Abstract
Rationale: Cough is the most frequent reason for consultation with a family doctor, or with a general or respiratory physician. Treatment options are limited and one meta-analysis concluded that over-the-counter remedies are ineffective. There is also increasing concern about their use in children. Environmental irritants such as air pollution and cigarette smoke are thought to evoke cough by stimulating airway sensory nerves; however, how this occurs is not fully understood.
Objectives: We hypothesized that the TRPA1 (transient receptor potential cation channel, subfamily A, member 1) receptor may have a role as a novel target for tussive agents given that many potential irritants have been shown to activate this channel.
Methods: We investigated the effect of TRPA1 ligands on vagal sensory nerve activity in vitro and in guinea pig and human tussive challenge models.
Measurements and Main Results: We demonstrated that TRPA1 agonists such as acrolein activate cloned human TRPA1 channels in HEK293 cells and also vagal sensory nerves in murine, guinea pig, and human tissues. A role for TRPA1 was confirmed, using specific inhibitors and tissue from Trpa1−/− gene–deleted animals. Finally, TRPA1 ligands evoked reproducible tussive responses in both a guinea pig model and normal volunteers.
Conclusions: This study identifies the TRPA1 receptor as a promiscuous receptor, activated by a wide range of stimuli, making it a perfect target for triggering cough and as such one of the most promising targets currently identified for the development of antitussive drugs.
Keywords: sensory nerves, cough, vagus
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
TRPA1 ligands are known to activate airway sensory nerves. However, there is little knowledge concerning the functional effects of these agents. No data currently exist profiling these ligands in clinical studies in the lung.
What This Study Adds to the Field
TRPA1 ligands evoked reproducible tussive responses in an animal model and in humans.
Cough is the most frequent reason for consultation with a family doctor (1), or with a general or respiratory physician. Patients with chronic cough probably account for 10–38% of respiratory outpatient practice in the United States (2). Chronic cough, of various etiologies, is a common presentation to specialist respiratory clinics and is reported as a troublesome symptom by 7% of the population (3). Treatment options are limited. A meta-analysis concluded that over-the-counter cough remedies are ineffective (4), and there is increasing concern about the use of over-the-counter therapies in children. Despite its importance our understanding of the mechanisms that provoke cough is poor.
Ion channels of the transient receptor potential (TRP) class have been implicated in the afferent sensory loop of the cough reflex (5–7) and in the heightened cough sensitivity seen in disease (8). Agonists of the TRPV1 (transient receptor potential cation channel, subfamily V, member 1) capsaicin receptor, such as vanilloids and protons, are among the most potent chemical stimuli that cause cough (5–7). Another TRP receptor, TRPA1 (transient receptor potential cation channel, subfamily A, member 1), which is not activated by capsaicin, has been shown to bind ligands such as acrolein, which is present in air pollution and the acrid smoke from organic material (9). We hypothesized that TRPA1 may have a role as a novel target for tussive agents.
TRPA1 is a Ca2+-permeant nonselective channel with 14 ankyrin repeats in its amino terminus, which belongs to the larger TRP family (10). TRPA1 has been characterized as a thermoreceptor that is activated by cold temperature (11). In addition, TRPA1 channels are activated by a range of natural products such as allyl isothiocyanate, allicin, and cannabinol, found in mustard oil, garlic, and cannabis, respectively (12–14). The channel is also activated by a multitude of environmental irritants such as isothiocyanate, cinnamaldehyde, and acrolein. The latter is present in air pollution, vehicle exhaust, and cigarette smoke (9, 15–20). TRPA1 is expressed primarily in small-diameter, nociceptive neurons where its activation probably contributes to the perception of noxious stimuli and the phenomena known as inflammatory hyperalgesia and neurogenic inflammation (9, 17, 19).
The respiratory tract is innervated by sensory afferent nerves including the myelinated, rapidly adapting receptors and the nonmyelinated, chemosensitive C-fibers, which are activated by mechanical and chemical stimuli (21, 22). Activation of these vagal sensory afferents leads to central reflexes including cough. It has been demonstrated that stimulating TRPA1 channels activates vagal bronchopulmonary C-fibers in the guinea pig and rodent lung (23–25).
The aim of these experiments was to employ a series of preclinical and clinical studies to determine, for the first time, whether activation of the TRPA1 channel can evoke a tussive response. Evidence to this effect could result in a deeper understanding of the pathogenesis of cough and lead to the development of novel treatment modalities.
METHODS
Functional Characterization of Cloned TRPA1 Expressed in HEK293 Cells
TRPA1-expressing HEK293 cells and the method used to measure increases in intracellular calcium levels in this study have been described previously (26; and see the online supplement).
Animals
Male Dunkin-Hartley guinea pigs (300–500 g) were housed in a temperature-controlled (21°C) room with food and water freely available for at least 1 week before commencing the experiment. The experiments were performed in accordance with the U.K. Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986.
Effect of TRPA1 Ligands on Isolated Vagal Nerve Preparation
To demonstrate a functional response of TRPA1 channel activation in native tissue we used our fully characterized isolated vagal nerve preparation as described in previous publications (27, 28). Sensory nerve responses to the two TRPA1 agonists, acrolein and cinnamaldehyde, were determined. For these experiments only one response to one agonist was obtained in each segment of vagus. We also performed experiments with the selective TRPA1 inhibitor, HC-030031, or vehicle (dimethyl sulfoxide, 0.1% [vol/vol]) (17). To demonstrate antagonist selectivity HC-030031 was also tested against the TRPV1 agonist capsaicin, using a parallel protocol. To provide additional proof-of-concept data an alternative antagonist was also used, AP-18 (29).
To further confirm that the response observed was via activation of the TRPA1 channel we performed parallel agonist (acrolein) experiments using vagal tissue from wild-type and Trpa1−/− gene-deleted mice (Jackson Laboratory, Bar Harbor, ME). We confirmed the deletion of the Trpa1−/− gene in the knockout mice, and not the wild type, using standard genotyping techniques.
Effect of TRPA1 Ligand Acrolein in Guinea Pig Conscious Cough Model
Cough was detected both by pressure change and by sound and recorded with a Buxco cough analyzer (Buxco, Wilmington, NC) as previously described (28). Guinea pigs received an aerosol of vehicle (0.9% sterile saline, n = 12) or acrolein (10, 30, 100, or 300 mM, n = 12, concentration derived from a preliminary study) for 5 minutes and coughs were counted during this period and for a further 5 minutes with the Buxco cough analyzer.
Having established a submaximal dose of acrolein, we confirmed the role of TRPA1 using the selective inhibitor HC-030031. Guinea pigs were dosed with vehicle (0.5% methyl cellulose in sterile saline at 1 ml/kg, administered intraperitoneally, n = 12) or HC-030031 (300 mg/kg, dose selected from data published by McNamara and colleagues [17]). One hour later the guinea pigs were exposed to a submaximal dose of TRPA1 agonist and cough was monitored as outlined previously.
In Vitro Functional Characterization of TRPA1 in Isolated Human Vagal Tissue
Briefly, human trachea, with branches of the cervical vagus still attached, was obtained from three unused donor tissue samples surplus to clinical requirement (two males, 32–55 yr of age) collected for heart–lung transplantation. Relevant approvals were obtained from the next of kin and the Royal Brompton and Harefield Trust Ethics Committee. The isolated human vagus was exposed to a TRPA1 agonist and antagonist as described previously for the guinea pig experiments.
Characterization of Tussive Response to Inhalation of TRPA1 Agonist in Human Volunteers
Healthy male and female, nonsmoking volunteers with normal lung function were entered into a randomized, crossover study of inhalational cough challenge of three tussive agents. Informed written consent was obtained from all the volunteers and the study was approved by the Hull and East Yorkshire Ethics Committee. Volunteers were randomized to inhalational cough challenge with the TRPA1 agonist diluent or cinnamaldehyde or capsaicin or citric acid. At Visit 1 a baseline cough challenge was performed and repeated 1 hour later with the same agent. The subjects were asked to return on two occasions 2–3 days apart when the protocol was repeated with an alternative cough stimulus. Each subject was recalled 2–3 days later for Visit 4 when a subthreshold dose (i.e., the dose below that evoking cough) of cinnamaldehyde or diluent was administered followed by a repeat cough challenge to capsaicin and citric acid, which was performed 1 hour later. This was repeated 2–3 days later at Visit 5 with the alternative subthreshold inhalation. Safety assessments were performed at all these visits.
Nebulizations were performed with a MEFAR MB3 dosimeter (Mefar Elletromedicale, Bovezzo, Italy) with a Respironics nebulizer chamber and mouthpiece according to our previously described methodology (30). The dosimeter was set to nebulize for 1 second and 2 ml of the agent was instilled into the nebulizer chamber. Coughs induced were assessed during the subsequent 15 seconds after the nebulization. Serial dilutions from stock (1 M citric acid, 1 mM capsaicin) were made with 0.9% saline whereas 50% ethanol was used as the diluent for cinnamaldehyde (800 mM).
Compounds and Materials
Fluo-3 acetoxymethyl ester and LipofectAMINE 2000 were purchased from Invitrogen (Paisley, UK). Penicillin–streptomycin, fetal calf serum, Dulbecco's modified Eagle's medium, sodium pyruvate, and geneticin (G418) were all bought from GIBCO (Paisley, UK). The TRPA1 inhibitor HC-030031 was purchased from ChemBridge (San Diego, CA). All other agents were purchased from Sigma-Aldrich (Poole, Dorset, UK). All other details are in the online supplement.
Data Analysis and Statistics
Antagonism of TRPA1 agonists was analyzed by two-tailed paired t test, comparing responses to agonist (in the same piece of vagus nerve) in the absence and presence of antagonist. Responses to TRPA1 ligands in gene-deleted mice were analyzed by Kruskal-Wallis test for multiple comparisons with Dunn's post-hoc test, comparing the responses in each TRPA1 receptor knockout with those of the wild-type control. Inhibition of acrolein-induced cough was analyzed by Mann-Whitney U test for nonparametric data. Data are presented as means ± SEM and statistical significance is denoted as *P < 0.05.
In the clinical study end points were taken as the concentration at which two or more coughs were induced (C2) and at which five or more coughs were induced (C5). For the purposes of statistical analysis the data were log transformed. SPSS version 13.0 was used for statistical analysis. Paired t tests or Wilcoxon analysis was performed when applicable. A P value of 0.05 or less was considered statistically significant.
RESULTS
In Vitro Functional Characterization of TRPA1 Ligands on Cloned Human TRPA1 Channels in HEK293 Cells
The TRPA1 receptor was successfully cloned from primary human fibroblasts and permanently expressed in HEK293 cells. Reverse transcription-polymerase chain reaction showed that HEK293 cells do not endogenously express TRPA1 mRNA (see Figure E1 in the online supplement).
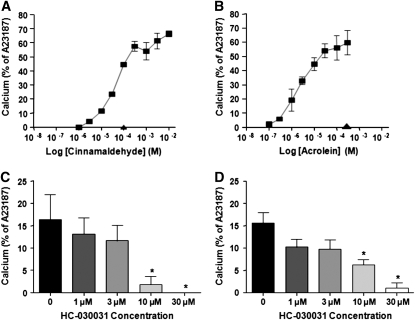
Calcium signaling was used to assess agonist-induced activation of TRPA1-HEK cells (Figure 1). hTRPA1-HEK cells responded to both cinnamaldehyde and acrolein in a concentration-dependent manner (Figure 1). None of the agonists tested stimulated an observable increase in calcium in nontransfected or mock-transfected HEK cells or in hTRPV1-HEK cells (Figure 1). These results also demonstrated that HC-030031 caused inhibition of both cinnamaldehyde- and acrolein-evoked responses in a concentration-dependent manner.
Figure 1.
Calcium signaling was used to assess agonist induced activation of TRPA1-HEK cells and agonist-induced activation of TRPA1-HEK cells after preincubation with the antagonist HC-030031. (A) Cinnamaldehyde concentration–response curve (squares) in hTRPA1-HEK cells. hTRPV1-HEK cells failed to respond to 100 μM cinnamaldehyde (triangle). (B) Acrolein concentration–response curve (squares) in hTRPA1-HEK cells. hTRPV1-HEK cells failed to respond to 300 μM acrolein (triangle). (C) Effect of increasing HC-030031 concentrations on cinnamaldehyde (30 μM)-evoked calcium responses. (D) Effect of increasing HC-030031 concentrations on acrolein (3 μM)-evoked calcium responses. Results are expressed as means ± SEM of up to seven experiments, each performed in duplicate. *Statistical significance (P < 0.05) from vehicle-treated group.
Effect of TRPA1 Ligands on Isolated Guinea Pig Vagal Nerve Preparation
Tussive agents such as capsaicin, low-pH solutions, and prostaglandin E2 (PGE2) are known vagal sensory nerve stimulants and as such isolated guinea pig, murine, and human vagus nerve preparations have been shown to elicit nerve depolarization responses to these stimulants (27, 28, 31). Furthermore, these agents are also known tussigenic agents in human and animal studies (5, 6, 32). These data suggest that the isolated vagus nerve is a useful and predictive preparation for conducting comprehensive pharmacological assessments of agents that may activate or inhibit sensory nerve function and thus the cough reflex. Previous data also suggest that the information generated is broadly predictive of data generated in single afferent nerve fiber recording studies, further validating the use of the isolated vagus preparation.
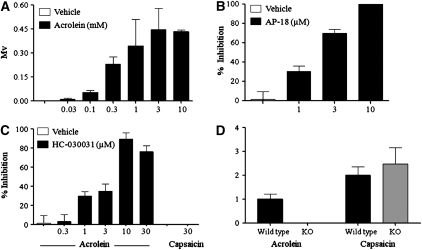
Therefore, to demonstrate a functional role for the TRPA1 channel in a native tissue, we demonstrated a concentration-related increase in the depolarization of the guinea pig isolated vagus (indicative of sensory nerve activation) with acrolein (Figure 2A). Furthermore, data obtained with cinnamaldehyde produced a similar pattern (see Figure E2 in the online supplement). To further confirm a role for TRPA1, we demonstrated that the selective TRPA1 inhibitors AP-18 and HC-030031 blocked depolarization in response to acrolein (Figures 2B and 2C). However, HC-030031 did not affect responses evoked by capsaicin (Figure 2C). In addition, we have shown that the vagus from Trpa1−/− mice failed to respond to the TRPA1 agonist acrolein, but depolarized in response to a TRPV1 agonist, capsaicin. The wild-type preparations responded appropriately to these stimuli (Figure 2D).
Figure 2.
(A) Characterization of the depolarization (mV) responses elicited by isolated guinea pig vagus nerve preparations in response to the TRPA1 agonist acrolein. (B and C) Effect of TRPA1 channel blockers (AP-18, 1–10 μM; and HC-030031, 0.3–30 μM) on acrolein (300 μM)- or capsaicin (1 μM)-induced depolarization of isolated guinea pig vagus nerve preparations. Values are presented as means ± SEM of the percentage inhibition of depolarization before and after drug superfusion (n = 4). (D) Depolarization (mV) in response to TRPA1 (acrolein, 1 mM)/TRPV1 (capsaicin, 1 μM) agonists in vagal nerve tissue from wild-type and Trpa1−/− gene-deleted mice. Results are expressed as the mean ± SEM of four to six experiments. KO, knockout.
Effect of TRPA1 Ligand Acrolein in a Conscious Guinea Pig Cough Model
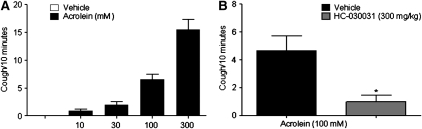
To demonstrate that the observations in the isolated tissue translate in vivo we exposed guinea pigs to aerosolized acrolein. As can be seen in Figure 3, the TRPA1 agonist caused a dose-related increase in coughs. To confirm that the response was through the TRPA1 channel we performed experiments in which the guinea pigs were pretreated with the TRPA1 inhibitor before exposure to acrolein. In these experiments HC-030031 significantly inhibited the tussive response to acrolein (Figure 3).
Figure 3.
(A) Dose response to inhaled acrolein in conscious male guinea pigs (n = 12). (B) Blockade of the tussive response to acrolein with the TRPA1 ion channel blocker HC-030031 (300 mg/kg). Guinea pigs were dosed with vehicle or HC-030031 one hour before the tussive challenge (n = 12). Statistical significance was determined by Mann-Whitney U test for nonparametric data. Data are presented as means ± SEM and statistical significance is denoted as *P < 0.05.
In Vitro Functional Characterization of Response to TRPA1 in Isolated Human Vagal Tissue
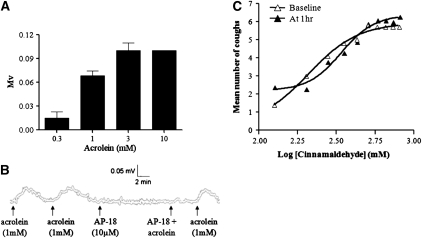
To demonstrate that the observations in guinea pigs and mice translated to humans we paralleled the key vagal experiments with tissue collected from donor lung samples. Figure 4 shows that the human vagus is activated by acrolein (Figure 4A) and that this response is inhibited by a TRPA1 antagonist (Figure 4B).
Figure 4.
(A) Characterization of depolarization (mV) responses elicited by isolated human vagus nerve preparations in response to the TRPA1 agonist acrolein. Results are expressed as means ± SEM of three experiments. (B) A representative trace describing depolarization of isolated human vagus evoked by acrolein (1 mM) and inhibition in the presence of the TRPA1 antagonist AP-18 (10 μM). The response to acrolein is recovered after washout. (C) Concentration–response curve of cinnamaldehyde inhaled at baseline and repeated 1 hour later in human volunteers. Nebulizations were performed with the MEFAR MB3 dosimeter with a Respironics nebulizer chamber and mouthpiece. The dosimeter was set to nebulize for 1 second and 2 ml of the agent was instilled into the nebulizer chamber. Coughs induced were assessed during the subsequent 15 seconds after the nebulization.
Demonstration of Tussive Response to TRPA1 Agonist in Human Volunteers
Ten subjects (6 males; mean age, 33.5 yr) were studied. Inhalation of nebulized cinnamaldehyde (but not diluent) induced cough in all subjects. One subject did not achieve C5 (the concentration inducing five or more coughs) at the highest concentration. One subject became nauseous at C2 (the concentration causing two or more coughs) and was withdrawn from further study. There was a distinct concentration–response relationship to inhaled cinnamaldehyde (Figure 4C). The median C2 for the baseline cinnamaldehyde challenge was 200 mM (range, 125–not achieved) with no significant tachyphylaxis seen with the second challenge (median C2, 275 mM [range 125–800]). The median C5 was 312.5 mM (range, 125–not achieved) compared with 387.5 mM (range, 125–not achieved) at 1 hour from baseline. These values are compared with citric acid and capsaicin challenge in the same subject in Table 1. Citric acid was the only agent that showed significant tachyphylaxis (P = 0.02) for C5. The prior inhalation of a subthreshold dose of cinnamaldehyde or of the diluent did not have any effect on subsequent cough challenge. Inhalation of cinnamaldehyde was well tolerated with no adverse effects other than mild nausea in one person.
TABLE 1.
RESPONSE THRESHOLD FOR THE VARIOUS TUSSIVE CHALLENGES IN 10 NORMAL SUBJECTS
| Tussive Agent | Response | Baseline | At 1 Hour | After Subthreshold Cinnamaldehyde | After Diluent |
|---|---|---|---|---|---|
| Cinnamaldehyde (mM) | C2 | 200 | 275 | n/a | n/a |
| C5 | 313 | 388 | n/a | n/a | |
| Citric acid (mM) | C2 | 125 | 125 | 125 | 94 |
| C5 | 200 | 250 | 250 | 250 | |
| Capsaicin (μM) | C2 | 4 | 4 | 8 | 4 |
| C5 |
12 |
16 |
16 |
16 |
Shown are median C2 and C5 values at baseline and 1 hour after baseline for cinnamaldehyde, citric acid, and capsaicin as well as median C2 and C5 values after inhalation of subthreshold TRPA1 agonist and diluent. C2 is the concentration inhaled at which two or more coughs were recorded and C5 is the concentration at which five or more coughs were recorded. In the citric acid group the concentration needed to induce cough was significantly greater at 1 hour (P = 0.02).
DISCUSSION
Inhalational exposure to numerous irritating gases, fumes, dusts, vapors, chemicals, and endogenous mediators can lead to the development of cough. It has long been established that TRPV1 receptor activation can elicit a cough response in both animal models and in humans (5–7). This receptor is polyvalent, and is activated by vanilloids (e.g., capsaicin), noxious heat (≥42°C), extracellular protons (pH ≤ 5.9), and endogenous lipids (e.g., anandamide) and eicosanoids [e.g., leukotriene B4, 12-(S)-hydroperoxyeicosatetraenoic acid, and 15-(S)-hydroperoxyeicosatetraenoic acid]. However, it does not respond to many irritants known to initiate cough. The mechanism whereby a range of seemingly diverse irritants initiate acute and chronic cough has remained a mystery. These data have identified, for the first time, an alternative target for antitussive therapies that responds to known environmental irritants.
We began by cloning human TRPA1 and permanently expressing it in HEK293 cells and demonstrated that the reported TRPA1 agonists activated this channel, confirming previous data (19, 24). In house we have extensively characterized robust and clinically relevant preclinical systems in which to test potential sensory nerve modulators (27, 28). As the guinea pig is the only small animal that possesses a cough reflex that resembles the human response we used the isolated guinea pig vagus in conjunction with an in vivo conscious guinea pig cough model (28). Using the isolated guinea pig vagus preparation we demonstrated that two different TRPA1 agonists were able to activate sensory nerves and that this response could be attenuated with selective TRPA1 inhibitors. Similar data demonstrating inhibition of the TRPA1 channel with these inhibitors have been generated by other investigators (17–19, 29). To strengthen this pharmacological proof of concept we performed experiments with knockout mice. We demonstrated that TRPA1 and TRPV1 agonists both activate vagal sensory nerves in the wild-type mice; but that depolarization was achieved only by the TRPV1 agonist capsaicin, on the isolated vagal preparations from mice from which the Trpa1 gene was deleted. Although the vagus is a useful and predictive preparation to use for a comprehensive pharmacological assessment of the activity of TRPA1 ligands on vagal sensory nerve activation the data should be viewed with some caution because pharmacological agents are applied to the axon of the isolated vagus nerve in vitro and not the nerve ending. The advantages and limitations of this preparation have been discussed at length in our previous publications (27, 28) however, evidence to date suggests that the isolated vagus preparation is predictive of data generated with single-fiber electrophysiological preparations and data generated in the conscious guinea pig cough mode (33). Furthermore, it is consistent with electrophysiological evidence suggesting that functional TRPA1 channels exist in respiratory nociceptive sensory neurons and their terminals (24). Next, we were able to show a dose–response relationship between increasing challenges with a TRPA1 agonist and the number of coughs in a guinea pig model. What is more, we were able to attenuate this tussive response with a selective TRPA1 inhibitor. To demonstrate that the preclinical data generated in animal in vitro and in vivo models actually translated to humans, we paralleled the previous experiments, using human vagal tissue.
With proof-of-concept data in an animal in vivo model and in human tissue in vitro we were able to move on with confidence to study normal human volunteers. The data from this study clearly show that a TRPA1 agonist causes a concentration-dependent cough in humans. The human cough response appears to be reproducible and without significant tachyphylaxis in this small data set. Agonizing TRPA1, using this protocol, does not appear to impact on cough responses to other tussive agents, suggesting a direct and specific response.
The TRPA1 receptor is activated by a number of irritant chemicals including mustard oil (i.e., isothiocyanate), acrolein, garlic, formalin, allicin, wasabi, cinnamon oil, diallyl disulfide, allyl isothiocyanate, 4-hydroxynonenal, and 4-oxononenal (12–14, 16–18, 20, 23, 25). The mechanism of action would appear to be through covalent modification of cysteine residues within the cytosolic N terminus of TRPA1 by reactive electrophilic molecules (16). This mode of action could be significant in pathological situations given that oxidant stress induced by either an inflammatory response or by exogenous irritants, such as cigarette smoke, can generate reactive electrophilic molecules including acrolein and 4-hydroxynonenal. This also suggests that the TRPA1 receptor is a promiscuous receptor that can be activated by a wide range of stimuli, thus making it a perfect target for triggering protective cough reflexes. However, more work will be required to determine which of these putative agonists are sufficiently potent to stimulate cough in vivo.
Interestingly, inflammation can lead to the formation of endogenous electrophilic compounds in vivo (34). Examples of such compounds are the cyclopentenone ring-containing A and J series prostaglandins, which are formed as nonenzymatic dehydration products of PGE2 and PGD2, respectively. Prostanoids that contain one or two electrophilic carbons such as 15PGJ2, Δ12-PGJ2, 8-iso-PGA2, and PGA2 are therefore able to activate nociceptive neurons via direct interaction with TRPA1 (25). Furthermore, prostaglandins such as PGE2 and the inducible form of cyclooxygenase-2 are elevated in respiratory disease states at the site of inflammation (35–37). Taken together, this information would suggest that reactive prostanoids and other endogenous TRPA1 ligands, which are produced in greater amounts during inflammation or oxidant stress, could evoke the cough seen in conditions such as asthma and chronic obstructive pulmonary disease.
It is still unclear whether there is cooperation between TRPV1 and TRPA1 channels. Both are activated by tussive agents and so it could be possible that these TRP channels act in concert to elicit functional responses. The dependence of TRPA1 on Ca2+ may result in the activation of TRPA1 channels by an overflow of Ca2+ in the locale of other activated channels without ever being modified by a reactive ligand. Furthermore, TRPA1 channels may also act as an amplifier of other Ca2+-mobilizing pathways, including activation of TRPV1 (38). However, whether this sort of cooperation exists in generating a cough reflex has yet to be determined.
In conclusion, we have shown for the first time that activation of the TRPA1 channel can evoke a tussive response in humans. This novel and exciting finding could have major implications for understanding the pathogenesis of respiratory diseases and for the treatment of cough, which presents as a significant unmet medical need. Because of their central role and activation by a wide range of irritant and chemical substances, either by exogenous agents, endogenously produced mediators during inflammation, or by oxidant stress, we suggest TRPA1 channels should be considered as one of the most promising targets currently identified for the development of novel antitussive drugs.
Supplementary Material
Supported by project grants from the Medical Research Council (MRC, UK) (M.A.B., G0800196; S.A.M. and M.G., G0800195). V.F.M. was funded by a European Respiratory Society Fellowship (no. 154). L.S. was funded by a grant from Proctor & Gamble. The human tissue work was funded by an Experimental Medicine Grant (G0502019) from the MRC, UK. The human tissue experiments described in this article were undertaken with the support of the NIHR Biomedical Research Unit in Advanced Lung Disease at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London and partly funded by the NIHR Biomedical Research Unit funding scheme.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200905-0665OC on September 3, 2009
Author Disclosure Statement: M.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G.B. received $5,001–$10,000 from GlaxoSmithKline, $5,001–$10,000 from Sound Pharma, and $5,001–$10,000 from AstraZeneca in consultancy fees, $1,001–$5,000 from Vectura, $1,001–$5,000 from GlaxoSmithKline, and $1,001–$5,000 from Schering Plough in advisory board fees, and $10,001–$50,000 from AstraZeneca and $10,001–$50,000 from Novartis in industry-sponsored grants. M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.S.'s institution received more than $100,001 from Procter & Gamble in sponsored grants. S.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.F.-M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H.M. received $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from AstraZeneca, and $5,001–$10,000 from Boehringer Ingelheim as an employee, $1,001–$5,000 from GlaxoSmithKline and $5,001–$10,000 from Procter & Gamble, $1,001–$5,000 from Procter & Gamble for serving on an advisory board. A.H.M.'s institution received a $50,001–$100,000 P&G grant.
References
- 1.McCormick A, Fleming DM, Charlton J. Office of population censuses and surveys. In: Morbidity statistics from general practice, fourth national study 1991–1992. Series MB5 no 3. London: HMSO; 1995.
- 2.Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis 1981;123:413–417. [DOI] [PubMed] [Google Scholar]
- 3.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006;61:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroeder K, Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ 2002;324:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol 1993;6:171–175. [DOI] [PubMed] [Google Scholar]
- 6.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol 1995;79:1082–1087. [DOI] [PubMed] [Google Scholar]
- 7.Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 2004;59:769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 2004;170:1276–1280. [DOI] [PubMed] [Google Scholar]
- 9.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006;124:1269–1282. [DOI] [PubMed] [Google Scholar]
- 10.Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol 2005;1:85–92. [DOI] [PubMed] [Google Scholar]
- 11.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003;112:819–829. [DOI] [PubMed] [Google Scholar]
- 12.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004;427:260–265. [DOI] [PubMed] [Google Scholar]
- 13.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004;41:849–857. [DOI] [PubMed] [Google Scholar]
- 14.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 2005;15:929–934. [DOI] [PubMed] [Google Scholar]
- 15.Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, Gigli PM, Catinella S, Civelli M, Patacchini R. α,β-Unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol 2007;37:617–623. [DOI] [PubMed] [Google Scholar]
- 16.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007;445:541–545. [DOI] [PubMed] [Google Scholar]
- 17.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 2007;104:13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trevisani M, Siemens J, Materazz S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 2007;104:13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. Cigarette smoke–induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 2008;118:2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brône B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, Gijsen HJ. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol 2008;231:150–156. [DOI] [PubMed] [Google Scholar]
- 21.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 1984;99:1–110. [DOI] [PubMed] [Google Scholar]
- 22.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 2004;557:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 2008;118:1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 2008;586:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autocoid 4-oxononenal. J Physiol 2008;586:3447–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadofsky LR, Campi B, Trevisani M, Compton SJ, Morice AH. Transient receptor potential vanilloid-1-mediated calcium responses are inhibited by the alkylamine antihistamines, dexbrompheniramine and chlorpheniramine. Exp Lung Res 2008;34:681–693. [DOI] [PubMed] [Google Scholar]
- 27.Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ, Yacoub MH, Belvisi MG. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol 2003;140:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belvisi MG, Patel HJ, Freund-Michel Hele DJ, Crispino N, Birrell MA. Inhibitory activity of the novel CB2 receptor agonist, GW833972A, on guinea-pig and human sensory nerve function in the airways. Br J Pharmacol 2008;155:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 2007;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morice AH, Kastelik JA, Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol 2001;52:365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterisation of prostanoid receptor–evoked responses in rat sensory neurones. Br J Pharmacol 1998;124:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello JF, Dunlop LS, Gardiner PJ. Characteristics of prostaglandin-induced cough in man. Br J Clin Pharmacol 1985;20:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox AJ, Barnes PJ, Venkatesan P, Belvisi MG. Activation of large conductance potassium channels inhibits the afferent and the efferent function of airway sensory nerves in the guinea-pig. J Clin Invest 1997;99:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterisation of electrophilic lipid–protein interactomes. Ann N Y Acad Sci 2006;1091:548–570. [DOI] [PubMed] [Google Scholar]
- 35.Nemoto T, Aoki H, Ike A, Yamada K, Kondo T. Serum prostaglandin levels in asthmatic patients. J Allergy Clin Immunol 1976;57:89–94. [DOI] [PubMed] [Google Scholar]
- 36.Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax 2003;58:585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Profita M, Sala A, Bonanno A, Riccobono L, Siena L, Melis MR, Di Giorgi R, Mirabella F, Gjomarkaj M, Bonsignore G, et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol 2003;112:709–716. [DOI] [PubMed] [Google Scholar]
- 38.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience 2008;154:1467–1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.