, Humans
1. Introduction
The concept of working memory has undergone a dramatic evolution since first formally introduced more than 50 years ago (Miller, 1956). Although not entirely consistent with the most contemporary descriptions of the process, the description of working memory by Baddeley and Hitch (Baddeley & Hitch, 1974) probably best captures the sentiments of most modern theorists. Baddely and Hitch assessed subject’s performance on list learning, retrieval, and comprehension tasks under conditions of high and low interference. Under conditions that imposed high interference, reasoning, comprehension, and learning were all impaired by otherwise insignificant memory loads. Based on these observations, Baddeley and Hitch proposed that working memory was a limited resource system with both storage and processing components which were traded off as a function of task demands. Accordingly, working memory has come to be viewed as a flexible system that both maintains current information while supporting the simultaneous execution of higher cognitive functions.
“Higher cognitive functions” (such as reasoning, comprehension, and learning) are the hallmark of contemporary intelligence test batteries, and form common colloquial descriptions of “intelligence”. Thus it is not surprising that “working memory” (or at least some of its sub-components) has come to be viewed by some as the potential latent factor which underlies general (fluid) cognitive abilities, i.e., intelligence (e.g., Mackintosh, 1999). Accordingly, variations in (components of) working memory efficacy have been proposed to regulate individual differences in intelligence.
Here we will briefly review evidence from the human literature that supports the assertion that specific components of the working memory system establish an individual’s level of performance across diverse tests of cognitive ability. Next, we will review recent evidence from our laboratory which indicates that genetically heterogeneous laboratory mice express a trait that is both quantitatively and structurally similar to “intelligence” in humans. It is noted that many studies of “animal intelligence” have historically taken a different approach than the one described here, often focusing on proof of concept demonstrations in non-human animals of abilities akin to “cognitive capacities”, “correlates of mind”, tool use, or language in humans (for reviews, see (Shettleworth, 2009); (Jerison, 1985). Here we will focus on a recent series of experiments which takes a different approach in which the aggregate performance of laboratory mice is assessed across diverse learning and attentional tasks, a strategy that is qualitatively and quantitatively analogous (in some respects) to the characterization of individual differences human intelligence. Following this strategy, our use of animal subjects has provided the opportunity for a comparative analysis of the role of working memory in the establishment of general cognitive abilities. Furthermore, we will describe our efforts to modulate the processing efficiency of working memory, allowing us to assess the causal relationship between working memory and general cognitive abilities (a test that is not easily implemented with human subjects). Lastly (and based in part on our analysis of aged animals), we describe a theoretical framework for understanding the role of working memory in the establishment of general cognitive abilities across the lifespan.
2. Working Memory and Intelligence in Humans
In a seminal paper, Underwood (Underwood, 1975) argued that psychological theories could be best assessed by tests of individual differences, that is, if Mechanism “A” is believed to underlie Process “B” than individual differences in Mechanism “A” should predict corresponding differences in Process “B”. It has largely been this approach that has led to the common assertion that processing components of the working memory system might serve as the latent factor which underlies variations in intelligence.
In their more recent formulation, Baddeley and Logie (Baddeley & Logie, 1999) suggested that working memory was a system that retains information (primarily from recent experience), and thus supports the acquisition of new knowledge, aids in problem solving, and provides information with which to formulate and act on current goals. It is these storage components of the working memory system that determine the duration of the memory trace as well as the amount of information that can be maintained (simple span) prior to displacement to some secondary storage device. However, storage alone is not sufficient to accomplish the diverse functions attributed to working memory. To this end, the storage system is controlled by processing devices, the primary purpose of which are to oversee decisions (regarding the transfer of information from primary [limited] and secondary storage sites) and to direct attention in a controlled manner (i.e., selective attention).
Decades of research were directed at establishing relationships between physical characteristics (e.g., head size, transmission speed, reflex latencies, degree of myopia, etc) and performance on standardized tests of intelligence. Although weak correlations between these physical characteristics and intelligence have often been reported, the research was somewhat un-systematic and the results less than compelling, such that no unifying principals emerged. In contrast, during the past decade much attention has been paid to the role of working memory in the establishment of intelligence. It is beyond the scope of the present paper to present a comprehensive review of this literature (for an extensive recent review, see(Unsworth & Engle, 2007a). However, a brief summary is warranted.
Although measures of list retention abilities have appeared in intelligence test batteries since their earliest descriptions (see (Dempster, 1981)), Daneman and Carpenter reported in 1980 that simple span (i.e., “memory span” or the ability to accurately recall a list of items, in this case, words) was uncorrelated with reading comprehension (on a Scholastic Aptitude Test, a task thought to be representative of intelligence). In contrast, complex span (the ability to retain and recall the last words in a series of related sentences) was strongly correlated with reading comprehension, although the actual list of words was identical in the simple and complex span tasks. While both simple and complex span each tax storage abilities, only complex span is believed to reflect processing abilities, i.e., the capacity to retain information while simultaneously using that information to complete a directed task. Thus Daneman and Carpenter proposed that processing components of working memory were more critical to the establishment of intelligence than were simple storage abilities. Numerous behavioral studies have supported this contention (e.g., (Ackerman, 2005; Colom et al., 2004; Conway & Engle, 1996; Engle et al., 1999; Sub et al., 2002). In converging support, brain image analyses have indicated that a wide range of higher cognitive tests (i.e., ones that simultaneously tax storage and processing abilities) engage areas of the frontal cortex (particularly the dorsolateral prefrontal cortex; see Section 4 below) which are thought to be critical for the efficient implementation of working memory (Gray et al., 2003; Haier et al., 2004), and activity in the dorsolateral prefrontal cortex during intelligence testing is predictive of overall performance on those tests (Conway et al., 2003).
There is no doubt that simple span and complex span measure overlapping processes, and performance on tasks that nominally isolate these two processes are typically correlated (Unsworth & Engle, 2007b). Nevertheless, it is presumed (consistent with(Daneman & Carpenter, 1980) that performance on a complex span task recruits processes beyond those required for simple span performance. It is this additional processing that is thought to account for the superiority of complex span performance in the prediction of higher cognitive abilities.
In its most rudimentary form, a complex span task requires subjects to not only maintain information, but to maintain that information in spite of competing demands or task-relevant distraction. This is clearly exemplified in the reading comprehension/word list recall task of (Daneman & Carpenter, 1980) described above. However, the conclusion that the correlation between complex span performance and higher cognitive abilities (e.g., intelligence) is specific to the processing demands of the complex span task has been challenged by a series of recent results. For instance, Unsworth and Engle (Unsworth & Engle, 2006) reported that at low memory loads (2–4 items), complex span performance was a moderate predictor (r ≈ .45) of fluid intelligence, while simple span performance was not (r ≈ .12). However, at higher memory loads (> 6 items), both simple and complex span performance were equally good predictors, with the correlation between simple span and fluid intelligence rising to ≈ .45 (also see (Colom et al., 2008; Oberauer et al., 2008).
A similar complication arises when one considers the nature of human memory. Perhaps uniquely so, humans typically rely on verbal processes to implement many memory tasks, and the phonological processes that overlay human performance on any memory tasks complicates their interpretation. Unsworth and Engle (Unsworth & Engle, 2007a) have argued that when phonological processing is minimized, humans’ performance on both simple and complex (working) memory span tasks may be equally predictive of intelligence test scores (provided that memory loads are sufficiently high).
Based on these recent observations and there interpretation, it is reasonable to ask what unique processing ability is taxed by a complex span task that is absent from a simple span task. One candidate for this unique process is controlled or selective attention, i.e., the ability to focus on task-relevant goals to the exclusion of salient distracters. A common task that isolates controlled attention to exclusion of simple memory demands is the Stroop test (Stroop, 1935). In a typical Stroop test, a subject is required to identify the color of a word that is briefly (e.g., 50 ms) presented. In the simple form of this test, subjects’ accuracy is normally quite good. However, if the color of the font conflicts with the meaning of the word, e.g., if a red font spells the word “BLUE” (i.e., BLUE), performance degrades such that the latency to respond is extended and/or response errors begin to accrue. Performance on the Stroop test is strongly correlated with intelligence test scores, i.e., more intelligent individuals perform better than their less intelligent counterparts under conditions of interference. The degree of this correlation often exceeds that between complex span tasks and intelligence, and is presumed to do so despite only trivial memory loads. Even so, interpretation of this data is not without complication, since the effect is eliminated simply by obscuring part of the word (Besner & Stolz, 1999; Vecera et al., 2000), a result which suggests a phonological influence on performance of this seemingly rudimentary task. Some of these (and related) complications are mitigated in studies of laboratory animals.
3. General Cognitive Abilities in Genetically Heterogeneous Mice
In part to overcome some of the complications described above (particularly those related to phonological confounds), we recently developed a testing and analysis regimen with which to assess the general cognitive abilities of laboratory mice. In our initial analysis (Matzel et al., 2003) mice were tested in a battery of five common learning tasks, each of which made unique sensory, motor, and information processing demands on the animals. The tasks in this battery were rudimentary in nature (associative fear conditioning, operant avoidance, path integration, odor discrimination, and spatial navigation) such that all animals could eventually acquire the target responses with equal efficiency, but did so at different rates. A positive correlation of individuals’ performance across all tasks was observed, and principal component analysis indicated that 38% of the variance across tasks was attributable to a single factor, which we described as “general learning ability”. However, in published commentaries on this article, the performance of these mice was described as being qualitative and quantitatively analogous to the trait that is described in humans as “intelligence” (Blinkhorn, 2003). In subsequent reports, we have determined that the general cognitive abilities of mice are not merely a function of variations in native levels of stress reactivity or emotionality (Matzel et al., 2006; Grossman et al., 2007). Furthermore, although general learning abilities are strongly correlated with exploration and novelty-seeking, these processes do not appear to be causally related (Grossman et al., 2007) (Light et al., 2008). Since the time of these earlier reports, similar results have been obtained in mice tested on as many as nine cognitive tasks (Matzel et al., 2008), and in a comprehensive test of 256 mice, Kolata et al. (Kolata et al., 2008) reported a hierarchical structure (where a general factor influenced domain-specific factors) of the general cognitive abilities of mice similar to that thought to underlie human intelligence test performance. Adding to the generality of these results, similar batteries to assess general cognitive performance in mice have been reported elsewhere (Locurto et al., 2003; Locurto et al., 2006; Locurto et al., 2003; Galsworthy et al., 2002; Galsworthy et al., 2005).
3.1 The Relationship of Working Memory to General Cognitive Abilities in Mice
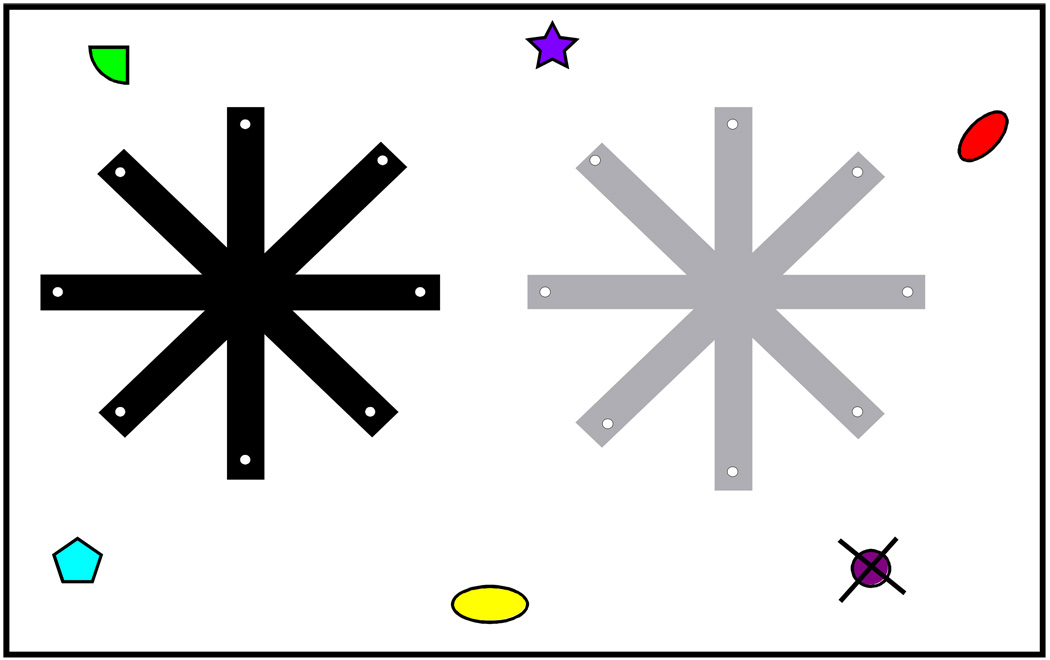
Having established a conceptually and quantitatively sound method with which to assess the general cognitive abilities of laboratory mice, we began to assess the relationship of working memory to these abilities, absent the complications associated with similar work in humans (e.g., phonological processes or prior experience). To begin to assess this relationship, we tested mice on a procedure adapted from an earlier one described by Roberts and Dale (Roberts & Dale, 1981). In this task, animals were first tested in the learning battery described above. These animals were then trained to asymptotic levels of performance in two separate eight-choice radial arm mazes (Fig 1; a task where the animal can collect eight pieces of food in distinct locations radiating out from a central hub. A return to a location from which food had already been obtained was scored as an error). After stable performance had been established in both mazes, two aspects of working memory were assessed. First, after several choices in one of the mazes, animals were confined to the central hub for varying periods of time before being allowed to make further choices in that maze. Errors following confinement increased as a function of the length of confinement, a result thought to reflect the decay of information in short-term storage. However, individual differences in the degree of disruption after confinement were only weakly related to animals’ general performance on the cognitive test battery. This result is comparable to that obtained with similar manipulations in humans, where it has been reported that short-term memory duration is weakly or inconsistently correlated with performance on standardized intelligence tests. In a second manipulation in this task, mice were required to concurrently operate in each of two mazes, i.e., several choices in one maze alternated with choices in a second maze. Since the spatial cues used to guide the animals’ choice were shared across the two mazes, this manipulation was thought to tax a process more analogous to working memory capacity, i.e., information from one task had to be retained for subsequent use while performing in a second, overlapping task. As anticipated, these competing demands promoted an increase in errors (with a non-linear increase in errors as the number of choices increased). The number of errors committed by individual animals was strongly related to their aggregate performance on the cognitive test battery. This led to the conclusion that working memory capacity, but not short-term memory duration, was related to the animals’ performance on the learning test battery.
FIGURE 1.
Radial arm mazes (grey and black) were used to assess composite working memory. Animals were trained independently in each of two mazes. The end of each of the arms on the mazes was baited with a desirable food. After initial training, the mice’s pattern of choices in each maze becomes very efficient, i.e., when started in the center hub, the animal will navigate down the arms of the maze (collecting food) in a nonsystematic order (they do not adapt a simple algorithmic strategy) and will rarely enter an arm from which food has already been retrieved (i.e., it will make no errors). Upon collection of the final food pellet, a trial is terminated. The errorless performance in such mazes reflects the implementation of a spatial strategy based on the locations of extra-maze cues. (These cues are depicted here as assorted geometric shapes, although more and more complex visual cues are present in the actual test environment.) After animals’ performance in each maze had reached stable levels, animals underwent a procedure where on occasional trials, they would begin to alternate between mazes after collecting only three food pellets in the starting maze. After three choices in the alternate maze, they were returned to the original maze where they collected three more food pellets. The animals alternated between mazes once more, until all food in each maze had been collected. This “alternating maze” version of the task requires that the animals maintain a list of locations (those which still hold food) while performing a second, related, memory task. That is, each of the maintained lists (one for each maze) shares an overlapping set of visual cues. Unlike the simple version of this task, this version is thought to tax several aspects of working memory, including span, duration, and capacity and/or selective attention. Typically, errors begin to rise precipitously after three to six pieces of food have been collected. Individual differences in the efficiency of an animal’s performance on latter choices are thought to reflect variations in composite working memory.
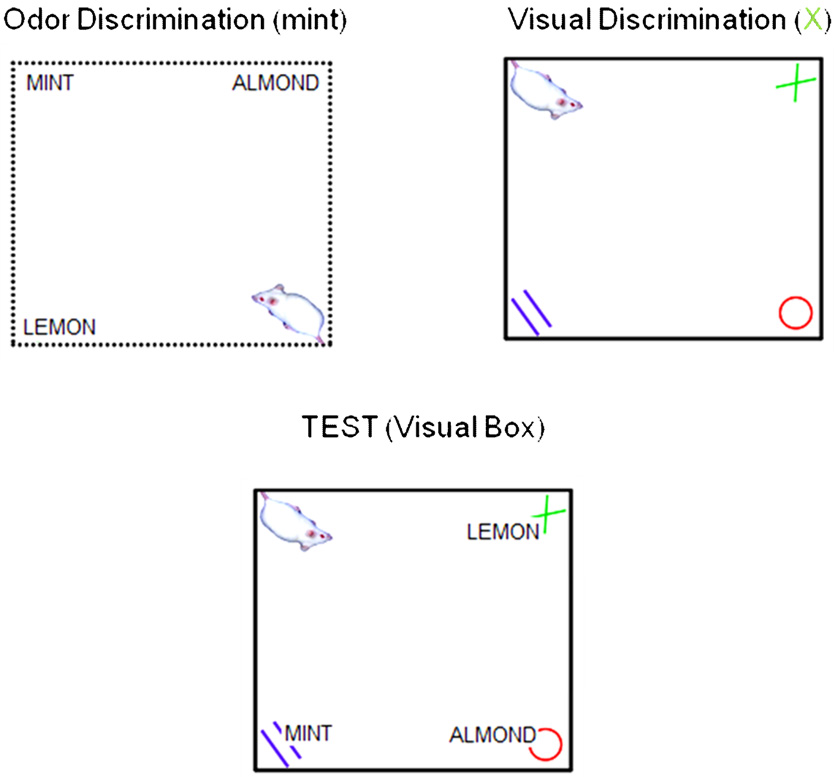
As noted above, working memory is not a singular process, but instead encompasses both the storage of information as well as the processing and integration of information (Baddeley, 2003);(Jarrod & Towse, 2008). The above experiment could not discern the relative contribution of these different aspects of the working memory system to the correlation with general cognitive abilities, and a second series of experiments (Kolata et al., 2007) was designed to assess these relative contributions. First, simple span abilities were assessed by requiring mice to maintain the memory of up to six visual symbols associated with food rewards. A moderate correlation (r = .38) was observed between this measure of simple span and individual animals’ aggregate performance in a battery of six learning tasks. A second task was employed with which we could assess the efficacy of these animals’ selective attention. This task was modeled after the Stroop test described above. In this task (Fig 2), mice learned a three-choice visual discrimination in a context referred to as “A” (Vis-A) and a three-choice olfactory discrimination in a context referred to as “B” (Olf-B). After stable performance had been attained in both tasks, animals were occasionally tested under conditions of high interference, i.e., both odor and visual cues were simultaneously presented Context A (Vis/Olf-A; the context which signaled the relevance of visual cues) or in Context B (Olf/Vis-B; the context which signaled the relevance of olfactory cues). Absent these interference conditions, animals’ performance on both the visual and olfactory discriminations were nearly perfect. However, when the task-relevant distracters were added to the test context (e.g., olfactory cues were present in the visual discrimination context), errors began to accumulate. The degree to which an animal committed errors under conditions of interference was strongly correlated (r = .50) with their aggregate performance in the cognitive test battery. Although no task can be asserted to be process-pure, this animal analog of the Stroop task makes no nominal demands on either short-term memory duration or simple span abilities, and instead requires the animal to ignore a task-relevant distracter in order to perform efficiently.
Figure 2.
The efficacy of animals’ selective attention was assessed in a test analogous to a human Stroop test. In each of two distinct boxes, animals received odor (in the odor discrimination box) or visual (in the visual discrimination box) discrimination training. In the individual tasks (not depicted), the animal could collect food at a location marked by a single discriminative stimulus, e.g., the MINT odor in the odor discrimination box or the green X in the visual discrimination box. (Note that in the actual test, distinct LED arrays served as the visual stimulus.) On each trial, the location of the cues was rearranged, but the identity of the target cue remained constant. Mice are quick to learn these discriminations, and will usually attain errorless performance (i.e., they do not approach non-target cues) within four trials. After the completion of training in the simple individual tasks, animals were occasionally tested in the visual discrimination box with odor cues present as salient distracters, or in the odor discrimination box with visual cues present as salient distracters. (These complex discriminations are depicted in the figure above. Note that the target visual and odor cue never appeared in the same location.) Unlike the simple discrimination tasks, the presence of salient distracters resulted in an increase in errors (as determined by incorrect choices for the discrimination cue by the test box). Errors in the presence of salient distracters are thought to reflect failures of selective attention, and the number of errors committed by individual animals varied widely.
A principal component analysis was performed to assess the entire data set described above. One factor accounted for 44% of the total variance in cognitive performance across the six learning tasks. On this factor, short-term memory duration loaded at a negligible level (.14), simple span abilities loaded at a moderate level (.50), and our measure of selective attention loaded strongly (.78). Given the good separation of simple span and selective attention that we believe these procedures support, these results (consistent with that from the human literature) suggest that simple span and controlled attention may act in unison (but to varying extents) to regulate the relationship between working memory and general cognitive abilities.
3.2 Modulation of Working Memory and General Cognitive Abilities
Prevailing theory is based, at least in part, on an assumption that the relationship between working memory and intelligence is causal in nature, although by design, the factor analytic techniques that underlie this assertion are correlational. Thus it was of interest to determine whether the observed relationship between aspects of working memory and general cognitive ability were merely correlational or if a causal influence was being revealed by this relationship.
Despite the colloquial (c.f. commercial) contention that “brain exercises” and “smart drugs” can enhance fluid intelligence in normal adults, these claims have rarely been subjected to empirical test, beyond the observation that such treatments have small task-specific benefits. (It is noted that many of the commercial “brain exercise” programs that are marketed to the public make claims of effectiveness based on improvement of performance on a common pre- and post-test of cognitive function, a result that is attributable to a simple practice effect.) In fact, decades of rigorous empirical research has found little evidence that environmental variables influence intelligence test performance in any systematic way (Gray et al., 2003).
Although seemingly insensitive to environmental variables, we have recently questioned whether the general learning abilities of mice could be modulated by extensive training on a task that taxed working memory functions. In work currently under review, Light et al. (Light et al., 2008) provided mice with complex working memory “exercise” by training them repeatedly (over a period of weeks) in the dual-radial arm maze task described above (Kolata et al., 2005). This training promoted superior performance when the animals were later tested in our animal analog of the Stroop task, i.e., working memory exercise promoted an improvement in animals’ selective attention. This was not merely an effect of working memory span exercise, as animals that spent comparable time performing in a single eight-arm radial maze did not exhibit the same increase in selective attention performance. Importantly, the animals that had undergone complex working memory exercise exhibited superior aggregate performance in a six-task learning battery. More so than the previous demonstrations of a correlation between working memory capacity/selective attention and general learning abilities, these results suggest the possibility of a causal relationship between the efficacy of working memory and general intelligence. This conclusion is partially supported by the recent report of beneficial effects of complex working memory training on human intelligence test performance (Jaeggi et al., 2008), although this later work has been questioned on methodological reasons (Moody, 2009). Nevertheless, we must reiterate that intelligence is not likely a unitary phenomenon (Ackerman, 2005; Conway et al., 2003); (Heitz et al., 2008), and these results should not be taken to indicate that intelligence and working memory are synonymous, but rather, that working memory may constitute at least some percentage of that trait that we describe as “intelligence”.
Our preferred interpretation of the above described relationship between working memory capacity and general learning abilities notwithstanding, those trained in experimental psychology would be quick to point out that “causal relationships” are never as easy to confirm as they are to infer. That said, one might ask if the effect of working memory training on general cognitive abilities is specific, or if the modulation of any co-variate with general cognitive abilities might have a similar beneficial impact. Early in our work with mice we observed a consistent positive correlation between various measures of exploration and the aggregate performance of mice on learning test batteries (Matzel et al., 2003; Matzel et al., 2006). Various measures of simple activity did not bear the same relationship with general learning abilities. We hypothesized that animals of high native exploratory tendencies might make quicker contact with those environmental contingencies upon which learning was based, and thus exploration might causally promote general cognitive abilities. We assessed this possibility using a procedure that was conceptually related to the one described above to promote more efficacious selective attention. Here, animals were repeatedly exposed to novel environments, a manipulation that had a long-lasting (at least months, including from pre-pubescence into adulthood) and profound effects on various exploratory behaviors, i.e., exposure to novelty promoted broad increase in exploration. However, this up-regulation of exploratory behaviors had little or no impact on performance on individual measures of learning, and did not promote an increase in the aggregate performance of mice on our learning test batteries (Light et al., 2008). Thus despite the correlation between the propensity for exploration and general learning abilities, and a conceptually logical expectation that a causal relationship might exist between these variables, no such causal relationship could be detected (for a related pharmacological manipulation, see (Grossman et al., 2007). This set of null results makes the observed relationship between working memory “exercise” and general cognitive performance that much more striking.
3.3 Working Memory and Age-Related Cognitive Declines
Broad learning deficits are a defining feature of the cognitive phenotype of elderly humans. Performance on human test batteries indicate that age-related learning deficits transcend specific domains of learning and are commonly expressed independent of the sensory, motor, motivational, or information processing demands of individual tasks. This is particularly important as the proportion of variance between individuals that is accounted for by general learning abilities increases across the lifespan, and “fluid” (i.e., domain-independent) intelligence tends to decline earlier than task-specific abilities (Li et al., 2004). (Although estimates of the amount of variability in cognitive performance vary, estimates of this variance increase from approximately 40% in young individuals to as much as 80% among the elderly.) Based on these observations, it is of great concern to begin to elucidate the basis for age-related declines in general cognitive abilities.
We recently assessed the performance of young (3–5 month old) and “elderly” (19–21 month old) mice on a battery of seven learning tasks and several components of the working memory system (Matzel et al., 2008). Aged animals exhibited significant deficits in five of the seven learning tasks, and ranked significantly lower than their young counterparts in general learning abilities. When cognitive performance was assessed by a principal component analysis, a “general” factor accounted for 33% of the total variance in performance in young animals, and 43% of the variance in old animals. This result suggests that aging adds variability to common core performance (i.e., that which underlies general learning abilities) and that an increase in the variability of aged animals’ performance on all learning tasks (as was observed) was attributable to increasing variability in general learning abilities.
Given the above result, it was critical to understand those variables that impact of common core performance of aged mice on this battery of cognitive tasks. Of particular relevance to the present discussion was the performance of young and old animals on tests of working memory ability. As described previously (Kolata et al., 2005; Kolata et al., 2007), here animals were tested on a task in which they were required to perform either separately or concurrently in two eight-choice radial arm mazes that required that animals use overlapping spatial cues to perform in each maze. By confining animals between choices, it was possible to assess the rate of decay and span of simple short-term memories, and by switching animals between mazes in the interim between subsequent choices, it was possible to assess the efficacy of working memory capacity (i.e., resistance to interference between independent short-term memories and/or selective attention). Separate factor analyses were conducted for young and old animals, and these analyses included as variables all seven of the learning tasks as well as these two measures relevant to the storage and processing components of working memory. Consistent with prior work (Kolata et al., 2005; Kolata et al., 2007), in young animals, short-term memory duration/span loaded only moderately (0.36) on the extracted primary factor, whereas working memory capacity loaded relatively heavily (0.73). In contrast, among old animals, short-term memory duration/span and capacity each loaded at similarly high levels (0.88 and 0.81, respectively). The most parsimonious explanation for this increasing co-variation of general cognitive abilities and short-term memory/span capacity is that our measure of working memory capacity (concurrent operation on two lists maintained within a limited storage device) makes demands on a single storage device. In young animals, this reliance on the storage components of working memory is normally insufficient to overly tax that device. However, as animals age, perturbations of the storage device impact working memory capacity to a degree not observed in young animals. This conclusion is consistent with that offered by Unsworth and Engle (Unsworth & Engle, 2007b) to describe the interaction of the storage and processing components of working memory in the determination of general intelligence in humans (see above). Of course it is also possible that as animals age, specific cognitive abilities become less differentiable, either as a real consequence of the aging process or owing to an artifact of our testing regimens (which to large extent have been developed for testing young animals). Regardless, each of these interpretations make similar functional predictions, i.e., that working memory span becomes increasingly dominant contributor to general cognitive abilities across the lifespan. Although further separation of the storage and processing components of working memory in aged animals will be necessary to fully elucidate the contributions of variations in these processes to age-related cognitive declines, these results are suggestive of a critical role for working memory in the determination of these cognitive failures.
4. Neuroanatomical and Neurophysiological Substrates of General Cognitive Abilities
The individual difference approach described above has provided a framework to both describe and characterize intelligence. As work with humans (and now animals) has evolved, this same approach has begun to be applied to studies of the co-variation of brain activity with common measures of intelligence, working memory, and selective attention. Of course much of this literature is subject to the same caveats as the work described above, i.e., it is correlational in nature. Nevertheless, it is useful to review this work as it provides another source of converging evidence in support of the critical relationship between working memory and intelligence. Before examining the relationship of activation of brain circuits putatively involved in the working memory/selective attention systems and performance on common measures of intelligence, it will first be necessary to briefly review the working memory networks of the mammalian brain. Most of the relevant data has been derived recently from the application of imaging techniques (e.g., fMRI) in human populations, although relevant neurophysiological data has also been obtained from non-human primates.
4.1. Network analysis of the working memory system
It was once believed that all aspects of working memory, including short-term maintenance of information, were instantiated in the prefrontal cortex. However, a new picture of working memory has since emerged in which it is assumed that domain-specific sensory information is transiently stored in modality-specific brain structures and the processing and attentional components of working memory are instantiated in the prefrontal cortex (although this region may also serve some storage functions). Evidence for this model comes mostly from imaging studies that demonstrate that spatial working memory tasks activate memory systems in both the parietal cortex as well as executive-attentional networks located in the prefrontal cortex (Cohen et al., 1997). Specifically, Cohen et al. observed that during a spatial working memory task the temporal activation dynamics in relevant brain regions could be divided into two categories. Parietal cortex, as well as some pre-frontal regions, expressed sustained activation during a working memory retention period (indicative of short-term maintenance of information) while most prefrontal regions expressed only transient activation whose peak varied with the extent of the memory load (indicative of executive-attentional components of working memory). Similarly, Rowe et al. (2000) showed that in a spatial working memory task, the dorsal lateral prefrontal cortex was activated during the selection of items from memory but not during the maintenance of those items. This was contrasted with the intraparietal cortex which was only active during the maintenance phase and not during selection. Todd et al. (2004) further established the role of the parietal cortex in short-term storage of visual items. They demonstrated that the amount of information that can be maintained in visual short-term memory is correlated with activity in the parietal cortex as measured by fMRI. Furthermore they demonstrated that the parietal activity during visual short-term memory tasks occurred during the encoding and maintenance period of the task but not at the time of retrieval.
The above data suggests that there is a disassociation between those areas involved short-term maintenance of information and those regions involved in processing of that information. However, there is significant overlap between maintenance and processing as evidenced by studies showing that spatial working memory is impaired when, during a delay period, subjects are prevented from attending to the memorized locations of relevant objects (Awh et al., 2001). Similarly, Gazzaley et al. (2005) demonstrated that the magnitude and the speed of neural processing in the visual association cortex are modulated by modality-independent top-down attentional networks in the prefrontal cortex. These frontal networks could enhance or suppress perceptual baseline visual association cortex activity depending on whether relevant stimuli were being attended to or ignored. Therefore, it appears that the entirety of “working memory” is not represented in any one region of the brain but that instead it involves a complex interplay between many networks located throughout the brain including the parietal lobe and the prefrontal cortex, the later of which appears primarily relevant to the processing of information during working memory tasks.
4.2. Network analysis of general intelligence
Moving from understanding general intelligence on purely a behavioral level to a deeper neural anatomical level of analysis is a holy grail of intelligence research. Witness Jensen, who stated “The highest priority in g research…is to discover how certain anatomical structures in the brain…cause individual differences in g.” (Jensen, 1998, pg. 579). In search of this elusive goal, Jung and Haier (2007) conducted a comprehensive review of imaging studies that attempted to locate brain regions involved in general intelligence and concluded that the cortical networks in the prefrontal cortex, parietal cortex, and the occipital lobe are all equally involved in general intelligence tasks. The authors described these results as fitting a parietal-frontal integration theory of intelligence (P-FIT). However, perhaps a more parsimonious explanation of these results is to state that these are the very same regions most commonly associated with working memory. In fact, given the high degree to which general intelligence and working memory are related, it is not surprising that the networks involved in working memory are also engaged by general intelligence tasks.
This putative relationship between working memory and intelligence brain networks is highlighted by the results of a study by Gray et al. (2003). In that study, the authors measured the general intelligence scores of 48 subjects using Raven’s Advanced Progressive Matrix task (which is a good predictor of general intelligence). They then used fMRI to image the networks engaged by a working memory task with a high selective attention demand. Not surprisingly, they found that individuals with higher intelligence scores performed better on the working memory task. However, they also found that activity in the prefrontal and parietal cortex mediated the relationship between intelligence and working memory performance. These are the brain regions most commonly associated with both intelligence and working memory.
Imaging studies investigating general intelligence seem to suggest that both the regions associated with domain-specific short-term maintenance of working memory information, such as parietal and occipital cortex, as well as regions associated with the processing component of working memory (i.e., prefrontal cortex) are all engaged by intelligence tasks. At a rudimentary level, this may seem paradoxical, since by definition, general intelligence is domain-independent. However, one may conclude from these studies that all of the sub-tasks in the intelligence batteries impinged on some of the same domain-specific abilities (e.g., visual information and processing), accounting for the common activation of parietal and occipital regions. Although it would be premature to make any definitive statement, this work as a whole suggest the possibility that the unifying brain region orchestrating all intelligence tasks, regardless of the information being processed, is the prefrontal cortex, as the prefrontal cortex appears to be the common mediator of both “intelligence” and selective attention. Although too preliminary to describe here, ongoing work in our laboratory has revealed a unique pattern of gene expression in the mouse prefrontal areas (but not other brain regions) that distinguishes animals of high and low general cognitive abilities. It is worth noting that this work, based on RNA transcription of ~25,000 genes, is not technically feasible with human subjects, indicative of another point of converging operations necessitating both human and animal work.
5. Summary
Human and animal test results have suggested that working memory and intelligence may be commonly constrained. Accumulating evidence suggests that this constraint may arise from limitations on both storage and processing (e.g., selective attention) components of the working memory system, and that the relative contributions of storage and processing capacities to higher cognitive function may depend on the degree to which these processes interact to influence the completion of any particular task. In contrast to the extensive body of evidence that has emerged from studies with human subjects, our work with laboratory mice is in a relative stage of infancy. Nevertheless, certain principles have emerged from these studies with animals. These conclusions have been consistent with many of those derived from human research, and in some instances, has allowed conclusions that go beyond that which could be derived from studies of humans.
First, we have observed that the parameters of both storage and processing components of a system analogous to working memory are correlated with animals aggregate performance on a battery of behavioral tests designed to assess a range of learning abilities. Although working memory capacity (or resistance to interference) was a more consistent and reliable predictor of general cognitive abilities than was simple span or resistance to decay, these storage components of working memory do have some predictive validity, and their predictive abilities appear to increase as a function of the degree to which they are taxed (as is always the case with aged animals). Thus we have concluded that both storage and processing aspects of the working memory system may play a role in the establishment of individual differences in higher cognitive abilities, depending on task demands and the nature of the test. This conclusion is similar to that which has emerged from studies with humans (Halford et al., 2007; Unsworth & Engle, 2007a). It has been suggested that the role of the storage and processing components of working memory in the establishment of higher cognitive abilities may arise from their common reliance on (and limitations of) attention (Cowan et al., 2006). As Cowan et al. have enumerated, it is often difficult to separate these processes in tests among humans owing to their reliance on mnemonic strategies such as rehearsal and grouping. These strategies (particularly those reliant on verbal processing) are at least partially mitigated with animal subject. In this regard, it is notable that the performance of mice on an analog of the Stroop test (in which the animals must focus on a target stimulus against a background of relevant distracters) was highly correlated with aggregate performance on a learning test battery. Since there is no obvious storage requirement for efficient performance on this task, this result suggests that processing aspects of working memory (i.e., selective attention) may have at least some unique relationship to the expression of higher cognitive abilities. Relatedly, we have determined that manipulations (e.g., working memory “exercise”) that promote the more efficacious utilization of selective attention have commensurate effects on general cognitive performance, suggestive of a causal relationship between these variables.
In total, available data suggests a conservation of the structure and determinants of “intelligence” in both human and non-human animals. The correspondence of humans and animals provides the opportunity for complimentary lines of research, and animals provide a practical opportunity to address lines of inquiry that are not always tractable in humans (e.g., Kolata et al., 2008b).
Acknowledgements
This work was supported by grants from the National Institute of Aging (R01AG029289 and AG022698) and the Busch Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman PL. Individual differences in skill learning: an integration of psychometric and information processing perspectives. Psych Bull. 1987;102:3–27. [Google Scholar]
- Ackerman PL. Ability determinants of individual differences in skilled performance. In: Sternberg RJ, Pretz JE, editors. Cognition and Intelligence: Identifying the Mechanisms of the Mind. Cambridge: Cambridge University Press; 2005. pp. 142–159. [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley AD. Working memory looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working Memory. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baddeley AD, Logie RH. Working memory: The multiple component model. In: Miyake A, Priti S, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Besner D, Stolz JA. What kind of attention modulates the Stroop effect? Psychonomic Bulletin & Review. 1999;6:99–104. doi: 10.3758/bf03210815. [DOI] [PubMed] [Google Scholar]
- Blinkhorn S. Neuroscience: Of mice and mentality. Nature. 2003;24:1004–1005. doi: 10.1038/4241004a. [DOI] [PubMed] [Google Scholar]
- Cowan N, Fristoe NM, Elliot EM, Brunner RP, Saults JS. Scope of attention, control of attention, and intelligence in children and adults. Memory and Cognition. 2006;34:1754–1768. doi: 10.3758/bf03195936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N, Elliott EM, Saults JS, Morey CC, Mattox S, Hismjatullina A, Conway ARA. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognitive Psychology. doi: 10.1016/j.cogpsych.2004.12.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Abad FJ, Quiroga MA, Shih PC, Flores-Mendoza C. Working memory and intelligence are highly related constructs, but why? Intelligence. 2008;36:584–606. [Google Scholar]
- Colom R, Rbello I, Palacios A, Juan-Espinosa M, Kyllonen PC. Working memory is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Conway AR, Cowan N, Bunting M, Therriault DJ, Scott RB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30:163–183. [Google Scholar]
- Conway AR, Engle RW. Working memory and retrieval: a resource-dependent inhibition model. J Exp Psychol Gen. 1995;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7 doi: 10.1016/j.tics.2003.10.005. 547-522. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980:450–466. [Google Scholar]
- Dayan P, Kakade S, Montague PR. Learning and selective attention. Nat Neurosci. 2000;3:1218–1223. doi: 10.1038/81504. [DOI] [PubMed] [Google Scholar]
- Dempster FN. Memory span: Sources of individual and developmental differences. Psychological Bulletin. 1981;89:63–100. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Paya-Cano JL, Monleon S, Plomin R. Evidence for general cognitive ability (g) in heterogeneous stock mice and an analysis of potential confounds. Genes Brain Behav. 2002;1:88–95. doi: 10.1034/j.1601-183x.2002.10204.x. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Grossman H, Hale G, Light K, Kolata S, Matzel LD. Pharmacological Manipulation of Stress Reactivity Dissociates its Role in the Determination of the Relationship of Exploration and General Cognitive Abilities. Behavioral Neuroscience. 2007;121:949–964. doi: 10.1037/0735-7044.121.5.949. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Buchsbaum MS, Abel L, Tang C, Siegel B. Intelligence and changes in the regional cerebral glucose metabolic rate following learning. Intelligence. 2004;16:415–426. [Google Scholar]
- Heitz RP, Redick TS, Hambrick DZ, Kane MJ, Conway ARA, Engle RW. WM, EF, and gF are not the same. Behavioral and Brain Sciences. in press. [Google Scholar]
- Halford GS, Cowan N, Andrews G. Separating cognitive capacity from knowledge: a new hypothesis. Trends in Cognitive Science. 2007;11:236–242. doi: 10.1016/j.tics.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi SM, BuschKuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Science USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrod C, Towse JN. Individual differences in working memory. Neurosci. 2006;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The g factor: The science of mental ability. Wesport, CT: Praegar; 1998. [Google Scholar]
- Jung RE, Haier RJ. The parietal-frontal integration theory (P-FIT) if intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kolata S, Light K, Townsend DA, Hale G, Grossman HC, Matzel LD. Variations in working memory capacity predict individual differences in general learning abilities among genetically diverse mice. Neurobiol Learn Mem. 2005;84:241–246. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Kolata S, Light K, Hale G, Matzel LD. Selective Attention is the Primary Determinant of the Relationship of Working Memory to General Cognitive Ability in Outbred Mice. Learning and Memory. 2007;14:22–28. doi: 10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata S, Light K, Matzel LD. General and Domain-Specific Cognitive Abilities in Heterogeneous Stock Mice. Intelligence. 2008a;36:619–629. doi: 10.1016/j.intell.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata S, Schachner M, Matzel LD. Conditional CHL1 deletion produces specific impairments in working memory span but spares general cognitive abilities. Journal of Neuroscience. 2008b;28:13505–13510. doi: 10.1523/JNEUROSCI.2127-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light K, Kolata S, Townsend D, Grossman H, Hale G, Matzel LD. Adaptation to novelty promotes exploration but not general learning abilities in pre-adolescent and adult outbred mice. Neurobiology of Learning and Memory. 2008 doi: 10.1016/j.nlm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Light K, Kolata S, Matzel LD. Complex working memory practice promotes increases in general cognitive abilities in outbred mice. Manuscript under review. 2009 [Google Scholar]
- Locurto C, Fortin E, Sullivan R. The structure of individual differences in heterogeneous stock mice across problem types and motivational systems. Genes Brain Behav. 2003;1:40–55. doi: 10.1034/j.1601-183x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- Locurto C, Scanlon Individual differences and a spatial learning factor in two strains of mice (mus musculus) J Comp Psychol. 2006 1998;112:344–352. [Google Scholar]
- Mackintosh NJ. IQ and human intelligence. Oxford: Oxford University Press; 1998. [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a “general” learning ability in mice. J Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in Outbred Mice Covaries with General Learning Abilities Irrespective of Stress Reactivity, Emotionality, and Physical Attributes. Neurobiology of Learning and Memory. 2006;86:228–240. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend DA, Kolata S. Variations in Age-Related Declines in General Cognitive Abilities of Balb/C Mice are Associated with Disparities in Working Memory Span/Capacity, and Body Weight. Learning and Memory. 2008;15:733–746. doi: 10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Moody DE. Can intelligence be increased by training on a task of working memory? Intelligence. 2009;37:327–328. [Google Scholar]
- Oberauer K, Süβ HM, Wilhelm O, Wittmann WW. Which working memory functions predict intelligence? Intelligence. 2008;36:641–652. [Google Scholar]
- Roberts WA, Dale RH. Remembrance of places past: Proactive inhibition and patterns of choice in rat spatial memory. Learning and Motivation. 1981;12:261–281. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–652. [Google Scholar]
- Süβ HM, Oberauer K, Wittmann WW, Wilhelm O, Schulze R. Working memory capacity explains reasoning ability – and a bit more. Intelligence. 2002;30:261–288. [Google Scholar]
- Todd JJ, Marois R. Capacity limits of short-term memory in the human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: response selection or maintenance with working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Underwood BJ. Individual differences as a crucible in theory construction. American Psychologist. 1975;30:128–134. [Google Scholar]
- Unsworth N, Engle RW. Simple and complex memory spans and their relation to fluid abilities: Evidence from list-length effects. Journal of Memory and Language. 2006;54:68–80. [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex span abilities and their relation to higher order functions. Psychological Bulletin. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Behrmann M, McGoldrick J. Selective attention to the parts of an object. Psychonomic Bulletin & Review. 2000;7:301–308. doi: 10.3758/bf03212985. [DOI] [PubMed] [Google Scholar]