Abstract
Rationale: Individuals with Hermansky-Pudlak syndrome type 1 (HPS-1), an autosomal recessive disorder characterized by defective biogenesis of lysosome-related organelles, develop an accelerated form of progressive fibrotic lung disease. The etiology of pulmonary fibrosis associated with HPS-1 is unknown.
Objectives: To investigate the potential pathogenesis of pulmonary fibrosis in HPS-1, lung cells and proteins from individuals with HPS-1 were studied.
Methods: Forty-one subjects with HPS-1 with and without pulmonary fibrosis were evaluated with pulmonary function tests, high-resolution computed tomography scan, and bronchoscopy. Bronchoalveolar lavage cells and analytes were analyzed.
Measurements and Main Results: Concentrations of total bronchoalveolar lavage cells and alveolar macrophages were significantly higher in epithelial lining fluid from subjects with HPS-1 with and without pulmonary fibrosis compared with healthy research volunteers. Concentrations of cytokines and chemokines (i.e., monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and granulocyte-macrophage colony-stimulating factor) in alveolar epithelial lining fluid were significantly higher in subjects with HPS-1 with and without pulmonary fibrosis compared with healthy research volunteers (P < 0.001). In vitro, HPS-1 pulmonary fibrosis alveolar macrophages, which did not express HPS1 mRNA, secreted significantly higher concentrations of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and regulated upon activation, normal T cell expressed and secreted (RANTES) protein compared with normal cells (P = 0.001, P = 0.014, and P = 0.011, respectively). Pirfenidone suppressed HPS-1 alveolar macrophage cytokine and chemokine secretion in vitro in a dose-dependent manner.
Conclusions: In HPS-1, alveolar inflammation predominantly involves macrophages and is associated with high lung concentrations of cytokines and chemokines. HPS-1 alveolar macrophages provide a model system in which to study the pathogenesis and treatment of HPS pulmonary fibrosis.
Keywords: inflammation, cytokines, chemokines, bronchoalveolar lavage, pirfenidone
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Limited information is available regarding the pathogenesis of Hermansky-Pudlak syndrome pulmonary fibrosis, a progressive and often fatal disease.
What This Study Adds to the Field
This study demonstrates that high concentrations of activated alveolar macrophages, cytokines, and chemokines are found in individuals with Hermansky-Pudlak syndrome type 1. Dysregulation of alveolar macrophages may contribute to the pathogenesis of Hermansky-Pudlak syndrome pulmonary fibrosis.
Hermansky-Pudlak syndrome type 1 (HPS-1), a rare autosomal recessive disease, occurs commonly in northwest Puerto Rico but can also affect individuals without Puerto Rican heritage (1–3). Clinical manifestations include oculocutaneous albinism, platelet dysfunction due to a storage pool defect, granulomatous colitis, and fibrotic lung disease, which typically leads to death in the fourth or fifth decade of life (1–3). The pulmonary fibrosis of HPS-1 is largely unresponsive to conventional medical treatment, although patients with mild to moderate disease may respond to treatment with pirfenidone (4). In patients with HPS-1 and advanced pulmonary fibrosis, lung transplantation has been successfully performed (5, 6).
To date, eight genetic subtypes of HPS with various phenotypes have been identified in humans, and six subtypes are not associated with fibrotic lung disease (3). Although the exact function of most HPS proteins remains unknown, they can interact to form complexes that regulate biogenesis of lysosome-related organelles. Individuals with HPS-2 have mutations in AP3B1, which encodes the β3A subunit of the adaptor protein-3 complex (7, 8). Patients with HPS-3, HPS-5, and HPS-6 have mutations resulting in abnormalities in biogenesis of lysosome-related organelles complex-2 (BLOC-2), and those with HPS-7 and HPS-8 have mutations affecting BLOC-1 (3, 9–11).
Individuals with HPS-1 or HPS-4 can develop pulmonary fibrosis (3, 12). Patients with HPS-1 have mutations in the HPS1 gene, located on chromosome 10q23.1–23.3 (13). Virtually all Puerto Rican individuals with HPS-1 are homozygous for a 16-base pair duplication in exon 15 of HPS1 [c.1472_1487dup16; p.H497QfsX90 (ex 15)], but other mutations in HPS1 have been reported, and many are associated with progressive pulmonary fibrosis (1, 2, 14–18). The HPS1 gene encodes HPS1, a 79.3-kD cytosolic and membrane-bound protein involved in biogenesis of lysosome-related organelles (19). The HPS4 gene encodes HPS4, a 76.9-kD protein; HPS1 associates with HPS4 to form BLOC-3, a protein complex that regulates the intracellular localization of lysosomes and endosomes (20, 21).
Although basic mechanisms causing pulmonary fibrosis in HPS-1 are unknown, findings in lung pathology specimens and murine models of HPS have provided insights into the disease pathogenesis. Distinctive histopathologic features of the HPS pulmonary fibrosis include accumulation of ceroid lipofuscin within alveolar macrophages and proliferation of type II alveolar epithelial cells that appear foamy due to the formation of giant lamellar bodies (22, 23). These characteristics have also been described in the lungs of murine models of HPS. Pale ear mice, which have mutations in Hps1, and pearl mice, which have mutations in Ap3b1, serve as models for HPS-1 and HPS-2, respectively (24–29). Pale ear mice develop giant lamellar bodies in type II alveolar epithelial cells, interstitial inflammation, and foamy alveolar macrophages (26, 29). Furthermore, constitutive activation of macrophages in the lung, but not in the blood or peritoneum, occurs in pale ear and pearl mice (28). Notably, pale ear mice do not develop spontaneous fibrosis, but exposure to silica induces persistent alveolar accumulation of activated macrophages and collagen fibers (26, 27). In addition, intratracheal administration of bleomycin to pale ear or pearl mice causes diffuse pulmonary fibrosis, deposition of extracellular matrix protein, and apoptosis of type II cells (29). Taken together, these findings suggest that dysregulation of lung immune and epithelial cells, in combination with certain environmental exposures, may contribute to the pulmonary fibrosis in HPS.
To investigate the potential pathogenesis of lung disease in HPS-1, we studied cells and proteins in the bronchoalveolar lavage fluid of affected individuals. We report that individuals with HPS-1 accumulate increased numbers of total bronchoalveolar lavage cells and macrophages in alveolar fluid, with accompanying elevations in cytokine and chemokine concentrations. We also show that alveolar macrophages cultured from individuals with HPS-1 secrete high concentrations of cytokines, and that pirfenidone down-regulates this cytokine secretion in vitro.
METHODS
Subjects
Forty-one adult subjects with HPS-1, evaluated at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD), were enrolled in protocols 95-HG-0193, 97-HG-0085, and 04-H-0211, which were approved by the Institutional Review Boards of the National Institute of Child Health and Human Development, National Human Genome Research Institute, or National Heart, Lung, and Blood Institute. HPS was diagnosed by the presence of oculocutaneous albinism and the absence of platelet dense bodies; the diagnosis of HPS-1 was confirmed by molecular analysis of the HPS1 gene (GenBank NM_000195) (1, 19). Eighty-five healthy research volunteers without lung disease were enrolled in protocols 96-H-0100 and 99-H-0068, which were approved by the National Heart, Lung, and Blood Institute Institutional Review Board. Written informed consent was obtained from all subjects. For patients with HPS, high-resolution computed tomography scan, pulmonary function tests, and bronchoscopy were performed during the same visit.
High-resolution Computed Tomography
High-resolution computed tomography (HRCT) scans of the chest were performed without intravenous contrast during end-inspiration in the prone position, using a 1-mm collimation at 30-mm intervals and a high-spatial-frequency reconstruction algorithm (General Electric Medical Systems, Milwaukee, WI) (2). Pulmonary fibrosis was diagnosed by characteristic findings on HRCT scans of the chest, as previously described (2). Briefly, HRCT scores were based on the following scheme: 0 = normal CT; 1 = mild disease (1 to 15 thickened interlobular septa per segment, 1 to 5 patches of reticulation, subpleural cysts, and pleural/parenchymal scars); 2 = moderate disease (moderate reticulation, peribronchovascular thickening, traction bronchiectasis, tracheal retraction); 3 = severe disease (areas of parenchymal consolidation, diffuse areas of peribronchovascular thickening, traction bronchiectasis, and patches of reticulation).
Pulmonary Function Testing
Measurements were made with standard equipment according to American Thoracic Society recommendations (Collins Medical, Inc., Braintree, MA; SensorMedics, Yorba Linda, CA) (30). FEV1, FVC, total lung capacity (TLC), and diffusion capacity (DlCO) were expressed as percentages of predicted values.
Fiberoptic Bronchoscopy with Bronchoalveolar Lavage
Bronchoscopy with lavage as well as isolation of alveolar macrophages and bronchoalveolar lavage (BAL) fluid were performed as described (31–33). Briefly, subjects were sedated and received 1% lidocaine for topical anesthesia. A fiberoptic bronchoscope was inserted into the proximal airways, and up to four 25- to 30-ml aliquots of sterile 0.9% normal saline were instilled into the right middle lobe medial or lateral segments and/or the lingula. The BAL fluid was collected by suction into sterile specimen containers, filtered through gauze, and centrifuged at 1,000 × g for 10 minutes at 4°C to pellet the BAL cells. Supernatant was aliquoted, stored at −80°C, and thawed immediately before analysis. Pelleted cells were washed twice in RPMI 1640, centrifuged at 1,000 × g for 10 minutes at 4°C, dispersed in RPMI 1640, transferred to sterile polystyrene tissue culture dishes, and incubated for 2 hours at 37°C in a humidified atmosphere containing 5% CO2. Adherent cells (about 95% alveolar macrophages) were analyzed further in the experiments described later. A blood sample was obtained after bronchoscopy, and urea concentrations in BAL fluids and plasma were measured to calculate dilution of BAL fluids (Sigma Diagnostics, St. Louis, MO). Differential cell counts of BAL cells were performed on Cytospin slides stained with Diff-Quik, using a hemocytometer, and concentrations of BAL cells were calculated (Thermo Fisher Scientific, Inc., Waltham, MA).
Northern Blot Analysis and Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was isolated from alveolar macrophages of a subject with HPS-1 and a healthy research volunteer as well as from normal fibroblasts. Northern blots containing 20 μg of mRNA were analyzed with a 32P-labeled human HPS1 cDNA as previously described (16). Reverse transcriptase-polymerase chain reaction (RT-PCR) amplification was performed with total RNA isolated from alveolar macrophages and peripheral blood mononuclear cells (PBMCs) from three different healthy research volunteers as well as from PBMCs from three different subjects with HPS-1. cDNA was generated with the SuperScript first strand synthesis system for RT-PCR in accordance with the manufacturer's instructions (Invitrogen, Carlsbad, CA). A 414-bp fragment of HPS1 was amplified in a 25-μl reaction mixture containing cDNA and primers (5′-CCTGTTTGGAGAATGCCTGTTC-3′ and 5′-CCAAGCTGCTGGCATTCTACTC-3′), using standard PCR methods. A 353-bp RT-PCR product of β-actin was generated in accordance with the manufacturer's instructions (Invitrogen). GeneRuler 100-bp DNA ladder (Fermentas, Inc., Glen Burnie, MD) and PCR products were subjected to electrophoresis on a 1.5% agarose gel and stained with ethidium bromide.
Quantification of Cytokines and Growth Factors
Concentrations of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), epidermal growth factor (EGF), granulocyte colony-stimulating factor (G-CSF), regulated upon activation, normal T cell expressed and secreted (RANTES), transforming growth factor (TGF)-β1, and platelet-derived growth factor (PDGF)-AB were measured in BAL fluid specimens that were concentrated 10-fold by lyophilization. ELISA kits were used in accordance with manufacturers' instructions (Amersham Biosciences, Piscataway, NJ; Biosource International, Camarillo, CA; R&D Systems, Minneapolis, MN). Concentrations of IL-4, IL-6, IL-10, IL-12p40, MIP-4, IFN-γ, tumor necrosis factor (TNF)-α, PDGF-AA, and PDGF-BB were measured by multiplex ELISA (SearchLight; Pierce Biotechnology, Inc., Woburn, MA). Final epithelial lining fluid (ELF) concentrations were determined by the urea method (31–33).
After bronchoscopy, alveolar macrophages (AMs, 1 × 106/ml) were cultured in cell culture medium (RPMI, 1× glutamine, 1× penicillin–streptomycin). Plates were incubated in 5% CO2 at 37°C. After 2 hours, AMs were washed twice with fresh medium, and then cultured with medium with or without pirfenidone. Concentrations of pirfenidone were 200, 600, 1,200, and 2,400 μg/ml. Conditioned medium was isolated after 24 hours, and immediately stored in a liquid nitrogen cryotank. Samples were thawed once, and concentrations of MCP-1, MIP-1α, RANTES, M-CSF, MIP-4, GM-CSF, IL-12p40, and IFN-γ were measured by multiplex ELISA (SearchLight; Pierce Biotechnology, Inc.).
Statistical Analysis
The Student t test was used to determine significant differences (P < 0.05) between the mean values of age, pulmonary function test measurements, BAL cell concentrations, and BAL analyte concentrations. Data are reported as mean values ± standard error of the mean.
RESULTS
Subject Characteristics
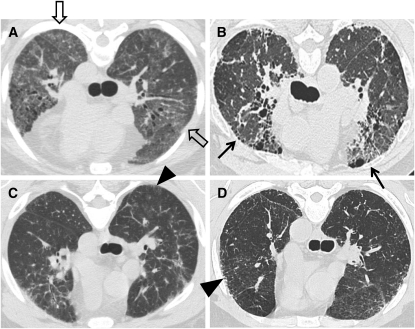
Forty-one subjects with HPS-1 and 85 healthy research volunteers participated in this study. PCR amplification of genomic DNA demonstrated that 35 of 41 subjects with HPS-1 were homozygous for a 16-base pair duplication in exon 15 of HPS1 [c.1472_1487dup16; p.H497QfsX90 (ex 15)]. Six of 41 subjects had 5 different truncating HPS1 mutations, including [c.1744-2A > C (IVS16)], [c.1375delA; c.1388C > A; p.S459VfsX16 (ex 14)], [c.97_100delTCAG; p.S33RfsX18 (ex 3)], [c.972dupC; p.M325HfsX128 (ex 11)], and [c.1189delC; p.Q397SfsX2 (ex 13)]. In 34 of 41 subjects with HPS-1, pulmonary fibrosis was diagnosed by characteristic findings on HRCT scan of the chest, including ground glass opacification in 20 (59%) (Figure 1). Seven subjects with HPS-1 did not have interstitial infiltrates on their HRCT scan images.
Figure 1.
(A–D) High-resolution computed tomography (HRCT) scan findings in subjects with Hermansky-Pudlak syndrome type 1 (HPS-1) pulmonary fibrosis. Representative HRCT scan images from four individual subjects with HPS-1 pulmonary fibrosis are shown. Reticulation (arrowheads), honeycombing (arrows), and ground glass opacification (open arrows) are found in subjects with HPS-1 pulmonary fibrosis.
The mean age of subjects with HPS-1 pulmonary fibrosis, 38.6 ± 1.5 years, did not differ significantly from that of subjects with HPS-1 without pulmonary fibrosis, 31.0 ± 4.2 years (P = 0.054) or our healthy research volunteers, 38.8 ± 1.4 years (P = 0.947) (Table 1). The most common symptoms of lung disease included dyspnea on exertion and cough. Six (86%) of 7 subjects with HPS-1 and 7 (21%) of 34 with HPS-1 pulmonary fibrosis were asymptomatic. One subject with HPS-1 without pulmonary fibrosis experienced dyspnea on exertion at age 43 years, and 27 (79%) of 34 subjects with HPS-1 with pulmonary fibrosis developed symptoms at approximately 34.9 ± 1.77 years of age. A few subjects in this cohort were being treated with inhaled steroids with or without a β-agonist, including one subject with HPS-1 and four subjects with HPS-1 pulmonary fibrosis. Eleven (32%) of 34 subjects with HPS-1 pulmonary fibrosis, 5 (71%) of 7 with HPS-1, and 55 (65%) of 85 healthy research volunteers were male. No subjects with HPS-1 with or without pulmonary fibrosis and no healthy research volunteers were current smokers, and 3 (8.8%) of 34 subjects with HPS-1 pulmonary fibrosis were former smokers who had stopped smoking for at least 2.5 years and had a mean history of smoking for 17.7 ± 1.20 pack-years. Many subjects with HPS-1 were unemployed or were receiving disability benefits, largely due to their visual impairment, including 4 of 7 with HPS-1 and 13 of 34 with HPS-1 pulmonary fibrosis. Among the subjects who were employed, one individual with HPS-1 pulmonary fibrosis was exposed to wood dust in the construction industry.
TABLE 1.
SUBJECT CHARACTERISTICS
|
P Value |
||||||
|---|---|---|---|---|---|---|
| HPS-1 (n = 7) | HPS-1 PF (n = 34) | NV (n = 85) | HPS-1 vs. HPS-1 PF | HPS-1 vs. NV | HPS-1 PF vs. NV | |
| Age, yr | 31.0 ± 4.2 | 38.6 ± 1.5 | 38.8 ± 1.4 | 0.054 | 0.134 | 0.947 |
| Sex, M/F | 5 / 2 | 11 / 23 | 55 / 30 | |||
| Current smokers | 0 | 0 | 0 | |||
| Former smokers | 0 | 3 | 6 | |||
| Dyspnea or cough | 1 | 27 | 0 | |||
| FEV1, % of predicted | 87.5 ± 4.3 | 85.6 ± 3.4 | 106.7 ± 1.9 | 0.803 | 0.004 | <0.001 |
| FVC, % of predicted | 82.1 ± 5.1 | 79.5 ± 3.4 | 101.5 ± 1.6 | 0.738 | <0.001 | <0.001 |
| TLC, % of predicted | 84.9 ± 5.6 | 78.8 ± 3.3 | 99.5 ± 1.5 | 0.478 | 0.006 | <0.001 |
| DlCO, % of predicted |
85.8 ± 7.7 |
75.0 ± 3.3 |
102.8 ± 1.8 |
0.186 |
0.009 |
<0.001 |
Definition of abbreviations: DlCO = diffusion capacity for carbon monoxide; F = female; HPS-1 = Hermansky-Pudlak syndrome type 1; HPS-1 PF = Hermansky-Pudlak syndrome type 1 with pulmonary fibrosis; M = male; NV = healthy research volunteer; TLC = total lung capacity.
Analysis of pulmonary function tests revealed that measurements of FEV1, FVC, TLC, and DlCO were significantly lower in subjects with HPS-1 with and without pulmonary fibrosis than in healthy research volunteers. Although no statistically significant differences were found between subjects with HPS-1 with and without pulmonary fibrosis, pulmonary function tests indicated that subjects with HPS-1 pulmonary fibrosis had mild restriction and a mild defect in gas exchange, and subjects with HPS-1 without pulmonary fibrosis had normal lung function.
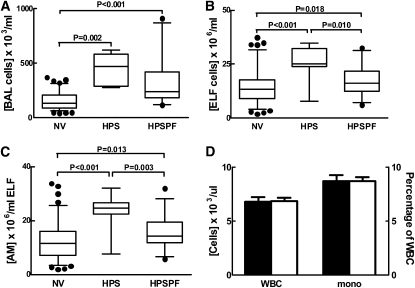
High Numbers of Total Alveolar Cells and Alveolar Macrophages in HPS-1 with and without Pulmonary Fibrosis
On fiberoptic bronchoscopy, subjects with HPS-1 without pulmonary fibrosis had significantly higher concentrations of total cells in BAL fluid (4.47 [±0.52] × 105 cells/ml) and ELF (25.2 [±3.29] × 106 cells/ml) compared with healthy research volunteers (1.57 [±0.08] × 105 and 14.1 [±0.77] × 106 cells/ml, respectively) (P = 0.002 and P < 0.001, respectively) (Figures 2A and 2B). To a lesser degree, subjects with HPS-1 pulmonary fibrosis had significantly higher concentrations of total cells in BAL fluid (3.13 [±0.34] × 105 cells/ml) and ELF (17.5 [±1.12] × 106 cells/ml) compared with healthy research volunteers (P < 0.001 and P = 0.018, respectively). In addition, differential cell counts revealed that concentrations of alveolar macrophages, but not lymphocytes or polymorphonuclear leukocytes, in ELF were significantly higher in subjects with HPS-1 without and with pulmonary fibrosis (23.4 [±2.87] and 15.5 [±0.96] × 106 cells/ml, respectively) than in healthy research volunteers (12.3 [±0.70] × 106 cells/ml) (P < 0.001 and P = 0.013, respectively) (Figure 2C). Compared with subjects with HPS-1 without pulmonary fibrosis, subjects with HPS-1 pulmonary fibrosis had significantly lower concentrations of total cells and alveolar macrophages in ELF (P = 0.010 and P = 0.003, respectively). In contrast, total white blood cells and percentage of monocytes in peripheral blood did not differ significantly in subjects with HPS-1 with and without pulmonary fibrosis (Figure 2D), and there were no significant differences in percentages of peripheral blood polymorphonuclear leukocytes, lymphocytes, or eosinophils between these two groups (data not shown).
Figure 2.
Increased alveolar concentrations of total cells and alveolar macrophages (AMs) in subjects with Hermansky-Pudlak syndrome type 1 (HPS-1). (A–C) Concentrations of total cells in bronchoalveolar lavage (BAL) fluid (× 103 cells/ml) and alveolar epithelial lining fluid (ELF) (× 106 cells/ml) were significantly higher in subjects with HPS-1 (HPS) and HPS-1 pulmonary fibrosis (HPSPF) compared with healthy research volunteers (NV). In ELF, concentrations (× 106 cells/ml) of AMs were significantly higher in subjects with HPS-1 and HPSPF compared with NV subjects. Subjects with HPS-1 had significantly higher concentrations of BAL cells and AMs in ELF compared with subjects with HPSPF. (D) No significant differences were found in peripheral blood concentrations of total leukocytes (WBC) or percentage of monocytes (mono) in subjects with HPS-1 (solid columns) and those with HPSPF (open columns).
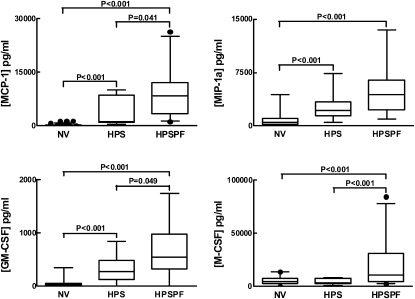
High Alveolar Concentrations of Cytokines and Chemokines in HPS-1 with and without Pulmonary Fibrosis
In subjects with HPS-1 pulmonary fibrosis, concentrations of MCP-1, MIP-1α, GM-CSF, and M-CSF in ELF were significantly higher (9,116 ± 1,122, 4,641 ± 498.0, 673 ± 77.0, and 19,190 ± 3,629 pg/ml, respectively) compared with the concentrations in healthy research volunteers (261 ± 29.0, 839 ± 108, 53 ± 7.0, and 5,318 ± 795.0 pg/ml, respectively) (P < 0.001) (Figure 3). Concentrations of MCP-1, MIP-1α, and GM-CSF were also significantly higher in subjects with HPS-1 without pulmonary fibrosis (3,668 ± 1,519, 2,663 ± 843.3, and 316.7 ± 104.9 pg/ml, respectively) than in healthy research volunteers (P < 0.001). Furthermore, concentrations of MCP-1α, GM-CSF, and M-CSF were significantly higher in subjects with HPS-1 pulmonary fibrosis compared with those in subjects with HPS-1 without pulmonary fibrosis (P = 0.041, P = 0.049, and P < 0.001, respectively). No significant differences in concentrations of IL-4, IL-6, IL-10, IL-12p40, MIP-4, IFN-γ, TNF-α, EGF, G-CSF, RANTES, TGF-β1, PDGF-AA, PDGF-AB, or PDGF-BB in ELF were found between subjects with HPS-1 with and without pulmonary fibrosis and healthy research volunteers (data not shown).
Figure 3.
High alveolar concentrations of cytokines and chemokines in subjects with Hermansky-Pudlak syndrome type 1 (HPS-1) with and without pulmonary fibrosis. Concentrations (pg/ml) of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein-1α (MIP-1a), granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF) were significantly higher in alveolar epithelial lining fluid (ELF) from subjects with HPS-1 pulmonary fibrosis (HPSPF) compared with healthy research volunteers (NV), and concentrations of MCP-1, MIP-1α, and GM-CSF were significantly higher from subjects with HPS-1 (HPS) compared with NV subjects. Subjects with HPSPF had significantly higher concentrations of MCP-1, GM-CSF, and M-CSF compared with subjects with HPS-1.
To determine whether alveolar concentrations of MCP-1, MIP-1α, GM-CSF, and/or M-CSF correlate with severity of HPS-1 pulmonary fibrosis, data from subjects with HPS-1 pulmonary fibrosis were segregated into those with mild or moderate/severe disease and compared with subjects with HPS-1 without pulmonary fibrosis. We found that concentrations of MCP-1 correlated with HPS-1 pulmonary fibrosis disease severity (see Figure E1 in the online supplement). In contrast, concentrations of MIP-1α and GM-CSF were significantly higher in subjects with HPS-1 with mild, and not moderate/severe, pulmonary fibrosis compared with those without lung disease (P = 0.041 and P = 0.033, respectively). Although concentrations of M-CSF were significantly higher in subjects with HPS-1 with some degree of pulmonary fibrosis compared with subjects with HPS-1 without lung disease (P = 0.002 and P = 0.035, respectively), levels of M-CSF did not correlate with disease severity.
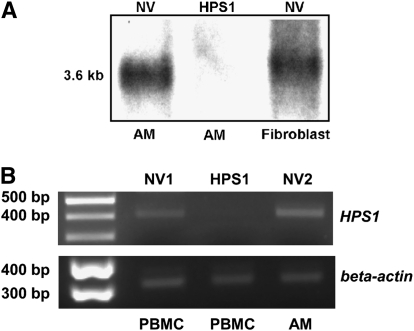
Cellular Expression of HPS1 in Healthy Research Volunteers, But Not in Subjects with HPS-1
Northern blot analysis demonstrated that alveolar macrophages from subjects with HPS-1 do not express HPS1 mRNA, whereas the HPS1 transcript is expressed in both alveolar macrophages and fibroblasts from healthy research volunteers (Figure 4A). In agreement with these data, amplification of cDNA by RT-PCR demonstrated that an HPS1 PCR product is amplified in alveolar macrophages and PBMCs from healthy research volunteers, but not in PBMCs from subjects with HPS-1 (Figure 4B).
Figure 4.
Cellular expression of HPS1 in healthy research volunteers, but not in subjects with Hermansky-Pudlak syndrome type 1 (HPS-1). (A) Northern blot analysis revealed that alveolar macrophages (AM) and fibroblasts from healthy research volunteers (NV), and not alveolar macrophages from subjects with HPS-1, express HPS1 mRNA. (B) Amplification of cDNA by reverse transcriptase-polymerase chain reaction (RT-PCR) using primers for HPS1 demonstrated that a 414-base pair (bp) HPS1 PCR product is expressed in peripheral blood mononuclear cells (PBMCs) and alveolar macrophages (AM) from two representative healthy volunteers (NV1 and NV2), but not in PBMCs from a representative subject with HPS-1. A 353-bp RT-PCR product was generated using primers for human β-actin.
Pirfenidone-induced Suppression of Cytokine Secretion by Alveolar Macrophages from Subjects with HPS-1
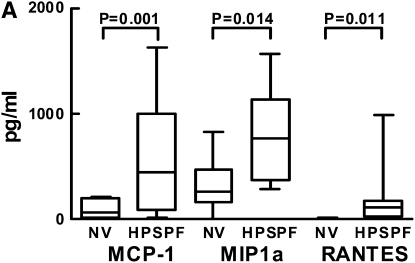
To determine whether absence of HPS1 transcript in alveolar macrophages is associated with cellular dysregulation, we cultured these cells and measured the concentrations of analytes secreted in vitro (Figure 5A). Concentrations of MCP-1, MIP-1α, and RANTES were significantly higher in conditioned medium from alveolar macrophages cultured for 24 hours from subjects with HPS-1 pulmonary fibrosis (548.1 ± 114.3, 787.3 ± 93.2, and 203.4 ± 69.6 pg/ml, respectively) compared with healthy research volunteers (96.5 ± 38.2, 318.7 ± 111.4, and 3.62 ± 2.17 pg/ml, respectively) (P = 0.001, P = 0.014, and P = 0.011, respectively).
Figure 5.
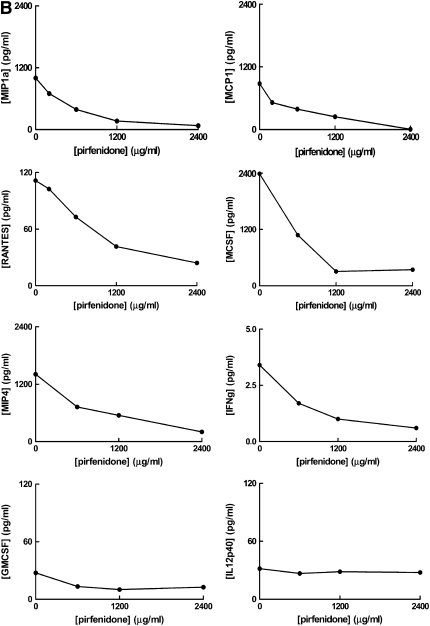
Cytokine secretion by Hermansky-Pudlak syndrome type 1 (HPS-1) and normal cultured alveolar macrophages and suppression by pirfenidone in HPS-1 cells. (A) Concentrations (pg/ml) of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein-1α (MIP-1a), and regulated upon activation, normal T cell expressed and secreted (RANTES) were significantly higher in conditioned medium from alveolar macrophages cultured for 24 hours from subjects with HPS-1 pulmonary fibrosis (HPSPF) (n = 18) compared with healthy research volunteers (NV) (n = 6). (B) Concentrations of MIP-1α, MCP-1, RANTES, macrophage colony-stimulating factor (M-CSF), macrophage inflammatory protein (MIP)-4, and IFN-γ (IFNg), but not granulocyte-macrophage colony-stimulating factor (GM-CSF) or IL12p40, in conditioned medium from alveolar macrophages from subjects with HPS-1 pulmonary fibrosis (n = 6) cultured for 24 hours were lower with increasing concentrations of pirfenidone.
To determine the effect of the antiinflammatory drug pirfenidone on alveolar macrophage cytokine and chemokine secretion, alveolar macrophages from subjects with HPS-1 pulmonary fibrosis were cultured with increasing concentrations of pirfenidone for 24 hours. The alveolar macrophages incubated with pirfenidone secreted lower amounts of MIP-1α, MCP-1, RANTES, M-CSF, MIP-4, and IFN-γ in conditioned medium in a dose-dependent manner, whereas concentrations of GM-CSF and IL-12p40 remained essentially unchanged (Figure 5B).
DISCUSSION
Alveolar inflammation may be associated with high concentrations of lung cytokines and chemokines. Indeed, we found this in patients with HPS, who also had higher concentrations of total BAL cells and alveolar macrophages in ELF. We also found that pirfenidone suppresses chemokine secretion by cultured HPS-1 alveolar macrophages in a dose-dependent manner. Finally, HPS-1 alveolar macrophages and PBMCs do not express HPS1 mRNA, whereas normal cells do.
Several attributes of HPS-1 make this a uniquely suitable disease in which to study pulmonary fibrosis. Individuals with HPS-1 comprise a relatively homogeneous cohort, because most are homozygous for the same 16-bp duplication in the HPS1 gene. This mutation is associated with absence of HPS1 protein and, thus, dysregulation of biogenesis of lysosome-related organelles is found in the cells of individuals with HPS-1. In addition, patients with HPS-1 are distinguishable from the general population because of their oculocutaneous albinism, and there is nearly universal development of progressive pulmonary fibrosis in these individuals. To our knowledge, there are no other populations that share such characteristics, except possibly for the few people with HPS-4 (12, 34).
Despite progress in the understanding of genetic, molecular, and cellular aspects of HPS, the etiology of pulmonary fibrosis in HPS-1 remains unknown. Our findings of high concentrations of activated alveolar macrophages, cytokines, and chemokines in individuals with HPS-1 provide evidence that immune cells and proinflammatory proteins contribute to the pathogenesis of pulmonary fibrosis. We demonstrate that concentrations of MCP-1 correlate with severity of pulmonary fibrosis in HPS-1. We also found that concentrations of MIP-1α, GM-CSF, and M-CSF are high in subjects with mild HPS-1 pulmonary fibrosis, and it is possible that these proteins may contribute to the pathogenesis of early, and not advanced, HPS-1 pulmonary fibrosis. Overall, these findings are consistent with studies focusing on lung disease in murine models of HPS, which demonstrate lung inflammation and constitutive activation of alveolar macrophages (26, 28).
Although the role of inflammation in the pathogenesis of pulmonary fibrosis is controversial, several reports indicate that dysregulation of immune cells contributes to the development of lung fibrosis. Specifically, high alveolar concentrations of activated lymphocytes were found in first-degree relatives of probands with familial pulmonary fibrosis with asymptomatic lung disease, suggesting that early stages of fibrotic lung disease are associated with accumulation of abnormal immune cells in the lung (32). In addition, impairment of general transcription, associated with decreased concentrations of transcription factor II-H, has been reported in alveolar macrophages of patients with idiopathic pulmonary fibrosis (33). High concentrations of MCP-1, MIP-1α, and M-CSF were found in BAL fluid from patients with idiopathic pulmonary fibrosis, and concentrations of MCP-1 correlated negatively with pulmonary function measurements (35, 36). Furthermore, mice deficient in MCP-1 or M-CSF had decreased lung fibrosis and inflammatory cell accumulation after receiving bleomycin, and anti–MCP-1 gene therapy attenuated lung disease in a murine model of pulmonary fibrosis (26, 37).
In agreement with these reports, several publications demonstrate a key etiologic role for macrophages in extrapulmonary fibrosis. For example, a decrement of macrophages or absence of macrophage-derived galectin-3 ameliorated renal fibrosis in a murine model of progressive renal fibrosis (38). Also, selective depletion of macrophages in an animal model of hepatic inflammation significantly attenuated liver fibrosis (39). Furthermore, in vivo and in vitro studies of wound healing demonstrated that macrophage-derived PDGF contributed to expression of fibroblast osteopontin, which may be partially responsible for the development of wound fibrosis (40). Taken together, these data confirm the importance of macrophages in the pathogenesis of fibrosis. Our findings in this cohort of individuals with HPS-1 are consistent with these reports, and further substantiate the hypothesis that inflammation and macrophages contribute to the pathogenesis of pulmonary fibrosis.
Although treatment options for HPS-1 are limited, oral pirfenidone has been shown to slow the rate of lung function decline in some individuals with HPS-1 (4). Other studies have demonstrated that pirfenidone reduced the incidence of acute exacerbations and stabilized lung function in patients with idiopathic pulmonary fibrosis (41, 42). In animal models of pulmonary fibrosis, pirfenidone ameliorated lung disease (43). The mechanism of action of pirfenidone is undefined, but it inhibits TGF-β, PDGF, and TNF-α and has antioxidant effects (44–47). Our results indicate that pirfenidone reduced alveolar macrophage cytokine secretion in vitro. It is unlikely that cytotoxic effects of pirfenidone contributed to these findings, because stable concentrations of GM-CSF and IL-12p40 indicate that the alveolar macrophages remained viable at these levels of pirfenidone. Notably, a limitation of this study is that plasma concentrations of pirfenidone achieved with therapeutic doses are lower than concentrations of pirfenidone used for these experiments (4, 48). Although lung epithelial lining fluid concentrations of pirfenidone are unknown, it is likely that the concentrations of pirfenidone used for these in vitro experiments are higher than those achieved locally in the alveolar space. These data suggest, however, that down-regulation of alveolar macrophage activation is a possible mechanism contributing to the clinical response to pirfenidone in HPS-1 pulmonary fibrosis, and this supports results of animal models indicating that pirfenidone has both antiinflammatory and antifibrotic properties (43–47, 49).
Our data indicate that lung inflammation and alveolar macrophage activation in HPS-1 are associated with high lung concentrations of cytokines and chemokines, including MCP-1, MIP-1α, and GM-CSF. Although alveolar fluid concentrations of TGF-β1 were not significantly different in HPS-1, it is unclear whether TGF-β1 activity is altered in this disease. Our findings also indicate that alveolar macrophages may be a potential source of cytokines and chemokines and, thus, may contribute to a self-perpetuating proinflammatory cycle in the HPS-1 lung. Because alveolar macrophages derive from the bone marrow, additional research using murine models of HPS is indicated to further explore the potential utility of bone marrow transplantation for treatment of pulmonary fibrosis in HPS-1 (50, 51).
Our findings provide a basis for future investigations into the causes and treatment of the pulmonary fibrosis of HPS. Can down-regulation of HPS1 expression in normal alveolar macrophages recapitulate the HPS phenotype, that is, increased cytokine secretion? Can transfection of HPS-1 alveolar macrophages with normal HPS1 reverse the abnormal cellular phenotype? Can HPS-1 alveolar macrophages be used to test new drug therapies, using reduced cytokine secretion as an outcome parameter? BAL cells and fluid hold promise in answering these questions.
Supplementary Material
Acknowledgments
The authors thank their patients with Hermansky-Pudlak syndrome and the Hermansky-Pudlak Syndrome Network for their contributions to this research. The authors thank Darryl Leja, NHGRI Intramural Publication Support Office, for assistance in preparing the figures.
Supported by the Intramural Research Program of the National Human Genome Research Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Child Health and Human Development, National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200901-0023OC on September 3, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gahl WA, Brantly M, Kaiser-Kupfer MI, Iwata F, Hazelwood S, Shotelersuk V, Duffy LF, Kuehl EM, Troendle J, Bernardini I. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome). N Engl J Med 1998;338:1258–1264. [DOI] [PubMed] [Google Scholar]
- 2.Brantly M, Avila NA, Shotelersuk V, Lucero C, Huizing M, Gahl WA. Pulmonary function and high-resolution CT findings in patients with an inherited form of pulmonary fibrosis, Hermansky-Pudlak syndrome, due to mutations in HPS-1. Chest 2000;117:129–136. [DOI] [PubMed] [Google Scholar]
- 3.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet 2008;9:359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, Cardona H, Calis KA, Gochuico B. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab 2002;76:234–242. [DOI] [PubMed] [Google Scholar]
- 5.Lederer DJ, Kawut SM, Sonett JR, Vakiani E, Seward SL Jr, White JG, Wilt JS, Marboe CC, Gahl WA, Arcasoy SM. Successful bilateral lung transplantation for pulmonary fibrosis associated with the Hermansky-Pudlak syndrome. J Heart Lung Transplant 2005;24:1697–1699. [DOI] [PubMed] [Google Scholar]
- 6.Thomas de Montpréville V, Mussot S, Dulmet E, Dartevelle P. Pulmonary fibrosis in Hermansky-Pudlak syndrome is not fully usual. Ann Pathol 2006;26:445–449. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor. Mol Cell 1999;3:11–21. [DOI] [PubMed] [Google Scholar]
- 8.Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, Gahl WA. Nonsense mutations in ADTB3A cause complete deficiency of the β3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr Res 2002;51:150–158. [DOI] [PubMed] [Google Scholar]
- 9.Di Pietro SM, Falcón-Pérez JM, Dell'Angelica EC. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic 2004;5:276–283. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 2003;35:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan NV, Pasha S, Johnson CA, Ainsworth JR, Eady RA, Dawood B, McKeown C, Trembath RC, Wilde J, Watson SP, et al. A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8). Am J Hum Genet 2006;78:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson PD, Huizing M, Claassen DA, White J, Gahl WA. Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum Genet 2003;113:10–17. [DOI] [PubMed] [Google Scholar]
- 13.Wildenberg SC, Oetting WS, Almodovar C, Krumwiede M, White JG, King RA. A gene causing Hermansky-Pudlak syndrome in a Puerto Rican population maps to chromosome 10q2. Am J Hum Genet 1995;57:755–765. [PMC free article] [PubMed] [Google Scholar]
- 14.Oh J, Bailin T, Fukai K, Feng GH, Ho L, Mao JI, Frenk E, Tamura N, Spritz RA. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat Genet 1996;14:300–306. [DOI] [PubMed] [Google Scholar]
- 15.Oh J, Ho L, Ala-Mello S, Amato D, Armstrong L, Bellucci S, Carakushansky G, Ellis JP, Fong CT, Green JS, et al. Mutation analysis of patients with Hermansky-Pudlak syndrome: a frameshift hot spot in the HPS gene and apparent locus heterogeneity. Am J Hum Genet 1998;62:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shotelersuk V, Hazelwood S, Larson D, Iwata F, Kaiser-Kupfer MI, Kuehl E, Bernardini I, Gahl WA. Three new mutations in a gene causing Hermansky-Pudlak syndrome: clinical correlations. Mol Genet Metab 1998;64:99–107. [DOI] [PubMed] [Google Scholar]
- 17.Horikawa T, Araki K, Fukai K, Ueda M, Ueda T, Ito S, Ichihashi M. Heterozygous HPS1 mutations in a case of Hermansky-Pudlak syndrome with giant melanosomes. Br J Dermatol 2000;143:635–640. [DOI] [PubMed] [Google Scholar]
- 18.Hermos CR, Huizing M, Kaiser-Kupfer MI, Gahl WA. Hermansky-Pudlak syndrome type 1: gene organization, novel mutations, and clinical-molecular review of non–Puerto Rican cases. Hum Mutat 2002;20:482. [DOI] [PubMed] [Google Scholar]
- 19.Dell'Angelica EC, Aguilar RC, Wolins N, Hazelwood S, Gahl WA, Bonifacino JS. Molecular characterization of the protein encoded by the Hermansky-Pudlak syndrome type 1 gene. J Biol Chem 2000;275:1300–1306. [DOI] [PubMed] [Google Scholar]
- 20.Nazarian R, Falcón-Pérez JM, Dell'Angelica EC. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): a complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. Proc Natl Acad Sci USA 2003;100:8770–8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martina JA, Moriyama K, Bonifacino JS. BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J Biol Chem 2003;278:29376–29384. [DOI] [PubMed] [Google Scholar]
- 22.Shotelersuk V, Gahl WA. Hermansky-Pudlak syndrome: models for intracellular vesicle formation. Mol Genet Metab 1998;65:85–96. [DOI] [PubMed] [Google Scholar]
- 23.Nakatani Y, Nakamura N, Sano J, Inayama Y, Kawano N, Yamanaka S, Miyagi Y, Nagashima Y, Ohbayashi C, Mizushima M, et al. Interstitial pneumonia in Hermansky-Pudlak syndrome: significance of florid foamy swelling/degeneration (giant lamellar body degeneration) of type-2 pneumocytes. Virchows Arch 2000;437:304–313. [DOI] [PubMed] [Google Scholar]
- 24.Feng GH, Bailin T, Oh J, Spritz RA. Mouse pale ear (ep) is homologous to human Hermansky-Pudlak syndrome and contains a rare 'AT-AC' intron. Hum Mol Genet 1997;6:793–797. [DOI] [PubMed] [Google Scholar]
- 25.Feng L, Novak EK, Hartnell LM, Bonifacino JS, Collinson LM, Swank RT. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS2 genes independently contribute to the production and function of platelet dense granules, melanosomes, and lysosomes. Blood 2002;99:1651–1658. [PubMed] [Google Scholar]
- 26.Tang X, Yamanaka S, Miyagi Y, Nagashima Y, Nakatani Y. Lung pathology of pale ear mouse (model of Hermansky-Pudlak syndrome 1) and beige mouse (model of Chediak-Higashi syndrome): severity of giant lamellar body degeneration of type II pneumocytes correlates with interstitial inflammation. Pathol Int 2005;55:137–143. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka Y, Kumasaka T, Ishidoh K, Kominami E, Mitani K, Hosokawa Y, Fukuchi Y. Inflammatory response and cathepsins in silica-exposed Hermansky-Pudlak syndrome model pale ear mice. Pathol Int 2004;54:322–331. [DOI] [PubMed] [Google Scholar]
- 28.Young LR, Borchers MT, Allen HL, Gibbons RS, McCormack FX. Lung-restricted macrophage activation in the pearl mouse model of Hermansky-Pudlak syndrome. J Immunol 2006;176:4361–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young LR, Pasula R, Gulleman PM, Deutsch GH, McCormack FX. Susceptibility of Hermansky-Pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. Am J Respir Cell Mol Biol 2007;37:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 31.Ren P, Rosas IO, MacDonald SD, Wu HP, Billings EM, Gochuico BR. Impairment of alveolar macrophage transcription in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;175:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP Jr, May RM, Wu HP, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald SD, Travis WD, Stylianou MP, Rosas IO. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008;168:159–166. [DOI] [PubMed] [Google Scholar]
- 34.Bachli EB, Brack T, Eppler E, Stallmach T, Trüeb RM, Huizing M, Gahl WA. Hermansky-Pudlak syndrome type 4 in a patient from Sri Lanka with pulmonary fibrosis. Am J Med Genet 2004;127:201–207. [DOI] [PubMed] [Google Scholar]
- 35.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM Jr, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capelli A, Di Stefano A, Gnemmi I, Donner CF. CCR5 expression and CC chemokine levels in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:701–707. [DOI] [PubMed] [Google Scholar]
- 37.Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L1038–L1044. [DOI] [PubMed] [Google Scholar]
- 38.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 2008;172:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 2008;205:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–1047. [DOI] [PubMed] [Google Scholar]
- 42.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am J Respir Crit Care Med 1999;159:1061–1069. [DOI] [PubMed] [Google Scholar]
- 43.Iyer SN, Margolin SB, Hyde DM, Giri SN. Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin–hamster model. Exp Lung Res 1998;24:119–132. [DOI] [PubMed] [Google Scholar]
- 44.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-β gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999;291:367–373. [PubMed] [Google Scholar]
- 45.Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol 1999;276:L311–L318. [DOI] [PubMed] [Google Scholar]
- 46.Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 2002;446:167–176. [DOI] [PubMed] [Google Scholar]
- 47.Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem 2000;204:119–126. [DOI] [PubMed] [Google Scholar]
- 48.Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 2002;41:1118–1123. [DOI] [PubMed] [Google Scholar]
- 49.Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin–hamster model of lung inflammation. Inflammation 2000;24:477–491. [DOI] [PubMed] [Google Scholar]
- 50.Virolainen M. Hematopoietic origin of macrophages as studied by chromosome markers in mice. J Exp Med 1968;127:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.