Abstract
Rationale: The pathogenesis of nasal polyps in chronic rhinosinusitis is poorly understood.
Objectives: These studies seek to implicate a functional role for vascular endothelial growth factor (VEGF) in perpetuating primary nasal epithelial cell overgrowth, a key feature of hyperplastic polyps.
Methods: Comparison of VEGF and receptor expression was assessed by ELISA of nasal lavage, immunohistochemistry of sinus tissue, flow cytometry of nasal epithelial cells, and ELISA of supernatants. VEGF-dependent cell growth and apoptosis were assessed with blocking antibodies to VEGF, their receptors, or small interfering RNA knockdown of neuropilin-1 by cell proliferation assays and flow cytometric binding of annexin V.
Measurements and Main Results: VEGF protein was sevenfold higher in nasal lavage from patients with polyposis compared with control subjects (P < 0.001). We also report elevated expression of VEGF (P < 0.012), receptors VEGFR2 and phospho-VEGFR2 (both P < 0.04), and identification of VEGF coreceptor neuropilin-1 in these tissues. Nasal epithelial cells from patients with polyps demonstrated faster growth rates (P < 0.005). Exposure of cells to blocking antibodies against VEGF resulted in inhibition of cell growth (P < 0.05). VEGF receptor blockade required blockade of neuropilin-1 (P < 0.05) and resulted in increased apoptosis (P < 0.001) and inhibition of autocrine epithelial VEGF production (P < 0.05).
Conclusions: These data demonstrate that VEGF is a novel biomarker for chronic rhinosinusitis with hyperplastic sinonasal polyposis that functions in an autocrine feed-forward manner to promote nasal epithelial cell growth and to inhibit apoptosis. These findings implicate a previously unrecognized and novel role of VEGF functioning through neuropilin-1 on nonneoplastic primary human airway epithelial cells, to amplify cell growth, contributing to exuberant hyperplastic polyposis.
Keywords: chronic rhinosinusitis with nasal polyposis, vascular endothelial cell growth factor, epithelial cell growth, apoptosis, neuropilin-1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Chronic rhinosinusitis with nasal polyposis is a disease characterized by recurrent and recalcitrant hyperplastic polyp growth, the pathogenesis of which is poorly understood.
What This Study Adds to the Field
These studies define a novel role for vascular endothelial growth factor as an autocrine epithelial cell mitogen and prosurvival factor that drives the epithelial cell hyperplasia observed in hyperplastic chronic rhinosinusitis with nasal polyposis.
Although chronic rhinosinusitis (CRS) is a widespread disease affecting about 15% of the US population, the pathogenesis is poorly understood (1). One of the most severe forms is CRS with hyperplastic sinonasal polyposis (CRSwNP) (2). The presence of hyperplastic polyps in the sinuses is an ominous clinical feature signifying the presence of recalcitrant disease for which there is no effective lasting treatment (3, 4). Mucosal hyperplasia, a hallmark of the tissue remodeling observed in CRSwNP, results in chronic disease that becomes refractory to either medical or surgical management (2). Despite the significant morbidity of recurrent disease, central mechanisms regarding the pathogenesis of sinonasal polyposis remain poorly understood. Histological features of CRSwNP resemble that of asthmatic airways with end-stage polyps displaying signs of helper T cell type-2 inflammation characterized by infiltration with eosinophils, thickening of the basement membrane, and hyperplasia of the epithelium and are strikingly reminiscent of the histopathology of severe asthmatic airways (5–8). Therefore understanding factors controlling aberrant epithelial cell growth may provide critical insights into therapeutic strategies in the treatment of chronic rhinosinusitis with nasal polyposis as well as asthma.
Despite the fact that epithelial hyperplasia is a key feature of sinonasal polyps, there is a surprising paucity of literature on studies of growth factors in the pathogenesis of epithelial hyperplasia in sinonasal polyps. Growth factors implicated in remodeling of asthmatic airways such as transforming growth factor (TGF)-β and fibroblast growth factor (FGF) were found to be increased in nasal polyp tissue. Messenger RNA for TGF-β1 (9) and FGF (10) are increased in tissue homogenates of polyps. Immunohistochemical analysis localized TGF-β1 to the extracellular matrix and stroma of nasal polyps, where eosinophils reside (11, 12). There have been many studies of other selected growth factors in nasal polyposis, such as insulin-like growth factor (13–16), FGF (13, 17), platelet-derived growth factor (18–20), and TGF-β (21). However, the effects of TGF and FGF on sinus tissue remodeling have not been established. Epidermal growth factor (EGF) is thought to play a key role in epithelial proliferation, growth, and repair in asthma (22, 23). EGF receptor overexpression in bronchial epithelial cells has been found to correlate with asthma severity and steroid refractoriness (24). However, the role of EGF in the development of sinonasal polyps has not been explored. Interestingly, TGF-α (25), TGF-β (26), FGF (27), and EGF (28) have all been shown to induce expression of vascular endothelial growth factor (VEGF), a known potent endothelial cell mitogen and vascular permeability factor (29). Work by Homer and Elias has established a role for VEGF as an inducer of remodeling in asthma (30) by enhancing helper T cell type 2–mediated antigen sensitization and inflammation in the lung and by increasing the number of activated DC2 dendritic cells (31). VEGF has been shown to be increased in asthmatic airways (32, 33) and correlates directly with disease activity and inversely with airway caliber and airway responsiveness (34–36). Similarly, we hypothesize that VEGF may mediate upper airway remodeling observed in the development of sinonasal polyposis.
VEGF, as an endothelial cell mitogen, has been implicated in the development of nasal polyps. Immunohistochemical analysis of nasal polyps from children showed increased VEGF staining within the vascular endothelium and increased mean blood vessel count, both of which correlated with size of nasal polyps (37). Others have also shown that enhanced VEGF and its receptor expression were localized to the endothelium, the basement membranes, perivascular spaces, and epithelium of polyps (38–41). These studies indicate that the epithelium is a significant, but not the sole, source of VEGF in polyp tissue (39). However, there have been no investigations to date on the role of VEGF as an epithelial mitogen in sinus disease. Therefore we postulated that VEGF is a central pathway by which airway epithelial cell growth is regulated. To test this hypothesis we used the following in vivo and in vitro human experimental models for our studies: (1) nasal lavage aspirates, (2) surgical sinonasal tissue, and (3) cultured primary nasal airway epithelial cells. Some of the results of these studies have been previously reported in the form of abstracts (42, 43).
METHODS
Human Subjects
All subjects studied were enrolled after obtaining informed consent under a Johns Hopkins Medicine Institutional Review Board–approved human subjects research protocol. The patients with CRSwNP were defined on the basis of historical, endoscopic, and radiographic criteria, and by meeting the definition of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) Chronic Rhinosinusitis Task Force (44). Specifically, patients with CRS alone had continuous symptoms of rhinosinusitis, as defined by the task force report, for greater than 12 consecutive weeks, associated with computed tomography of the sinuses revealing isolated or diffuse sinus mucosal thickening and/or air fluid level. A subject with CRSwNP was defined by endoscopic examination findings of polyps and posttreatment computerized tomographic scan confirmation of persistent bilateral and diffuse paranasal sinus mucosal thickening. Surgery for patients with CRSwNP was performed only if a patient's symptoms and radiographic findings failed to resolve despite at least 6 weeks of treatment with oral antibiotics, topical corticosteroids, decongestants, and/or mucolytic agents in accordance with the accepted standards of medical care. However, subjects with CRSwNP or CRS alone, and normal control subjects who were chosen for these studies, had no immediate preoperative steroids within 14 days of obtaining any specimen. In addition, subjects with CRSwNP had no intranasal glucocorticoid exposure during the immediate 1-month postoperative period. Normal control subjects were defined as those individuals failing to meet criteria for CRS as defined previously and having no evidence of sinus disease. These subjects were normal healthy volunteers. Normal control sinus tissue was obtained as discarded sinus tissue from non-CRS patients who were undergoing endoscopic surgery for transphenoidal hypophysectomy or cerebrospinal fluid leak repair. As a secondary characteristic, atopic status was defined by puncture skin test positivity, using 22 allergens as previously described (45). These included cat, dog, mouse, and rat danders, short ragweed, mugwort, rye grass, Bermuda grass, oak, birch, dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), cockroach (Blattella germanica, Periplaneta americana, and Blatta orientalis), and mold (Alternaria alternata, Aspergillus fumigatus, Cladosporium herbarum, Curvularia, Dreschella, Fusarium, and Rhizopus) (ALK Laboratories, Wallingford, CT). Phosphate-buffered saline (PBS) and histamine was used as negative and positive controls, respectively. The skin test response was measured 15 minutes after application on the forearm by puncture with a bifurcated needle. An imprint of the perimeter of the wheal and erythema was made with Transpore tape (3M, St. Paul, MN) transferred onto a sheet of paper for permanent documentation. The two cross-diameters were measured to quantitate the size of the wheal and the erythema. A positive test was defined as an average wheal diameter greater than or equal to 3 mm relative to the saline control. Subjects with asthma were defined as those who (1) had a physician's diagnosis of asthma as described in the National Heart, Lung, and Blood Institute (NHLBI, National Institutes of Health, Bethesda, MD) Expert Panel Report 3 (46), where subjects with asthma displayed an FEV1/FVC ratio less than 0.7 measured before optimization of asthma control and either one of the following conditions after administration of a bronchodilator: (a) a greater than 12% improvement in FEV1 (or FVC) or (b) an absolute improvement of more than 0.2 L; and (2) had been administered prescription medication for asthma (such as bronchodilators, inhaled steroids, or oral steroids). In addition, subjects with CRSwNP and asthma were further classified with respect to level of asthma control by (1) FEV1 values measured within 1 month of enrollment and (2) number of exacerbations requiring oral systemic corticosteroid treatment to control asthma symptoms as defined in the NHLBI Expert Panel 3 Report (46).
Collection of Nasal Scrapings
Nasal epithelial cells were collected from the inferior nasal turbinate by curettage with a sterile nasal cytology brush (Wampole, Harrisburg, PA) and were rinsed twice in a sterile Eppendorf tube containing 5 ml of lactated Ringer's solution as previously described (47). The cells were centrifuged at 300 × g for 5 minutes at room temperature. Each nasal scraping specimen yielded 1–2 × 106 cells, of which more than 95% were epithelial cells by Wright stain cell count.
Collection of Nasal Lavage Samples
Nasal lavage was collected according to previously described methods (48). Lavages were performed with sterile saline solution prewarmed to 37°C. Briefly, with the patient's head extended, 5 ml of saline was instilled into each nasal cavity with a pipette while the patient held his/her breath for 10 seconds. The patient was then asked to tilt his/her head forward to allow the saline to drip into a collection basin. The lavage fluid was processed by spinning at 3,600 rpm for 15 minutes and aliquots were processed and frozen at −80°C for detection of VEGF, other growth factors, and total protein.
Culture of Primary Nasal Airway Epithelial Cells
Epithelial cells cultured from nasal scrapings were cultured in bronchial epithelial basal medium (Biosource, Camarillo, TX) on collagen-coated plates as previously described (47, 49, 50). Cultures of primary nasal airway epithelial cells (PNECs) were routinely 99 to 100% positive for cytokeratin staining at the time of harvest. PNECs were routinely used for all in vitro studies at first or second passage only. For functional studies measuring cell growth and apoptosis, the cells were incubated for various lengths of time with recombinant VEGF (100 ng/ml; R&D Systems, Minneapolis, MN) (51) or antibody that had been previously shown to functionally block its target: anti-VEGF blocking antibody (1 μg/ml; R&D Systems), anti–neuropilin-1 (NP1) blocking antibody (1 μg/ml; Miltenyi Biotec, Auburn, CA) (52), anti–vascular endothelial cell growth factor receptor-1 (VEGFR1) blocking antibody (10 μg/ml; R&D Systems) (53), anti-VEGFR2 blocking antibody (1 μg/ml; R&D Systems) (54), recombinant EGF (50 ng/ml; R&D Systems) (55), or irrelevant isotype control antibody (eBioscience, San Diego, CA).
Flow Cytometry
The monoclonal antibodies against VEGF (R&D Systems) and NP1 (Miltenyi Biotec) used for flow cytometry were analyzed as previously described with a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest software (47, 49, 50). The viability of PNECs at the time of cell harvest was assessed by propidium iodide exclusion. Fluorescence was determined for all cells in each sample after debris, dead cells, and aggregates were excluded by forward angle and side scatter gating. Mean fluorescence intensity was compared with control staining, using an irrelevant isotype-matched mouse monoclonal antibody. For each sample, at least 10,000 events were collected, and histograms were generated. Data are usually expressed as means and SEM.
Immunohistochemistry
Surgical sinonasal tissue was immediately fixed in 4% formaldehyde in PBS (4°C, 4 h) and then rinsed with PBS. Antibody to VEGF (R&D Systems), NP1 (Miltenyi Biotec), VEGFR1 (R&D Systems), VEGFR2 (R&D Systems), phospho-VEGFR2 (Santa Cruz Biotechnology, Santa Cruz, CA), or irrelevant IgG isotype (eBioscience) control was performed as previously described (47, 49, 50). To ensure that detection of positive staining was performed in a standardized and uniform manner between tissue samples, staining was routinely performed in sets of tissues, using a specimen from each of the two patient groups. Each round of staining was exposed to diaminobenzidine for a fixed duration to standardize the time for color development. The slides were evaluated with a bright-field microscope (BX-50; Olympus, Center Valley, PA) equipped with a camera (Retiga EXi [QImaging, Surrey, BC, Canada] or Spot ET-3 CCD [Diagnostic Instruments, Sterling Heights MI]) and a micrograph field of view of the entire stained section. Image-Pro Lab imaging software (Media Cybernetics, Silver Spring, MD) was used to analyze areas of positive staining in each digitized micrograph. All epithelial cells were selected (from the basement membrane to the lumenal surface) as the region of interest (ROI) in each image of the immunohistochemically stained (e.g., VEGF) section. A standard size of ROI surface area was used and applied to all images. Simple bilevel thresholding, based on criteria for positive staining, was set by trained personnel blinded to the specimen phenotype. This threshold window was set and applied to all analyzed images; the number of nuclei was also counted in the ROI. The software measurement of the area of positive immunostaining and number of nuclei in the ROI were transferred to an Excel spreadsheet (Microsoft, Redmond, WA) for statistical analysis and determination of the average intensity area per cell (total area of positive immunostaining divided by number of nuclei). The data are expressed as intensity of staining per cell.
ELISA for Growth Factors
VEGF, EGF, and TGF-β1 from nasal lavages and cell supernatants were measured with ELISA kits from R&D Systems according to the manufacturer's instructions. The minimal detectable concentration was typically 5.0 pg/ml. The assay of each sample was performed in triplicate. Data are expressed per microgram of total protein, which was measured by Bradford assay (Bio-Rad, Hercules, CA).
Determination of PNEC Growth to Culture Confluence
Two hundred thousand cells per well were plated onto collagen-coated 6-well plates. Attainment of culture confluency was assessed by ×40 power phase-contrast light microscopy. An estimate of confluency was determined by averaging inspection of five separate fields: 0, 90, 180, and 270 degrees and the center of each well. Confluency was defined when a minimal average of 90% had been reached. Each sample assay was performed in triplicate and the analysis was blinded to the subject group.
CyQUANT Cell Proliferation Assay
Cell proliferation was assayed with the CyQUANT cell proliferation assay (Molecular Probes/Invitrogen, Carlsbad, CA). Cells were seeded at 5 × 103 cells per well and grown to 50% confluence in 96-well plates in serum-free bronchial EC growth medium (Cambrex, East Rutherford, NJ), deprived of EGF for 24 hours before challenge to synchronize cell growth, and subsequently stimulated according to the experimental protocols. As per the manufacturer's instructions, at the end of the experiment, the cell supernatants were aspirated and the cells were lifted by trypsinization. Cell lysis was performed by two sequential freeze–thaw cycles. Standard curves were executed with each run of the assay according to the manufacturer's instructions. Cells were then incubated for 5 minutes at room temperature with CyQUANT lysis buffer containing the CyQUANT-GR fluorescent dye. Fluorescence was measured with a Cytofluor 4000 fluorescence reader (Applied Biosystems, Foster City, CA). Each experimental condition was assessed in quadruplicate.
Small Interfering RNA Transfection
PNECs was grown to 75% confluence and transfected with the indicated concentration of target gene NP1 (100 nM), negative control, or rhodamine-tagged control small interfering RNA (siRNA) (Qiagen, Valencia, CA), using RNAiMAX transfection reagent made up in OptiMEM buffer (1:1, vol/vol) as directed by the vendor (all reagents from Invitrogen). PNECs were then exposed to siRNA/RNAiMAX in cell culture medium without antibiotics (1:3) for 24 hours at 37°C. Viability was monitored by light microscopy. Transfection efficiency was assessed by measuring the average percentage of rhodamine-positive cells per field (four sampled fields) at ×10 and ×40 magnification, using fluorescence microscopy.
Statistical Analysis
All data are expressed as means and SEM. Comparison between the phenotypic classes and control groups was analyzed by Kruskal-Wallis test. Comparison of in vitro quantitative data between multiple treatment conditions was done by analysis of variance and post-hoc Bonferroni test.
RESULTS
VEGF Is Elevated in Nasal Lavages of CRSwNP
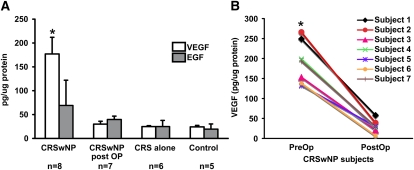
To begin to examine the role of growth factors in upper airway remodeling observed in sinonasal polyposis, we compared the levels of VEGF, EGF, and TGF-β1 in nasal lavage aspirates from CRSwNP, CRS alone, and normal control patient groups. Patient characteristics are given in Table 1. All subjects had a negative history of glucocorticoid use (either intranasal or oral) for at least 2 weeks before analysis. In addition, subjects with CRSwNP had no glucocorticoid exposure during the immediate 1-month postoperative period. Antihistamines were withheld 48 hours before skin testing and then resumed as medically indicated. All other medications, as listed in Table 1, including inhaled corticosteroids (indicated for asthma) and leukotriene antagonists (indicated for asthma or rhinitis), were continued through the study. The specific monoclonal antibodies used in this assay (R&D Systems) have been shown to detect all soluble human VEGF [including VEGF(165) and VEGF(121)], EGF, and TGF-β1, respectively. Results in Figure 1A demonstrate that subjects with CRSwNP displayed significantly greater than sevenfold higher levels of VEGF protein in nasal lavages (177 ± 35 pg/μg protein) compared with normal control subjects (24 ± 3 pg/μg) or subjects with CRS without sinonasal polyposis (25 ± 2 pg/μg). In addition, removal of polyps and achievement of quiescent disease in subjects with CRSwNP resulted in a dramatic reduction of VEGF levels comparable to that of normal control values (see Figure 1B). Total protein levels in nasal lavages did not differ significantly between the three patient groups (our unpublished data). In contrast to VEGF, there was no significant difference in the level of EGF measured in nasal lavages between the three patient groups. In addition, we failed to detect any measurable soluble TGF-β1 in nasal lavage aspirates (our unpublished data).
TABLE 1.
CHARACTERISTICS OF HUMAN SUBJECTS
| Bronchodilator Challenge |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Phenotype | Sex | Age (yr) | Skin Test | Medications at Time of Enrollment | Asthma | FEV1/FVC | Pre FEV1 (L) | Post FEV1 (L, % improvement) | Oral Corticosteroid (doses/yr) |
| 1 | CRSwNP | M | 55 | − | − | 0.85 | 3.79 | 3.83 (1%) | 0 | |
| 2 | CRSwNP | M | 43 | + | ICS/LABA, antihistamines | + | 0.64 | 4.37 | 5.02 (16%) | 2 |
| 3 | CRSwNP | F | 20 | + | ICS/LABA, antihistamines, montelukast, albuterol | + | 0.7 | 3.2 | 3.78 (18%) | 6 |
| 4 | CRSwNP | M | 46 | + | ICS/LABA, antihistamines, montelukast, albuterol | + | 0.65 | 2.83 | 3.20 (13%) | 4 |
| 5 | CRSwNP | M | 80 | − | ICS/LABA, montelukast | + | 0.55 | 2.65 | 3.00 (13%) | 5 |
| 6 | CRSwNP | F | 32 | − | − | 0.87 | 2.2 | 2.10 (0%) | 0 | |
| 7 | CRSwNP | M | 40 | − | ICS/LABA | + | 0.57 | 3.85 | 4.08 (6%) | 1 |
| 8 | CRSwNP | F | 50 | − | ICS/LABA | + | 0.68 | 1.83 | 2.10 (15%) | 8 |
| 9 | CRS | F | 68 | − | Antihistamines | |||||
| 10 | CRS | M | 46 | − | Antihistamines, montelukast (for rhinitis) | |||||
| 11 | CRS | M | 73 | − | ||||||
| 12 | CRS | M | 66 | − | ||||||
| 13 | CRS | M | 43 | − | ||||||
| 14 | CRS | F | 29 | + | Antihistamines, montelukast (for rhinitis) | |||||
| 15 | Control | M | 35 | − | ||||||
| 16 | Control | F | 58 | − | ||||||
| 17 | Control | M | 27 | − | ||||||
| 18 | Control | M | 30 | − | ||||||
| 19 |
Control |
F |
24 |
– |
||||||
Definition of abbreviations: CRS = chronic rhinosinusitis; CRSwNP = chronic rhinosinusitis with hyperplastic sinonasal polyposis; F = female; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; M = male.
Figure 1.
Comparison of soluble growth factors in nasal lavages of patients with untreated chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP), patients with CRSwNP 1 month postoperatively, patients with CRS alone, and normal control subjects. Vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) in nasal lavages were assayed according to the manufacturer's instructions (antibodies and kits from R&D Systems). The lower limit of detection was 5 pg/ml. Values represent the means and SEM. *P < 0.001 versus control, CRS alone, and CRSwNP postoperative (PostOP) subjects by Kruskal-Wallis test. Human subject data are shown in Table 1.
Examination of secondary characteristics of subjects with CRSwNP revealed that only three of eight subjects with CRSwNP were skin test positive. However, the majority of the subjects with CRSwNP (six of eight) examined had asthma. Furthermore, five of eight subjects with CRSwNP had poorly controlled asthma as defined by (1) FEV1 <60% predicted or (2) at least two doses per year of oral corticosteroid treatment for asthma exacerbations.
VEGF Is Overexpressed in Vivo and in Vitro in CRSwNP
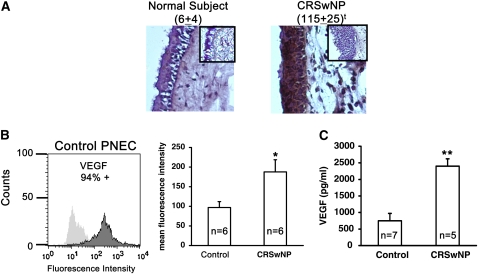
To confirm the presence of VEGF on epithelium in sinonasal polyps, immunohistochemical staining was performed on sinus tissue surgical samples, using monoclonal antibodies against VEGF and isotype control IgG. Sinonasal tissue samples from subjects with CRSwNP (maxillary sinus polyp) and normal control subjects (maxillary or sphenoid sinus) were tested. The staining intensity of digitized images of epithelial cells was objectively assessed with Image-Pro software and expressed as intensity units per epithelial cell, using similar sized sampling areas. First, Figure 2A (and also see Figure 3) shows that epithelial cell hyperplasia, which is characteristic of CRSwNP, is uniquely present in CRSwNP, and absent from normal control subjects. Data show that VEGF was detected in representative human sinonasal tissue. The irrelevant IgG isotype negative control with secondary antibody shown in the inset demonstrated no nonspecific brown staining in the epithelial layer in either subject group. As additional controls, staining performed in the absence of primary or secondary antibody yielded no significant signal (our unpublished data). The intensity of staining within the epithelium varied between subject groups and the sinonasal polyp from subjects with CRSwNP demonstrated significantly increased staining intensity for VEGF, especially within the epithelial cell layer as compared with sinonasal tissue from maxillary sinuses of control subjects (P < 0.012). Not surprisingly, VEGF staining was observed in the epithelial cell layer, but not exclusively.
Figure 2.
Vascular endothelial growth factor (VEGF) is overexpressed by epithelial cells in sinonasal tissue. (A) Surgical sinonasal tissue from chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) mucosa (right, n = 5) and normal control sinus mucosa (left, n = 5) were immediately placed in 4% paraformaldehyde and processed for immunohistochemical staining of VEGF as described in Methods. Score shown in parentheses is the brown-colored staining intensity expressed as mean intensity × 103 per cell ± SEM, determined with Image-Pro software. Each inset represents the matching IgG control. Data are representative of n = 5 subjects in each group and are shown at an original magnification of ×10. tP < 0.012 by Kruskal-Wallis test. (B) Flow cytometric analysis of cell surface VEGF on cultured primary nasal airway epithelial cells (PNECs) from normal control subjects and subjects with CRSwNP. *P < 0.05 versus control subjects by Kruskal-Wallis test. (C) Soluble VEGF (isoforms 165 and 121) in cell supernatants of PNECs were measured by ELISA according to the manufacturer's instructions (R&D Systems). Lower limit of detection was 5 pg/ml. **P < 0.02 versus control subjects by Kruskal-Wallis test.
Figure 3.
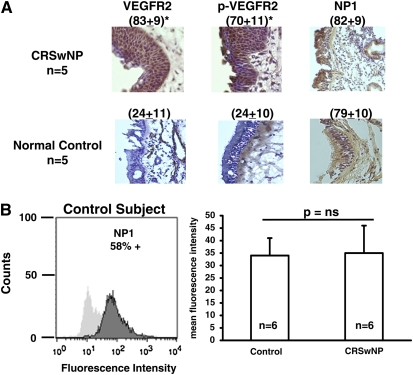
Receptors for vascular endothelial growth factor (VEGF) are abundantly expressed by epithelial cells in sinonasal tissue. (A) Expression of VEGF receptor-2 (VEGFR2), phospho-VEGFR2, and neuropilin-1 (NP1) by epithelial cells in sinonasal tissue. Surgical sinonasal tissue from chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) mucosa (n = 5) and normal control sinus mucosa (n = 5) were immediately placed in 4% paraformaldehyde and processed for immunohistochemical staining of VEGFR2, phospho-VEGFR2, and NP1, as described in Methods. Scores shown in parentheses represent the brown-colored staining intensity expressed as mean intensity × 103/cell ± SEM determined with Image-Pro software. Data are shown at an original magnification of ×10. *P < 0.04 versus control by Kruskal-Wallis test, n = 6 for each group. (B) Flow cytometric analysis of cell surface NP1 expression on primary nasal airway epithelial cells (PNECs) from normal control subjects and subjects with CRSwNP.
Because we had found VEGF to be highly expressed on epithelial cells in vivo, we wanted to know if VEGF was detectable on the surface of nasal epithelial cells in vitro. VEGF is known to exist on the cell surface residing within an extracellular pool (56, 57). In addition, VEGF is known to exist in a membrane-bound form. The results shown in Figure 2B (left) display flow cytometric findings using specific monoclonal antibodies to VEGF on nasal epithelial cells taken from inferior turbinates that had been expanded in cell culture from a normal control subject. The anti-VEGF antibody used detects all isoforms of VEGF, including membrane-bound VEGF(189). The results demonstrate fairly unimodal detection of robust levels of cell surface expression of VEGF (94% positive) on PNECs from a normal control subject. Figure 2B (right panel) compares VEGF cell surface expression on PNECs taken from the inferior turbinates of control subjects and subjects with CRSwNP. The data demonstrate that cell surface expression of VEGF from subjects with CRSwNP is increased by about twofold (P < 0.05). VEGF is also known to be secreted by cells in the form of two soluble isoforms: VEGF(165) and VEGF(121). To examine whether PNECs from subjects with CRSwNP produce elevated levels of soluble VEGF, we compared soluble VEGF [both VEGF(165) and VEGF(121)] released into PNEC supernatants from subjects with CRSwNP and control subjects. Wells from a 6-well plate were seeded with identical cell numbers of PNECs from individual subject donors and grown to 90% confluence. Cell supernatants were assayed for VEGF by ELISA. The results in Figure 2C demonstrate that PNECs from subjects with CRSwNP expressed, produced, and released greater than threefold higher levels of soluble VEGF into the cell supernatant as compared with PNECs from normal control subjects (P < 0.02). These data demonstrate that both soluble and cell surface VEGF are overexpressed by PNECs from subjects with CRSwNP.
VEGFR2, Phospho-VEGFR2, and NP1 Are Abundantly Expressed by Epithelial Cells in Sinonasal Tissue
Because VEGF was found to be so abundantly present in epithelium of sinonasal polyp tissue, we hypothesized that VEGF has a biological function on these cells. We hypothesized that the receptors for VEGF must also be present on nasal epithelium. VEGFR2 is known to exhibit robust tyrosine kinase activity and auto- and transphosphorylation when activated by VEGF ligand binding (58, 59). To examine whether activation of the VEGF signaling pathway occurs in sinonasal epithelium in vivo, we looked for the presence of phosphorylation of VEGFR2 by performing immunostaining for VEGFR2 and phospho-VEGFR2. Figure 3A demonstrates that VEGFR2 was detected in human sinonasal tissue from subjects with CRSwNP, but was barely detectable in normal control subjects (P < 0.04). In addition, there was markedly elevated staining of phospho-VEGFR2 in polyps of subjects with CRSwNP as compared with specimens from normal control subjects (P < 0.04). Interestingly, we detected robust constitutive staining of neuropilin-1 (NP1), the coreceptor for VEGF, in sinonasal epithelium from both patient groups. These studies are the first to report expression of NP1 on human airway epithelial cells. In vitro studies, shown in Figure 3B, demonstrated that cell surface NP1 is expressed at high constitutive levels by PNECs (58% positive) derived from control subjects. Furthermore, both in vivo and in vitro expression of NP1 appears to be unaffected by disease phenotype. These data show that not only is the ligand VEGF abundantly present in diseased sinonasal epithelium, but at least one of the receptors that is necessary for signaling is also present in active form. In addition, NP1 has been identified as being abundantly expressed on airway epithelial cells of sinonasal tissue in both patient groups.
PNECs from Subjects with CRSwNP Display Elevated Growth Rates in Vitro
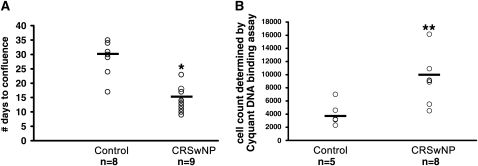
During the course of expanding PNECs in culture from nasal scrapings, we observed that epithelial cells harvested from subjects with CRSwNP displayed a faster rate of growth compared with normal control cells, consistent with their hyperplastic histologic appearance. Therefore we undertook efforts to systematically examine the growth and survival of nasal cells derived from subjects with CRSwNP and to compare them with nasal epithelial cells derived from control subjects. We standardized culture conditions to optimize recovery of cells by minimizing time from harvest to seeding as well as the initial cell seeding concentration and assessed the number of days to culture confluency. The results in Figure 4A demonstrate that sinonasal epithelial cells derived from subjects with CRSwNP have a 2.3-fold increase in rate of cell growth to confluency as compared with normal control PNECs, when grown under identical seeding concentrations and conditions (P < 0.008). To examine this further, we directly quantitated net cell growth, using CyQUANT dye fluorometric quantification of DNA (Figure 4B). After 96 hours in culture, PNECs from inferior turbinates of subjects with CRSwNP displayed a greater than twofold faster growth rate as compared with PNECs from control subjects (P < 0.005). In subjects with CRSwNP, PNECs obtained directly from the polyps by nasal brushing (cell count at 96 h: 10,584 ± 1,249, n = 5) demonstrated growth rates similar to those of PNECs harvested from the inferior turbinate (see Figure 4B) (9,162 ± 1,331, n = 8) and demonstrated elevated growth rates compared with PNECs from control subjects (P < 0.006; 4,049 ± 919, n = 5). Because an elevated growth rate of epithelial cells from subjects with CRSwNP was similarly observed in cells harvested from either polyps or inferior turbinates, comparison of in vitro PNEC behavior between subjects with CRSwNP and control subjects was performed, using PNECs from inferior turbinates so that matched comparison of cell types could be made.
Figure 4.
Primary nasal airway epithelial cells (PNECs) from subjects with chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) display faster growth rates in vitro. (A) Comparison of time to confluence of cultures of PNECs from normal control subjects and subjects with CRSwNP. PNECs from normal control subjects and subjects with CRSwNP were cultured as described in Methods. Results are expressed as number of days to confluency of culture from day of seeding into 6-well plates and were assessed in triplicate. (B) Comparison of cell proliferation rates of PNECs from normal control subjects and subjects with CRSwNP. Cell number was determined by normalization of DNA content to a standard curve, using the CyQUANT cell proliferation assay as described in Methods. Each circle indicates an experiment from a single donor. Bars represent the mean. *P < 0.008 or **P < 0.005 versus control group by Kruskal-Wallis test.
VEGF Drives Human Nasal Epithelial Cell Growth
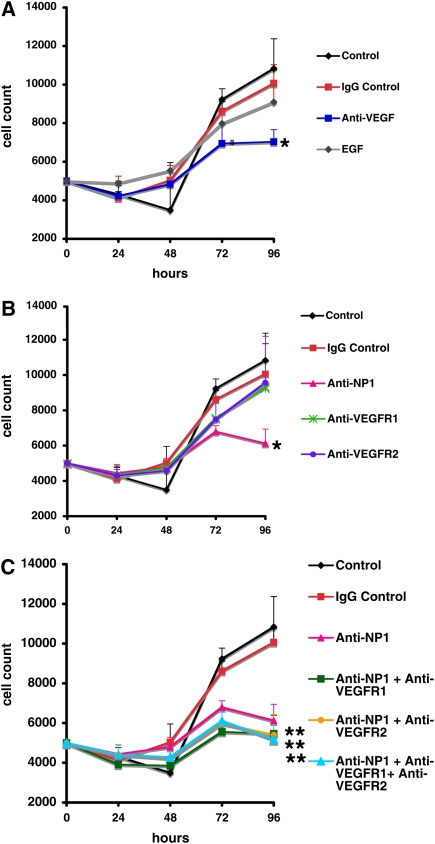
Given the excessive levels of VEGF from sinonasal epithelium derived from subjects with CRSwNP, we hypothesized that VEGF may be acting in an autocrine fashion to increase the growth rate of epithelium from subjects with CRSwNP. To address this notion, we examined the effect of functional blocking antibodies to VEGF and to components of the VEGF signaling pathway on proliferation rates of PNECs in vitro. Synchronization of the cell cycle was first achieved by withdrawal of basal EGF from the medium. All conditions had no exogenous VEGF supplementation. Figure 5A shows that exposure of blocking antibody to VEGF ligand for 96 hours resulted in 34% inhibition of cell growth (P < 0.05). Exposure to blocking antibody against VEGFR1 or VEGFR2 alone resulted in a modest 12% inhibition of cell proliferation rates. However, exposure to functional blocking antibody against coreceptor NP1 resulted in a 43% inhibition of cell growth (Figure 5B) (P < 0.05). The combination of blocking antibody to NP1 and anti-VEGFR1 and/or anti-VEGFR2 antibody resulted in a greater decline in cell proliferation to 50% (Figure 5C, P < 0.02). Exposure to IgG control antibody had no effect. Thus the effect of exposure to multiple blocking antibodies to VEGF receptors was additive. In addition, exposure to recombinant EGF had no significant effect on cell growth up to 96 hours. The results of this analysis were highly reproducible in that the standard deviation of quadruplicate measurements obtained with this assay was small (<10% of measured values). In addition, the standard deviation between the experiments (n = 5 donor subjects) ranged between 12 and 27% of the mean growth rates. Cell proliferation at 96 hours for each condition was also assessed on the basis of bromodeoxyuridine incorporation (Calbiochem, San Diego, CA) and resulted in identical patterns of cell growth (unpublished data). These results suggest that VEGF functions in an autocrine manner to promote epithelial cell growth.
Figure 5.
Sinonasal epithelial cell growth is vascular endothelial growth factor (VEGF) dependent. (A) Effects of anti-VEGF antibody and recombinant epidermal growth factor (EGF) exposure on growth rates of primary nasal airway epithelial cells (PNECs) from subjects with chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP). (B) Effects of anti–neuropilin-1 (NP1), anti–VEGF receptor-1 (VEGFR1), and anti-VEGFR2 antibody exposures on growth rates of PNECs from subjects with CRSwNP. (C) Effects of combination of anti-NP1, anti-VEGFR1, and anti-VEGFR2 antibody exposures on growth rates of PNECs from subjects with CRSwNP. PNECs from subjects with CRSwNP were seeded at 5,000 cells per well of a 96-well plate and exposed to the designated blocking antibodies, recombinant EGF (R&D Systems), or IgG control antibody as described in Methods. Cell number was determined after normalization of DNA fluorescence to a standard curve, using the CyQUANT cell proliferation assay as described in Methods. Each curve represents the mean of five experiments, each from an individual donor with CRSwNP. Each condition and time point was measured in quadruplicate. The SEM ranged between 12 and 27% of the mean. *P < 0.05, **P < 0.02, versus control condition at 96 hours by analysis of variance and post-hoc Bonferroni test.
VEGF Inhibits Apoptosis through NP1
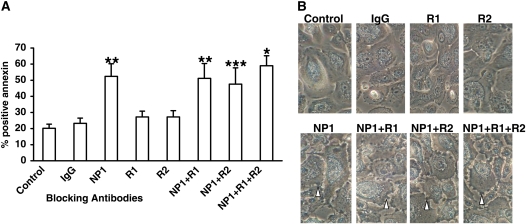
To examine whether VEGF may also function as a prosurvival factor, we examined the effect of functional blocking antibodies to components of the VEGF pathway on induction of apoptosis of PNECs in vitro. Apoptosis was determined by flow cytometric detection of annexin V–fluorescein isothiocyanate staining. Figure 6A shows that 48 hours of exposure to blocking antibody to NP1 resulted in a significant 2.5-fold increase in apoptosis of PNECs from subjects with CRSwNP (P < 0.02). Exposure to blocking antibody to receptor VEGFR1 or VEGFR2 resulted in no significant increase in apoptosis of PNECs. The combination of blocking antibody to NP1 with anti-VEGFR1 and/or anti-VEGFR2 antibodies resulted in the same level of apoptosis observed with anti-NP1 alone (P < 0.01, P < 0.02, and P < 0.05, respectively). Figure 6B shows the matching light microscopic appearance of cells under blocking antibody conditions. Cells exposed to anti-NP1 or the combination of anti-NP1 with anti-VEGFR1 and/or anti-VEGFR2 demonstrated significant cell membrane blebs indicative of morphologic evidence of increased cell death, as compared with control conditions or with cells exposed to IgG control antibody, or to blocking antibody to receptors VEGFR1 or VEGFR2 alone. These results demonstrate that VEGF functions to inhibit apoptosis in PNECs through NP1.
Figure 6.
Functional blocking of neuropilin-1 (NP1) results in apoptosis. Primary nasal airway epithelial cells (PNECs) from subjects with chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) (n = 5) were grown to 90% confluence and incubated for 48 hours with blocking antibodies to NP1 (1 μg/ml), vascular endothelial growth factor receptor-1 (VEGFR1) (R1, 10 μg/ml), VEGFR2 (R2, 1 μg/ml), IgG control (1 μg/ml), or medium control and processed for flow cytometric analysis of annexin V–fluorescein isothiocyanate staining (R&D Systems). Results in (A) are expressed as mean (and SEM) percentage of cells staining positive for annexin. (B) Light microscopic views of PNECs (original magnification, ×20). Open arrowheads point to cell membrane blebs. *P < 0.001, **P < 0.02, ***P < 0.05 versus control condition by analysis of variance and post-hoc Bonferroni test.
VEGF Functions to Autoregulate Its Own Expression in PNECs
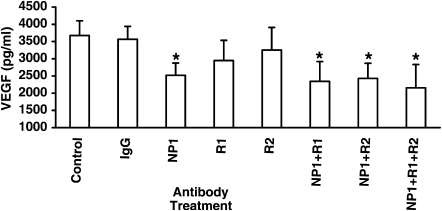
We proposed that an additional key function of VEGF from nasal epithelial cells in subjects with CRSwNP is to regulate its own expression in an autocrine manner. To test this hypothesis, we examined the effect of blocking VEGF receptor function on soluble VEGF produced and secreted by PNECs in vitro. VEGF levels in cell supernatants were measured by ELISA as described in Methods. Figure 7 shows that PNECs from subjects with CRSwNP produce a remarkable level of constitutive soluble VEGF in the nanomolar range. In addition, Figure 7 shows that exposure of PNECs from subjects with CRSwNP to blocking antibodies against VEGFR1 or VEGFR2 alone resulted in minimal change (20 and 12% decrease) in soluble VEGF from cell supernatants. However, exposure to anti-NP1 resulted in an enhanced (significant) 33% inhibition of soluble VEGF expressed by PNECs (P < 0.05). Addition of anti-VEGFR1 and/or anti-VEGFR2 to anti-NP1 produced no further inhibition of soluble VEGF expression (42, 36, and 34% decline, respectively; P < 0.05 for each condition). Thus receptor blockade of VEGF function resulted in inhibition of autocrine VEGF production in PNECs.
Figure 7.
Functional blocking of neuropilin-1 (NP1) results in inhibition of autocrine vascular endothelial growth factor (VEGF) expression by primary nasal airway epithelial cells (PNECs). PNECs from subjects with chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) (n = 3) were incubated for 48 hours with blocking antibodies to NP1 (1 μg/ml), VEGF receptor-1 (VEGFR1) (R1, 10 μg/ml), VEGFR2 (R2, 1 μg/ml), IgG control (1 μg/ml), or medium control. Cell supernatants were then harvested and assayed for VEGF by ELISA as described in Methods. *P < 0.05 versus control condition by analysis of variance and post-hoc Bonferroni test.
To explore this further, we examined the effect of silencing NP1 by siRNA knockdown on VEGF expression. To this end, we developed optimal conditions for siRNA knockdown of NP1 in PNECs from normal control subjects. Our conditions optimized transfection efficiency to greater that 90%, as assessed by control siRNA tagged with rhodamine and fluorescence microscopy. As shown in Figure 8B, all PNECs examined in culture were successfully transfected with siRNA tagged with rhodamine. All transfection conditions were performed in the absence of exogenous mitogenic stimuli. Cell viability was greater than 90% (Figure 8B). Three independent nonoverlapping NP1 siRNA sequences were used in parallel to transfect 6-well plates of PNECs as described in Methods. As a control, nonspecific moderate GC content scrambled siRNA was also used. Knockdown of target genes was verified by mRNA analysis using real-time polymerase chain reaction for NP1 and by assessing cell surface expression by flow cytometry. Figure 8C shows that transfection of PNECs with three separate siRNA sequences for NP1 resulted in 70, 75, and 60% knockdown of mRNA for NP1 (P < 0.02 vs. medium control for each siRNA sequence tested). This was confirmed by flow cytometric analysis of cell surface NP1, which demonstrated 75% (P < 0.05 for siRNA 1), 86% (P < 0.05 for siRNA 2), and 60% (P < 0.4 for siRNA 3) silencing of NP1 protein expression (Figure 8D), showing significant knockdown by two of the three siRNA sequences tested. Scrambled negative control siRNA had no effect on mRNA levels of target genes. In addition, cell surface HLA-ABC expression was used as an irrelevant target control to monitor for nonspecific effects and was found to be unchanged (our unpublished data, n = 3). Also, absolute levels of the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene were unchanged pre- and postexposure to siRNA (our unpublished data). The results in Figure 8A show that effective siRNA knockdown of NP1 in PNECs resulted in a significant 37% inhibition of cell surface VEGF expression, similar to results obtained using antibody blockade of NP1 to inhibit autocrine VEGF expression (Figure 7). These results further support the autoregulatory role of VEGF to up-regulate its own expression through NP1.
Figure 8.
Small interfering RNA (siRNA) knockdown of neuropilin-1 (NP1) results in inhibition of autocrine vascular endothelial growth factor (VEGF) expression by primary nasal airway epithelial cells (PNECs). PNECs from subjects with chronic rhinosinusitis with hyperplastic sinonasal polyposis (CRSwNP) were transfected with three nonoverlapping siRNA sequences against NP1 or scrambled negative control for 24 hours as described in Methods. Untransfected medium control was also used (Control). (A) Flow cytometric analysis of cell surface VEGF. Data represent the mean fluorescence intensity (MFI) and SEM of n = 4 experiments. *P < 0.05 versus medium control by ANOVA with post-hoc Bonferroni test. (B) Fluorescence and matching light microscopy micrographs of PNECs transfected with rhodamine-tagged control siRNA. (C) Real-time polymerase chain reaction analysis of NP1 mRNA. (D) Flow cytometric analysis of cell surface NP1. Data represent means and SEM of n = 3 experiments. Analysis of variance with post-hoc Bonferroni test resulted in **P < 0.02 for all three NP1 siRNAs versus medium control or negative control siRNA (siControl). ***P < 0.05 for siRNA1 (siNP1 #1)or siRNA2 (siNP #2) for NP1 versus medium control. +P < 0.05 for siRNA2 for NP1 versus negative control siRNA.
DISCUSSION
This study presents several novel observations directly relevant to the diagnosis and pathophysiology of chronic sinusitis with nasal polyps. This evidence can be summarized as follows. In addition to confirming previous observations that VEGF is overabundantly produced by the upper airways of subjects with CRSwNP (38–41), we have found that (1) VEGF levels in nasal lavage correlate with disease phenotype, being elevated in only those sinusitis patients with polyposis; (2) the coreceptor for VEGF, NP1, is highly expressed in nasal airway epithelial cells; (3) sinonasal epithelium from subjects with CRSwNP displays an intrinsically increased growth rate in vitro as compared with cultured epithelial cells from normal control subjects, mimicking their in vivo behavior; (4) the increase in growth rate of the epithelial cells derived from subjects with CRSwNP can be reversed by blocking antibodies targeted against either VEGF or NP1, the coreceptor for VEGF; (5) VEGF not only promotes cell growth, but also inhibits apoptosis in epithelial cells; and (6) VEGF functions to autoregulate its own production in a positive feed-forward manner through NP1, in nonneoplastic primary human airway epithelial cells. These results support the hypothesis that nasal epithelial VEGF may serve as a useful “biomarker” for this disease, and that its actions promote the development of epithelial cell hyperplasia, one of the key features of polyposis observed in CRSwNP.
We found that the soluble forms of VEGF [VEGF(121) and VEGF(165)] measured in nasal lavage were increased specifically in subjects with CRSwNP and demonstrated that overexpression of soluble VEGF in nasal lavage specifically correlated with the presence of polyposis. By contrast, growth factors EGF and TGF-β1 were not elevated in CRSwNP nasal lavage aspirates. The failure to detect differences in EGF and TGF-β1 in nasal lavage may indicate that these other growth factors may not be secreted into the airway lumen or may function locally within the tissue. VEGF measured in nasal lavage aspirates is a reflection of the VEGF produced and released from the sinonasal airway tissues, the source of which could be epithelial, endothelial, or of inflammatory cell origin. Our immunohistochemical analysis of sinonasal tissue supports the conclusion that that the epithelium is a major source of VEGF production in the nasal sinus. The results represent composite staining of VEGF within the epithelial layer from all subcellular areas including cell surface VEGF bound tightly to the extracellular matrix, cell surface VEGF bound to its receptors, and VEGF within the epithelial cell. It is well known that endoscopic sinus surgery is not curative for hyperplastic polyposis, as polyp recurrence ultimately ensues (2). Endoscopic sinus surgery that resulted in temporary reduction of polyp load normalized VEGF production in subjects with CRSwNP postoperatively, indicating that polyps are the major source of the increased VEGF in this disease. All subjects with CRSwNP were controlled for absence of intranasal or oral steroids within 2 weeks of obtaining nasal lavage and throughout the 1-month postoperative period. Medications such as antihistamines and leukotriene antagonists taken by these subjects did not affect the level of VEGF in nasal lavage. That VEGF in nasal lavage appears to track with the presence of polyps supports the hypothesis that it may be a useful biomarker of hyperplastic polyposis with respect to both disease phenotype and disease activity.
These studies also emphasize that CRS is a heterogeneous disease. Subjects with CRS without polyposis displayed a distinctly different phenotype as compared with subjects with CRSwNP, in that they expressed lower levels of VEGF in nasal lavage, similar to normal control subjects. Phenotypic characterization of the subjects with CRSwNP shows that the majority, but not all, of these patients also have asthma (six of eight subjects), consistent with previously published studies (60–63). Furthermore, our data indicate that most of these patients (five of eight) displayed poorly controlled asthma, requiring repeated courses of oral corticosteroids for treatment of asthma exacerbations. These limited data preliminarily suggest that the CRSwNP phenotype may correlate with the presence of asthma. However, as to whether nasal lavage VEGF also serves as a biomarker for asthma, remains to be determined.
A substantive finding presented here is that the nasal epithelial cells from subjects with CRSwNP retained their capacity to overexpress VEGF and maintain their pathological phenotype in vitro when cultured at low passage number (P1 or P2). Both soluble and cell surface VEGF were overexpressed by PNECs from subjects with CRSwNP as compared with normal control PNECs. In addition, these cells showed a distinctly elevated spontaneous growth rate to culture confluency and proliferation rates as compared with PNECs from normal control subjects, mimicking their in vivo hyperplastic behavior. This is analogous to observations that bronchial epithelial cells isolated from asthmatic airways can maintain their “abnormal” phenotype when cultured in vitro (64). The results from nasal lavages obtained presurgery and postsurgery indicated that, based on mass, the bulk of soluble VEGF associated with CRSwNP is derived from the polyp tissue. The nasal epithelial cells taken from subjects with CRSwNP, however, were obtained from epithelial cell brushings of the inferior turbinate within the nasal cavity (similar to PNECs from control subjects), not from the polyp tissue. Therefore, the pathological abnormality we describe here, a VEGF-overexpressing hyperplastic epithelium, is related generally to the PNECs and not specifically to the polyp. This may provide a clue as to why surgical removal of polyps may provide temporary relief of polyposis and reduction of soluble VEGF in nasal lavage, but does not cure the disease. The recurrence of the polyps at some time postsurgically may be due to the underlying abnormality of the PNECs. These observations also do not preclude the possibility that the nasal epithelial cells present postoperatively (and preoperatively) in subjects with CRSwNP possess the capacity for overexpression of nonsoluble forms of VEGF, with autocrine binding and function, which may not be readily detected by ELISA. Indeed, we observed elevated levels of nonsoluble, cell surface VEGF on PNECs from subjects with CRSwNP (see Figure 2). Further studies are needed to address the precise roles of the various isoforms of VEGF in this disease. Regardless, VEGF represents an important growth factor–related biomarker for sinonasal polyposis identified by our studies.
The data obtained from blocking antibodies indicates that VEGF is the growth factor responsible for this hyperplastic behavior. When the cells were treated with either anti-VEGF or anti-NP1 antibodies, the growth rate was normalized. This occurred in the absence of exposure to exogenous VEGF. These data support the contention that elevation in VEGF may be more than merely a biomarker for this disease; it may also be pivotal in driving and maintaining a key pathological feature of the disease, epithelial hyperplasia. However, as to whether the role of VEGF as a perpetrator of epithelial cell hyperplasia is a consequence versus a cause of polyposis remains a question requiring further studies. To our knowledge, this is the first report of VEGF functioning in an autocrine manner to control nonneoplastic human epithelial cell growth.
We found expression of the receptor VEGFR2 in sinonasal polyp tissue. Moreover, we demonstrated expression of an activated form of this receptor, phospho-VEGFR2, on nasal epithelial cells from diseased polyp tissue. These studies confirm previous findings of the expression of VEGF and VEGFR2 on upper airway epithelium (38–41). VEGFR2 is known to mediate proliferative effects in endothelial cells (65). Its presence in a phosphorylated and presumably activated form within the epithelium of hyperplastic sinonasal polyp tissues in situ suggests that VEGF mediates biologically significant function in sinonasal epithelial cells. Our functional studies show, however, that blockade of VEGFR2 (or VEGFR1) alone had little effect on the growth rate of the epithelial cells. These negative data should be interpreted with caution until repeated with other selective VEGFR2 antagonists.
NP1 is a cell surface receptor that has multiple functions. Relevant to the present study are findings that NP1 functions as a potent endothelial cell mitogen and regulates vasculature formation (66). NP1 is thought to function to increase the binding affinity of VEGF to VEGFR2, by serving as a docking site for ligand binding (67). NP1 occurs in a wide variety of tissues, including epithelial cells and tumors. Our finding that blocking antibodies targeted against NP1 virtually mimicked the inhibitory effect of blocking VEGF ligand itself supports the idea that activation of NP1 is essential for the VEGF-mediated increase in epithelial cell proliferation seen in subjects with CRSwNP.
The increase in cell growth observed with CRSwNP epithelium may be explained by a decrease in cell death due to the presence of a survival factor such as autocrine VEGF. Because activation of both VEGF and NP1 has been shown to have an antiapoptotic function (68, 69), we sought to determine whether VEGF contributes to cell survival by inhibiting apoptosis. The results in Figure 6 suggest that VEGF functions to inhibit apoptosis through activation of NP1. Several studies have demonstrated that NP1 mediates VEGF-induced human breast cancer cell survival in the absence of VEGFR1 and VEGFR2 (68, 70). Barr and colleagues have shown that in a breast cancer cell line that expresses constitutive VEGF and NP1, but lacks VEGF receptor expression, NP1 blockade using a peptide antagonist induces tumor cell apoptosis (68). Thus VEGF has a potential to act as a prosurvival factor on cells expressing NP1 in the absence of VEGFR1 and VEGFR2, a concept at least consistent with the findings presented here.
In addition to implicating VEGF as an epithelial cell mitogen, we asked whether VEGF could function in an autocrine feedback manner to autoregulate its own expression in PNECs. Blockade of NP1 by antibody neutralization resulted in inhibition of VEGF production. These results were corroborated by effective siRNA silencing of NP1 in PNECs, which resulted in inhibition of VEGF production. In addition to the novel role of VEGF as an autocrine epithelial cell mitogen, to our knowledge this is the first report of VEGF functioning in an autocrine manner to regulate its own expression in nonneoplastic, nontransformed primary human airway epithelial cells.
In summary, we have demonstrated evidence of VEGF as a potential biomarker for sinonasal polyposis and have implicated a novel role for VEGF as an epithelial cell mitogen and prosurvival factor that functions in a positive feed-forward manner. In addition, we have identified that this function is dependent on NP1, which we report as being constitutively expressed in human upper airway epithelial cells. Given the central role of the epithelium in orchestrating innate and adaptive immune responses of the airways, understanding the factors that promote growth and survival of these cells may provide key insights into controlling aberrant inflammatory responses emanating from the epithelium in CRSwNP and other airway diseases such as asthma (71, 72). Future studies are needed to further understand the potential contribution of VEGF in the development of sinonasal polyposis. These studies support the contention that targeting the VEGF pathway in upper airway epithelial cells, in an anti-VEGF or anti-NP1 clinical trial, may provide new therapeutic avenues for treatment of this recalcitrant disease.
Acknowledgments
The authors thank Drs. Bruce Bochner and Brad Undem for their helpful critique of this manuscript.
Supported by National Institutes of Health grant AI57400 and the Flight Attendant Medical Research Institute.
Originally Published in Press as DOI: 10.1164/rccm.200905-0740OC on September 17, 2009
Conflict of Interest Statement: H.S.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.M. received more than $100,001 from Sanofi-Aventis for a department-wide general research contract and up to $1,000 from Blackwell Scientific Publication in royalties; J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Benson V, Marano MA. Current estimates from the National Health Interview Survey, 1995. Vital Health Stat 10 1998;199:1–428. [PubMed] [Google Scholar]
- 2.Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, Hamilos DL. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol 2007;21:19–26. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004;114:155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L, Chinchilli VM, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol 2006;118:S17–S61. [DOI] [PubMed] [Google Scholar]
- 5.Bachert C, Patou J, Van Cauwenberge P. The role of sinus disease in asthma. Curr Opin Allergy Clin Immunol 2006;6:29–36. [DOI] [PubMed] [Google Scholar]
- 6.Mygind N, Dahl R, Bachert C. Nasal polyposis, eosinophil dominated inflammation, and allergy. Thorax 2000;55:S79–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harlin SL, Ansel DG, Lane SR, Myers J, Kephart GM, Gleich GJ. A clinical and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol 1988;81:867–875. [DOI] [PubMed] [Google Scholar]
- 8.Stierna P, Carlsoo B. Histopathological observations in chronic maxillary sinusitis. Acta Otolaryngol 1990;110:450–458. [DOI] [PubMed] [Google Scholar]
- 9.Watelet JB, Claeys C, Perez-Novo C, Gevaert P, Van Cauwenberge P, Bachert C. Transforming growth factor-β1 in nasal remodeling: differences between chronic rhinosinusitis and nasal polyposis. Am J Rhinol 2004;18:267–272. [PubMed] [Google Scholar]
- 10.Mahfouz ME, Elsheikh MN, Ghoname NF. Molecular profile of the antrochoanal polyp: up-regulation of basic fibroblast growth factor and transforming growth factor β in maxillary sinus mucosa. Am J Rhinol 2006;20:466–470. [DOI] [PubMed] [Google Scholar]
- 11.Ohno I, Lea RG, Flanders KC, Clark DA, Banwatt D, Dolovich J, Denburg J, Harley CB, Gauldie J, Jordana M. Eosinophils in chronically inflamed human upper airway tissues express transforming growth factor β1 gene (TGF β1). J Clin Invest 1992;89:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haruna S, Nakanishi M, Otori N, Moriyama H. Histopathological features of nasal polyps with asthma association: an immunohistochemical study. Am J Rhinol 2004;18:165–172. [PubMed] [Google Scholar]
- 13.Zaravinos A, Soufla G, Bizakis J, Spandidos DA. Expression analysis of VEGFA, FGF2, TGF-β1, EGF and IGF1 in human nasal polyposis. Oncol Rep 2008;19:385–391. [PubMed] [Google Scholar]
- 14.Zhang PJ, Weber R, Liang HH, Pasha TL, LiVolsi VA. Growth factors and receptors in juvenile nasopharyngeal angiofibroma and nasal polyps: an immunohistochemical study. Arch Pathol Lab Med 2003;127:1480–1484. [DOI] [PubMed] [Google Scholar]
- 15.Hartnell A, Heinemann A, Conroy DM, Wait R, Sturm GJ, Caversaccio M, Jose PJ, Williams TJ. Identification of selective basophil chemoattractants in human nasal polyps as insulin-like growth factor-1 and insulin-like growth factor-2. J Immunol 2004;173:6448–6457. [DOI] [PubMed] [Google Scholar]
- 16.Hansson HA, Brandsten C, Lossing C, Petruson K. Transient expression of insulin-like growth factor I immunoreactivity by vascular cells during angiogenesis. Exp Mol Pathol 1989;50:125–138. [DOI] [PubMed] [Google Scholar]
- 17.Ebbens FA, Georgalas C, Luiten S, van Drunen CM, Badia L, Scadding GK, Hellings PW, Jorissen M, Mullol J, Cardesin A, et al. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: a multicenter randomized controlled study. Laryngoscope 2009;119:401–408. [DOI] [PubMed] [Google Scholar]
- 18.Coste A, Wang QP, Roudot-Thoraval F, Chapelin C, Bedbeder P, Poron F, Peynegre R, Escudier E. Epithelial cell proliferation in nasal polyps could be up-regulated by platelet-derived growth factor. Laryngoscope 1996;106:578–583. [DOI] [PubMed] [Google Scholar]
- 19.Kouzaki H, Seno S, Fukui J, Owaki S, Shimizu T. Role of platelet-derived growth factor in airway remodeling in rhinosinusitis. Am J Rhinol Allergy 2009;23:273–280. [DOI] [PubMed] [Google Scholar]
- 20.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O'Byrne P, Dolovich J, Jordana M, Tamura G, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Respir Cell Mol Biol 1995;13:639–647. [DOI] [PubMed] [Google Scholar]
- 21.Little SC, Early SB, Woodard CR, Shonka DC Jr, Han JK, Borish L, Steinke JW. Dual action of TGF-β1 on nasal-polyp derived fibroblasts. Laryngoscope 2008;118:320–324. [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST. Epithelial damage and response. Clin Exp Allergy 2000;30:37–41. [DOI] [PubMed] [Google Scholar]
- 23.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 1999;29:90–95. [DOI] [PubMed] [Google Scholar]
- 24.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000;14:1362–1374. [DOI] [PubMed] [Google Scholar]
- 25.Gille J, Swerlick RA, Caughman SW. Transforming growth factor-α–induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2–dependent DNA binding and transactivation. EMBO J 1997;16:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-β in fibroblastic and epithelial cells. J Biol Chem 1994;269:6271–6274. [PubMed] [Google Scholar]
- 27.Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, Nagai S, Izumi T. Vascular endothelial growth factor mrna and protein expression in airway epithelial cell lines in vitro. Eur Respir J 2002;20:1449–1456. [DOI] [PubMed] [Google Scholar]
- 28.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes: implications for normal and impaired wound healing. J Biol Chem 1995;270:12607–12613. [DOI] [PubMed] [Google Scholar]
- 29.Milanini J, Vinals F, Pouyssegur J, Pages G. p42/p44 MAP kinase module plays a key role in the transcriptional regulation of the vascular endothelial growth factor gene in fibroblasts. J Biol Chem 1998;273:18165–18172. [DOI] [PubMed] [Google Scholar]
- 30.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. [DOI] [PubMed] [Google Scholar]
- 31.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy 2003;33:595–599. [DOI] [PubMed] [Google Scholar]
- 33.Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol 2002;110:571–575. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001;107:1034–1038. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001;107:295–301. [DOI] [PubMed] [Google Scholar]
- 36.Lee YC, Lee HK. Vascular endothelial growth factor in patients with acute asthma. J Allergy Clin Immunol 2001;107:1106. [DOI] [PubMed] [Google Scholar]
- 37.Hu KH, Lee FP, Cheng YJ, Huang HM. Vascular endothelial growth factor and children featuring nasal polyps. Int J Pediatr Otorhinolaryngol 2007;71:23–28. [DOI] [PubMed] [Google Scholar]
- 38.Bateman ND, Shahi A, Feeley KM, Woolford TJ. Vascular endothelial growth factor in nasal polyps: a comparison of asthmatic and non-asthmatic patients. Clin Otolaryngol Allied Sci 2004;29:677–681. [DOI] [PubMed] [Google Scholar]
- 39.Gosepath J, Brieger J, Lehr HA, Mann WJ. Expression, localization, and significance of vascular permeability/vascular endothelial growth factor in nasal polyps. Am J Rhinol 2005;19:7–13. [PubMed] [Google Scholar]
- 40.Wittekindt C, Hess A, Bloch W, Sultanie S, Michel O. Immunohistochemical expression of VEGF and VEGF receptors in nasal polyps as compared to normal turbinate mucosa. Eur Arch Otorhinolaryngol 2002;259:294–298. [DOI] [PubMed] [Google Scholar]
- 41.Vento SI, Wolff CH, Salven PJ, Hytonen ML, Ertama LO, Malmberg CH. Vascular permeability factor/vascular endothelial growth factor in nasal polyps. Acta Otolaryngol Suppl 2000;543:170–174. [DOI] [PubMed] [Google Scholar]
- 42.Kim J. Overexpression of VEGF from sinonasal epithelium is a biomarker for chronic rhinosinusitis with nasal polyposis [abstract]. J Allergy Clin Immunol 2009;123:724–725. [Google Scholar]
- 43.Lee H, Myers A, Kim J. Involvement of VEGF in upper airway epithelial cell dysfunction in chronic rhinosinusitis with nasal polyposis subjects [abstract]. Presented at the Keystone Symposium on Molecular and Cellular Biology. J1: Allergy and Asthma. January 20–25, 2009, Keystone Resort, Keystone, CO.
- 44.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003;129:S1–S32. [DOI] [PubMed] [Google Scholar]
- 45.Creticos PS. In vivo provocative testing for IgE-mediated disease. New York: Marcel Dekker; 1999.
- 46.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Myers AC, Chen L, Pardoll DM, Truong-Tran QA, Lane AP, McDyer JF, Fortuno L, Schleimer RP. Constitutive and inducible expression of B7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol 2005;33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naclerio RM, Meier HL, Kagey-Sobotka A, Adkinson NF Jr, Meyers DA, Norman PS, Lichtenstein LM. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis 1983;128:597–602. [DOI] [PubMed] [Google Scholar]
- 49.Heinecke L, Proud D, Sanders S, Schleimer RP, Kim J. Induction of B7-H1 and B7-DC expression on airway epithelial cells by the Toll-like receptor 3 agonist double-stranded RNA and human rhinovirus infection: in vivo and in vitro studies. J Allergy Clin Immunol 2008;121:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Sanders SP, Siekierski ES, Casolaro V, Proud D. Role of NF-κB in cytokine production induced from human airway epithelial cells by rhinovirus infection. J Immunol 2000;165:3384–3392. [DOI] [PubMed] [Google Scholar]
- 51.Conn G, Bayne ML, Soderman DD, Kwok PW, Sullivan KA, Palisi TM, Hope DA, Thomas KA. Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci USA 1990;87:2628–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, DiPietro LA. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol 2005;167:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- 54.Giuliani N, Colla S, Lazzaretti M, Sala R, Roti G, Mancini C, Bonomini S, Lunghi P, Hojden M, Genestreti G, et al. Proangiogenic properties of human myeloma cells: production of angiopoietin-1 and its potential relationship to myeloma-induced angiogenesis. Blood 2003;102:638–645. [DOI] [PubMed] [Google Scholar]
- 55.Beck LA, Tancowny B, Brummet ME, Asaki SY, Curry SL, Penno MB, Foster M, Bahl A, Stellato C. Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J Immunol 2006;177:3344–3354. [DOI] [PubMed] [Google Scholar]
- 56.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 1992;267:26031–26037. [PubMed] [Google Scholar]
- 57.Plouet J, Moro F, Bertagnolli S, Coldeboeuf N, Mazarguil H, Clamens S, Bayard F. Extracellular cleavage of the vascular endothelial growth factor 189-amino acid form by urokinase is required for its mitogenic effect. J Biol Chem 1997;272:13390–13396. [DOI] [PubMed] [Google Scholar]
- 58.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994;269:26988–26995. [PubMed] [Google Scholar]
- 59.Ito N, Huang K, Claesson-Welsh L. Signal transduction by VEGF receptor-1 wild type and mutant proteins. Cell Signal 2001;13:849–854. [DOI] [PubMed] [Google Scholar]
- 60.Isik SR, Karakaya G, Celikel S, Demir AU, Kalyoncu AF. Association between asthma, rhinitis and NSAID hypersensitivity in chronic urticaria patients and prevalence rates. Int Arch Allergy Immunol 2009;150:299–306. [DOI] [PubMed] [Google Scholar]
- 61.Staikuniene J, Vaitkus S, Japertiene LM, Ryskiene S. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Medicina (Kaunas) 2008;44:257–265. [PubMed] [Google Scholar]
- 62.Larsen K. The clinical relationship of nasal polyps to asthma. Allergy Asthma Proc 1996;17:243–249. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol 2003;24:159–168. [DOI] [PubMed] [Google Scholar]
- 64.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med 2006;12:1023–1026. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J 2001;20:2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998;92:735–745. [DOI] [PubMed] [Google Scholar]
- 67.Whitaker GB, Limberg BJ, Rosenbaum JS. Vascular endothelial growth factor receptor-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF(165) and VEGF(121). J Biol Chem 2001;276:25520–25531. [DOI] [PubMed] [Google Scholar]
- 68.Barr MP, Byrne AM, Duffy AM, Condron CM, Devocelle M, Harriott P, Bouchier-Hayes DJ, Harmey JH. A peptide corresponding to the neuropilin-1–binding site on VEGF(165) induces apoptosis of neuropilin-1–expressing breast tumour cells. Br J Cancer 2005;92:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998;273:13313–13316. [DOI] [PubMed] [Google Scholar]
- 70.Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 2001;61:5736–5740. [PubMed] [Google Scholar]
- 71.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003;8:432–446. [DOI] [PubMed] [Google Scholar]
- 72.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int 2008;57:1–10. [DOI] [PubMed] [Google Scholar]