Abstract
The pathogenesis of sepsis is mediated in part by the pathogen-associated molecular pattern molecule bacterial endotoxin, which stimulates macrophages to sequentially release early (e.g., TNF-α, IL-1β) and late (e.g., high-mobility group box [HMGB] 1 protein) proinflammatory mediators. The recent discovery of HMGB1 as a late mediator of lethal sepsis has prompted investigation into development of several new experimental therapeutics that limit release, either blocking HMGB1 itself or its nominal receptors. Quercetin was recently identified as an experimental therapeutic that significantly protects against oxidative injury. Here, we report that quercetin attenuates lethal systemic inflammation caused by endotoxemia, even if treatment is started after the early TNF response. Quercetin treatment reduced circulating levels of HMGB1 in animals with established endotoxemia. In macrophage cultures, quercetin inhibited release as well as the cytokine activities of HMGB1, including limiting the activation of mitogen-activated protein kinase and NF-κB, two signaling pathways that are critical for HMGB1-induced subsequent cytokine release. Quercetin and autophagic inhibitor, wortmannin, inhibited LPS-induced type-II microtubule-associated protein 1A/1B–light chain 3 production and aggregation, as well as HMGB1 translocation and release, suggesting a potential association between autophagy and HMGB1 release. Quercetin delivery, a strategy to pharmacologically inhibit HMGB1 release that is effective at clinically achievable concentrations, now warrants further evaluation in sepsis and other systemic inflammatory disorders.
Keywords: quercetin, high-mobility group box 1, sepsis, autophagy
CLINICAL RELEVANCE
Quercetin delivery, a strategy to pharmacologically inhibit high-mobility group box 1 release that is effective at clinically achievable concentrations, now warrants further evaluation in sepsis and other systemic inflammatory disorders.
Sepsis, a lethal systemic inflammatory response to infection, affects nearly 750,000 patients in the United States annually, and has a mortality of 20 to 40% (1). The incidence is rising at rates between 1.5 and 8% per year (1, 2). Cytokines, such as TNF-α, IL-1β, and high-mobility group box (HMGB) 1 protein, produced during inflammation, are critical mediators of sepsis-related tissue injury and death (3, 4). Significant advances have been made in understanding the role of proinflammatory mediators in the pathogenesis of sepsis, but effective therapies that target these inflammatory mediators have not yet entered clinical practice (5). A major difficulty in developing therapeutics that target cytokines (e.g., TNF-α and IL-1β) is that they are released early in the development of a systemic inflammatory response. This leaves a narrow therapeutic window for administration of antagonists, and inhibitors of TNF-α and IL-1β that are not effective when delivered after the acute cytokine response has occurred (6).
HMGB1, a highly conserved nuclear protein, is secreted by activated macrophages/monocytes (4, 7–10), and functions as a crucial “late” mediator of lethal endotoxemia and sepsis (4, 11). Circulating HMGB1 levels are elevated in a delayed fashion (after 16–32 h) in septic mice (4, 12) and patients (4). Administration of recombinant HMGB1 to mice recapitulates many of the clinical signs of sepsis, including fever, derangement of intestinal barrier function, tissue injury, and multiple organ failure. Extracellular HMGB1 can stimulate the release of TNF-α, IL-1β, and other inflammatory products from macrophages/monocytes (13), induce chemotaxis and proliferation of smooth muscle cells (14), and promote dendritic cell maturation (15). Administration of anti-HMGB1 antibodies or inhibitors (e.g., ethyl pyruvate, nicotine, stearoyl lysophosphatidylcholine, or tanshinone IIA sodium sulfonate) significantly protects mice against LPS-induced acute tissue injury (16, 17) and lethal endotoxemia (4, 18–20). Notably, these anti-HMGB1 reagents confer significant protection against delayed endotoxin lethality, even when applied at a time after the acute-phase cytokine responses have peaked and resolved (4, 18, 19, 21, 22), suggesting that HMGB1-targeted therapeutic strategies might be useful.
Quercetin (3,3′,4′,5,7-pentahydroxyflavone dihydrate), one of the most widely distributed flavonoids in the plant kingdom, is ingested as a major constituent in the diet (23, 24). Quercetin has a broad range of activities within cells (23). As an antioxidant, it prevents oxidation of low-density lipoproteins and the expression of metalloproteinase 1, thus inhibiting the disruption of atherosclerotic plaques and contributing to plaque stabilization (25). In tumor cells, it exerts antiproliferative effects and arrests human leukemic T cells in late G1 phase of the cell cycle (26). Quercetin has anti-inflammatory effects, regulating nitric oxide, IL-6, and TNF-α release (27–33), thereby alleviating oxidative damage in the tissue (28, 34) and inhibiting the LPS-induced delay in spontaneous apoptosis and activation of neutrophils (35). Its role in preventing lethality in animals with established lethal sepsis and systemic inflammation, ability to inhibit HMGB1 release, and proinflammatory function has not been previously demonstrated. We evaluated its capacity to inhibit the release and cytokine activities of HMGB1 in animal models of lethal endotoxemia.
MATERIALS AND METHODS
Animal Model of Endotoxemia
This study was approved and performed in accordance with the guidelines for the care and use of laboratory animals at Central South University, Changsha, China. Endotoxemia was induced in Balb/C mice (male, 7–8 wk old, 20–25 g weight) by intraperitoneal injection of bacterial endotoxin (10 mg/kg LPS, Escherichia coli LPS 0111:B4; Sigma, St. Louis, MO), as previously described (4, 19, 36). Blood was collected at different times after LPS administration, allowed to clot for 2 hours at room temperature, and then centrifuged for 15 minutes at 1,500 × g. Serum samples were stored at −20°C before analysis. Mortality was recorded for up to 3 weeks after injection to ensure that no additional late deaths occurred.
Cell Culture and Treatment
Murine macrophage-like RAW 264.7 cells were obtained from the Shanghai Type Culture Collection (Shanghai, China), and cultured in RPMI medium 1,640 (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated FBS, 2 mM glutamine, and antibiotic–antimycotic mix in a humidified incubator with 5% CO2 and 95% air. At 70% confluency, RAW264.7 cells were removed mechanically and resuspended in serum-free Opti-MEM I medium (Life Technologies). After preincubation for 2 hours, RAW264.7 cells were treated with LPS, recombinant TNF-α protein (Peprotech, Rocky Hill, NJ) or recombinant HMGB1 protein (37) (kindly provided by Dr. Kevin J. Tracey, Feinstein Institute for Medical Research, Manhasset, NY).
Quercetin and Wortmannin Solution
In experiments, the quercetin and wortmannin (Sigma) were prepared in DMSO, as previously described (38, 39).
Cell Viability Assay
Cells were plated at a density of 104 cells/well on 96-well plates in 100 μl RPMI. Cell viability was evaluated using the conventional MTT reduction assays. For the MTT reduction assay, cells of each micro well were incubated with 20 μl 0.5% MTT for 2 hours at 37°C, and the reaction was stopped by adding 150 μl DMSO. The amount of MTT formazan product was determined by measuring absorbance using a micro-plate reader (Bio-Rad, Hercules, CA) at a test wavelength of 570 nm and a reference wavelength of 630 nm.
Beclin1 shRNA
Beclin-1 short hairpin RNA (shRNA) and control shRNA (Sigma) were transfected into cells using Lipofectamine 2,000 reagent (Life Technologies) according to the manufacturer's instructions. At the end of the shRNA transfection (48 h), the medium over the cells was changed before any other treatment.
Preparation of Cellular Extracts
At the indicated time points after treatment, cells were harvested and washed twice with cold PBS; nuclear and cytoplasmic extracts were prepared as previously described (8, 9, 40).
Western Blotting Analysis
Proteins in the whole-cell lysate, subcellular fractions, or concentrated cell culture supernatants were resolved on 10% SDS-PAGE gel, and transferred to a polyvinylidene fluoride membrane. After blocking, the membrane was incubated for 2 hours at 25°C with various primary antibodies specific for HMGB1, proliferating cell nuclear antigen (PCNA) (BD Biosciences, San Jose, CA), β-actin, glyceraldehyde phosphate dehydrogenase (KangChen Biotechnology, Shanghai, China), β-tubulin (Sigma), phospho-p38 (T180/Y182; R&D Systems, Minneapolis, MN), phospho–extracellular signal-regulated kinase 1/2 (Thr202/Tyr204; Upstate, Lake Placid, NY), phospho–c-Jun NH2-terminal kinase 1/2 (Thr183/Tyr185; Cell Signaling Technology, Danvers, MA), NF-κB p65, inhibitor of NF-κB (IκBα) (Santa Cruz Biotechnology, Santa Cruz, CA), and microtubule-associated protein 1A/1B–light chain 3 (LC3; NOVUS, Littleton, CO). After incubation with peroxidase-conjugated secondary antibodies for 1 hour at 25°C, the signals were visualized by 3,3′-diaminobenzidine (DAB) detection (Boster Biotech, Wuhan, China) according to the manufacturer's instructions. The relative band intensity was quantified by using the Gel-pro Analyzer software (Media Cybernetics, Bethesda, MD).
Cytokine Measurements
Levels of TNF-α and IL-1β in the culture medium were determined in ELISA kits (Boster Biotech) according to the manufacturer's instructions. Levels of TNF-α and IL-1β mRNA were assayed by RT-PCR analysis, as previously described (41).
Electrophoretic Mobility Shift Assay for NF-κB Activation
Nuclear extracts were prepared, and electrophoretic mobility shift assay was performed using biotin-labeled oligonucleotides to measure NF-κB DNA binding activity (Pierce, Rockford, IL). In brief, 10 μg nuclear extract was incubated with biotin-labeled double-stranded DNA fragment corresponding to the NF-κB (sense strand, 5′-AGTTGAGGGGACTTTCCCAGGC-3′) at 4°C in the presence or absence of 100 × unlabeled NF-κB and subjected to electrophoresis at 110 V for 4 hours at 4°C on a 5% polyacrylamide gel. The gels were transferred to a nylon membrane and subjected to cross-link with an ultraviolet lamp, and the signals were detected by chemiluminescence. For supershift experiments, NF-κB p65 antibody (Santa Cruz Biotechnology) was incubated with the binding reaction mixture for 30 minutes at room temperature before addition of the labeled oligonucleotide.
HMGB1 Western Blotting Analysis
Levels of HMGB1 in the culture medium or serum were determined by Western blotting analysis, as previously described (4, 8, 9). The relative band intensity was quantified by using the Gel-pro Analyzer software.
HMGB1 Immunostaining
At 12 hours after LPS or TNF-α stimulation, cellular HMGB1 was immunostained with anti-HMGB1 polyclonal antibodies, and nuclear morphology was analyzed with the fluorescent dye, Hoechst 33,258 (Sigma). Images were acquired using a fluorescence microscope (Eclipse 80i; Nikon, Tokyo, Japan), as previously described (8, 9).
Analysis of Autophagic Activities by ArrayScan
Autophagy (self-eating) is a process responsible for the bulk degradation of intracellular material that is evolutionarily conserved between all eukaryotes. During autophagy, a cytoplasmic form of LC3-I (18 kD) is proteolysed (near the C terminus) and conjugated to phosphatidylethanolamine to form a 16-kD product (LC3-II), which is recruited to autophagosomal membranes. Thus, detecting LC3-II by immunoblotting or immunofluorescence has become a reliable method for monitoring autophagic activities. Specifically, RAW 264.7 cells were seeded in 96-well plates and cultured in the presence of stimulus for a given time, then fixed with 3% paraformaldehyde and stained with HMGB1 and LC3 antibody (NOVUS). Secondary antibodies were goat Ig conjugated with either Alexa 488 or Alexa 680 fluorochromes. Nuclear morphology was analyzed with the fluorescent dye, Hoechst 33258. Image data were collected with an ArrayScan HCS 4.0 imaging cytometer with a 20× objective (Cellomics, Pittsburgh, PA). Arrayscan is an automated fluorescent imaging microscope that collects information about the spatial distribution of fluorescently labeled components in cells placed in 96-well microtiter plates. The Spot Detector BioApplication was used to acquire and analyze the images after optimization. Images of 1,000 cells for each group treatment were analyzed to obtain the average nuclear and cytosolic HMGB1 intensity and LC3 fluorescence spot number per cell, as previously described (42, 43).
Statistical Analysis
Data are expressed as means (±SEM) of three independent experiments performed in triplicate. One-way ANOVA was used for comparison among the different groups. When the ANOVA was significant, post hoc testing of differences between groups was performed using the least significant difference test. The Kaplan-Meyer method was used to compare the differences in mortality rates among groups. A P value of less than 0.05 was considered significant.
RESULTS
Pretreatment with Quercetin Prevents Endotoxin Lethality and Inhibits Release of TNF-α and HMGB1
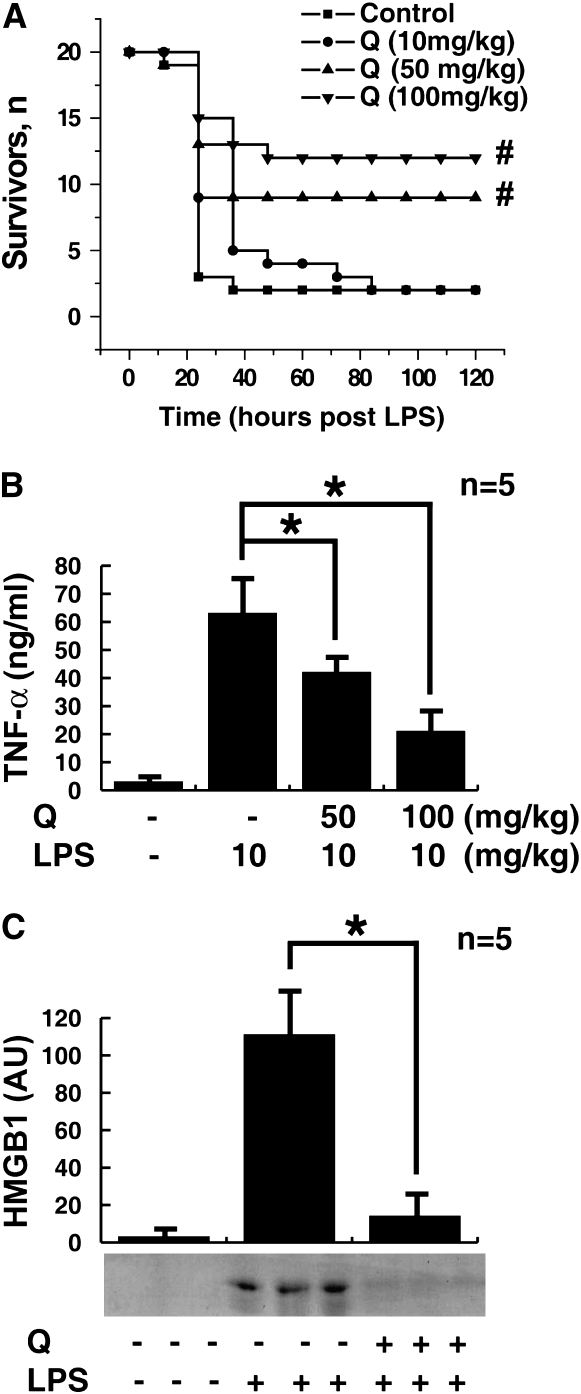
We conducted an initial evaluation of quercetin as a therapeutic agent in a standard model of murine endotoxemia. Balb/C mice received a single dose of quercetin (100, 50, or 10 mg/kg, intraperitoneally), followed 30 minutes later by an injection of LPS (10 mg/kg LPS, intraperitoneally). Pretreatment with a single dose of quercetin (100 or 50 mg/kg, intraperitoneally) conferred significant protection from lethal endotoxemia (survival in quercetin-treated mice = 12/20 or 9/20 compared with survival in vehicle-treated mice = 2/20; P < 0.05) (Figure 1A). Pretreatment with this dose of quercetin prevented the development of clinical manifestations of endotoxin morbidity, including decreased activity, lethargy, diarrhea, piloerection, and huddling. Late deaths in quercetin-treated animals were not observed during the 3 weeks after endotoxin injection, indicating that quercetin treatment conferred complete protection against lethal endotoxemia, and did not merely delay the onset of lethal pathology. A lower dose of quercetin (10 mg/kg, intraperitoneally) provided no protection (2/20). Because endotoxin induces systemic TNF-α accumulation (peaking between 1 and 2 h) before HMGB1 (peaking after 24 h) (3, 4), we determined the effects of quercetin on circulating TNF-α and HMGB1 at 1 and 20 hours, respectively. Pretreatment of endotoxemic mice with quercetin (100 or 50 mg/kg, intraperitoneally) significantly attenuated the serum levels of both TNF-α at 1 hour (Figure 1B) and HMGB1 at 20 hours (Figure 1C) after LPS infusion.
Figure 1.
Quercetin pretreatment prevents endotoxin lethality, attenuating TNF-α and high-mobility group box (HMGB) 1 release in vivo. (A) Mice (n = 20 per group) were injected with a single dose of quercetin (Q) as indicated, followed 30 minutes later by a lethal infusion of endotoxin (LPS, 10 mg/kg, intraperitoneally). Quercetin conferred significant protection against lethality (#different from vehicle control group, P < 0.05). The Kaplan-Meyer method was used to compare the differences in mortality rates between groups. (B) In a parallel group of quercetin-treated animals, circulating TNF levels were measured by ELISA of sera prepared at 1 hour after LPS injection. Quercetin pretreatment (50 and 100 mg/kg, intraperitoneally) significantly attenuated the release of serum TNF in response to LPS (*P < 0.05). (C) Circulating levels of HMGB1 20 hours after LPS infusion (LPS, 10 mg/kg, intraperitoneally) were measured in a parallel experimental group of endotoxemic animals by Western blot analysis. Pretreatment with quercetin (100 mg/kg, intraperitoneally) attenuated serum HMGB1 levels at 20 hours after LPS.
Slight Delay in Quercetin Administration Still Prevents Endotoxemic Lethality and Inhibits HMGB1 Release
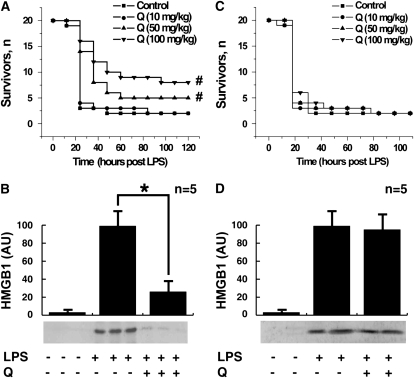
We next assessed the therapeutic efficacy of quercetin when first administered after the onset of endotoxemia. Treatment with quercetin was initiated 4 hours after the onset of endotoxemia, a time at which clinical signs of LPS-induced toxicity, including diarrhea, piloerection, and depressed spontaneous activity already were evident. Notably, the first dose of quercetin was administered well after the early peak in serum TNF-α, which occurs within the first 1 to 2 hours after the onset of endotoxemia (44). Delayed treatment with quercetin (100 or 50 mg/kg, intraperitoneally) beginning 4 hours after LPS injection protected mice from lethal systemic inflammation as compared with treatment with vehicle (survival with quercetin treatment = 8/20 or 5/20, respectively; survival with vehicle alone = 2/20; P < 0.05) (Figure 2A). Because treatment with quercetin began after the early, acute-phase response to endotoxin (Figure 1B), the significant protection conveyed by quercetin suggests that it might target late-acting mediators of lethal systemic inflammation. HMGB1 is a late mediator of endotoxin lethality (4), and treatment of endotoxemic mice with quercetin beginning 4 hours after LPS injection significantly attenuated the systemic release of HMGB1 measured at 20 hours after the onset of endotoxemia (Figure 2B). However, treatment of endotoxemic mice with quercetin beginning 12 hours after LPS injection failed to protect mice from lethal systemic inflammation, and did not attenuate the systemic release of HMGB1 at 20 hours after the onset of endotoxemia (Figures 2C and 2D).
Figure 2.
Effects of delayed administration of quercetin on the lethality of endotoxemia and serum HMGB1 level. (A) Mice (n = 20 per group) received a lethal infusion of endotoxin (LPS, 10 mg/kg, intraperitoneally) and were treated with quercetin (as indicated) 4, 8, 12, 24, and 36 hours later. Quercetin conferred significant protection against lethality (#different from vehicle control group, P < 0.05). The Kaplan-Meyer method was used to compare the differences in mortality rates between groups. (B) In a parallel set of endotoxemic mice, HMGB1 release was analyzed by Western blot of serum collected at 20 hours. Slight delayed treatment with quercetin (100 mg/kg, intraperitoneally) inhibited the release of serum HMGB1. (C) Mice (n = 20 per group) received a lethal infusion of endotoxin (LPS, 10 mg/kg, intraperitoneally) and were treated with quercetin (as indicated) 12, 24, and 36 hours later. Quercetin conferred no protection against lethality (P > 0.05). The Kaplan-Meyer method was used to compare the differences in mortality rates between groups. (D) In a parallel set of endotoxemic mice, HMGB1 release was analyzed by Western blot of serum collected at 20 hours.
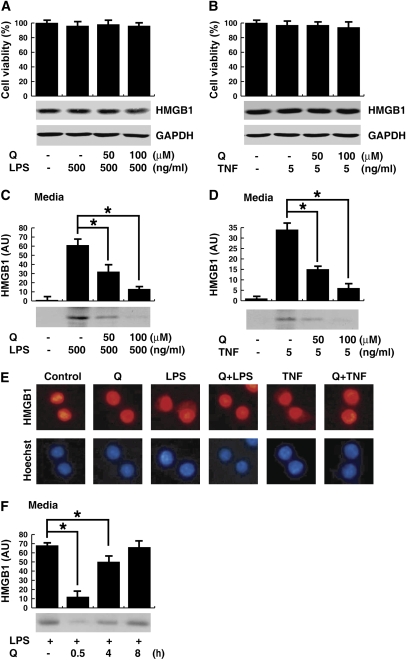
Quercetin Inhibits the Cytoplasmic Translocation and Release of HMGB1 in Macrophage Cultures
To determine the effect of quercetin on HMGB1 release by macrophages, murine macrophage line RAW 264.7 cells were stimulated with LPS or TNF-α for 24 hours, and HMGB1 measured in the conditioned medium. Administration of LPS or TNF-α or quercetin, or both (Figures 3A and 3B) did not affect the total levels of HMGB1 protein. However, quercetin pretreatment significantly inhibited LPS- and TNF-induced HMGB1 secretion (Figures 3C and 3D). HMGB1 translocates from the nucleus to the cytoplasm as the first step for its extracellular secretion (7, 9, 18). Accordingly, we next analyzed whether quercetin inhibited LPS- or TNF-induced HMGB1 cytoplasmic translocation (Figure 3E). In agreement with previous studies, fluorescence microscopy showed that HMGB1 localized predominantly in the nucleus of macrophages (7, 9, 18). LPS and TNF-α induced HMGB1 translocation to the cytoplasm, the earliest known stage leading to its extracellular secretion. Quercetin inhibited LPS- and TNF-induced HMGB1 cytoplasmic translocation and preserved its nuclear localization, suggesting that quercetin specifically inhibits the release of HMGB1 without affecting its protein levels. Although concurrent administration of quercetin was maximally effective in inhibiting LPS-induced HMGB1 release, significant inhibition was still achieved when it was added 0.5 to 4 hours after LPS (Figure 3F).
Figure 3.
Effects of quercetin on LPS- and TNF-α-induced HMGB1 expression, release and translocation in macrophage. (A–D) RAW 264.7 macrophages were pretreated for 1 hour with quercetin at the indicated dose before stimulation with LPS (A and C) or TNF-α (B and D) at the indicated doses for 24 hours, cell viability was determined by MTT assay, and expressed as the mean (±SEM) of four experiments in duplicate. In parallel experiments, HMGB1 levels in the whole-cell lysate (A and B) or culture medium (C and D) were determined by the relative optical intensity (in arbitrary units [AU]) of the immunoreactive bands on Western blots, and expressed as the mean (±SEM) of three experiments in duplicate. *P < 0.05. (E) RAW 264.7 macrophages were pretreated for 1 hour with quercetin (100 μM) before stimulation with LPS (500 ng/ml) or TNF-α (5 ng/ml) for 12 hours, and monitored for HMGB1 cytoplasmic translocation by immunocytochemistry (E). Red, HMGB1; blue, nuclei; original magnification, ×400. (F) RAW 264.7 cells were stimulated with LPS, and quercetin (100 μM) was added at 0.5, 4, and 8 hours after LPS stimulation. Levels of HMGB1 in the culture medium were determined at 20 hours after LPS stimulation, and expressed (AU) as mean (±SEM) of three experiments in duplicate.
Quercetin Inhibits Cytokine Activities of HMGB1 in Macrophage Cultures
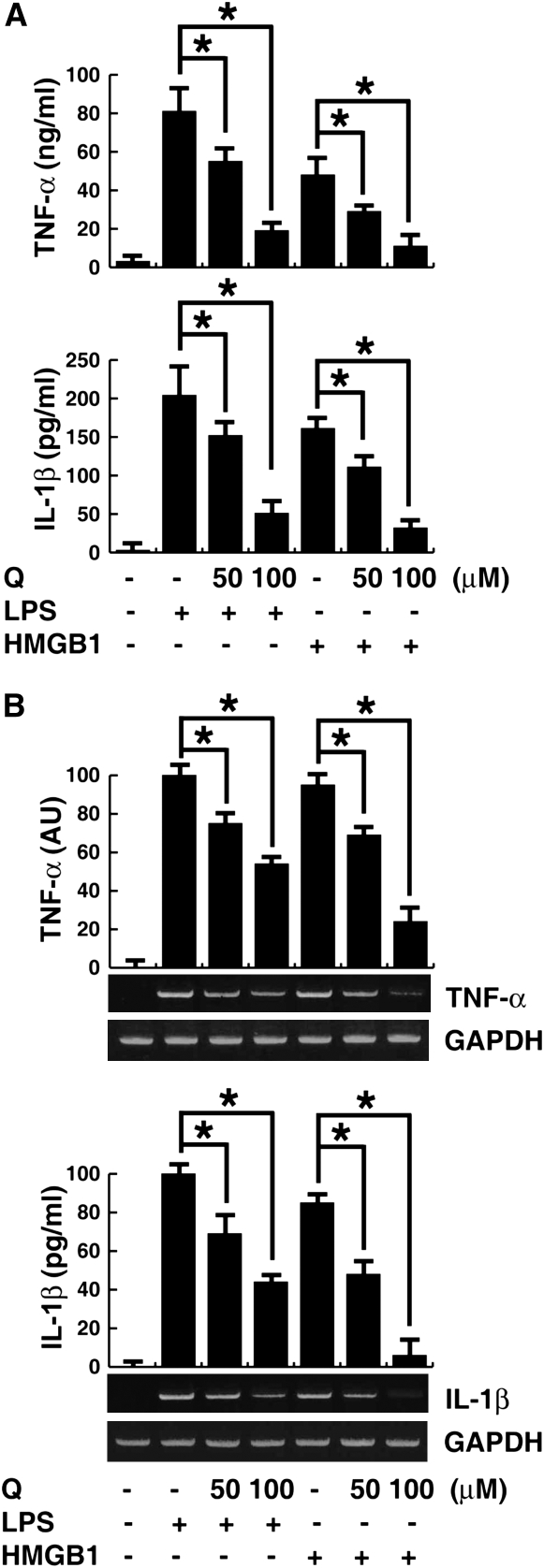
HMGB1 can be actively secreted by activated macrophages/monocytes, and passively released by damaged and necrotic cells, thereby mediating an inflammatory response (11, 45, 46). HMGB1 migration to organs/tissue sites induces several inflammatory cytokines, including TNF-α, IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-8, macrophage inflammatory protein (MIP)-1α, and MIP-1β, thereby contributing to the inflammatory cascade (13, 47). To determine the effect of quercetin on HMGB1 cytokine activites, murine macrophage-like RAW 264.7 cells were stimulated with LPS for 1 hour or HMGB1 for 4 hours, and TNF-α and IL-1β measured in the conditioned medium. Quercetin significantly inhibited HMGB1-induced TNF-α and IL-1β release in macrophage cultures in a concentration-dependent manner (Figure 4A). Quercetin also significantly reduced intracellular levels of TNF-α and IL-1β mRNA (Figure 4B), indicating that quercetin not only inhibits HMGB1 release, but also suppresses the proinflammatory activities of HMGB1. Similar to the results of previous studies (28, 30), quercetin inhibited LPS-induced expression and release of TNF-α (Figures 4A and 4B).
Figure 4.
Effects of quercetin on LPS- and HMGB1-induced TNF-α and IL-1β release and expression in macrophages. (A) RAW 264.7 macrophages were pretreated for 1 hour with quercetin at the indicated dose before stimulation with LPS (500 ng/ml) for 1 hour or HMGB1 (1 μg/ml) for 4 hours, and TNF-α and IL-1β levels in the culture medium were detected by ELISA analysis. Values are means (±SEM) of three experiments in duplicate. *P < 0.05. (B) In parallel experiments, the mRNA expression level of TNF-α and IL-1β were detected by RT-PCR analysis. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a loading control. Values are means (±SEM) of three experiments in duplicate. *P < 0.05.
Quercetin Inhibits Signal Transduction via Mitogen-Activated Protein Kinase and NF-κB
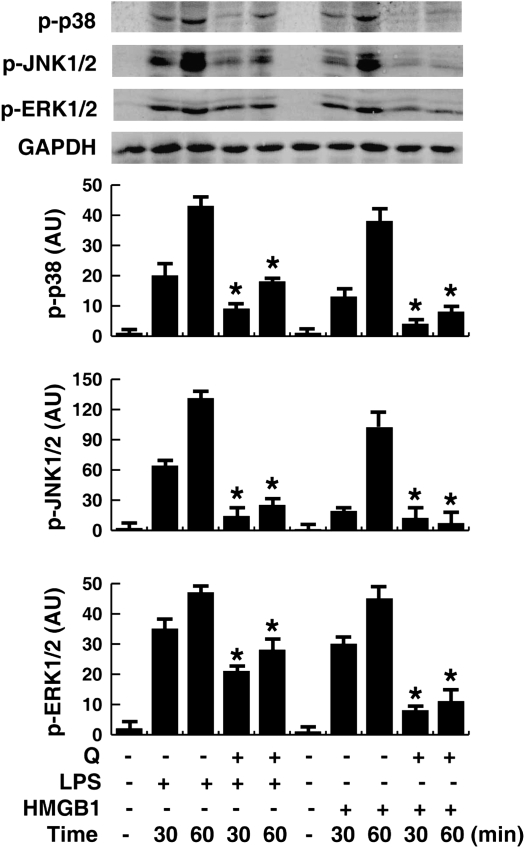
Quercetin-mediated inhibition of HMGB1-induced TNF-α and IL-1β mRNA expression suggests that quercetin might modulate intracellular signaling events that coordinate the activity of proinflammatory cytokines. Activation of mitogen-activated protein kinase (MAPK) signaling cascades is an important upstream step in HMGB1-induced expression and release of cytokines such as TNF-α and IL-1β in macrophages, neutrophils, and endothelial cells (14, 47–49). We investigated whether quercetin inhibits HMGB1-induced cytokine release partly through interfering with MAPK pathways. HMGB1 or LPS time-dependently induced phosphorylation of p38, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase MAPK in macrophages. Pretreatment with quercetin significantly inhibited HMGB1- or LPS-induced phosphorylation of each kinase (Figure 5).
Figure 5.
Effects of quercetin on LPS- and HMGB1-induced MAPKs phosphoration in macrophage cultures. RAW 264.7 macrophages were pretreated with quercetin (100 μM) for 1 hour before stimulation with LPS (500 ng/ml) or HMGB1 (1 μg/ml) for 30–60 minutes, and phosphorylated p38 (P-p38), c-Jun NH2-terminal kinase (JNK) 1/2 (P-JNK1/2), or extracellular signal-regulated kinase (ERK) 1/2 (P-ERK1/2) protein levels in the whole-cell lysate were detected by Western blotting analysis. GAPDH was used as a loading control. Values are means (±SEM) of three experiments in duplicate. *P < 0.05 versus LPS group or HMGB1 group.
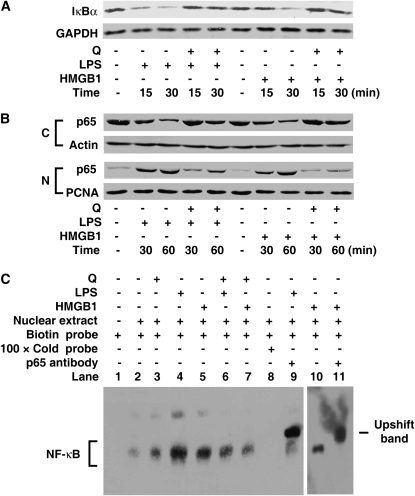
In addition to MAPK activation, the NF-κB signal transduction pathways is also involved in HMGB1-induced cellular activation, and NF-κB–dependent transcriptional activity is important for cytokine expression (49, 50). In quiescent cells, NF-κB factors (p50 and p65) are sequestered as inactive trimers in the cytosol through interaction with IκBα, the most important member of the IκB family (51). In response to HMGB1 or LPS stimulus, rapid IκBα degradation (by 15 min) (Figure 6A) preceded p65 (Figure 6B) and p50 (date not shown) translocation into the nucleus (by 30 min) in RAW 264.7 cells. However, pretreatment with quercetin significantly inhibited IκBα degradation, and NF-κB p65 nuclear translocation (Figures 6A and 6B). Thus, after stimulation with HMGB1 or LPS, p65, the key activator of NF-κB–regulated transcription, becomes available to κB-regulated genes in the nucleus, and nuclear localization is effectively inhibited by quercetin.
Figure 6.
Quercetin inhibits LPS- and HMGB1-induced inhibitor of NF-κB (IκBα) degradation, NF-κB p65 nuclear translocation, and NF-κB DNA binding activation in macrophage cultures. (A and B) Cell pretreatment with quercetin (100 μM) for 1 hour before stimulation with LPS (500 ng/ml) or HMGB1 (1 μg/ml) for indicated times, and IκBα level (A) in cytosolic fractions, and p65 level (B) in cytosolic (C) or nuclear (N) fractions were detected by Western blotting analysis. GAPDH, actin, or PCNA was used as a loading control. (C) Activities of transcriptional factor NF-κB in cells pretreated with quercetin (100 μM) for 1 hour in the absence or presence of LPS (500 ng/ml) or HMGB1 (1 μg/ml). At 1 hour after LPS or HMGB1 stimulation, NF-κB activities were determined by electrophoretic mobility shift assay with biotin-labeled NF-κB probe, and unlabeled (Cold) NF-κB probe. Specificity was determined by addition of p65 antibody to the nuclear extracts. All blots shown are representative of three experiments with similar results.
We further observed the effect of quercetin on NF-κB DNA binding activity. Indeed, HMGB1 or LPS stimulation of RAW 264.7 cells led to a dramatic increase in NF-κB DNA-binding activity assayed by electrophoretic mobility shift assay (Figure 6C, lanes 4, 5, and 10). The DNA-binding activities were specific, as indicated by competition with cold κB probe (Figure 6C, lane 8), and the presence of p65 in the NF-κB–DNA complexes was confirmed by a dramatic “supershift” in the presence of p65-specific antibodies (Figure 6C, lanes 9 and 11). Notably, HMGB1- or LPS-inducible DNA binding activity of NF-κB was markedly inhibited by quercetin (Figure 6C, lanes 6 and 7). It remains unknown whether NF-κB activation is critical in LPS-induced HMGB1 release, and whether quercetin inhibits LPS-induced HMGB1 release in an NF-κB–dependent mechanism.
Quercetin and Autophagic Inhibitor, Wortmannin, Concurrently Inhibited LPS-Induced LC3-II Production/Aggregation and HMGB1 Translocation/Release
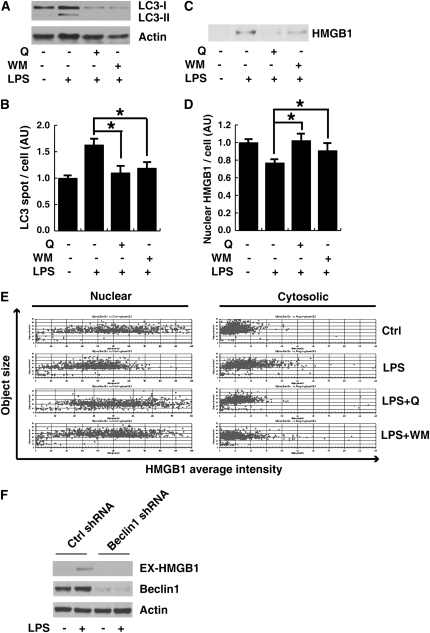
Autophagy has recently been shown to be an important component of the innate immune response to LPS treatment (39, 52). A critical step in the autophagy process is the ligation of LC3 to phosphatidylethanolamine, resulting in aggregates (spots) of LC3 (42). Incubation of RAW 264.7 cells with LPS led to the increase in LC3 spots and type-II LC3 (Figures 7A and 7B). Quercetin, as well as autophagic inhibitor, wortmannin, inhibited type-II LC3 production and aggregation (Figures 7A and 7B). Notably, wortmannin and quercetin inhibited LPS-induced HMGB1 translocation and release (Figures 7C–7E). Moreover, knockdown beclin-1, a key regulator of autophagy (53), inhibited LPS-induced HMGB1 release (Figure 7F). These data suggest that quercetin inhibits LPS-induced HMGB1 release, potentially through interfering with LPS-induced autophagy.
Figure 7.
Quercetin and wortmannin inhibited microtubule-associated protein 1A/1B–light chain 3 (LC3) expression and aggregation as well as HMGB1 translocation and release after treatment with LPS in macrophage cultures. RAW 264.7 macrophages were pretreated with quercetin (Q; 50 μM) or wortmannin (WM; 100 nM) for 1 hour before stimulation with LPS (500 ng/ml) for 24 hours, and LC3 protein levels in the whole-cell lysate (A) and HMGB1 release (C) were detected by Western blotting analysis. In parallel experiments, average LC3 spots (B) and nuclear and cytosole HMGB1 intensity (D) per cell were detected by ArrayScan assay. Values are means (±SEM). *P < 0.05. (E) HMGB1 translocation, determined by imaging analysis, was plotted against HMGB1 nuclear and cytosolic area. Each point represents a single cell. (F) RAW 264.7 cells were treated with Beclin1 shRNA, then stimulated with LPS (500 ng/ml) for 24 hours. Extracellular HMGB1 (Ex-HMGB1) was assayed by Western blotting analysis.
DISCUSSION
The pathogenesis of lethal sepsis remains obscure, but is associated with dysregulated inflammatory responses, tissue injury, and multiple organ dysfunction. The inflammatory response is mediated in part by bacterial endotoxin (54), which stimulates macrophages/monocytes to sequentially release early (TNF-α and IL-1β) and late (HMGB1) proinflammatory cytokines. Although early cytokines may be protective against infection (6), dysregulated inflammatory response sustained by late-acting mediators (such as HMGB1) may contribute to the development of tissue injury and organ dysfunction at the late stage of lethal sepsis. Therefore, agents capable of selectively attenuating systemic HMGB1 accumulation may hold potential in the treatment of lethal sepsis. Here, we demonstrated that an experimental pharmacological agent, quercetin, significantly inhibits the systemic release and proinflammatory function of both early (TNF-α and IL-1β) and late (HMGB1) cytokines that mediate lethality of systemic inflammation.
HMGB1 has recently been identified as a proinflammatory cytokine–like molecule participating in lethal systemic inflammation (e.g., endotoxemia and sepsis), arthritis, meningitis, and local inflammation (e.g., hepatic injury after ischemia–reperfusion and LPS-induced acute lung injury) (11, 46, 55–57). Activated macrophages release HMGB1 after a significant lag compared with TNF, and a similar delayed kinetic is observed in the serum after lethal endotoxemia (16). Targeting HMGB1 has been successful in experimental models of diverse infectious and inflammatory diseases, and these findings have renewed the clinical interest of specific cytokine inhibitors and methods (58). Here, we observed that quercetin significantly inhibited HMGB1 release from macrophages and prevented the accumulation of serum HMGB1 levels in mice with lethal endotoxemia.
Once HMGB1 is released into the extracellular space, it may interact with a wide range of exogenous (bacterial endotoxin, CG sequence–DNA) (59, 60) or endogenous (IL-1β) (61, 62) proinflammatory mediators, thereby amplifying a rigorous inflammatory response, which contributes to the pathogenesis of diverse disorders, including sepsis, cardiovascular shock, rheumatoid arthritis, diabetes, and cancer (58). Although HMGB1 itself may not be injurious to animals, it may act to amplify the LPS-induced shock response, because HMGB1 may facilitate innate recognition of bacterial endotoxin by CD14 (61), and synergistically increase endotoxin-induced animal lethality (4). The mechanisms underlying quercetin-mediated protection against lethal endotoxemia may be multiple: ranging from heme oxygenase–1 induction to scavenging of reactive oxygen species (63), to increasing production of IL-10 (64), to LPS-induced release of proinflammatory cytokines, such as TNF, IL-6, and HMGB1.
We further observed the effects of quercetin on HMGB1's proinflammatory function in vitro. As shown previously, HMGB1 induced the release of TNF-α and IL-1β in macrophages (13). However, quercetin inhibited HMGB1- or LPS-induced cytokine synthesis and release. It now appears that quercetin can be explored further in the development of treatments for critical illness and sepsis.
We further investigated the possible mechanism of quercetin's anti-inflammatory function by determining whether quercetin interferes with activation of MAPK and NF-κB signal transduction pathways. Macrophage activation by endotoxin, cytokines (e.g., TNF-α and HMGB1), and products of cell injury leads to the phosphorylation of MAPK (which increases cytokine translation efficiency [e.g., TNF-α and HMGB1] [19, 47–49, 65–67]), and nuclear translocation of NF-κB, a transcription factor that enhances the transcription of TNF-α and HMGB1 products of the activated macrophage (19, 49, 50, 68). The activity of NF-κB is mainly regulated by IκBα and p50/p65 (69). Quercetin inhibits the phosphorylation of the IκBα induced by LPS treatment in bone marrow–derived macrophages, hence inhibiting activation of the NF-κB pathway (27). Here, we demonstrate that quercetin inhibits activity of the NF-κB pathway partly by inhibiting HMGB1- or LPS-induced IκBα degradation, p65 nuclear translocation, and NF-κB DNA-binding activity in macrophages.
Autophagy (self-eating) is a highly evolutionarily conserved process in virtually all eukaryotic cells. It involves the sequestration of regions of the cytosol within double membrane–bound compartments, and delivery of the contents to the lysosomes for degradation. Autophagy has recently been shown to be an important component of the innate immune response (39, 52), and can be induced by LPS in primary human macrophages and murine macrophage RAW 264.7 cells in the absence of cell death (52). Here, we demonstrate that LPS-induced macrophage autophagy is associated with HMGB1 cytoplasmic translocation and release. Quercetin and phosphoinositide 3-kinase (PI3K) inhibitor, wortmannin, concurrently inhibited LPS-induced type-II LC3 production/aggregation and LPS-induced HMGB1 translocation/release. Indeed, class-III PI3K has been shown to be required for both autophagic vesicle formation and vesicular transport to the lysosome (70). As a broad-spectrum protein kinase inhibitor, quercetin can bind to PI3K ATP site and block PI3K activation (71). In light of the observation that HMGB1-containing secretory endolysosomes can be fused with the cytoplasmic membrane (72), it will be important to determine whether PI3K activation plays a role in the regulation of HMGB1 release.
Thus, quercetin may have therapeutic potential for diseases mediated by systemic release of the proinflammatory cytokines, TNF-α and HMGB1. The molecular target of quercetin action is enigmatic, but it is an effective inhibitor of HMGB1 release and proinflammatory function in macrophage cultures in vitro and in animal models of lethal endotoxemia in vivo. The doses of quercetin used here, which were not associated with toxicity, significantly inhibited serum HMGB1 release and conferred protection against endotoxemia lethality. Indeed, quercetin is a relatively nontoxic food compound, widely studied as an antioxidant, and the protective effects occurred in therapeutically achievable, safe doses. It will be of interest to assess the pharmacological activity of quercetin as an inhibitor of HMGB1 release in other models of local and systemic inflammation, including arthritis and sepsis induced by cecal ligation and puncture.
Acknowledgments
The authors thank Adam M. Farkas (University of Pittsburgh) for help with the ArrayScan assay.
This work was supported by National Natural Sciences Foundation of China grants 30500485 (D.T.) and 30330280 (X.X.), Major National Basic Research Program of China grant G2000056908 (X.X.), Specialized Research Fund for the Doctoral Program of Higher Education of China grant 20060533009 (X.X.), and Innovative Program of Central South University for Post-Graduate Research grant 2005-75239 (D.T.), and in part by National Institutes of Health (National Institute of General Medical Sciences) grants R01GM063075 and R01GM070817 (H.W.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0119OC on March 5, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979. through 2000. N Engl J Med 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ III, Zentella A, Albert JD, et al. Shock and tissue injury induced by recombinant human cachectin. Science 1986;234:470–474. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest 2003;112:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 1992;148:2724–2730. [PubMed] [Google Scholar]
- 7.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol 2003;170:3890–3897. [DOI] [PubMed] [Google Scholar]
- 8.Tang D, Shi Y, Jang L, Wang K, Xiao W, Xiao X. Heat shock response inhibits release of high mobility group box 1 protein induced by endotoxin in murine macrophages. Shock 2005;23:434–440. [DOI] [PubMed] [Google Scholar]
- 9.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 2007;81:741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol 2007;178:7376–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331–342. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 2004;101:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 2001;152:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 2004;173:307–313. [DOI] [PubMed] [Google Scholar]
- 16.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000;165:2950–2954. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 2004;170:1310–1316. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004;10:1216–1221. [DOI] [PubMed] [Google Scholar]
- 19.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA 2002;99:12351–12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, et al. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol 2007;178:3856–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 2006;203:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Li W, Li J, Rendon-Mitchell B, Ochani M, Ashok M, Yang L, Yang H, Tracey KJ, Wang P, et al. The aqueous extract of a popular herbal nutrient supplement, Angelica sinensis, protects mice against lethal endotoxemia and sepsis. J Nutr 2006;136:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjeldanes LF, Chang GW. Mutagenic activity of quercetin and related compounds. Science 1977;197:577–578. [DOI] [PubMed] [Google Scholar]
- 24.Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 1997;51:305–310. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Xu M, Lopes-Virella MF, Huang Y. Quercetin inhibits matrix metalloproteinase-1 expression in human vascular endothelial cells through extracellular signal-regulated kinase. Arch Biochem Biophys 2001;391:72–78. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, Yamamoto M, Nikaido T. Quercetin arrests human leukemic T-cells in late G1 phase of the cell cycle. Cancer Res 1992;52:6676–6681. [PubMed] [Google Scholar]
- 27.Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol 2005;35:584–592. [DOI] [PubMed] [Google Scholar]
- 28.Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr 2005;135:2299–2304. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Li X, Yue Y, Li J, He T, He Y. The inhibitory effect of quercetin on IL-6 production by LPS-stimulated neutrophils. Cell Mol Immunol 2005;2:455–460. [PubMed] [Google Scholar]
- 30.Manjeet KR, Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-alpha production in murine macrophages. Int J Immunopharmacol 1999;21:435–443. [DOI] [PubMed] [Google Scholar]
- 31.Kim BH, Lee IJ, Lee HY, Han SB, Hong JT, Ahn B, Lee CK, Kim Y. Quercetin 3-O-beta-(2″-galloyl)-glucopyranoside inhibits endotoxin LPS-induced IL-6 expression and NF-kappa B activation in macrophages. Cytokine 2007;39:207–215. [DOI] [PubMed] [Google Scholar]
- 32.Kumazawa Y, Kawaguchi K, Takimoto H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr Pharm Des 2006;12:4271–4279. [DOI] [PubMed] [Google Scholar]
- 33.Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 2005;521:9–20. [DOI] [PubMed] [Google Scholar]
- 34.Abd El-Gawad HM, Khalifa AE. Quercetin, coenzyme Q10, and L-canavanine as protective agents against lipid peroxidation and nitric oxide generation in endotoxin-induced shock in rat brain. Pharmacol Res 2001;43:257–263. [DOI] [PubMed] [Google Scholar]
- 35.Liu JJ, Song CW, Yue Y, Duan CG, Yang J, He T, He YZ. Quercetin inhibits LPS-induced delay in spontaneous apoptosis and activation of neutrophils. Inflamm Res 2005;54:500–507. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res 2005;46:623–627. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 38.Chander V, Singh D, Chopra K. Reversal of experimental myoglobinuric acute renal failure in rats by quercetin, a bioflavonoid. Pharmacology 2005;73:49–56. [DOI] [PubMed] [Google Scholar]
- 39.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007;450:1253–1257. [DOI] [PubMed] [Google Scholar]
- 40.Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol 2007;179:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Tu Z, Tang D, Zhang H, Liu M, Wang K, Calderwood SK, Xiao X. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock 2006;26:277–284. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Yu AD, Xie X, Ma D, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci USA 2007;104:19023–19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vakkila J, DeMarco RA, Lotze MT. Imaging analysis of STAT1 and NF-kappaB translocation in dendritic cells at the single cell level. J Immunol Methods 2004;294:123–134. [DOI] [PubMed] [Google Scholar]
- 44.Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MA Jr, Cerami A, Shires GT, Lowry SF. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet 1988;166:147–153. [PubMed] [Google Scholar]
- 45.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191–195. [DOI] [PubMed] [Google Scholar]
- 46.Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, Wang H, Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med 2008;36:291–295. [DOI] [PubMed] [Google Scholar]
- 47.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 2003;101:2652–2660. [DOI] [PubMed] [Google Scholar]
- 48.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 2003;284:C870–C879. [DOI] [PubMed] [Google Scholar]
- 49.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004;279:7370–7377. [DOI] [PubMed] [Google Scholar]
- 50.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005;61:1–9. [DOI] [PubMed] [Google Scholar]
- 51.Senftleben U, Karin M. The IKK/NF-kappaB pathway. Crit Care Med 2002;30:S18–S26. [PubMed] [Google Scholar]
- 52.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007;27:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999;402:672–676. [DOI] [PubMed] [Google Scholar]
- 54.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 2005;4:854–865. [DOI] [PubMed] [Google Scholar]
- 55.Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ. The role of high mobility group box-1 protein in severe sepsis. Curr Opin Infect Dis 2006;19:231–236. [DOI] [PubMed] [Google Scholar]
- 56.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol 2005;26:381–387. [DOI] [PubMed] [Google Scholar]
- 57.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia–reperfusion. J Exp Med 2005;201:1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantell LL, Parrish WR, Ulloa L. HMGB-1 as a therapeutic target for infectious and inflammatory disorders. Shock 2006;25:4–11. [DOI] [PubMed] [Google Scholar]
- 59.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 2007;110:1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007;8:487–496. [DOI] [PubMed] [Google Scholar]
- 61.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol 2008;180:5067–5074. [DOI] [PubMed] [Google Scholar]
- 62.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 2008;180:2531–2537. [DOI] [PubMed] [Google Scholar]
- 63.Lin HC, Cheng TH, Chen YC, Juan SH. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology 2004;71:107–112. [DOI] [PubMed] [Google Scholar]
- 64.Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, de Medina FS, Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow–derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol 2006;72:1010–1021. [DOI] [PubMed] [Google Scholar]
- 65.Dong Z, Qi X, Fidler IJ. Tyrosine phosphorylation of mitogen-activated protein kinases is necessary for activation of murine macrophages by natural and synthetic bacterial products. J Exp Med 1993;177:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 1995;267:682–685. [DOI] [PubMed] [Google Scholar]
- 67.Hambleton J, McMahon M, DeFranco AL. Activation of Raf-1 and mitogen-activated protein kinase in murine macrophages partially mimics lipopolysaccharide-induced signaling events. J Exp Med 1995;182:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishikawa Y, Mukaida N, Kuno K, Rice N, Okamoto S, Matsushima K. Establishment of lipopolysaccharide-dependent nuclear factor kappa B activation in a cell-free system. J Biol Chem 1995;270:4158–4164. [PubMed] [Google Scholar]
- 69.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998;16:225–260. [DOI] [PubMed] [Google Scholar]
- 70.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov 2007;6:304–312. [DOI] [PubMed] [Google Scholar]
- 71.Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 2000;6:909–919. [DOI] [PubMed] [Google Scholar]
- 72.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002;3:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]